Abstract
p53 is required for DNA damage-induced apoptosis, which is central to its function as a tumour suppressor. Here, we show that the apoptotic defect of p53-deficient cells is nearly completely rescued by inactivation of any of the three subunits of the DNA repair holoenzyme DNA-dependent protein kinase (DNA-PK). Intestinal crypt cells from p53 nullizygous mice were resistant to radiation-induced apoptosis, whereas apoptosis in DNA-PKcs/p53, Ku80/p53 and Ku70/p53 double-null mice was quantitatively equivalent to that seen in wild-type mice. This p53-independent apoptotic response was specific to the loss of DNA-PK, as it was not seen in ligase IV (Lig4)/p53 or ataxia telangiectasia mutated (Atm)/p53 double-null mice. Furthermore, it was associated with an increase in phospho-checkpoint kinase 2 (CHK2), and cleaved caspases 3 and 9, the latter indicating engagement of the intrinsic apoptotic pathway. This shows that there are two separate, but equally effective, apoptotic responses to DNA damage: one is p53 dependent and the other, engaged in the absence of DNA-PK, does not require p53.
Keywords: p53-independent apoptosis, DNA-PK, DNA damage, radiation
Introduction
The tumour suppressor p53 has a crucial function in the cellular response to DNA damage. After DNA damage, p53 is rapidly phosphorylated by the ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3 related (ATR) protein kinases leading to its stabilization, induction of its transcriptional targets and cell-cycle arrest or apoptosis (Vousden & Lu, 2002; Shiloh, 2003). p53-deficient cells fail to undergo G1 cell-cycle arrest or apoptosis in response to DNA damage, emphasizing the crucial function of p53 in these responses. The apoptotic resistance of p53-deficient cells could have clinical implications, as p53 is frequently mutated in human tumours and p53-deficient tumour models have reduced sensitivity to radio- or chemotherapy ( Johnstone et al, 2002). However, in some settings, p53-deficient cells can undergo apoptosis (Strasser et al, 1994; Merritt et al, 1997; Roos & Kaina, 2006), suggesting that there are alternative cell death pathways. Understanding p53-independent cell death pathways and using them to enhance the sensitivity of tumour cells to chemo- or radiotherapy could have therapeutic benefits (Brown & Attardi, 2005).
The DNA double-strand break (DSB) is the main DNA lesion that activates p53-dependent apoptosis (Nelson & Kastan, 1994). In addition to triggering programmed cell death, DSBs activate the repair machinery to rejoin the breaks through either homologous recombination or non-homologous end joining (NHEJ; Shrivastav et al, 2008). During NHEJ, two subunits, Ku70 and Ku80, form a heterodimer that binds to the broken DNA ends, which then recruit the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs; Smith & Jackson, 1999). DNA breaks are synapsed together by DNA-PKcs (DeFazio et al, 2002; Spagnolo et al, 2006) and processed to remove the overhanging 3′ or 5′ ends so that they can be rejoined by the DNA ligase IV (LIG4)–XRCC4 heterodimer (XRCC4 for X-ray repair complementing defective repair in Chinese hamster cells 4; Lees-Miller & Meek, 2003; Spagnolo et al, 2006).
Mutations in any one of these NHEJ components lead to impaired DSB repair and increased sensitivity to ionizing radiation (Zhu et al, 1996; Gu et al, 1997; Gao et al, 1998). Similar to ATM and ATR, DNA-PKcs is a phosphoinositol-3 kinase-related protein kinase (PIKK). DNA-PK can phosphorylate p53 in vitro (Lees-Miller et al, 1992), but the function of p53 and apoptosis in the radiosensitive phenotype conferred by DNA-PK mutation is unclear. The finding of a synthetic lethal interaction between DNA-PKcs and ATM provided a clue to the mechanism by which DNA-PK impinges on cell death pathways. Severe combined immunodeficient (SCID) mice, which lack DNA-PK activity owing to a mutation in DNA-PKcs (Blunt et al, 1996) and Atm-null mice are born at normal frequencies, whereas scid/scid Atm−/− compound mutant embryos die early in development (Gurley & Kemp, 2001). This shows that the function of ATM is required for the survival of cells with loss of NHEJ capacity. As p53 is a direct target of ATM signalling, we investigated whether simultaneous loss of DNA-PKcs and p53 synergized to affect cell death pathways. scid/scid p53−/− mice are viable (Gurley et al, 1998); however, here we report that although cells from p53-null mice are resistant to ionizing radiation-induced apoptosis, scid/scid p53−/− compound mutant cells are highly sensitized to apoptosis. This identifies a role for DNA-PK in the suppression of p53-independent apoptosis and a cellular mechanism by which loss of DNA-PK sensitizes cells to the lethal effects of radiation.
Results
As DNA-PK can phosphorylate p53 in vitro, we investigated first whether p53 induction or apoptosis was impaired in the absence of DNA-PK activity. Epithelial cells within the crypts of the small intestine from Scid, DNA-PKcs−/−, Ku70−/− and Ku80−/− null mice showed no apparent defects in ionizing radiation-induced p53 expression or apoptosis. Whole body radiation (4 Gy) induced apoptosis to a similar extent in both wild-type and all four DNA-PK-deficient strains (Fig 1A,D), and in some cases the apoptotic response in the absence of DNA-PK was enhanced compared with the wild-type response. p53−/− mice showed little or no increase in this early wave of apoptosis, as reported previously (Merritt et al, 1994). The number, staining intensity and localization of cells that stained for nuclear p53 following radiation were also similar between wild-type and DNA-PK mutant mice (Fig 1A). Colonic crypt epithelial cells and hair follicle epithelial cells from the dorsal skin of DNA-PKcs-null mice were also sensitive to ionizing radiation-induced apoptosis, whereas these same cell types from p53-null mice were resistant (data not shown). To determine whether DNA-PK was required for apoptosis in tumour cells, we crossed SCID mice with adenomatosis polyposis coli (ApcMin) mutant mice, which spontaneously develop intestinal adenomas (Su et al, 1992). Intestinal adenomas that developed in scid/scid ApcMin mice also showed a rapid apoptotic response to ionizing radiation, comparable to that seen in tumours from ApcMin mice with intact DNA-PK (Supplementary Fig 1 online). T-cell lymphomas that developed in SCID mice also showed a robust apoptotic response to DNA damage (Gurley et al, 1998). These in vivo results, obtained from several normal and neoplastic cell types using two mutant alleles of DNA-PKcs, as well as all three components of the DNA-PK holoenzyme, show that DNA damage-induced p53 activation and apoptosis do not require DNA-PK activity.
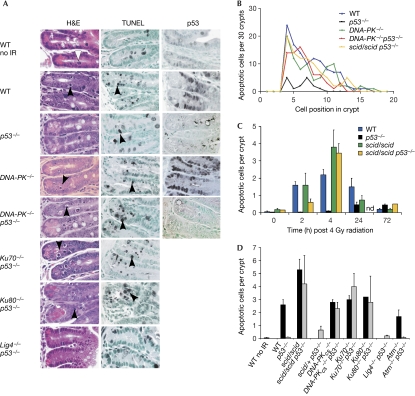
Figure 1.
Elimination of DNA-PK rescues the apoptotic defect in p53-null mice. (A) H&E-stained sections of small intestinal crypts from unirradiated (no IR) or irradiated (4 h post 4 Gy) mice. Mitotic cells (white arrowhead) and apoptotic cells (black arrowheads) are indicated. TUNEL assays and p53 immunostaining are also shown. Note the spatial coincidence of p53-expressing cells and apoptotic cells. (B) Spatial distribution of apoptotic cells within the crypt. Note that the distribution of apoptosis is similar between wild-type (WT), DNA-PK−/− and DNA-PK−/−p53−/− mice, peaking at cell positions 4–7. (C) Time course of small intestinal crypt cell apoptosis after 4 Gy IR in DNA-PK- and p53-deficient mice (nd, not done). (D) Quantification of apoptotic cells in small intestinal crypts from irradiated mice (4 h post 4 Gy; ten × 40 fields were counted per mouse, approximately 5 crypts per field and approximately 50 cells per crypt). Bars are the mean values plus standard deviations from 3–8 mice per genotype except for Ku80−/− (n=1). Atm, ataxia telangiectasia mutated; DNA-PK, DNA-dependent protein kinase; H&E, haematoxylin and eosin; IR, ionizing radiation; Lig4, ligase IV; Scid, severe combined immunodeficient; TUNEL, TdT-mediated dUTP nick-end labelling.
DNA-PK-deficient cells are highly sensitive to radiation and apoptosis is one factor that can contribute to radiosensitivity. By contrast, p53-deficient cells are resistant to apoptosis and, to a certain extent, radioresistant (Gudkov & Komarova, 2003). To determine whether DNA-PK and p53 interact genetically in the radiation response, we examined apoptotic sensitivity in DNA-PK p53 compound mutant mice. Strikingly, the apoptotic response in these double mutants was quantitatively similar to wild type, indicating that the apoptotic defect of p53-null cells was nearly completely rescued by the concomitant mutation of DNA-PK. At 4 h post 4 Gy, the number of apoptotic cells per crypt in scid/scid p53−/− mice was similar to that in scid/scid mice and substantially greater than that in p53−/− mice or unirradiated wild-type mice (Fig 1; Supplementary Table I online). On a per cell basis, DNA-PK mutation increased the apoptotic sensitivity of p53-null cells around 40-fold. Apoptotic cells were identified by using the morphological criteria of cytoplasmic shrinkage, chromatin condensation and nuclear fragmentation, and confirmed by TdT-mediated dUTP nick-end labelling (Fig 1A) and active caspase 3 staining (Fig 2). p53-null mice remained resistant to rapid-onset apoptosis at doses as high as 20 Gy (data not shown). Time-course analysis showed that apoptosis was slightly delayed in scid/scid p53−/− mice (compare the 2 h time point in Fig 1C) compared with wild-type mice and that p53-null mice showed a slight increase in apoptosis at later time points of 24 and 72 h.
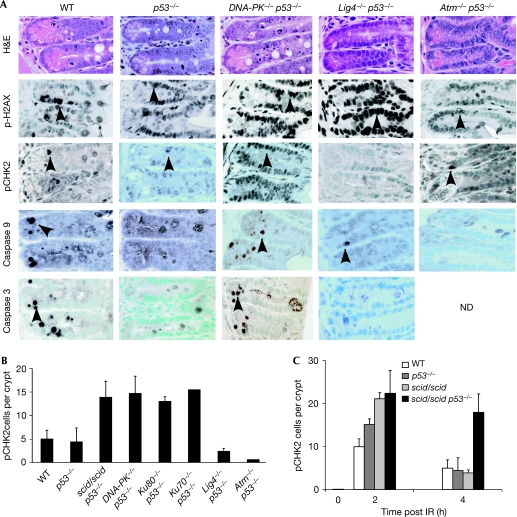
Figure 2.
Mechanism of apoptosis in DNA-PK and p53 mutant mice. (A) H&E-stained sections and immunostaining for the indicated proteins from intestinal crypts at 4 h post 4 Gy. Note apoptotic cells in wild-type (WT) and DNA-PK−/− p53−/− mice but not in Atm−/− p53−/−, Lig4−/− p53−/− or p53−/− mice. Increased p-H2AX is seen in both DNA-PK−/− and Lig4−/− crypts, but persistent pCHK2 staining is seen only in DNA-PK−/− p53−/− crypts, indicating that p53-independent apoptosis correlates with pCHK2 activation but not DNA damage per se. Arrowheads indicate positive stained cells. (B) Quantification of pCHK2 staining in intestinal crypts taken at 4 h post 4 Gy. (C) Time course of pCHK2 staining in crypts. Bars are the mean values plus standard deviations from 3–5 mice per genotype except for Lig4−/− p53−/− (n=2) and Atm−/− p53−/− (n=2). Note increased levels and persistent pCHK2 staining in scid/scid p53−/− mutant mice compared with other genotypes. Atm, ataxia telangiectasia mutated; DNA-PK, DNA-dependent protein kinase; H&E, haematoxylin and eosin; IR, ionizing radiation; Lig4, Ligase IV; ND, not done, pCHK2, phosphorylated checkpoint kinase 2; pH2AX, phosphorylated histone H2AX; Scid, severe combined immunodeficiency.
To exclude the possibility that the mutant DNA-PKcs protein in SCID mice acts in a gain-of-function manner to activate apoptosis, we examined DNA-PKcs knockout mice. DNA-PKcs−/− p53−/− compound null mice were also highly sensitized to apoptosis (Fig 1). Rescue of the apoptotic defect of p53-null cells by mutation of DNA-PKcs was seen on the C57BL/6, 129 × C57BL/6 and C3H × C57BL/6 genetic backgrounds (data not shown), showing that this phenotype is independent of strain background. We also observed a DNA-PKcs gene dosage effect on p53-independent apoptosis, as the number of apoptotic cells per crypt in irradiated scid/+ p53−/− mice (0.65±0.3) was sixfold greater than in littermate control p53−/− mice (0.1±0.01; Fig 1D).
In wild-type mice, ionizing radiation-induced apoptosis is not uniformly distributed through the small intestinal crypt, but is localized between cell positions 4–10, which overlap with the stem cell and proliferative compartment of this tissue (Barker et al, 2008). It has been shown previously that the distribution of p53-positive cells overlaps precisely with the distribution of apoptotic cells (Merritt et al, 1994; Fig 1). This observation and the absence of apoptosis in p53-null mice suggested that the apoptotic sensitivity of these cells is due to p53 and its regulation. However, analysis of the spatial distribution of apoptotic cells in DNA-PK−/− p53−/− mice showed a nearly identical profile as in wild-type mice (Fig 1B), indicating that the apoptotic sensitivity of these cells is not solely due to p53.
Next, we used a genetic analysis to address the mechanism of this p53-independent apoptotic response. To determine whether this was unique to the loss of DNA-PKcs or whether it could be triggered by deficiency in any component of the NHEJ pathway, we used a series of mouse mutants, all of which result in defective DNA DSB repair and radiosensitivity. Both Ku70−/− p53−/− and Ku80−/− p53−/− mice showed an apoptotic response virtually identical to wild-type mice and again much greater than their p53−/− littermates (Fig 1A,D). Therefore, disabling any component of the DNA-PK holoenzyme, Ku70, Ku80 or DNA-PKcs, equally and efficiently sensitizes cells to p53-independent apoptosis. Next, we tested the final component of the NHEJ pathway, LIG4. Similar to DNA-PK, deficiency in LIG4 results in the inefficient repair of DSBs, confirmed here by increased phosphorylated histone H2AX (p-H2AX) staining in irradiated crypt cells (Fig 2A). However, in marked contrast to DNA-PK/p53-deficient mice, intestinal crypt cells from Lig4−/− p53−/− mice were resistant to ionizing radiation-induced apoptosis (Figs 1, 2). This shows that p53-independent apoptosis is not triggered by deficiencies in all NHEJ components, but is specific to DNA-PK.
Deficiency in the PIKK ATM also confers a radiosensitive phenotype. To determine whether the loss of ATM engaged p53-independent apoptosis, we compared the apoptotic response of Atm−/− and Atm−/− p53−/− mice. Intestinal crypt cells from irradiated Atm−/− mice showed robust, nearly wild-type levels of apoptosis, as reported previously (Gurley & Kemp, 2007), whereas those from Atm−/− p53−/− mice were as resistant as p53−/− mice (Figs 1D, 2). Therefore, the loss of Ku70, Ku80 and DNA-PKcs, but not ATM or LIG4, sensitizes p53-null cells, indicating that p53-independent apoptosis is triggered specifically by the loss of DNA-PK.
Next, we examined possible mediators of this apoptotic response. The serine/threonine kinase, checkpoint kinase 2 (CHK2), is a crucial effector in the cellular response to DNA damage (Bartek & Lukas, 2003). CHK2 is phosphorylated at Thr 68 by ATM at the sites of DSBs (Shiloh, 2003) and can, in turn, phosphorylate p53, among other substrates. CHK2 has been shown to modulate apoptosis in p53-deficient cancer cell lines, suggesting it has p53-independent functions (Yang et al, 2002; Stevens et al, 2003; Urist et al, 2004). Radiation induced a rapid increase in phospho-CHK2 (pCHK2; Thr 68) in crypt cells that peaked at 2 h and was already reduced by 4 h (Fig 2C). Irradiated CHK2-null tissue showed no staining, confirming antibody specificity (Supplementary Fig 2 online). Irradiated Atm-null crypts showed reduced levels of pCHK2, indicating that ATM regulates CHK2 in this setting (Fig 2). By contrast, high levels of pCHK2 staining persisted in DNA-PKcs−/− p53−/−, Ku70−/− p53−/− and Ku80−/− p53−/− mice for 4 h and was still detected 24 h after irradiation. Prominent pCHK2 staining was not seen in Lig4−/−p53−/−, Atm−/− p53−/− or p53−/− mice, genotypes that are resistant to apoptosis. E2F transcription factor 1 (E2F1), promyelocytic leukaemia (PML) and p73 have been proposed to be mediators of CHK2-regulated apoptosis; however, expression patterns of these proteins in irradiated crypts did not correlate with genotype or apoptosis (data not shown).
Next, we investigated whether p53-independent apoptosis is mediated through the intrinsic apoptotic pathway. This pathway is instigated by the activation of BH3-only proteins, leading to BCL2-associated X protein (BAX)- and BCL2-antagonist/killer 1 (BAK)-mediated release of cytochrome c from the mitochondria (Danial & Korsmeyer, 2004). Cytosolic cytochrome c induces oligomerization of apoptotic peptidase activating factor 1 (APAF1) to form the apoptosome, which activates and cleaves pro-caspase 9, the initiator caspase in the intrinsic apoptosis pathway (Riedl & Salvesen, 2007). Active caspase 9, in turn, triggers the cleavage and activation of the executioner caspase 3, which is common to both intrinsic and extrinsic apoptotic pathways. Staining for cleaved caspases 9 and 3 was clearly detected in crypt cells undergoing apoptosis from both wild-type and DNA-PK p53-deficient mice at levels quantitatively similar to apoptosis, but was not detected in the apoptotic-resistant genotypes (Fig 2; Supplementary Table I online). This shows that p53-independent apoptosis, engaged by the loss of DNA-PK, is executed through the intrinsic mitochondrial pathway.
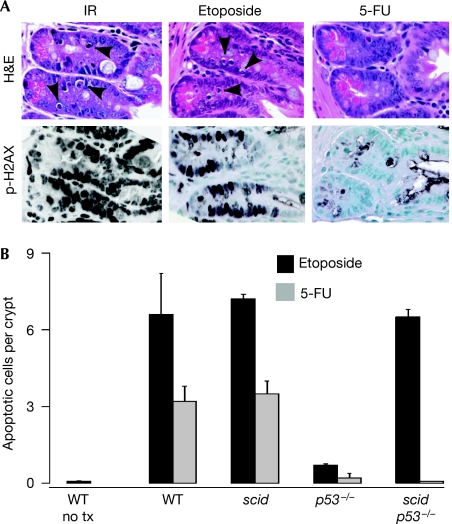
The chemotherapy agents etoposide and 5-fluorouracil also induce p53-dependent apoptosis, so it was of clinical interest to determine whether the absence of DNA-PK sensitized p53-null cells to these agents. Treatment of mice with the type II topoisomerase inhibitor etoposide induced crypt cell apoptosis at 4 h in wild-type, scid/scid and scid/scid p53−/− mice but not p53−/− mice (Fig 3). By contrast, the antimetabolite 5-fluorouracil induced apoptosis (peaking at 24 h) in wild-type and scid/scid mutant mice, but not in p53−/− or scid/scid p53−/− mice. Radiation and etoposide are thought to induce apoptosis through the creation of DSBs, whereas 5-fluorouracil induces apoptosis through alterations in DNA replication or RNA metabolism (Pritchard et al, 1997). Consistent with this, p-H2AX was prominent in crypt cells from radiation- and etoposide-treated mice, but not 5-fluorouracil-treated mice (Fig 3). This shows that the loss of DNA-PK does not rescue all modes of p53-dependent apoptosis, but is specific to DNA damage-initiated apoptosis.
Figure 3.
Role of DNA-PK in chemotherapy-induced apoptosis. (A) H&E-stained sections and immunostaining for p-H2AX of small intestinal crypts from irradiated (4 h post 4 Gy), etoposide (4 h) or 5-FU (24 h)-treated mice. (B) Quantification of apoptotic cells in small intestinal crypts after treatment with etoposide or 5-FU. Bars are the mean values plus standard deviations from 3–8 mice per genotype. DNA-PK, DNA-dependent protein kinase; H&E, haematoxylin and eosin; 5-FU, 5-fluorouracil; IR, ionizing radiation; p-H2AX, phosphorylated histone H2AX.
Discussion
Here, we show that DNA-PK is not required for the canonical p53-mediated apoptotic response to DNA damage. By contrast, DNA-PK has an anti-apoptotic function, suppressing a robust apoptotic response that is entirely p53 independent. By using a combination of genetic, chemical and immunohistochemical analyses, we characterized this DNA-PK-regulated apoptotic response. Previous studies have shown that p53 induction and apoptosis coincided precisely both spatially and temporally within the intestinal crypt, suggesting that the apoptotic sensitivity of these cells is attributed solely to p53 or its regulation. Apoptosis in DNA-PK/p53-null mice occurred in the same cell positions and, with the exception of a slight delay, with similar kinetics. This indicates that these cells are intrinsically sensitive and that apoptosis can be triggered either by p53-dependent or p53-independent pathways. This region contains the transit-amplifying compartment that is responsible for generating many of the differentiated cell types of the small intestine. The existence of redundant pathways to regulate apoptosis in these cells might act as a fail-safe mechanism to delete damaged cells and prevent neoplastic transformation.
Apoptosis in DNA-PK/p53-deficient mice was induced by ionizing radiation and etoposide, but not by 5-fluorouracil, implicating DNA damage as the initial apoptotic trigger. However, several lines of evidence indicate that DNA damage per se is insufficient to trigger rapid-onset p53-independent apoptosis. First, radiation doses as high as 20 Gy failed to induce rapid apoptosis in p53-null intestinal crypts. Second, the ionizing radiation-induced apoptosis that is seen in p53-null mice is modest, markedly delayed (>24 h after ionizing radiation) and coincides with the resumption of abundant mitotic activity. The apoptotic bodies at these late time points were larger and multinucleate, suggesting that they are undergoing G2/M cell death as an indirect consequence of the radiation damage (Merritt et al, 1997). By contrast, the rapid apoptosis (4 h after ionizing radiation) seen in DNA-PK−/− p53−/− mice is likely a direct response to ionizing radiation, arguing that the apoptotic responses in DNA-PK−/− p53−/− and p53−/− mice are fundamentally different. Third, although LIG4 and ATM deficiency leads to defective DNA repair and radiosensitivity, crypt cells from Lig4/p53 and Atm/p53 double-null mice remained resistant to radiation-induced apoptosis. Finally, the fact that deletion of Ku70, Ku80 or DNA-PKcs all showed a quantitatively equivalent and nearly complete rescue of apoptosis in a p53-null background implicate a direct role of the DNA-PK holoenzyme in the suppression of DNA damage-initiated p53-independent apoptosis. A common phenotype associated with the loss of Ku70, Ku80 and DNA-PKcs is the inability to activate the holoenzyme DNA-PK and to process DNA DSBs. Deficiency of LIG4 also results in unrepaired breaks, but with one important distinction: the activity of DNA-PK would not be impaired, and therefore the breaks would be synapsed and processed. Unlike any of the DNA-PK subunits, LIG4 deficiency leads to embryonic lethality, attributed to massive p53-dependent apoptosis (Frank et al, 2000). This lethality is rescued by p53 deletion, as Lig4−/− p53−/− mice are viable. This crucial phenotypic distinction among components of the NHEJ pathway is extended by our studies to the differential regulation of p53-independent apoptosis.
p53-independent apoptosis was tightly associated with increased pCHK2 and caspase 9 activation, suggesting that the absence of DNA-PK, either directly through the loss of kinase activity or indirectly through the presence of unprocessed DSBs, leads to hyperactivation of ATM signalling through CHK2 and/or other downstream targets resulting in engagement of the intrinsic apoptotic pathway.
Speculation
DNA DSBs activate two mutually exclusive responses: DNA repair, leading to cell survival, and apoptosis, leading to cell death; however, the regulation of the choice between these responses is poorly understood. It is now apparent that DNA-PK has a crucial function in both, in that it directly mediates DSB repair and suppresses apoptosis. Its role in tumour suppression (Kemp et al, 1999) or its usefulness as a target in cancer therapy (O'Connor et al, 2007) will likely depend on which of these responses predominate. Enhanced apoptosis in the absence of DNA-PK provides an explanation for the radiosensitive phenotype that has long been associated with DNA-PK. Importantly, p53 is not required for this cell death response, pointing to the NHEJ apparatus as a potential target to sensitize cancer cells.
Methods
Mice. DNA-PKcs knockout mice on a 129 × C57BL/6 background (Gao et al, 1998) or C57BL/6 (Prdkdcscid) mice (The Jackson Laboratory, Bar Harbor, ME, USA) were crossed with C57BL/6 p53 knockout mice (from L. Donehower) to generate DNA-PKcs−/− p53−/− or scid/scid p53−/− mice and control littermates. Atm−/− (Barlow et al, 1996), Ku80−/− (Zhu et al, 1996), Ku70−/− (Gu et al, 1997) and Lig4+/− (Frank et al, 2000) mice were similarly crossed with p53-null mice to generate Atm−/− p53−/−, Ku80−/− p53−/−, Ku70−/− p53−/− and Lig4−/− p53−/− mice. Genotyping was carried out using established PCR protocols and conditions, and are available on request. Here, 6- to 10-week-old mice were given 4 Gy of whole-body ionizing radiation using a Co60 source and killed at given times post-irradiation. Unirradiated age-matched littermate controls were also killed for tissues. Tumour-bearing APCMin mice (The Jackson Laboratory) were irradiated and tissues were taken 4 h post-ionizing radiation. For chemotherapy studies, mice were injected with etoposide (40 μg/g, i.p.) or 5-fluorouracil (40 μg/g, i.p.), and tissues were examined at 4 and 24 h, respectively.
Histology. Tissues were fixed, processed and stained for p53 (CM5; Novocastra Laboratories Ltd, Newcastle upon Tyne, UK), cleaved caspase 9 (Asp 353; ab52298; Abcam, Cambridge, MA, USA), cleaved caspase 3 (Asp175; Cell Signaling Technologies Inc, Danvers, MA, USA), pCHK2 (Thr 68; Abcam) and phospho-histone H2AX (S139; Cell Signaling) using a three-step streptavidin technique as described previously (Gurley & Kemp, 2007). Numbers of apoptotic figures or positive-staining cells were counted in intestinal crypts of ten × 40 fields or in ten × 100 fields of intestinal adenomas.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Fred Alt for providing DNA-PKcs and DNA ligase IV knockout mice, John Petrini and Noburo Motoyama for providing Chk2-null tissue, and S. Lawrence Bailey and Valerie Holcomb for technical assistance. This study was supported by grants from the National Institutes of Health (to C.J.K. and P.H.) and from the American Cancer Society (to Y.G.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Barker N, van de WM, Clevers H (2008) The intestinal stem cell. Genes Dev 22: 1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C et al. (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171 [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429 [DOI] [PubMed] [Google Scholar]
- Blunt T, Gell D, Fox M, Taccioli GE, Lehmann AR, Jackson SP, Jeggo PA (1996) Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci USA 93: 10285–10290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Attardi LD (2005) The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 5: 231–237 [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- DeFazio LG, Stansel RM, Griffith JD, Chu G (2002) Synapsis of DNA ends by DNA-dependent protein kinase. EMBO J 21: 3192–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, Manis JP, Horner J, DePinho RA, Alt FW (2000) DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell 5: 993–1002 [DOI] [PubMed] [Google Scholar]
- Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver DT, Alt FW (1998) A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity 9: 367–376 [DOI] [PubMed] [Google Scholar]
- Gu Y, Jin S, Gao Y, Weaver DT, Alt FW (1997) Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci USA 94: 8076–8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA (2003) The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 3: 117–129 [DOI] [PubMed] [Google Scholar]
- Gurley KE, Kemp CJ (2001) Synthetic lethality between mutation in Atm and DNA-PK(cs) during murine embryogenesis. Curr Biol 11: 191–194 [DOI] [PubMed] [Google Scholar]
- Gurley KE, Kemp CJ (2007) Atm is not required for p53 induction and apoptosis in irradiated epithelial tissues. Mol Cancer Res 5: 1312–1318 [DOI] [PubMed] [Google Scholar]
- Gurley KE, Vo K, Kemp CJ (1998) DNA double strand breaks, p53, and apoptosis during lymphomagenesis in scid/scid mice. Cancer Res 58: 3111–3115 [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW (2002) Apoptosis: a link between cancer genetics and chemotherapy. Cell 108: 153–164 [DOI] [PubMed] [Google Scholar]
- Kemp CJ, Vo K, Gurley KE (1999) Resistance to skin tumorigenesis in DNAPK-deficient SCID mice is not due to immunodeficiency but results from hypersensitivity to TPA-induced apoptosis. Carcinogenesis 20: 2051–2056 [DOI] [PubMed] [Google Scholar]
- Lees-Miller SP, Meek K (2003) Repair of DNA double strand breaks by non-homologous end joining. Biochimie 85: 1161–1173 [DOI] [PubMed] [Google Scholar]
- Lees-Miller SP, Sakaguchi K, Ullrich SJ, Appella E, Anderson CW (1992) Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol Cell Biol 12: 5041–5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Lane DP, Hall PA (1994) The role of spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53 deficient mice. Cancer Res 54: 614–617 [PubMed] [Google Scholar]
- Merritt AJ, Allen TD, Potten CS, Hickman JA (1997) Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after γ-irradiation. Oncogene 14: 2759–2766 [DOI] [PubMed] [Google Scholar]
- Nelson WG, Kastan MB (1994) DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol 14: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MJ, Martin NM, Smith GC (2007) Targeted cancer therapies based on the inhibition of DNA strand break repair. Oncogene 26: 7816–7824 [DOI] [PubMed] [Google Scholar]
- Pritchard CA, Watson AJ, Potten CS, Jackman AL, Hickman J (1997) Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluoruracil: evidence for the involvement of RNA perturbation. Proc Natl Acad Sci USA 94: 1795–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS (2007) The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 8: 405–413 [DOI] [PubMed] [Google Scholar]
- Roos WP, Kaina B (2006) DNA damage-induced cell death by apoptosis. Trends Mol Med 12: 440–450 [DOI] [PubMed] [Google Scholar]
- Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168 [DOI] [PubMed] [Google Scholar]
- Shrivastav M, De Haro LP, Nickoloff JA (2008) Regulation of DNA double-strand break repair pathway choice. Cell Res 18: 134–147 [DOI] [PubMed] [Google Scholar]
- Smith GC, Jackson SP (1999) The DNA-dependent protein kinase. Genes Dev 13: 916–934 [DOI] [PubMed] [Google Scholar]
- Spagnolo L, Rivera-Calzada A, Pearl LH, Llorca O (2006) Three-dimensional structure of the human DNA-PKcs/Ku70/Ku80 complex assembled on DNA and its implications for DNA DSB repair. Mol Cell 22: 511–519 [DOI] [PubMed] [Google Scholar]
- Stevens C, Smith L, La Thangue NB (2003) Chk2 activates E2F-1 in response to DNA damage 25. Nat Cell Biol 5: 401–409 [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Jacks T, Cory S (1994) DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 79: 329–339 [DOI] [PubMed] [Google Scholar]
- Su L-K, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF (1992) Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256: 668–670 [DOI] [PubMed] [Google Scholar]
- Urist M, Tanaka T, Poyurovsky MV, Prives C (2004) p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev 18: 3041–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lu X (2002) Live or let die: the cell's response to p53. Nat Rev Cancer 2: 594–604 [DOI] [PubMed] [Google Scholar]
- Yang S, Kuo C, Bisi JE, Kim MK (2002) PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nat Cell Biol 4: 865–870 [DOI] [PubMed] [Google Scholar]
- Zhu C, Bogue MA, Lim DS, Hasty P, Roth DB (1996) Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell 86: 379–389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information