Abstract
The predominant leukocyte population present in both human and murine peritoneal ovarian tumors is the Vascular Leukocyte (VLC). VLCs are recruited en masse to the ovarian tumor microenvironment whereupon they promote tumor progression. Importantly, the presence of VLCs is requisite for peritoneal ovarian cancer progression: selective elimination of VLCs inhibits tumor burden and ascites accumulation. Despite the critical importance of VLCs to ovarian tumors, their derivation and the mechanisms by which they facilitate tumor progression are not well understood. Here we demonstrate in vivo that the murine ID8 ovarian tumor model can usurp the host peritoneal macrophage pathway to elicit and recruit VLCs. Moreover, we demonstrate that VLCs express CD11b and Gr-1, a characteristic phenotype shared amongst heterogeneous populations of leukocytes referred to as myeloid-derived suppressor cells (MDSCs). In accord with their MDSC phenotype, both murine and human VLCs express arginase 1 (ARG1). Importantly, we demonstrate that the VLCs suppress both CD8+ and CD4+ T cells responses and that this immunosuppression is ARG1 –dependent, since blockade of VLC ARG1 activity with nor-NOHA reversed the immunosuppression. These data further characterize the tumor associated leukocytes in ovarian cancer and provide insights into the mechanisms by which they promote tumor growth.
Keywords: Ovarian Cancer, Arginase 1, VLC, Myeloid Derived Suppressor Cell
1. Introduction
Tumors require a fostering and permissive environment to support their growth and subsequent metastasis. To establish these favorable conditions, tumors commonly coordinate angiogenesis, the cytokine milieu, and immunosuppression within their microenvironment. Regarding the latter factor, emerging evidence supports that tumors can suppress host anti-tumor immune responses through a variety of mechanisms and can co-opt resident and recruited cells of the host’s immune system to play a critical and supportive role in tumor development (de Visser et al., 2006; Rabinovich et al., 2007). Indeed, an increased prevalence of both regulatory T cells and tumor associated macrophages (TAMs) is correlated with a poor prognosis (Bingle et al., 2002; Curiel et al., 2004). One such pivotal type of cell present within both human and murine ovarian cancers is the Vascular Leukocyte (VLC) (Conejo-Garcia et al., 2004; Conejo-Garcia et al., 2005).
VLCs are the most abundant infiltrating leukocyte present in the ascites of human and murine ovarian carcinomas, commonly exceeding 107 cells within the peritoneal ascites of a single mouse and comprising >75% of the CD45+ tumor-infiltrating leukocytes (Conejo-Garcia et al., 2004). VLCs express canonical markers of leukocytes (CD45, CD11c) and low levels of the endothelial markers VE-Cadherin and P1H12 (Conejo-Garcia et al., 2004; Conejo-Garcia et al., 2005). We recently demonstrated the importance of VLCs to ovarian tumor progression with the use of the murine ID8 model of ovarian cancer (Roby et al., 2000). ID8 cells are a transplantable, tumorigenic, murine ovarian carcinoma disease model that recapitulates the progression of the human disease, including slow progression, the development of late-stage ascites and, importantly, VLC recruitment and accumulation (Roby et al., 2000). Selective elimination of VLCs reduced ID8 peritoneal ovarian tumor burden and ascites volume, revealing a crucial dependence of the tumor upon VLCs (Bak et al., 2007). Despite the importance of VLCs to ovarian tumor progression, the cellular derivation of the VLC and the mechanisms by which VLCs enable peritoneal ovarian tumor growth are poorly understood.
Murine VLCs have previously been proposed to be a form of dendritic cell due to expression of cell surface markers (CD11c) and their ability to present antigen in vitro (Conejo-Garcia et al., 2004; Coukos et al., 2005). However, the vast numbers of VLCs within ovarian ascites, along with their anatomical peritoneal localization, led us to critically test which leukocyte populations can give rise to VLCs. Here we show that VLCs within the peritoneum of tumor bearing mice also express macrophage specific markers. We then provide in vivo genetic confirmation that VLCs are derived from the macrophage pathway with the use of a transgenic strain of mice that expresses GFP under the macrophage-specific c-fms (CD115) promoter: the presence of peritoneal ID8 tumors within these mice converts the GFP+CD11c− peritoneal macrophages to a GFP+CD11c+ VLC population. Interestingly, previous reports contrast in respect to CD11b expression on VLCs (Conejo-Garcia et al., 2005; McLean and Buckanovich, 2008). Here we show that both murine and human VLCs express CD11b, which led us to the observation that VLCs exhibit the CD11b+CD115+Gr01+ phenotype that is characteristic of myeloid-derived suppressor cells (MDSCs). MDSCs are reported to be derived from several heterogeneous leukocyte populations, but functional commonalities are that they are immunosuppressive and they are known to accumulate within both murine and human tumors (Almand et al., 2001; Bronte et al., 2001; Kusmartsev and Gabrilovich, 2002; Serafini et al., 2006a; Talmadge, 2007). Previous reports indicated that VLCs within solid tumor models support tumor progression through tumor neovascularization (Conejo-Garcia et al., 2004). Thus, given the lack of neovascularization necessary for a peritoneal ascitic tumor and based on the phenotype of the VLCs, we tested the hypothesis that VLCs recruited by peritoneal ID8 tumors represent a functionally immunosuppressive cell population. Here we show that both human and murine ovarian tumor-derived VLCs express arginase 1 (ARG1) and the VLCs functionally suppress both CD8+ and CD4+ T cell responses through ARG1 activity. These data identify VLCs as a leukocyte population with immunosuppressive function that are elicited and recruited by the ID8 ovarian tumor and specifically identify a role for arginase activity within the ovarian tumor microenvironment. These findings, and their implications, are discussed in relation to the roles VLCs play in ovarian tumor progression.
2. Materials and Methods
2.1 Mice
Female C57Bl/6 and CB6/F1 mice (4–6 weeks) were purchased from the National Cancer Institute (Fredricksburg, MD). MAFIA mice (Burnett et al., 2004) were purchased from Jackson Laboratories (Bar Harbor, ME) under agreement with Ariad Pharmaceuticals (Cambridge, MA). Animal Experiments were approved by the Dartmouth Medical School Institutional Animal Care and Use Committee.
2.2 Cells and Antibodies
ID8 cells transduced with Vegf-A and Defb29 (referred to as ID8 within this manuscript) were a generous gift of Dr. Jose Conejo-Garcia (Dartmouth Medical School), and were generated and maintained as previously described (Conejo-Garcia et al., 2004). Parental ID8 cells, human ovarian cancer samples, and human blood monocytes were the generous gift of Dr. Charles Sentman (Dartmouth). Anti-mouse Fc Block was purchased from BD Biosciences (San Jose, CA); anti-mouse CD45 (30-F11), anti-mouse F4/80 (BM8), anti-mouse CD3 (145-2C11), anti-mouse Gr-1 (RB6-8C5), anti-mouse CD11b (M1/70), anti-human CD3 (OKT3), anti-human CD11c (3.9), anti-human CD11b (ICRF44), and anti-human CD14 (61D3) antibodies from eBiosciences (San Diego, CA); anti-mouse CD11c (N481) antibodies from Biolegend (San Diego, CA, USA); anti-mouse calreticulin antibodies from Abcam (Cambridge, MA); anti-mouse VE-cadherin antibodies from Bender Medsystems (Burlingame, CA); anti-mouse CD8 (CT-CD8a) antibodies from Invitrogen (Carlsbad, CA); anti-mouse iNOS (N-9657) from Sigma (St. Louis, MO); and anti-human arginase 1 (H-52) from Santa Cruz Biotechnology (Santa Cruz, CA).
2.3 Generation of tumors and harvest of tumor associated leukocytes
Ovarian tumors were generated and harvested as previously described (Bak et al., 2007). Briefly, mice were injected i.p. with 5×106 ID8 cells. Approximately 5 weeks later peritoneal ascites were harvested. The cellular fraction was treated with ACK lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA) to remove red blood cells, and the remaining cells were resuspended in 0.5% BSA in PBS or media for analysis. In indicated experiments single cell suspensions of spleens were generated from naïve or tumor challenged mice by digestion with collagenase/DNAse and passage through a 70 M cell strainer (BD Biosciences, San Jose, CA).
2.4 Flow cytometry
Cells from murine ascites were resuspended at 1×106 cells/mL in 0.5% BSA in PBS with Fc-blocking antibody and subsequently stained with the indicated antibodies. Flow cytometry and cell sorting was performed at the NCCC Englert Cell Analysis Laboratory using a FACS Calibur or FACS Aria and analyzed with CellQuest.
2.5 iNOS and Arginase Expression
Bone marrow derived DCs (BMDCs) were generated as previously described (Amiel et al., 2007; Inaba et al., 1993). As a positive control for iNOS expression, BMDCs were incubated overnight in the presence of 1µg/ml LPS (Sigma, St. Louis, MO). ID8 ascites were processed as above and VLCs were isolated via positive selection using Miltenyi CD11c magnetic beads (Miltenyi Biotec, Auburn, CA) according to manufacturer’s protocol. Cell samples were suspended in sample buffer with β-mercaptoethanol, heated for 5min at 90°C, then run on a 12% SDS-PAGE gel. After transfer to a PVDF membrane and blocking, membranes were incubated overnight with primary anti-mouse iNOS antibody, washed, blocked, and incubated with HRP-conjugated secondary antibody. Bands were detected with ECL Plus Western Blotting Detection Reagent (Amersham Biosciences, Buckinghamshire, UK). Western analysis was conducted on CD11b-selected (Miltenyi beads) human ovarian carcinoma and blood monocyte samples, as above, using anti-human ARG1.
2.6 Reactive Oxygen Species Assay
C57Bl/6 mice were injected i.p. with 107 ID8 tumor cells 4–5 weeks prior to sacrifice to promote the recruitment of VLC's. ID8 ascites were collected from the peritoneum and treated with ACK lysis buffer for 10 min. at room temperature to remove RBC's. Remaining cells were centrifuged, washed, and resuspended in RPMI at a concentration of 106 cells /ml. Samples were then treated with 8µM of the ROS detection agent carboxy-H2DFDA (Molecular Probes, Eugene, OR) for 1h at 37C. Where indicated, experimental groups were pretreated with varying concentrations of the ARG1 inhibitor hydroxy-nor-l-arginine (nor-NOHA) (Calbiochem, San Diego, CA) for 1 hr at 37C. Cells were then labeled with anti-CD11c antibodies on ice for 30 min prior to FACS analysis.
2.7 T cell proliferation and activation assays
A variation on the Lyons-Parish Assay was used to assess T cell proliferation (Lyons and Parish, 1994). Splenocytes were derived from C57Bl/6 mice as described above and resuspended in 2.5µM CFSE in HBSS. Cells were incubated for 10min at 37°C and subsequently washed with HBSS. ID8 ascites were processed as above and VLCs were isolated via positive selection with the use of CD11c magnetic beads, as above, or FACS sorted for VE-Cadherin+CD11c+CD45+ cells. 106 splenocytes in the presence or absence of the indicated number of isolated VLCs were resuspended in medium and, as indicated, stimulated with 1µg of plate bound αCD3 antibody or a combination of 50nM PMA and 0.5µM ionomycin (Sigma, St. Louis, MO). After 72 hr the cells were spun down and supernatants removed for ELISA. Cells were then stained with anti-CD8 or CD4 antibody and CFSE dilution of the CD8+ cells assessed by FACS analysis. Supernatants from Lyons Parish assays were assayed for IFN-γ content using the murine DuoSet ELISA (R&D Systems Minneapolis, MN) according to manufacturer’s protocol. In indicated experiments CD11c+ cells were harvested as above and incubated in 1mM nor-NOHA for 1h at 37°C. Cells were washed 3x with HBSS and 1×105 cells were added to assays as described above.
3. Results
3.1 Phenotypic Analyses of Peritoneal Ovarian Tumor VLCs
VLCs constitute >75% of infiltrating leukocytes (CD45+ cells) within the peritoneal ascites of both human and murine ovarian cancer (Conejo-Garcia et al., 2004; Conejo-Garcia et al., 2005). VLCs were defined by their myeloid surface markers (CD11c) and endothelial markers (VE-Cadherin) (Fig. 1A and (Bak et al., 2007; Conejo-Garcia et al., 2004). Therefore VLCs were proposed to be a form of dendritic cell that promotes the formation of neo-vasculature in solid tumor models (Conejo-Garcia et al., 2004; Conejo-Garcia et al., 2005; Coukos et al., 2005; McLean and Buckanovich, 2008). However, within peritoneal ovarian tumor ascites these cells express the macrophage marker CD11b (Fig. 1B), previously undetected on VLCs in solid tumor samples (Conejo-Garcia et al., 2004; Conejo-Garcia et al., 2005).In tumor-free mice, macrophages (F4/80+CD11c−) are the majority of resident immune cells in the peritoneum (Fig. 1C, lower right quadrant). Moreover, the vast number of VLCs that are recruited to the peritoneum during ovarian cancer progression, routinely >107 per mouse, was reminiscent of elicited macrophages recruited by peritoneal inflammation (Bak et al., 2007; Conejo-Garcia et al., 2004; Leijh et al., 1984). Therefore, using the ID8 model of murine ovarian cancer (Roby et al., 2000), we investigated the relationship between peritoneal macrophages and VLCs during ovarian tumorogenesis. Analysis of the peritoneal CD11c+ VLCs revealed that they also robustly and uniformly express the macrophage cell-surface marker F4/80 (Fig. 1D, upper right quadrant). Just as striking is the observation that within ID8 ovarian cancer ascites there is a disappearance of the F4/80+CD11c− macrophage population (as seen in Fig. 1C, lower right quadrant) and an appearance of an F4/80+CD11c+ cellular population (compare Fig. 1C with 1D). Interestingly, while the F4/80+CD11c− cells are no longer present in the ascites, there is no apparent change in the F4/80−CD11c+ population. Thus, we hypothesized that VLCs were cells derived from the peritoneal macrophage pathway that now expressed CD11c, which results in the F4/80+CD11c+ population. To test this hypothesis in vivo we employed a genetic tool, the MAFIA mouse. The MAFIA mouse has the green fluorescent protein (GFP) transgene expressed by the Colony Stimulating Factor-1 Receptor (CSF-1R or c-fms or CD115) promoter which results in GFP transgene expression in all macrophages (Burnett et al., 2006; Burnett et al., 2004). To confirm the cellular phenotype in this genetic model, we first analyzed the GFP expression on cells derived from a peritoneal lavage of a naïve mouse. GFP+ cells derived from a peritoneal lavage express F4/80 (Fig. 1E) but not CD11c (Fig. 1F). Peritoneal ID8 tumors were then grown in MAFIA mice and the ascites analyzed. As expected there was no change in the GFP profile of F4/80+ cells from the ascites of tumor-bearing mice (Fig. 1G). However, the GFP+ cells present in the ascites were now also CD11c+ (Fig. 1H, as compared to Fig. 1F). F4/80, CD115 and CD11b expression on VLCs and the observation that VLCs replace the canonical peritoneal macrophage population in the ID8 tumor indicates that VLCs are derived from the peritoneal macrophage pathway and are likely amongst the same cells, as defined by CD11b expression, described by others as ID8-recruited TAM (Hagemann et al., 2008).
Figure 1. Vascular Leukocytes are derived from the macrophage pathway.

(A) The predominant leukocyte population within ID8 ascites is CD11c+VE-Cadherin+ VLCs (A, gated) and this population also expresses CD11b (B, black filled line; grey line isotype control). (C) Naïve, non- tumor bearing, mice have peritoneal macrophages that display a F4/80+CD11c− phenotype, whereas in ID8 tumor-bearing mice (D) there is a disappearance of F4/80+CD11c− cells in the peritoneal ascites and the appearance of a F4/80+CD11c+ cellular phenotype. (E–H) MAFIA GFP+ macrophages are converted in vivo to a CD11c+ phenotype by ID8 ovarian tumor growth. Peritoneal lavages from MAFIA mice that express GFP under the macrophage-specific c-fms (CD115) promoter were stained for CD11c and F4/80. Peritoneal macrophages from naïve MAFIA mice robustly express F4/80 and GFP (E) and do not express CD11c (F). The F4/80+ cellular population derived from the peritoneal ascites of ID8-bearing mice remained unchanged in GFP expression (G). However, these GFP+ cells now also express CD11c (H).
3.2 Human Ovarian Cancer VLCs are also a CD11c+CD11b+ Population
To determine if these new murine cell surface expression markers of VLCs were consistent with human ovarian cancer samples, and to reconcile previous discrepancies in CD11b expression on human VLCs (Conejo-Garcia et al., 2005; McLean and Buckanovich, 2008), we analyzed single-cell preparations of both solid and ascites human ovarian cancer samples. In agreement with our murine data, CD11c+ VLCs in both the solid (Fig. 2A) and ascites (Fig. 2C) portion of the ovarian tumors express CD11b+. As positive and negative controls for this analysis we demonstrate that these cells, respectively, consistently express CD14 (Fig. 2E and F) but are distinct from the CD3+ lymphocyte population (Fig. 2B and D). These data extend our murine findings to human ovarian tumor infiltrating leukocytes and likewise support that, based on cell surface markers, human ovarian tumor VLCs likely represent cells referred to as TAM by other groups (Kryczek et al., 2006).
Figure 2. Human ovarian tumor VLCs are CD11c+CD11b+ leukocytes.
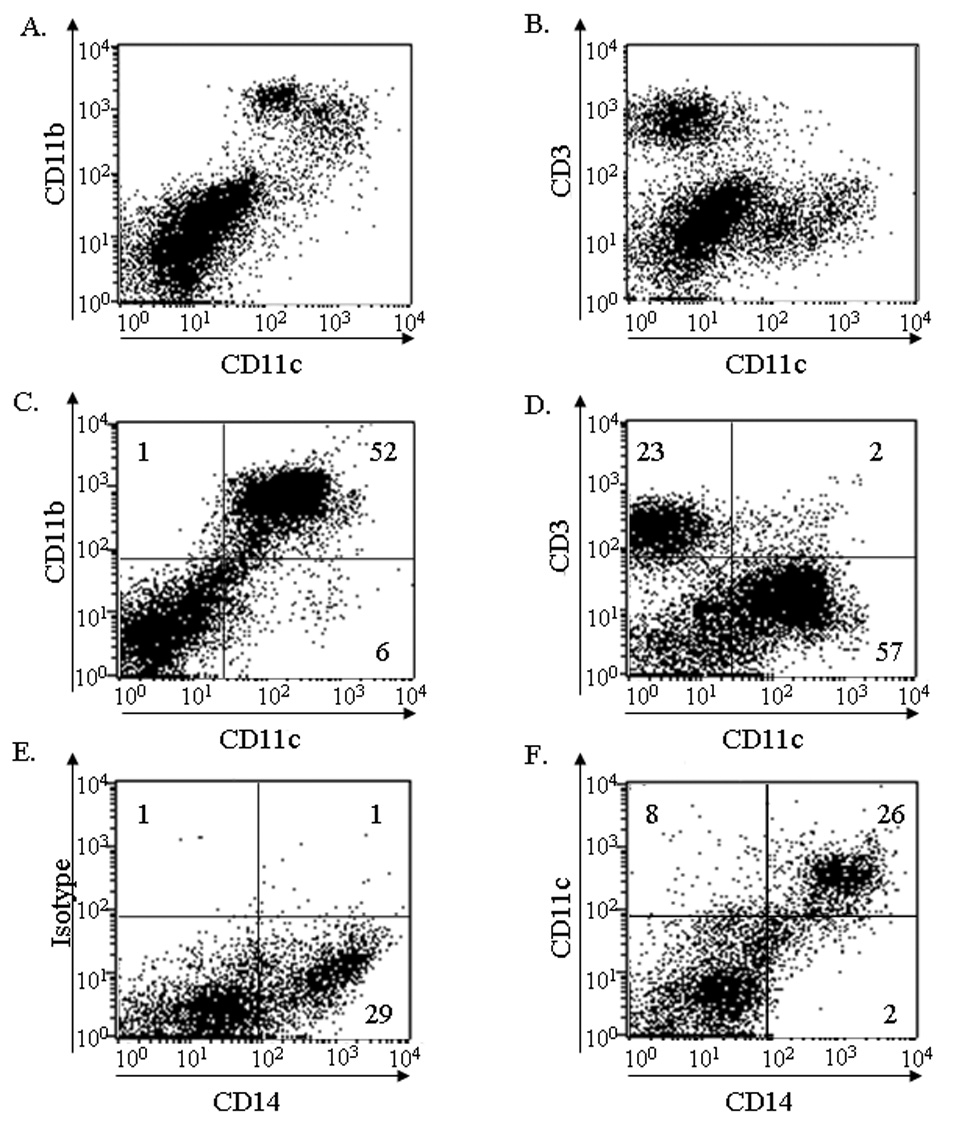
Cells from solid (A, B) and ascites (C, D) origin were stained for (A, C) CD11c and CD11b or, as a negative control, CD3 (B, D). (E and F) CD14 expression was used as a positive control for identification of CD11c+ cells.
3.3 VLCs are a CD11b+Gr-1+ Leukocyte Population within ID8 Ascites
Since VLCs are CD11b+CD115+ (Fig. 1), we asked if ovarian tumor-associated VLCs might constitute a population of MDSCs. MDSCs are a heterogeneous leukocyte population(s) that can facilitate immunosuppression within the tumor microenvironment, and thereby promote tumor growth and metastasis (Bronte et al., 2001; Bunt et al., 2006; Serafini et al., 2004). Murine MDSCs are commonly characterized by the expression of CD11b and Gr-1 as cell surface markers (Bronte et al., 2000; Gallina et al., 2006; Yang et al., 2006). FACS analysis revealed that, indeed, VLCs present within peritoneal ID8 ovarian tumor ascites express Gr-1 (Fig. 3A) and, consistent with the phenotypic representation of canonical MDSCs, this population is CD11b+Gr-1+ (Fig. 3B). Additionally, as previously shown in Fig. 1, ID8-derived VLCs are also CD115+ which is consistent with MDSCs found in other tumor models (Huang et al., 2006; Pan et al., 2008). To examine whether the peritoneal ID8 tumor induced systemic production of MDSCs we analyzed the spleens of naïve mice and ID8 tumor-bearing mice. Only a modest increase in the percentage of splenic CD11b+Gr-1+ cells was observed in ID8 tumor-bearing mice (Fig. 3C and D, respectively, and E). However, the total numbers of splenic CD11b+Gr-1+ cells within the tumor-bearing mice was greatly increased, likely due to splenomegaly (Fig. 3E). Thus, these data suggest that the ovarian tumor likely co-opts the macrophage pathway to produce a local VLC population and that these VLCs represent a variation of MDSCs.
Figure 3. VLCS exhibit cell surface markers of MDSCs.

Cells were harvested from peritoneal ID8 ascites (A and B) and from the spleens of tumor-bearing (C) or naïve (D) C57Bl/6 mice. The (A) F4/80+ and (B) CD11b+ VLC population (as in Fig. 1) in peritoneal ID8 ascites expresses Gr-1 (~20% of total cells). The spleens from ID8 tumor-bearing (C) and naive mice (D) contain similar percentages of CD11b+Gr-1+ cells, however the tumor-bearing mice contain a greater total number of splenic CD11b+Gr-1+ cells (E).
3.4 VLCs Suppress T Cell Division
The mechanism(s) by which VLCs support tumor growth is not well understood. Previous reports indicate that VLCs can function to promote tumor neo-vasculature in solid tumors, however this process is less critical to ascites tumors and therefore, with the emerging notion that VLCs share surface and functional markers with MDSCs, we hypothesized that VLCs may be immunosuppressive and thereby inhibit T cell -mediated immune responses (Gallina et al., 2006; Nagaraj et al., 2007; Rodriguez et al., 2003). To assess whether VLCs are functionally immunosuppressive we tested whether they could inhibit the activation of CD4+ and CD8+ T cells. CD11c+ VLCs from an ID8 peritoneal tumor were isolated and added to CFSE-labeled naïve splenocytes stimulated with αCD3 antibody or PMA and ionomycin. CD8+ T cells prolifically divided in response to both CD3 and PMA stimulation as assessed by CFSE dilution (Fig. 4A and B). These results were quantified and expressed as the percentage of cells having undergone at least one cell division compared to the parental CFSE peak. As seen in Figure 4C and D, VLCs inhibit the ability of CD4+ and CD8+ T cells to divide in response to two independent mitogens. As a control, CD11c+ cells from naïve spleens were isolated and included in the splenocyte stimulation reaction. CD11c+ cells from non-tumor (naïve) tissue had no effect on the ability of both T cell subsets to respond to mitogens (Fig. 4C and D). As an independent analysis that VLCs suppress T cell activity we assessed whether VLCs can inhibit the production of IFNγ following cellular stimulation. αCD3 antibody or PMA/ionomycin robustly stimulated the production of IFNγ by splenocytes, as assessed by ELISA. However, the presence of VLCs blocked production of IFNγ by >95% (Fig. 5A). Consistent with the data in Figure 4, the presence of CD11c+ splenocytes from a naïve mouse did not affect the ability of splenocytes to produce IFNγ. To confirm the immunosuppressive activity of CD11c-isolated cells more stringently we FACS-sorted VLCs (VE-Cadherin+CD11c+) from murine ID8 ascites and assayed for their ability to suppress IFNγ production by splenocytes. FACS-sorted VLCs likewise suppressed IFNγ production (Fig. 5B) and T cell division (data not shown). These data support the novel function of VLCs as an immunosuppressive MDSC-like cell population within the tumor microenvironment.
Figure 4. VLCs suppress CD8+ and CD4+ T cell responses.

(A) 5×105 C57Bl/6 CFSE-labeled splenocytes were incubated for 72h in the absence (Negative) or presence of either αCD3 antibody (αCD3) or PMA and ionomycin (PMA). After incubation the cells were stained with αCD8 antibody, and CFSE dilution, indicative of T cell division, was assessed by FACS. Data gated on CD8+ cells. (B) As in A, shown as histogram of stimulated (black line) and unstimulated (grey line) CD8+ cells. (C) Cells were treated as described for A and B and the percentages of CD8+ T cells having undergone cell division, as assessed by CFSE fluorescence, were analyzed. Where indicated, 1×105 CD11c+ VLCs from tumor bearing mice or 1×105 CD11c+ splenocytes from naïve mice were added. (D) Cells were prepared as in C, and CD4+ division was assessed by FACS. The standard deviation of three independent experiments is shown (C, D). The statistical significance (*p<0.05, **p<0.01, ***p<0.005) of stimulated cells with CD11c+ VLCs in comparison to stimulated controls with media alone was determined with the Student's t test.
Figure 5. VLCs inhibit splenocyte IFNγ production.

(A) Supernatants from splenocytes stimulated, as indicated, with media alone (neg),αCD3 antibody (αCD3), or PMA/ionomycin (PMA), in the presence (Tumor CD11c+) or absence (Media Alone) of CD11c+ VLCs, or CD11c+ splenocytes from naïve mice (Naive CD11c+), were quantitatively analyzed for the presence of IFNγ by ELISA. The results are derived from three independent experiments with the standard deviation shown. (B) FACS-sorted CD11c+VE-Cadherin+ cells were added to stimulated or unstimulated splenocytes, as above. Supernatants were then harvested and IFNγ assayed with ELISA. The results are derived from two independent experiments with the standard deviation shown. Statistical significance (*p<0.05) was determined with the paired Student’s t test (Stimulated cells with VLCs were compared against stimulated controls with media alone).
3.5 Confirmation of VLC Immunosuppressive Activity with the Parental ID8 Cell Line
VLCs were originally described from solid tumors and ascites generated from ID8 cells transduced with VEGF-A and β-Defensin-29 (Defb29) (Conejo-Garcia et al., 2004). Recently, VEGF-A has been shown to modulate CD11b+Gr-1+ cell infiltration and function in tumorogenesis (Shojaei et al., 2007). To confirm that previous experiments conducted with ID8-Vegf-Defb29 are consistent with those derived from the parental (untransduced) ID8 cell line, we performed phenotypic and functional analyses on ascites-infiltrating leukocytes from mice challenged with the parental ID8 cell line (Roby et al., 2000). Parental ID8 cells likewise generate leukocytes that express the CD11c and VE-Cadherin cell surface markers (Fig. 6A) and, consistent with Fig.1, this double positive population expresses CD11b (Fig 6B). Indeed, the majority of CD11c+ cells within the peritoneum express the macrophage marker CD11b (Fig. 6C). To confirm that these cells were also functionally similar to those generated from with ID8-Vegf-Defb29 tumors we harvested CD11c+ cells via magnetic bead separation and assessed their ability to suppress T cell activation. As previously described (Fig. 5), these CD11c+ cells likewise suppress IFNγ production (Fig. 6D) and T cell division (data not shown).
Figure 6. VLCs derived from ascites created by the parental ID8 cell line replicate the phenotype and immunosuppressive function of those from the ID8 cell line that expresses VEGF-A and Defb29.

(A) CD11c+VE-Cadherin+ VLCs are present in the peritoneal ascites from the parental ID8 cell line (not transduced for VEGF-A or Defb29) and (B) expresses CD11b (black filled line; grey line isotype control). (C) Ascites cells were stained for CD11c and CD11b to confirm the concomitant expression of both cell surface markers. (D) CD11c+ cells from the ascites were tested for their ability to suppress IFNγ secretion as in Fig. 5A. The results are derived from two independent experiments with the standard deviation shown. Statistical significance (**p<0.01) was determined with the paired Student’s t test (Stimulated cells with VLCs were compared against stimulated controls with media alone).
3.6 VLCs Express the MDSC-Associated Enzyme Arginase 1
To determine the mechanism by which VLCs inhibit T cell function, we assessed the expression of ARG1 by murine and human ovarian tumor VLCs. MDSCs commonly express ARG1 and this expression is inversely regulated with inducible Nitrous Oxide Synthase (iNOS) (Bunt et al., 2006; Kusmartsev and Gabrilovich, 2003; Rodriguez et al., 2004). ARG1 -mediated decreases in systemic L-arginine availability correlate with impaired T cell function and therefore expression of ARG1 by MDSCs is thought to be central to their ability to suppress T cell responses (Bronte et al., 2003; El-Gayar et al., 2003; McLean and Buckanovich, 2008; Rodriguez et al., 2002; Schaffer and Barbul, 1998).To test the ability of VLCs to express functional ARG1, we isolated CD11c+ cells from the ascites of ID8-bearing mice. Isolated VLCs did not express measurable iNOS by Western analysis (Fig. 7A, lanes 2–4); LPS-activated bone marrow –derived DCs were used as a positive control for iNOS expression (lane 1). To test for the production of reactive oxygen species (ROS) by VLCs we utilized the compound DCFDA. DCFDA fluoresces when oxidized by ROS, including the ROS produced by ARG1 activity (Bronte et al., 2003; Kusmartsev and Gabrilovich, 2003). CD11c+ VLCs induce DCFDA fluorescence indicative of the production of ROS (Fig. 7B). To directly test the contribution of ARG1 to the production of the ROS we utilized a specific inhibitor of ARG1 activity, nor-NOHA (Boucher et al., 1994; Sinha et al., 2005). The inclusion of nor-NOHA in the assay resulted in a dose-dependent reduction in the DCFDA fluorescence, with the presence of 4µM nor-NOHA resulting in >50% inhibition of the total ROS produced by the VLCs (Fig. 7C). To confirm these results in human ovarian cancer, we assayed human ovarian VLCs for the expression of ARG1 by western analysis. Human ovarian cancer VLCs robustly expressed ARG1, whereas naïve blood monocytes did not express measurable ARG1 protein (Fig. 7D). This data indicates that VLCs produce ARG1 that results in the production of ROS, and further supports the characterization and function of VLCs as MDSCs.
Figure 7. VLCs express ARG1 but not iNOS.

(A) CD11c-isolated VLCs from ID8 ascites were analyzed for iNOS expression by Western blot. iNOS expression was observed in LPS-stimulated BMDCs (positive control; lane 1) but not in 1×105, 5×104, and 1×104 VLCs (lanes 2–4, respectively). Calreticulin was used as a loading control (bottom panel). (B–C) Analysis of ROS production by VLCs. (B) CD11c+ VLCs express ROS, as indicated by the fluorescence in cells incubated with the ROS-indicator DCFDA (open black line) in comparison to cells analyzed in the absence of DCFDA (shaded line). (C) To assess the specific contribution of ARG1 to VLC ROS production, VLCs were incubated with DCFDA in the presence of the indicated concentration of the ARG1 inhibitor nor-NOHA. Cells were incubated for 1 hr at 37°C, and DCFDA fluorescence was analyzed by FACS. The standard deviation from three independent experiments is shown. (D) Human ovarian cancer CD11b+ cells (lane 1) or naïve blood monocytes (lane 2) were analyzed by western blot for arginase 1 expression (bottom panel); gp96 was used as loading control (top panel).
3.7 Blockade of Arginase 1 Alleviates VLC-Mediated Immunosuppression
Since VLCs were found to both inhibit T cell activity and to express immunosuppressive ARG1, we directly tested the contribution of ARG1 activity to the observed T cell suppression. Murine CD11c+ VLCs were isolated from ascites and incubated in the presence or absence of 1mM nor-NOHA for 1h at 37°C. T cell suppression assays, as in Fig. 6 and Fig. 7, were performed by stimulating cells with αCD3 or PMA/ionomycin in the presence of VLCs, or VLCs preincubated with 1mM nor-NOHA for 1h. While VLCs suppressed CD3-stimulated CD4 and CD8 T cell division by 66% and 70%, respectively (consistent with Fig. 4–Fig 6), nor-NOHA -treated VLCs failed to suppress the division of CD4+ and CD8+ T cells (Fig. 8A and B), restoring the CD4 and CD8 division to the levels (not statistically different) of control stimulation. To confirm these findings, we analyzed the supernatants from these suppression assays for IFNγ̣ (Fig. 8C). The production of IFNγ by splenocytes activated with αCD3 or PMA/ionomycin was reduced to 2% and 5% of the positive control when VLCs were present in the cultures. However, incubation of VLCs with nor-NOHA led to a resumption of IFNγ production that was similar (no statistical difference) to control treatments. These data identify that VLCs immunosuppression is mediated through ARG1 activity and that inhibitors of ARG1 can be used to alleviate the VLC-mediated immunosuppression.
Figure 8. Arginase 1 activity is required for VLC –mediated T cell suppression.

(A and B) Splenocytes were harvested and labeled as in Fig. 7. As indicated, cells were unstimulated or were stimulated with either PMA or plate bound αCD3; and this was done in presence of media alone, in the presence of 105 CD11c+ cells from ID8 tumor ascites, or in the presence of 105 CD11c+ cells from tumor ascites pre-incubated for 1h with 1mM nor-NOHA. Cells were incubated for 72h, and CD8+ and CD4+ T cell division was subsequently assessed by FACS. (C) Supernatants were harvested from the above assays and IFNγ concentration determined by ELISA. In all cases, data represent results from ≥3 independent experiments with the standard deviation shown. Statistical significance (*p<0.05, **p<0.01,***p<0.005, NS- No significance) was determined with the paired Student’s t test (Stimulated cells with CD11c+ VLCs or with nor-NOHA treated CD11c+ cells were each compared against stimulated controls with media alone).
4. Discussion
VLCs constitute the predominant population of ovarian tumor -associated leukocytes (Conejo-Garcia et al., 2004; Conejo-Garcia et al., 2005). Importantly, this cell type is critical to tumor progression since the selective elimination of VLCs impairs the growth of both solid flank tumors and peritoneal ovarian cancer (Bak et al., 2007; Conejo-Garcia et al., 2004). However, the origin of this cell type and how it fits into the spectrum of known leukocyte populations was unknown. In regard to VLCs recruited by ovarian cancer ascites, the number of VLCs (>107 per mouse) and the peritoneal localization led us to hypothesize that VLCs may result from an ability of the tumor to usurp the peritoneal macrophage pathway. Here we demonstrate that VLCs harvested directly from the tumor environment express canonical macrophage cell surface markers such as F4/80 and CD11b (Fig. 1). These data were then confirmed using a genetic model, the MAFIA strain of mice; these mice have GFP+ macrophages due to GFP gene expression by the c-fms (CD115) promoter. Resident peritoneal macrophages in naïve MAFIA mice are uniformly GFP+F4/80+CD11c−, but upon ID8 tumor growth in the peritoneum they then exhibit a GFP+F4/80+CD11c+ phenotype. Our data support that the well-characterized GFP+F4/80+CD11− peritoneal macrophage population acquires CD11c expression. Additionally we demonstrate that induction/recruitment of the VLC population drastically alters, and replaces, the macrophage (F4/80+) population while asymmetrically not markedly affecting the peritoneal population of CD11c+F4/80− DCs (Fig. 1C and D; upper left quadrants). The macrophage phenotype is additionally supported by the observation that VLCs bind folate, a previously described characteristic of tumor-associated macrophages that is not exhibited by DCs (unpublished results) (Gordon and Taylor, 2005; Turk et al., 2002; Turk et al., 2004). Human ovarian cancer samples support an origin for VLCs from the monocyte/macrophage pathway, since the CD11c+ cells also express CD11b and CD14 (Fig. 3). These data support that macrophages, and the macrophage pathway, are a source of ovarian-tumor VLCs in the peritoneum and, indeed, the CD11b expression indicates that these are the same cells that others isolate as TAM; whether VLCs recruited to solid tumors and to alternative anatomical locations are derived from different precursors or cellular pools remains to be evaluated.
Our observation that ovarian-tumor -derived VLCs represent a Gr-1+CD11b+CD115+ population led us to hypothesize that VLCs may be a variety of the previously-described MDSC tumor-associated leukocytes (Fig. 1 and Fig 2). MDSC can be derived from heterogeneous cell types, however in mice they are reported to consistently express a CD11b+Gr-1+ phenotype (Nagaraj and Gabrilovich, 2007). Our identification of VLCs as MDSCs was confirmed functionally by their immunosuppressive activity upon stimulated T cells. Using complementary assays, we found that the presence of VLCs drastically inhibited CD8+ and CD4+ T cell activity (Fig. 4), with IFNγ release inhibited >95% (Fig. 5). Interestingly, the VLCs were able to suppress T cell activity both when the T cells were stimulated through the T cell receptor and when the T cells were stimulated by PMA/ionomycin. Additionally, this VLC phenotype was not a result of ectopic expression of VEGF, as CD11c+ from untransduced cell lines were able to suppress T cells (Fig. 6). Various reports on other tumor systems have implicated the importance of ROS, IL-10, IL-13, cell contact, PDE5, and the induction of Treg cells in the generation of an immunosuppressive environment (Gabrilovich et al., 2007; Gallina et al., 2006; Huang et al., 2006; Serafini et al., 2006b; Sinha et al., 2007; Yang et al., 2006). Previous studies using the ID8 tumor model identified that blockade of CD80 on the MDSCs, or CD152 on the T cells, reduced T cell suppression (Yang et al., 2006). Here we demonstrate that ARG1 activity is an alternative mechanism, and therapeutic target, that is critical for the immunosuppressive activity of ID8 –derived VLCs (Fig. 7 and Fig 8). ARG1 activity depletes the local environment of L-arginine, causing a disregulation of T cell receptor (TCR) signaling and subsequent CD8+ T cell unresponsiveness (Nagaraj et al., 2007; Rodriguez et al., 2004; Rodriguez et al., 2003). Importantly, we demonstrate that the ability of VLCs to suppress CD8+ and CD4+ T cell division and IFNγ production is dependent on ARG1 activity and that inhibition with the ARG1-specific inhibitor nor-NOHA restores the ability of CD8 and CD4 T cells to respond to stimuli. Taken together with the work of Yang et al (Yang et al., 2006), these data suggest that ovarian cancer -associated MDSCs may have several mechanisms each required to orchestrate T cell immunosuppression. It is interesting to speculate that these limiting steps may be interwoven, and that ARG1 activity may alter CD80 expression or that, conversely, CD80 signaling may induce ARG1 activity. Additionally, since ARG1 is a downstream effector of PDE5 activity, we speculate that the nor-NOHA mediated effects we observe on the ovarian tumor-associated leukocytes may represent an analogous, and more specific, effect to those observed upon PDE5 inhibition with the CT26 colon tumor model (Serafini et al., 2006b).
Finally, the identification of ID8-derived VLCs as an immunosuppressive cell provides a plentiful source of murine MDSCs for researchers who require large quantities for experimental purposes. At later stages of peritoneal ID8 tumor growth it is facile to isolate >107 non-adherent VLCs per mouse which consistently express ARG1 (Figure 7). Additionally, the work presented here provides evidence for a convergence of multiple fields of tumor leukocyte study. We propose that there is likely a high degree of overlap in the cells being characterized and studied as VLCs, TAMs, MDSCs and as M2 macrophages in both ovarian cancer models and disparate tumor models (Balkwill et al., 2005; Gallina et al., 2006; Hagemann et al., 2006; Huang et al., 2006; Robinson-Smith et al., 2007; Rodriguez et al., 2004; Sinha et al., 2005; Yang et al., 2004).
In summary, these findings elucidate an origin for ovarian ascites resident VLCs and help to delineate a place for VLCs amongst the myriad of tumor-associated leukocyte populations. Additionally, the identification of functional mechanisms by which they suppress T cell activity may elucidate how they promote tumor progression. In light of these current findings, our previous report was the first effective and selective targeted in vivo depletion of ovarian tumor-associated MDSCs (Bak et al., 2007). As such, we now propose that VLC depletion from the tumor microenvironment alleviated the suppression of T cell activity and enhanced a T cell -mediated anti-tumor response that resulted in the observed tumor inhibition (Bak et al., 2007). Thus, future efforts will test the hypothesis that that targeted in vivo depletion of VLCs results in tumor inhibition by a T cell-dependent mechanism.
Acknowledgements
We thank the Norris Cotton Cancer Center Englert Cell Analysis Laboratory for assistance with FACS analysis, and the members of the Berwin Lab for helpful discussions. Grant Support: NIH COBRE P20RR016437 and NIH R01AI067405 (BB), a Dartmouth-Norris Cotton Cancer Center Nanotechnology grant (BB and MJT), and NIH training grant T32 AI07363 (SPB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;Vol. 166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- Amiel E, Nicholson-Dykstra S, Walters JJ, Higgs H, Berwin B. Scavenger receptor-A functions in phagocytosis of E. coli by bone marrow dendritic cells. Exp Cell Res. 2007;Vol. 313:1438–1448. doi: 10.1016/j.yexcr.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak SP, Walters JJ, Takeya M, Conejo-Garcia JR, Berwin BL. Scavenger receptor-A-targeted leukocyte depletion inhibits peritoneal ovarian tumor progression. Cancer Res. 2007;Vol. 67:4783–4789. doi: 10.1158/0008-5472.CAN-06-4410. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;Vol. 7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;Vol. 196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Boucher JL, Custot J, Vadon S, Delaforge M, Lepoivre M, Tenu JP, Yapo A, Mansuy D. N omega-hydroxyl-L-arginine, an intermediate in the L-arginine to nitric oxide pathway, is a strong inhibitor of liver and macrophage arginase. Biochem Biophys Res Commun. 1994;Vol. 203:1614–1621. doi: 10.1006/bbrc.1994.2371. [DOI] [PubMed] [Google Scholar]
- Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;Vol. 96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother (1997) 2001;Vol.24:431–446. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;Vol. 24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;Vol. 176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- Burnett SH, Beus BJ, Avdiushko R, Qualls J, Kaplan AM, Cohen DA. Development of peritoneal adhesions in macrophage depleted mice. J Surg Res. 2006;Vol. 131:296–301. doi: 10.1016/j.jss.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;Vol. 75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;Vol. 10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;Vol. 105:679–681. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;Vol. 92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;Vol. 10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;Vol. 6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- El-Gayar S, Thuring-Nahler H, Pfeilschifter J, Rollinghoff M, Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol. 2003;Vol. 171:4561–4568. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;Vol. 67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;Vol. 116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;Vol. 5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. "Re-educating" tumor-associated macrophages by targeting NF-{kappa}B. J Exp Med. 2008 doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;Vol. 176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;Vol. 66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A. 1993;Vol. 90:3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;Vol. 203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;Vol. 51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;Vol. 74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- Leijh PC, van Zwet TL, ter Kuile MN, van Furth R. Effect of thioglycolate on phagocytic and microbicidal activities of peritoneal macrophages. Infect Immun. 1984;Vol. 46:448–452. doi: 10.1128/iai.46.2.448-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;Vol. 171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- McLean K, Buckanovich RJ. Myeloid cells functioning in tumor vascularization as a novel therapeutic target. Transl Res. 2008;Vol. 151:59–67. doi: 10.1016/j.trsl.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells. Adv Exp Med Biol. 2007;Vol. 601:213–223. doi: 10.1007/978-0-387-72005-0_22. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;Vol. 13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;Vol. 111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;Vol. 25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM, Drew AF. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;Vol. 67:5708–5716. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;Vol. 21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;Vol. 64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;Vol. 277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;Vol. 171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- Schaffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg. 1998;Vol. 85:444–460. doi: 10.1046/j.1365-2168.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006a;Vol. 16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;Vol. 53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006b;Vol. 203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;Vol. 25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;Vol. 67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;Vol. 174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;Vol. 13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, Low PS. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2002;Vol. 46:1947–1955. doi: 10.1002/art.10405. [DOI] [PubMed] [Google Scholar]
- Turk MJ, Waters DJ, Low PS. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004;Vol. 213:165–172. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;Vol. 6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;Vol. 66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]


