Abstract
Success in functional neuroimaging has brought the promise of quantitative data in the form of brain images to the diagnosis of disorders of the central nervous system for which only qualitative clinical criteria have previously existed. Even though the translation of research to clinical neuroimaging for conditions such as major depression may not be available yet, rapid innovation along this trajectory of discovery to implementation compels exploration of how such information will eventually affect providers and patients. Clinical neuroethics is devoted to elucidating ethical challenges prior to and during the transfer of new research capabilities to the bedside. Through a model of proactive ethics, clinical neuroethics promotes the development of responsible social and public policies in response to new diagnostic and prognostic capabilities for the benefit of patients and their families, and for providers within the health care systems in which they practice. To examine views about the potential interaction of clinical neuroimaging and depression, we surveyed both mental health providers and outpatients and inpatients diagnosed with major depressive disorder. From responses of 52 providers and 72 patients, we found high receptivity to brain scans for treatment tailoring and choice, for improving understanding of and coping with disease, and for mitigating the effects of stigma and self-blame. Our results suggest that, once ready, roll out of the fully validated technology has significant potential to reduce social burden associated with highly stigmatized illnesses like depression.
Keywords: Depression, Functional neuroimaging, Neuroethics, Diagnosis, Treatment
1. Introduction
Depression is one of the most common public health problems worldwide, and estimates predict that one in four people will personally experience depression or another mental illness at some point in their lives (Gray, 2002). Diagnosis of depression is currently made on the basis of a constellation of factors that include mood, attention, and motivation and, despite the public health significance, it often goes inadequately detected and treated. Complexities of personal context, denial (Angermeyer and Matschinger, 2004; Haslam et al., 2005; Priest et al., 1996), lack of insight about disease severity (Simon et al., 2006), and pressures for rapid interactions and communication between providers and their patients exacerbate the complexities of diagnosis.
The introduction of imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and neurochemical, and neuropharmacologic functional methods such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) in the 1960s and 1970s revolutionized the study of the biology of psychiatric illness with potential implications for psychiatric practice. Today, structural MRI and its functional counterpart – fMRI – are widely used to define anatomy and hemodynamic changes associated with depression. While MRI had been the cornerstone of the neurology clinic for decades, its application to psychiatry came to the foreground in the 1990s when the first studies were published demonstrating a blood oxygenation level dependent effect (BOLD) in humans (reviewed in Kikka et al., 1996). The non-invasiveness and repeatability of the fMRI methods have made it a ubiquitous research tool (Ebmeier et al., 2006), superseding even radionuclide imaging methods (Mitterschiffhaler et al., 2006).
Nearly 2000 fMRI empirical studies of depression, bipolar disorder and schizophrenia can be retrieved from Pub-Med today. Another approximately 3000 focus on core cognitive functions disrupted by mental illness such as emotion, mood and arousal. Studies of major depression specifically have identified abnormalities in multiple areas of the prefrontal cortex (Drevets, 2000), the amygdala (Thomas et al., 2001), related parts of the striatum and thalamus (Caetano et al., 2001) and hippocampus (Videbech et al., 2001). Smaller fronto-temporal volumes have been measured in treatment-resistant depressed patients, and have been linked with impaired and short-term memory functioning (Simpson et al., 2001). Other studies (Sheline et al., 2001) have reported left amygdala hyperarousal in depressed patients, even when they were processing stimuli outside conscious awareness. Siegle et al. (2002) reported sustained amygdalar responses to emotional stimuli in depressed individuals with fMRI, and good predictive validity of the technique for recovery with cognitive behavior therapy (Siegle et al., 2006).
Taken together, neuroimaging studies implicate interconnected neural circuits that can be mapped both structurally and functionally. Clinical imaging may eventually reveal co-morbid disorders that share common biological substrates (Abou-Saleh, 2006), including shared genes and dysfunctional circuitry, and the combination of imaging and genomics (Hariri and Weinberger, 2003; Roffman et al., 2006) may eventually enable tailoring treatment of affected pathways in depressed patients. At the present time, imaging technology reveals the anatomical structure of and blood flow patterns in the brain but cannot directly provide information about behavior. Moreover, images are constructed using aggregate data and still have limited, albeit improving relevance for individual diagnosis (Canli, 2006; Canli et al., 2001). Specifically, advances in brain imaging technology are expected to impact diagnosis in depression (Davidson et al., 2002; Moras, 2006), as well as pretreatment prediction of, and ongoing evaluation of treatment response (Etkin et al., 2005). In a recent study using fMRI technology, Siegle et al. (2006) reported that response to an emotional processing task prior to treatment predicted response to cognitive-behavioral therapy for depression.
Thus, there is merit to acquiring anticipatory data and preparing to help guide the eventual translation of the technology, whether it is fMRI or another modality, by understanding the prospective place of neurotechnology in the lives of patients and their providers. Indeed, the framework for this study is that for modern neuroscience to be applied for maximum public health good, it is critical to identify and address the intersections of the research with ethics and society at its earliest stages. Little is known to date about the potential impact of brain images and other novel technology on the conceptualization of psychiatric illnesses, such as depression, by patients, their self-help efforts, treatment preferences and compliance. Similarly, few data exist regarding the attitudes of psychiatrists and psychologists on the potential impact of neuroimaging technology in the diagnosis and treatment of major depression. Indeed, patients and providers are often barraged with conflicting messages about mental illness and different alternatives for treatment from academic and commercial sources, the popular press, the Internet and support organizations (Berger et al., 2005). Given the scope of available information, how do patients view new types of diagnostic and therapeutic tools? How do providers expect to integrate the new information obtained from calculated norms and biological patterns, such as functional MRIs, especially when the neurobiology is still incompletely understood? How do patients, their advocates and their providers view the potential risks and rewards of having such additional tests, including impact on stigma, medication compliance, participation in psychotherapy, self-help efforts, insurability and privacy? These are some of the questions to which early answers will bring about maximum benefit of new technical capabilities as those with proven efficacy become available for clinical practice. These issues are addressed by the field of neuroethics, which seeks to explicitly and proactively link ethical, legal and social issues with advances in neuroscience.
This report describes findings gathered in one survey designed for psychiatrists and psychologists in both academic and clinical settings, and a separate survey given to patients diagnosed with major depressive disorder (MDD). To our knowledge, this is the first effort to examine attitudes toward the possible use of neuroimaging technologies in the diagnosis and treatment of depression.
2. Materials and methods
2.1. Participants
Sixty psychiatrists and psychologists active in both academic and clinical settings were invited to participate. The academic psychiatrists and psychologists were known for their research in the treatment of major depression, and had faculty appointments in departments of psychiatry and psychology. Those from clinical settings all practiced in the San Francisco Bay Area. They included MDs and PhDs practicing in the community, Veterans Administration Health Care System, and private practice settings. One was an EdD whose data were included with the PhDs.
All were recruited by email and then by telephone follow-up to maximize enrollment. In addition to including both MDs and PhDs from academic and clinical settings, the cohort was constructed to represent both genders, diverse backgrounds and ethnicities, and to have varying exposure to neuroimaging in their environment.
Patients were recruited from the Stanford University Department of Psychiatry and Behavioral Sciences. Inclusion criteria were: (1) men and women age 18–75 with a principal diagnosis of MDD; (2) no terminal medical illness; and (3) capacity to understand the nature of the study and provide written, informed consent. Exclusion criteria were diagnosis at the time of the study of: (1) schizophrenia or other psychotic disorder, (2) bipolar disorder, (3) dementia Alzheimer Type or related cognitive disorders; (4) alcohol or other substance-related dependence disorder (with the exception of nicotine dependence); (5) inability to read and write English. Twenty-seven (27) were participants in a National Institute of Mental Health (NIMH) randomized clinical trial that involved both medication and psychotherapy, 22 were recruited at the Stanford University Department of Psychiatry outpatient clinic, and 23 were recruited from Stanford’s inpatient psychiatry service. Patients in the NIMH studies were recruited by study coordinators. Clinic patients were recruited by providers. Patients were compensated $25 for their participation.
2.2. Surveys
Separate surveys for the providers and patients were constructed, piloted and finalized. The 27-question provider survey was divided into three major sections, querying for the anticipated impact of functional imaging on clinical practice, anticipated impact of functional imaging on choice of treatment and patient compliance, and perceived costs and benefits of neuroimaging for patients with major depression. Responses to these questions were made on a 5-point Likert Scale (1–5) signifying strong disagreement, disagreement, neutrality, agreement, and strong agreement. Where appropriate, not applicable was an option. Providers were also asked questions about their professional background, current practice patterns, and the extent of research in neuroimaging in their environment.
The 29-question patient survey was also divided into three major sections. It queried patients about their condition, the impact it has on them and others in their environment, and about their views on brain scan technology. Similar to the provider survey, responses were made on a 5-point Likert Scale. Patients also responded to questions about their background, including age, history of mental illness, treatment, education, ethnicity, type of health insurance, and previous experience with brain scans. All subjects provided written informed consent and the study was approved by the Stanford University Medical School Institutional Review Board.
To achieve a common level of understanding about the purpose of the study and nature of brain scans, the first page of the patient survey showed a coronal MRI with colorized activations superimposed on it, and presented the following question:
If your provider could use a brain scan like the one below to diagnose depression and assess the effectiveness of treatment, would you be receptive to having one?
The purpose of the survey, as specified on the questionnaires and articulated during consent, was to examine how emerging neurotechnology could affect patients, doctors, and others in the future.
Data were described qualitatively and main effects tested for significance using Fisher’s Exact tests unless specified otherwise. Answers of “agree” or “agree strongly” were collapsed into ‘agree’ or ‘positive’ responses; disagree or disagree strongly were collapsed into ‘disagree’ or ‘negative’ responses. The occasional responses between two labeled categories were coded according to whichever of the two categories was closer to neutral. The order of results mirrors the order of questions on the surveys.
3. Results
3.1. Demographics of respondents
Fifty-two (52) of the 60 providers responded (87% response rate; Table 1). We did not find significant differences on survey responses from the professional groups. The data were therefore collapsed for final analysis.
Table 1.
Provider demographics
| N (%) | N (%) | ||
|---|---|---|---|
| Background | Type of practice | ||
| MD | 22 (42) | Acad. Medical Ctr | 23 (43) |
| PhD, EdD, PsyD | 29 (56) | Private Practice | 26 (48) |
| Not reported | 1 (2) | Private hospital | 1 (2) |
| VA Medical Ctr | 2 (4) | ||
| Ethnicity | Other | 1 (2) | |
| Caucasian | 48 (92) | Not reported | 1 (2) |
| Asian | 3 (6) | ||
| African–American | 1 (2) | ||
| Hispanic/Latino | 0 (0) | ||
| Other | 0 (0) | ||
| Age | |||
| <39 | 8(15) | ||
| 40–49 | 17 (33) | ||
| 50–59 | 17 (33) | ||
| 60+ | 8 (15) | ||
| Undisclosed | 2 (4) | ||
Percentages may exceed 100 since some providers indicated more than one choice as an answer.
A total of 72 surveys were returned from patients for analysis (Table 2). The majority of responders were outpatients (n = 49; 68%); 23 (32%) were inpatients. Women represented 61% (n = 44) of the sample. Mean age across genders was 45 years (standard deviation = 13); 92% had completed high school, and 67% had completed college or graduate school. Of the group, 72% were Caucasian, 9% African–American, 9% Asian, 8% Hispanic, and 1% other. The majority of respondents (69%) earned above the median income in the United States for 2005. A large proportion of the cohort (69%) had been diagnosed with MDD more than 5 years before the study. Over half (56%) had a family history of neurological or mental disease. One subject had previously participated in a brain imaging study, and a total of 34 (47%) subjects indicated awareness that brain scan technology is being developed for improving the diagnosis of depression. Respondents reported that they used a variety of therapies to treat their depression; 90% were on medication with or without additional treatment; 60% were receiving psychotherapy.
Table 2.
Patient demographics
| N (%) | N (%) | ||
|---|---|---|---|
| Gender | Treatment type | ||
| Female | 44 (61) | Medication | 65 (90) |
| Male | 28 (39) | Psychotherapy | 44 (60) |
| Support Group | 9 (13) | ||
| Ethnicity | ECT | 4 (6) | |
| Caucasian | 55 (72) | No Treatment | 3 (4) |
| African–American | 7 (9) | Alt. Medicine | 3 (4) |
| Asian | 7 (9) | Self Education | 1 (1) |
| Hispanic/Latino | 6 (8) | ||
| Other | 1 (1) | ||
| Age | |||
| <39 | 25 (35) | ||
| 40–49 | 16 (22) | ||
| 50–59 | 21 (29) | ||
| 60+ | 10 (14) | ||
Percentages may exceed 100 since some patients indicated more than one choice as an answer.
Patients overwhelmingly reported that depression negatively affects their daily lives and alienated them from others (92% and 94% positive responses). Patients also reported that, by extension, their depression negatively affected their family and friends (53% positive response). Fifty-seven percent blamed themselves for their depression.
3.2. Receptivity to brain scans
Eighty-five percent (N = 44) of providers agreed or strongly agreed that such data would be a valuable adjunctive diagnostic tool for clinical evaluation. There was no difference on this measure by average number of years in practice (18 years). Twenty-five percent agreed or strongly agreed with the desirability of a brain scan as a primary means of diagnosing depression. The average number of years in practice for this cohort was 20 years. The average number of years in practice for those neutral or negative was 14.5 years. The majority of providers anticipated that fMRI data would improve the overall care of MDD patients (71% positive). Benefits were predicted to outweigh personal risks (71% positive), although the financial cost was a mitigating factor with 40% of providers indicating concern about this variable (40% positive response, 37% neutral and 23% negative).
Among the patients, 92% responded positively to wanting a brain scan to diagnose depression if it was available. Four subjects were neutral; none were entirely unreceptive to the possibility of a brain scan. On this measure and those that follow, results were highly consistent for age, gender and ethnicity, and across inpatient and outpatient respondents. Seventy-six percent of patients reported that a brain scan would help them accept their condition and 66% of the subjects responded that it would increase their confidence in a provider’s diagnosis. Sixty-four percent of patients disagreed that brain scans would cause them to worry more about their condition; 22% were neutral and 14% agreed.
3.3. Understanding, stigma and coping
Eighty-seven percent (N = 45) of providers agreed or strongly agreed that reliable and valid fMRI data would help with understanding of biological factors of depression. Their response to whether fMRI would improve understanding of psychosocial factors was more evenly distributed: 35% positive, 27% neutral and 28% negative. Differences in provider responses between their views on biological versus psychosocial factors were significant (Bowker’s test, p < 0.0001). Providers also agreed that fMRI data would help patients understand their condition (81% positive response). Sixty-five percent of providers did not predict an increase in stigma associated with MDD diagnosed with fMRI. On the contrary, the proportion of positive responses about stigma reduction was significantly greater than the proportion of neutral and negative responses (p < 0.019, exact one-sided binomial test; Fig. 1). The majority also did not anticipate any adverse effect on family interactions (86% negative response).
Fig. 1.
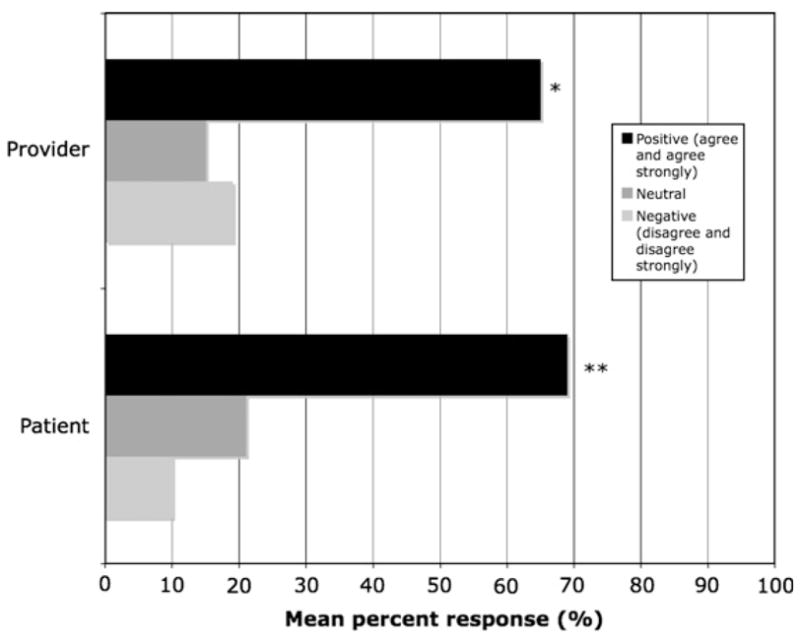
Provider and patient anticipation of reduced stigma associated with a functional brain scan for MDD. *p < 0.019; **p < 0.001.
Sixty-three percent of patients reported that brain scans would aid their families to accept their condition better. Ninety-two percent (92%) of the respondents from the patient group anticipated that brain scans would help them and others better understand the biology of the condition. In response to questions about stigma reduction, patients’ positive answers were significantly more frequent than neutral and negative ones (p < 0.001, exact one-sided binomial test; Fig. 1). Fifty-seven percent of the sample reported blaming themselves for being depressed. Those who agreed or strongly endorsed the self-blame item reported that a scan would have a significant positive impact on mitigating this sense of responsibility (71%). We found that those eager – strong agreement to have a scan – expected enhanced self-understanding of the disorder (95% responding positively) compared to those not eager (50%, p < 0.003). Similarly, patients reporting an eagerness for a scan were more likely to expect improved coping (71% responding positively) compared to those not eager (17%, p < 0.016). An absence of negative responses on this question suggests that while patients with depression who blame themselves expect that scans will be helpful, those who are not self-blaming at least do not anticipate adverse effects.
Providers and patients groups had similar positive responses associated with understanding of biological factors (Fisher’s exact test p = 0.384). Provider and patient views on the anticipated impact of fMRI on psychosocial understanding of MDD were significantly different (Fisher’s exact test p = 0.022; Fig. 2).
Fig. 2.
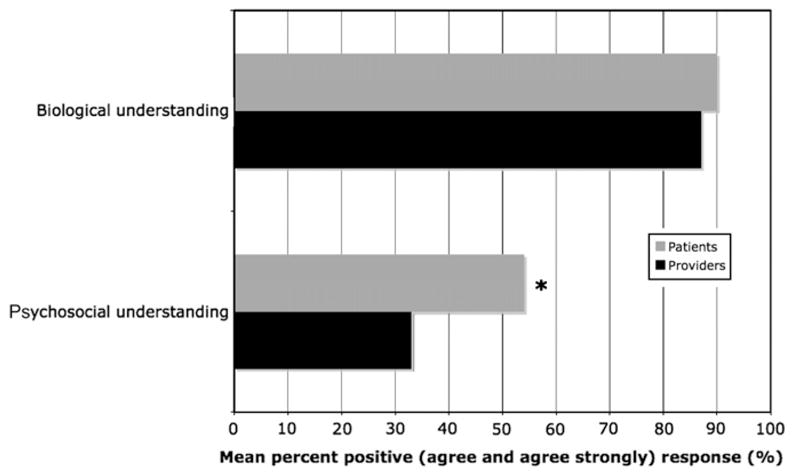
Anticipated impact of fMRI on biological and psychosocial understanding of MDD. *Fisher’s exact test p = 0.022.
Providers were not asked about coping. A majority of patients (n = 49, 69%), however, anticipated a positive impact on coping with the disease; 10% did not see a positive impact and 21% were neutral.
3.4. Impact on treatment and compliance
Eighty one percent responded that fMRI data consistent with MDD would encourage patients to pursue treatment. The most positive influences of fMRI on treatment and compliance of MDD as anticipated by providers was encouraging patients to try medication (84% positive), tolerate the side effects of medication (67% positive) and comply with medications prescribed (81% positive). Providers anticipated that patients undergoing imaging would be more inclined to try medication to treat their depression (84% positive) compared to psychotherapy (33% positive response; p < 0.001 by Bowker’s test). Providers generally did not foresee fMRI having an impact on patients’ motivation to examine their thinking and interpersonal patterns, willingness to complete homework assignments, or participation in non-medical self-help (Fig. 3).
Fig. 3.
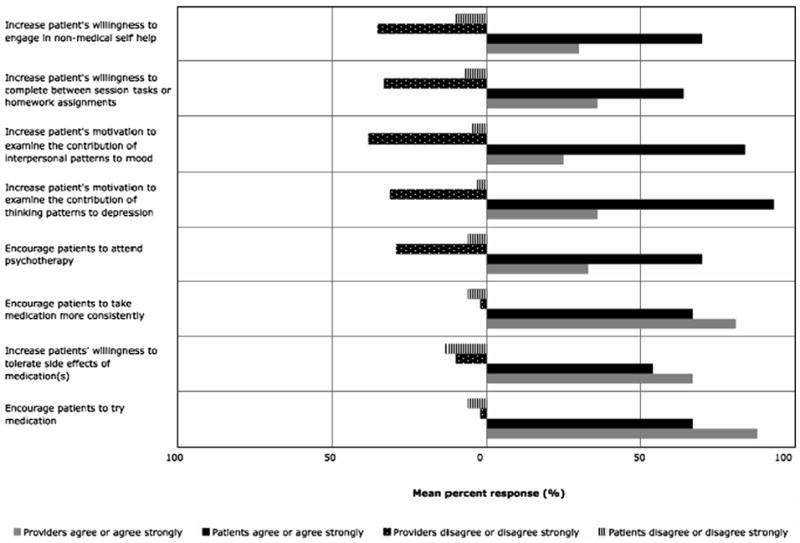
Percentage of positive (agree or agree strongly) and negative (disagree or disagree strongly) responses by providers and patients to the anticipated impact of neuroimaging on various dimensions of treatment and compliance.
Patients reported that brain scans would increase their interest in a range of treatment choices. Patients were also generally positive about the effects of imaging on taking medication consistently (67%). Twenty-six percent indicated that it would have no effect, and 6% a negative effect. We found the same distribution of responses when patients were asked if a brain scan consistent with signs of depression would increase their willingness to try medication. We reiterate, however, that 90% of patients were already being treated with medication.
Seventy percent reported that a brain scan showing signs of depression would make them more likely to attend psychotherapy. Patients also reported that such a scan would encourage them to complete homework (64%) and engage in non-medical self-help (70%). The most neutral (33%) and negative responses (13%) were to the questions about increased willingness to tolerate side effects of medication. Patients also predicted that brain scans would motivate them to examine their thinking patterns in the context of their depression, as well as to the contribution of interpersonal patterns to mood (93% and 84% positive responses).
Among the providers group, 54% anticipated adverse effects on patients’ health insurance. Fifty-five patients (77%) reported being concerned about the effects of brain scans on their insurance.
Queried for discrimination in the workplace, 58% (n = 30) of providers responded negatively to concern that a brain scan would increase discrimination; 29% (n = 15) were neutral; 13% (n = 7) responded positively to this question. More than 25% of each of the three questions concerning employment was left unanswered by patients. These results were not analyzed, therefore, since the data are incomplete for the measure.
4. Discussion
Historically, consideration of the ethical and policy implications of frontier neurotechnology has lagged the development and deployment of the technology itself. As non-invasive imaging methods are being used to examine the biology of an unprecedented range of cognitive and mental phenomena, today’s innovations make elimination of this lag a pressing challenge. Our results suggest that both patients and providers value the promise that a technique like fMRI holds for informing the management of major depression beyond the anatomo-functional information its provides. Early identification of challenges related to this receptivity to technology, especially to those technologies that address highly stigmatized conditions like depression (Sartorius, 2007), can maximize benefit and prevent false hope, mitigate hype, and curtail their premature use and even misuse in the private sector (Eaton and Illes, 2007).
The anticipated impact of neuroimaging was statistically undifferentiated between the patients we surveyed, a cohort that was ethnically diverse but representing greater education and affluence than the general population. Both patient and provider groups perceived a wide range of benefits that included increased confidence in the diagnosis by providers, and increased acceptance of their own condition. Attitudes toward the use of fMRI technology were similarly positive among provider groups in academic settings and those in practice settings. Although they were recruited to the study for their documented expertise in depression and familiarity with functional neuroimaging methods, we still found this result surprising. As scans could promote overemphasis on biological factors in mental illness, as opposed to learning or environmental influences (Linehan, 2007), we had expected that psychologists would be more skeptical than psychiatrists. However, we found no such differences. All provider groups perceived value in the use of fMRI for diagnosis as an adjunctive method of assessment assuming, as the survey stated, that research supported reliability and validity of the tool for individual patients. Surprisingly, fully 25% of providers reported willingness to use brain scans as a primary diagnostic tool given appropriate scientific validity, which perhaps reflects dissatisfaction with current descriptive diagnostic methods (Beckham et al., 1995) and the expectation that neuroimaging could potentially refine the diagnosis of depressed patients (Davidson et al., 2002). Overall, acceptability of this technology appears to be robust at this stage among all the groups we surveyed.
Patients and providers differed on the anticipated intersection between fMRI and non-medical types of treatment. Patients reported that image-based evidence of depression would encourage their participation in psychotherapy and other non-medical approaches to treatment. Providers, on the other hand, were significantly less likely to agree that the availability of neuroimaging data would increase participation in psychotherapy. Similarly, patients believed that brain scans would increase their willingness to explore thinking and interpersonal contributions to depression while providers were generally neutral on this issue.
One interpretation of the differences between patients and providers on the impact of scan data on participation in non-medical treatment and consideration of non-medical factors is that providers may view the availability of the data as promoting a biological explanation of depression while patients may be more focused on confirmation that the problem is real, eroding the sense of denial. The functioning of depressed patients has been shown to be significantly worse compared to patients with other chronic illnesses (Moussavi et al., 2007), yet access to treatment is more limited than for other illnesses (Andrews and Titov, 2007). Andrews and Titov (2007) have suggested that the lack of a laboratory test for depression may inhibit doctors from insisting on treatment adherence. It may also inhibit patients from taking more vigorous initiatives, both medical and non-medical, which might have a positive impact on their condition. Patients’ responses to our survey on the benefits of fMRI may reflect both their personal experience grappling with an illness that lacks a biomarker and the belief that the availability of such a marker would encourage a wider range of efforts to deal with it.
Given some of the limitations in the sensitivity and specificity of fMRI and the heterogeneity in the depression phenotype, one targeted use of this technology in the future may be for selecting courses of treatment. Our results show that patients and providers anticipate that fMRI will have a significant impact on treatment course, compliance and alternatives. Moreover, in the space given to comment on brain scan technology, a number of patients expressed hope for using the technology to tailor treatment and monitor progress.
A majority of our patients reported blaming themselves for their condition, reflecting the well-documented stigma attached to mental illnesses of all types (Sartorius and Schulze, 2005; Benbow, 2007). Our findings suggest that patients clearly perceived the potential of fMRI in the diagnosis and treatment of depression as a means of reducing stigma. Among those acknowledging self-blame, nearly 75% believed that a positive fMRI result would reduce it. Similarly, those expressing the greatest avidity to undergo a scan if it were available were significantly more likely to express belief that it would increase self-understanding and increase coping compared to those less eager to undergo such testing.
Given the importance of rumination in amplifying depressed mood (Nolen-Hoeksema, 1991; Roberts et al., 1998), we explored whether patients anticipated that scans might increase worry about their symptoms. A substantial majority of our sample disagreed that scan participation would have such an effect. This is consistent with the overall pattern we observed of patients’ receptivity and openness to the use of such technology, and their anticipation of benefit over risk. Perhaps, not surprisingly in this American sample, a large majority did express concerns about possible adverse impact on health insurance.
Our study had several limitations. For example, the majority of the patients were being treated either in a fee-for-service clinic or in various treatment studies at a single academic medical center. We do not know the extent to which these findings might generalize to a more diverse population of depressed patients, to those not in treatment, and even according to varying individual estimates of the potential reliability and accuracy of the technology. A high percentage of patients were treated with medication; it is possible that attitudes toward fMRI technology might be different among patients averse to medical treatment. Providers in both academic and practice settings were recruited by one of the co-authors (B.A.) and we do not know the extent to which they are representative of the universe of providers treating depressed patients. Finally, there is the possibility that in asking subjects to assume that the technology would be useful for diagnosis they assumed perfect sensitivity and specificity, however unlikely for any method, and of a social desirability effect despite assurance of anonymity. Generalizability is limited therefore, and invites further research with a larger cohort of providers and patients with depression and others with disorders that are on the early discovery path for imaging such as schizophrenia (Kubicki et al., 2007), bipolar disorder (Yurgelun-Todd and Ross, 2006), and ADHD (Bush et al., 2005).
Although fMRI is still a long way from being used in individuals, our findings suggest that both providers and patients are receptive to this eventuality, and believe it could both enhance compliance with treatment and mitigate stigma. The reliability of diagnostic measures such as fMRI varies. As the technology comes closer to being more fully validated, provider and patient attitudes are likely to change. Assessing the views of these two groups as a function of varying degrees of reliability would provide important information on how the public embraces technology as it develops and can inform how to best roll out these innovations.
We conclude that while paradigmatic factors having to do with standardization of brain data acquisition and analysis have yet to be resolved (Illes and Racine, 2005), in combination with other indices, fMRI promises significant benefit to the diagnostic process for major depression. Open dialogue among scientists, clinicians, patient stakeholders, and policymakers will further ensure that this potential is realized.
Acknowledgments
The authors gratefully acknowledge Pamela Schraedley-Desmond for statistical analysis, and the research assistance of Marisa Gallo and Allyson Mackey. The data were presented in part at the 2007 Society for Neuroscience Annual Meeting (November 3–7, 2007).
Role of Funding Source
Funding for this study was provided by NIH Grant NS#045831 (J.I.); the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
Authors Judy Illes and Bruce Arnow designed the study and wrote the manuscript. Author Sofia Lombera managed the data. Author Jarrett Rosenberg carried out the statistical analysis. Authors Sofia Lombera and Jarrett Rosenberg also contributed to the writing of the manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Abou-Saleh MT. Neuroimaging in psychiatry: an update. Journal of Psychosomatic Research. 2006;61:789–93. doi: 10.1016/j.jpsychores.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Andrews G, Titov N. Changing the face of mental health care through needs-based planning. Australian Health Review. 2007;31:S122–8. doi: 10.1071/ah07s122. [DOI] [PubMed] [Google Scholar]
- Angermeyer MC, Matschinger H. Public attitudes to people with depression: have there been any changes over the last decade? Journal of Affective Disorders. 2004;83:177–82. doi: 10.1016/j.jad.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Beckham EE, Leber WR, Youll LK. The diagnostic classification of depression. In: Beckham EE, Leber WR, editors. Handbook of depression. 2. New York: Guilford Press; 1995. [Google Scholar]
- Benbow A. Mental illness, stigma, and the media. Journal of Clinical Psychiatry. 2007;68:31–5. [PubMed] [Google Scholar]
- Berger M, Wagner TH, Baker LC. Internet use and stigmatized illness. Social Science and Medicine. 2005;6:1821–7. doi: 10.1016/j.socscimed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biological Psychiatry. 2005;1:1273–84. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Sassi R, Brambilla P, Harenski K, Nicoletti M, Mallinger AG, et al. MRI study of thalamic volumes in bipolar and unipolar patients and healthy individuals. Psychiatry Research. 2001;108:161–8. doi: 10.1016/s0925-4927(01)00123-8. [DOI] [PubMed] [Google Scholar]
- Canli T. When genes and brains unite: ethical implications of genomic neuroimaging. In: Illes J, editor. Neuroethics: defining the issues in theory, practice, and policy. Oxford: Oxford University Press; 2006. [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Forss J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000:48. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Eaton ML, Illes J. Commercializing cognitive neurotechnology – the ethical terrain. Nature Biotechnology. 2007;25:393–7. doi: 10.1038/nbt0407-393. [DOI] [PubMed] [Google Scholar]
- Ebmeier K, Rose E, Steele D. Cognitive impairment and fMRI in major depression. Neurotoxicology Research. 2006;10:87–92. doi: 10.1007/BF03033237. [DOI] [PubMed] [Google Scholar]
- Etkin A, Pittenger C, Polan HJ, Kandel ER. Toward a neurobiology of psychotherapy: basic science and clinical applications. Journal of Neuropsychiatry and Clinical Neuroscience. 2005;17:145–58. doi: 10.1176/jnp.17.2.145. [DOI] [PubMed] [Google Scholar]
- Gray AJ. Stigma in psychiatry. Journal of the Royal Society of Medicine. 2002;95:72–6. doi: 10.1258/jrsm.95.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri D, Weinberger DR. Imaging genomics. British Medical Bulletin. 2003;65:259–70. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Haslam C, Atkinson S, Brown SS, Haslam RA. Anxiety and depression in the workplace: effects on the individual and organization. Journal of Affective Disorders. 2005;88:209–15. doi: 10.1016/j.jad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Illes J, Racine E. Imaging or imagining? A neuroethics challenge informed by genetics. American Journal of Bioethics. 2005;5:5–18. doi: 10.1080/15265160590923358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikka JT, Belliveau JW, Hari R. Future of functional brain imaging. European Journal of Nuclear Medicine and Molecular Imaging. 1996;23:737–40. doi: 10.1007/BF00843700. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. Journal of Psychiatric Research. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. On being mad versus bad: cautionary comments on biology as a causal explanation of mental disorder. The Clinical Psychologist. 2007;60:1–3. [Google Scholar]
- Mitterschiffhaler MT, Ettinger U, Mehta MA, Mataix-Cols D, Williams SCR. Applications of functional magnetic resonance imaging in psychiatry. Journal of Magnetic Resonance Imaging. 2006;23:851–61. doi: 10.1002/jmri.20590. [DOI] [PubMed] [Google Scholar]
- Moras K. The value of neuroscience strategies to accelerate progress in psychological treatment research. Canadian Journal of Psychiatry. 2006;51:810–2. doi: 10.1177/070674370605101303. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. The Lancet. 2007;370:851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–82. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Priest RG, Vize C, Roberts A, Roberts M, Tylee A. Lay people’s attitudes to treatment of depression: results of opinion poll for defeat depression campaign just before its launch. British Medical Journal. 1996;313:858–9. doi: 10.1136/bmj.313.7061.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Gilboa E, Gotlib IH. Ruminative response style and vulnerability to episodes of dysphoria: gender, neuroticism, and episode duration. Cognitive Therapy and Research. 1998;22:401–23. [Google Scholar]
- Roffman JL, Weiss AP, Goff DC, Rauch SL, Weinberger DR. Neuroimaging-genetic paradigms: a new approach to investigate the patho-physiology and treatment of cognitive deficits in schizophrenia. Harvard Review of Psychiatry. 2006;14:78–91. doi: 10.1080/10673220600642945. [DOI] [PubMed] [Google Scholar]
- Sartorius N. Stigma and mental health. The Lancet. 2007;37:810–1. doi: 10.1016/S0140-6736(07)61245-8. [DOI] [PubMed] [Google Scholar]
- Sartorius N, Schulze H. Reducing the stigma of mental illness: a report from a global association. UK: Cambridge University Books; 2005. [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Simon D, Loh A, Wills CE, Harter M. Depressed patients’ perception of depression treatment decision-making. Health Expectations. 2006:10. doi: 10.1111/j.1369-7625.2006.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SW, Baldwin RC, Burns A, Jackson A. Regional cerebral volume measurements in late-life depression: relationship to clinical correlates, neuropsychological impairment and response to treatment. International Journal of Geriatric Psychiatry. 2001;16:469–76. doi: 10.1002/gps.364. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–163. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen AR, Egander A, Landbo B, Rasmussen NA, et al. The Danish PET/depression project: PET findings in patients with major depression. Psychological Medicine. 2001;31:1147–57. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Ross AJ. Functional magnetic resonance imaging studies in bipolar disorder. CNS Spectrums. 2006;11:287–97. doi: 10.1017/s1092852900020782. [DOI] [PubMed] [Google Scholar]


