Abstract
Arf (ADP-ribosylation factor) family small G proteins are crucial regulators of intracellular transport. The active GTP-bound form of Arf interacts with a set of proteins—effectors—which mediate the downstream signalling events of Arf activation. A well-studied class of Arf1 effectors comprises the coat complexes, such as the cis-Golgi-localized COPI (coat protein complex I) coat, and trans-Golgi network-endosomal clathrin coats. At least five different coats require Arf1-GTP to localize to organelle membranes. How a single Arf protein recruits different coat complexes to distinct membrane sites raises the question of how specificity is achieved. Here, we propose a molecular mechanism of this specificity for the COPI coat by showing a direct and specific interaction between a COPI subunit and a cis-Golgi localized subfamily of Arf guanine nucleotide exchange factors (GEFs) that takes place independently of Arf1 activation. In this way, a specific output on Arf1 activation can be programmed before the exchange reaction by the GEF itself.
Keywords: guanine nucleotide exchange factor (GEF), COPI, ADP-ribosylation factor (Arf), small G protein, Golgi
Introduction
Arf (ADP-ribosylation factor) family proteins act as molecular switches to control the organization of the secretory and endosomal pathways of eukaryotic cells (Gillingham & Munro, 2007). Regulatory proteins modulate the activation and inactivation cycle of Arf, with guanine nucleotide exchange factors (GEFs) promoting the formation of active Arf-GTP (Cox et al, 2004; Shin & Nakayama, 2004; Casanova, 2007; Gillingham & Munro, 2007), and GTPase-activating proteins (GAPs) hydrolysing the associated GTP to return Arf to its inactive GDP-bound state (Gillingham & Munro, 2007). In the Golgi and endosomal systems of mammalian cells, there are three Arf1 GEFs—Golgi-associated brefeldin A (BFA)-resistant GEF 1 (GBF1), BFA-inhibited guanine nucleotide exchange protein (BIG) 1 and BIG2—which have distinct localizations and functions (Shin & Nakayama, 2004; Casanova, 2007; Gillingham & Munro, 2007). These Arf GEFs are highly conserved in evolution: the yeast homologues of GBF1, the redundant Gea1p and Gea2p proteins (Gea for guanine nucleotide exchange on Arf), are localized to the cis-Golgi as is GBF1, and carry out similar functions. Sec7p, the yeast homologue of BIG1 and BIG2, localizes to and functions at trans-Golgi and endosomal membranes, similar to its mammalian counterparts (Zhao et al, 2002; Shin & Nakayama, 2004; Gillingham & Munro, 2007). The GBF/Gea and BIG/Sec7 GEFs are large multidomain proteins that share several common domains (Cox et al, 2004; Mouratou et al, 2005), as well as domains specific to each subfamily (Cox et al, 2004). The amino-terminal regions of these large Arf GEFs contain the common domains DCB (dimerization and cycophilin-binding), required for homodimerization, and HUS (homology upstream from Sec7), the function of which is unknown (Mouratou et al, 2005; Ramaen et al, 2007). We have recently shown that for GBF1, BIG1 and BIG2, the DCB and HUS domains interact both in vivo and in vitro (Ramaen et al, 2007).
The best-studied role of Arf1 is the recruitment and/or maintenance of coat complexes on organelle membranes (Bonifacino & Lippincott-Schwartz, 2003; Bethune et al, 2006). Arf1-GTP is responsible for the recruitment and/or maintenance of the coat protein complex I (COPI) coat on cis-Golgi membranes, and also maintains adaptor protein complex 1 (AP1)/clathrin, Golgi-localized, γ ear-containing, ARF-binding protein (GGA)/clathrin, AP3 and AP4 coats on trans-Golgi network and endosomal membranes. The fact that a single class of Arf proteins—Arf1 and Arf3 in mammalian cells, Arf1 and Arf2 in yeast—can recruit different coat complexes to distinct membrane sites within the cell raises the question of how specificity is achieved. An attractive hypothesis is that the Arf regulators are involved in accomplishing this task. Indeed, for AP3, it has been shown that an Arf GAP, AGAP1, is involved in specifically recruiting this coat to endosomal membranes, although the precise molecular mechanism has not been elucidated (Nie et al, 2003). For the COPI, AP1/clathrin and GGA/clathrin coats, there is evidence that the Arf GEFs are involved in the specificity of recruitment (Shin & Nakayama, 2004; Casanova, 2007; Gillingham & Munro, 2007). The fact that the GBF/Gea and BIG/Sec7 Arf GEFs are large, multidomain proteins suggest that they have many interacting partners that integrate spatial and temporal cues to regulate when and where Arf1 will be activated in cells. An interesting hypothesis is that the Arf GEFs could interact specifically with effectors to programme a particular output on Arf1 activation. Here, we show a direct interaction between GBF1/Gea Arf GEFs and the γ-COP subunit of the COPI coat. This interaction is evolutionarily conserved and is specific, as no interaction between γ-COP and BIG/Sec7 Arf GEFs was observed.
Results And Discussion
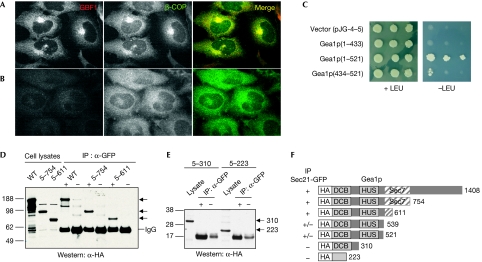
To explore the possibility that Arf GEFs are involved in the recruitment of specific coat complexes to membranes, we carried out depletion experiments using small interfering RNAs directed against GBF1, BIG1 and BIG2. We found that depletion of either BIG1 or BIG2, or both together had no effect on the localization of COPI to the Golgi, whereas depletion of GBF1 had a marked effect, causing complete relocation of COPI from the Golgi to the cytosol (Fig 1A,B; supplementary Fig S1 online). Therefore, GBF1, but not BIG1 or BIG2, is required in cells for the localization of COPI to Golgi membranes (see also supplementary information online).
Figure 1.
Functional and physical interaction between GBF/Gea Arf guanine nucleotide exchange factors and the COPI coat. (A,B) Specificity of recruitment of COPI by GBF1 in vivo. HeLa cells were transfected with short interfering RNAs targeting (A) BIG1+BIG2, or (B) GBF1 and prepared for immunofluorescence analysis using antibodies against GBF1 and β-COP. (C) Yeast two-hybrid interaction between the yeast GBF1 homologue, Gea1p, and yeast γ-COP, Sec21p. Bait plasmid carrying full-length Sec21p was co-transformed into yeast with prey plasmid alone or carrying the indicated regions of Gea1p. Cells were grown on control (+LEU) and selective medium (−LEU) to monitor the expression of the reporter. (D) Lysates from yeast strain CJY104 SEC21∷3xGFP carrying a low-copy plasmid expressing HA-Gea1p, HA-Gea1p(5–754) or HA-Gea1p(5–611), were prepared and incubated with GFP antibodies (+) or IgG alone (−). Bound material was analysed by Western blot using HA antibodies. (E) Same as (D) except CJY104 carried plasmids expressing HA-Gea1p(5–310) or HA-Gea1p(5–223). (F) Summary of co-IPs using indicated truncations of Gea1p. Arf, ADP-ribosylation factor; BIG, brefeldin A-inhibited guanine nucleotide exchange protein; co-IP, co-immunoprecipitation; COPI, coat protein complex I; DCB, dimerization and cycophilin-binding domain; GBF1, Golgi-associated brefeldin A-resistant guanine nucleotide exchange factor 1; Gea, guanine nucleotide exchange on Arf; GFP, green fluorescent protein; HA, haemagglutinin; HUS, homology upstream from Sec7 domain; Sec7, catalytic domain; WT, wild type.
Interaction between Gea1p and yeast γ-COP (Sec21p)
As a first step to testing the hypothesis that there is a physical association between GEFs and coats, we carried out yeast two-hybrid assays. We found that one subunit of COPI, γ-COP (Sec21p in yeast), interacted with the N-terminal region of Gea1p (the yeast homologue of GBF1; Fig 1C). A series of deletion mutants of this N-terminal region all failed to interact with Sec21p, except for one construct containing amino acids 434–521 of Gea1p, which showed a weak but reproducible interaction with Sec21p (Fig 1C; supplementary Fig S2 online). Next, we performed co-immunoprecipitation (co-IP) experiments to assess the association between Gea1p and Sec21p in yeast cells. We expressed haemagglutinin (HA)-tagged Gea1p in a strain in which the chromosomal copy of Sec21p was fused to three tandem copies of green fluorescent protein (GFP) at its carboxyl terminus (Park et al, 2005). A significant fraction of HA-Gea1p was present in a complex with immunoprecipitated Sec21p-GFP (Fig 1D); Gea2p-HA also co-immunoprecipitated with Sec21p-GFP (data not shown). We tested a series of C-terminal truncations for interaction with Sec21p, and confirmed the yeast two-hybrid result showing that the N-terminal region of Gea1p upstream from the Sec7 domain interacts with Sec21p (Fig 1D,F; supplementary Fig S2 online). Deletions past amino acid 520 did not interact detectably with Sec21p in co-IPs (Fig 1E,F).
Sec21p interaction with Gea1p and Gea2p is direct
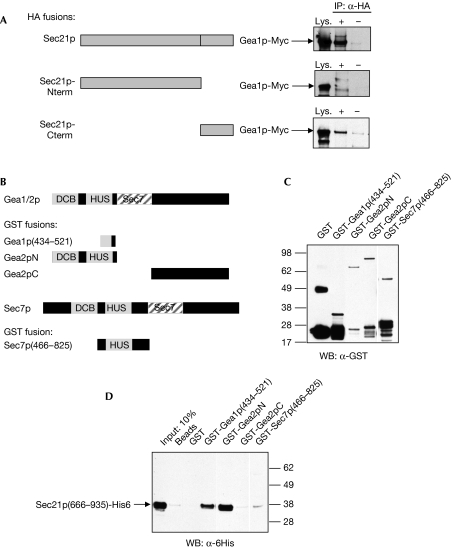
The γ-COP subunit of COPI is composed of two domains: a large N-terminal domain and a smaller C-terminal appendage domain (Bonifacino & Lippincott-Schwartz, 2003). The crystal structure of the latter domain has been solved and shows remarkable similarity to the appendage domains of the clathrin adaptors such as AP2 (Hoffman et al, 2003; Watson et al, 2004). To determine which domain of Sec21p interacts with Gea1p, we performed co-IPs in yeast expressing either the N or C terminus of Sec21p. We found no interaction between Gea1p and the N-terminal domain, but did observe co-IP of the C-terminal appendage domain with Gea1p (Fig 2A). These results indicate that Gea1p interacts with the C-terminal appendage domain of Sec21p.
Figure 2.
Gea1p interacts with the carboxy-terminal appendage domain of Sec21p. (A) Cell lysates were prepared from strain CJY110 GEA1∷13xMyc carrying plasmids expressing the indicated portions of Sec21p-HA (Sec21p-Nterm: aa 1–665; Sec21p-Cterm: aa 666–953), and immunoprecipitations (IP) were carried out with HA antibody (+) or IgG alone (−). Western blot (WB) analysis was performed using Myc antibody to detect endogenous Myc-tagged Gea1p. (B) Schematic diagram showing regions of Gea1p, Gea2p and Sec7p expressed as GST fusions in Escherichia coli. (C) Western blot analysis of GST fusion constructs expressed in E. coli. (D) The indicated GST fusion constructs were expressed in E. coli, purified on a glutathione-Sepharose column, and Sec21p(666–935)-His6 purified from E. coli was passed over the columns. Eluates were subject to western blot analysis with His6 antibodies. The first lane shows 10% of the input of Sec21p(666–935)-His6. DCB, dimerization and cycophilin-binding domain; Gea, guanine exchange factor on Arf; GST, glutathione S-transferase; HA, haemagglutinin; HUS, homology upstream from Sec7 domain.
To determine whether the interaction between Gea1/2p and Sec21p was direct, we expressed the interacting domains in Escherichia coli and tested the interaction between the purified proteins. The C-terminal appendage domain of Sec21p was tagged with six histidines (His6), and various domains of Gea1p or Gea2p were expressed as glutathione S-transferase (GST) fusions in E. coli (Fig 2B,C). The N-terminal portion of Gea2p retained a significant amount of the Sec21p C-terminal domain (approximately 10%), whereas the C-terminal domain of Gea2p showed no detectable interaction with Sec21p (Fig 2D). The region between amino acids 434 and 521 of Gea1p, which showed weak interaction with Sec21p in the yeast two-hybrid assay, showed some capacity to bind to the purified Sec21p appendage domain (Fig 2D).
If the GEF–coat interaction determines specificity of coat recruitment, other Arf GEFs should not interact with γ-COP. To test specificity of the Gea1/2p-Sec21p interaction, we purified an N-terminal region of the Arf GEF Sec7p fused to GST (Fig 2B). We could not detect any interaction between the N terminus of Sec7p and the appendage domain of Sec21p (Fig 2C,D). These results show that there is a direct interaction between the yeast Sec21p appendage domain and the N-terminal region of Gea1p and Gea2p, and that the interaction is specific to this subfamily of Arf GEFs.
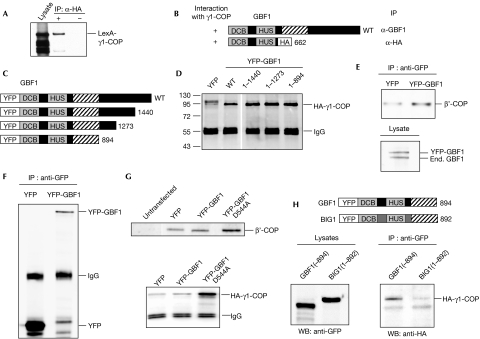
Arf GEF GBF1 interacts with γ-COP in mammalian cells
Given the evolutionary conservation of COPI and the Arf GEFs, we tested next the interaction between the single mammalian homologue of the Gea1p/Gea2p pair, GBF1, and mammalian γ-COP. First, we tested the interaction between human GBF1 and human γ1-COP, the main mammalian homologue of Sec21p (Wegmann et al, 2004), by the expression of both proteins in yeast followed by co-IP. The human proteins interacted in this assay and, as for Gea1p, the N terminus of GBF1 was sufficient for the interaction (Fig 3A,B).
Figure 3.
The amino-terminal region of human GBF1 interacts with mammalian γ1-COP. Yeast strain BY4742 carrying GBF1(1–662)-2 × HA (A,B) or YEp352-GBF1 (B) and pEG202-LexA-γ1-COP was subjected to immunoprecipitation (IP) analysis as described in Fig 1 with HA or GBF1 antibodies, respectively. Western blot (WB) analysis was carried out using antibodies against LexA. (C) Schematic diagram showing the series of carboxy-terminal truncations of human GBF1 tested for interaction with bovine HA-γ1-COP. (D) Lysates from COS7 cells expressing YFP alone or the indicated human Venus-GBF1 construct and bovine HA-γ1-COP were incubated with GFP antibodies. Immunoprecipitates were subjected to Western blot analysis using HA antibodies. (E) Co-IP experiments as in (D) were carried out with COS7 cells expressing YFP alone or human Venus-GBF1. Immunoprecipitates were subjected to Western blot analysis using β′-COP antibodies to detect endogenous COPI (upper panel). Lysate from cells expressing Venus-GBF1 was analysed by Western blot using GBF1 antibodies to detect YFP-GBF1 and endogenous (End.) GBF1 (lower panel). A representative blot of three independent experiments is shown. (F) Co-IP experiments as in (E) were carried out with COS7 cells expressing YFP alone or human Venus-GBF1, and the immunoprecipitated proteins were detected using GFP antibodies. A representative blot of three independent experiments is shown. (G) Co-IPs were carried out with cells expressing no YFP-tagged protein (untransfected), YFP alone, human Venus-GBF1 or Venus-GBF1-D544A, and endogenous β′-COP was detected in co-IPs using β′-COP antibodies (upper panel). Cells were co-transfected with human HA-γ1-COP and YFP alone, human Venus-GBF1 or Venus-GBF1-D544A, and Western blots were carried out with HA antibodies (lower panel). A representative blot of three independent experiments is shown. (H) The indicated human GBF1 or BIG1 constructs were co-expressed with human HA-γ1-COP in COS7 cells and co-IPs were carried out as in (D). BIG, brefeldin A-inhibited guanine nucleotide exchange protein; co-IP, co-immunoprecipitation; COPI, coat protein complex I; DCB, dimerization and cycophilin-binding domain; GBF1, Golgi-associated brefeldin A-resistant guanine nucleotide exchange factor 1; GFP, green fluorescent protein; HA, haemagglutinin; HUS, homology upstream from Sec7 domain; WT, wild type; YFP, yellow fluorescent protein.
Next, we carried out co-IPs in mammalian cells. Yellow fluorescent protein (YFP)-tagged human GBF1 (Niu et al, 2005) and HA-tagged bovine γ1-COP were co-expressed in COS7 cells. A significant fraction of γ1-COP was present in a complex with immunoprecipitated GBF1 (Fig 3C,D), indicating that in mammalian cells GBF1 and γ1-COP were physically associated. Next, we tested three YFP-tagged C-terminal truncations of GBF1 (Fig 3C) for their capacity to co-IP with γ1-COP and found that all of them interacted (Fig 3D), indicating that the N-terminal region of GBF1 was sufficient for the interaction. The conditions used for the co-IPs significantly affected the ability to detect the GBF1–γ1-COP interaction. Wash buffer containing detergent and a higher salt concentration led to the recovery of a significantly smaller amount of γ1-COP in the YFP-GBF1 immunoprecipitate (supplementary Fig S2C online). Hence the interaction between the full-length proteins was fairly weak, making it difficult to detect interaction between the endogenous proteins. However, we were able to detect an interaction between endogenous COPI and YFP-GBF1 expressed at the same level as the endogenous protein, using the β′-COP antibody (Fig 3E). The level of expression of YFP alone was much greater than YFP-GBF1 (Fig 3F), around 47±8 times higher. Normalizing to the amount of YFP or YFP-GBF1 protein present in cell lysates, 57±8-fold more β′-COP was co-immunoprecipitated with YFP-GBF1 compared with the YFP alone control (Fig 3E–G; see supplementary information online for details of quantifications). We have shown previously that a mutation in the highly conserved HUS box, GBF1-D544A, disrupts the interaction between the DCB and HUS domains of GBF1 (Ramaen et al, 2007). The expression level of the mutant YFP-GBF1-D544A was less than or equal to that of YFP-GBF1 (data not shown); however, a significantly higher fraction of either endogenous β′-COP (Fig 3G, upper panel) or co-expressed γ1-COP (Fig 3G, lower panel) co-immunoprecipitated with the GBF1-D544A mutant. The fact that the GBF1-D544A mutant shows a higher level of interaction with COPI than wild-type GBF1 suggests that the GEF–coat interaction is regulated, probably through conformational changes within GBF1 involving the DCB and HUS domains.
Next, we tested the specificity of the GBF1–γ1-COP interaction by performing experiments with the N-terminal region of BIG1. Although we clearly saw an interaction with the equivalent region of GBF1, no interaction was detected with BIG1 (Fig 3H). Hence the interaction between GBF1 and γ1-COP is specific to the GBF/Gea subfamily of Arf GEFs in both mammalian cells and yeast.
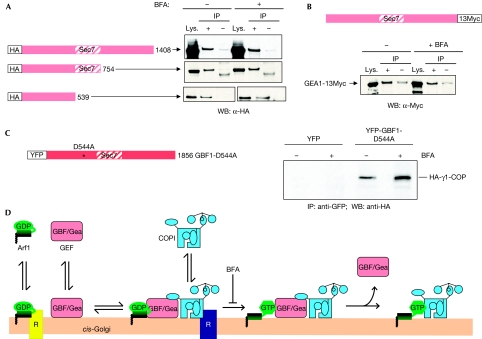
GEF–γ-COP interaction in the presence of brefeldin A
To determine whether Gea1p and Sec21p interact independently of Arf1 activation, we performed co-IPs in the presence of BFA. BFA acts as an uncompetitive inhibitor that binds to and traps an Arf1–GDP–GEF complex, thus blocking the exchange reaction (Peyroche et al, 1999; Mossessova et al, 2003; Renault et al, 2003). For full-length Gea1p, as well as two C-terminal truncations, the level of interaction was identical in BFA-treated and untreated cells (Fig 4A). To verify that this lack of effect of BFA was not due to resistance caused by overexpression of Gea1p (Peyroche et al, 1999), the experiment was repeated using a strain in which the endogenous copy of Gea1p was Myc-tagged; interaction between Gea1p-Myc and Sec21p was not affected by the addition of BFA (Fig 4B). In addition, the interaction between the N terminus of Gea1p (amino acids 1–539), which lacks the Sec7 domain and does not confer resistance to BFA, and Sec21p occured in the presence of the drug (Fig 4A).
Figure 4.
Interaction between Gea1p/GBF1 and γ-COP occurs independently of Arf activation. (A) CJY104 erg6Δ SEC21∷3xGFP cells expressing the indicated Gea1p construct were subjected to immunoprecipitations (IP) as described in Fig 1. Cells were treated with (+) or without (−) 100 μg/ml BFA for 10 min before the preparation of cell lysates, and 100 μg/ml BFA was maintained throughout the rest of the experiment. (B) CJY110 erg6Δ GEA1∷13xMyc expressing YEp352-SEC21-3 × HA cells in the absence or presence of 100 μg/ml BFA were subjected to immunoprecipitation analysis using HA antibodies, and the Western blot was probed with Myc antibodies to detect Gea1p. (C) COS7 cells expressing YFP alone or the Venus-GBF1-D544A and human HA-γ1-COP were treated with or without 25 μg/ml BFA for 5 min before the preparation of cell lysates, and 25 μg/ml BFA was maintained throughout the remainder of the experiment. Immunoprecipitation was performed with GFP antibodies, and Western blot analysis with HA antibodies. (D) Model for the interaction of the COPI coat with GBF/Gea Arf GEFs before Arf activation. Arf1, Arf GEFs and COPI all cycle rapidly between membranes and the cytosol. Receptors (R) such as p23/p24 have been identified for both Arf1-GDP and COPI (Bethune et al, 2006). Once associated with the membrane, Arf1-GDP, GBF/Gea and COPI form a complex. After nucleotide exchange, the Arf GEF is released and active Arf1-GTP stabilizes the association of COPI with the membrane. Arf, ADP-ribosylation factor; BFA, brefeldin A; COPI, coat protein complex I; GBF1, Golgi-associated BFA-resistant GEF1; Gea, guanine nucleotide exchange on Arf; GEF, guanine nucleotide exchange factor; HA, haemagglutinin; WB, Western blot; YFP, yellow fluorescent protein.
Next, we tested the interaction between human GBF1 and γ1-COP in mammalian cells in the presence of BFA. We used the GBF1-D544A mutant because its increased level of binding to γ1-COP provided a higher level of sensitivity to the assay. We found that GBF1 not only bound to γ1-COP in the presence of BFA but that the level of interaction was even higher. Around twofold more γ1-COP was recovered in the YFP-GBF1-D544A immunoprecipitate from BFA-treated cells compared with the untreated control (Fig 4C). Hence in mammalian cells, as in yeast, the interaction between GBF1 and γ1-COP can occur when the activation of Arf1 is blocked.
Our results provide a molecular explanation for the specificity of COPI coat recruitment by the GBF/Gea subfamily of Arf GEFs. We propose that the COPI coat interacts with the GBF1/Gea Arf GEF on membranes before GEF-mediated activation of Arf1, and hence that the coat is in place at the moment when Arf1-GTP is formed (Fig 4D). This model is distinct from the presently accepted idea that the activation of Arf and coat recruitment are sequential events. Only a fraction of each component is in the GEF–coat complex at any given time, and our data suggest that, at least in mammalian cells, the interaction is regulated. It is clear that GBF1 is not the sole localization determinant for COPI, as other membrane receptors for COPI have been identified (Bethune et al, 2006), and our data are consistent with the idea that Arf1-GDP, the GEF and COPI are recruited separately to membranes and interact transiently once all are present on the membrane. We have shown specificity of the GBF1/Gea–γ-COP interaction in terms of protein–protein interaction at the level of the Arf GEFs, but part of the specificity also comes from interactions of the coat with other receptors on the membrane site at which it is recruited. Indeed, a previous report suggests that GBF1 is involved in the recruitment of the GGA coat complex to Golgi membranes, indicating further levels of specificity than simply the GEF–coat interaction (Lefrancois & McCormick, 2007). The recruitment of GGA coats could be more complex than that of COPI because it has also been shown that BIG1 and BIG2 Arf GEFs are involved in their recruitment to the late Golgi (Manolea et al, 2008). An important question raised by our study is the mechanism by which GBF/Gea Arf GEFs are recruited to specific membrane sites, and evidence exists for determinants of localization in both the N- and C-terminal regions of the proteins (Gillingham & Munro, 2007). In conclusion, our results provide support for a new mechanism by which a specific output on Arf1 activation can be programmed before nucleotide exchange on Arf by the GEF itself.
Methods
Strains, plasmids and antibodies. See the supplementary information online.
GST pull-down and yeast two-hybrid assays. Purified GST or GST fusion proteins were immobilized on glutathione-Sepharose 4B beads (Amersham Biosciences, GE Health Care, Piscataway, NJ, USA). After blocking, the bound beads were incubated with purified His6-tagged protein in binding buffer at 4°C for 1 h. The beads were washed six times with washing buffer (20 mM HEPES pH 7.2, 200 mM KCl, 5 mM MgCl2, 1% Triton X-100). Bound proteins were resolved by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and shown by immunoblotting using His6 antibodies. For yeast two-hybrid assays, strain EGY48 was co-transformed with bait (HIS3) and prey (TRP1) plasmids, and reporter expression was assayed by growth on −LEU plates. Further details can be found in the supplementary information online.
Co-IP assays in yeast. Yeast cells were collected and cell lysates were prepared as described in the supplementary information online. Briefly, cleared cell lysates were diluted into lysis buffer and incubated with antibody or control IgG at 4°C for 1 h, followed by incubation with 50 μl pre-equilibrated Dynabeads Protein G (Dynal Biotech, Hamburg, Germany) at 4°C for 1 h. The resin was then washed six times with washing buffer (20 mM HEPES pH 7.2, 500 mM KCl, 5 mM MgCl2, 1% Triton X-100) and resuspended in SDS sample buffer. Bound proteins were resolved by SDS–PAGE and shown by immunoblotting. For experiments carried out in the presence of BFA, a final concentration of 100 μg/ml BFA was added 10 min before the preparation of cell lysates, and was maintained throughout the remainder of the co-IP procedure.
Co-IP assays in mammalian cells. Details of co-IP experiments in mammalian cells can be found in the supplementary information online. Briefly, a Venus-tagged version of human GBF1 or C-terminal truncations (Niu et al, 2005) and Bos taurus γ1-COP (Wu et al, 2000) with an HA epitope tag (Fig 3D) were co-expressed in COS7 cells. After 20 h, cells were washed and cell lysates were prepared. After centrifugation at 4°C, supernatants were incubated with 0.8 μg of GFP antibodies for 1.5 h at 4°C, followed by the addition of 20 μl of protein G Sepharose 4 Fast Flow and the mixtures were incubated at 4°C for 1.5 h. The resin was washed four times with 1 ml of buffer W100 (50 mM Tris–HCl pH 7.5, 100 mM NaCl and 1 mM EDTA), then twice with 1 ml PBS. Proteins were eluted in SDS–PAGE sample buffer, then subjected to SDS–PAGE and Western blotting using HA antibodies. In Fig 3E–G (upper panel), the resin was washed twice with W100, then once with PBS. In Fig 3G (lower panel), Figs 3H, 4C, COS7 cells were transfected with the indicated GBF1 constructs and Homo sapiens HA-γ1-COP. The resin was washed four times with W100, then twice with PBS.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to Anne Eugster and Rainer Duden for preliminary results indicating an interaction between Sec21p and Gea1p, and for pJG4-5-Sec21p, to Felix Wieland for β′-COP antibodies, to Ilya Serebriiskii for pEG202 and pJG4-5, to Juan Bonifacino for human γ1-COP, to Rick Cerione for bovine HA-γ1-COP, to George Patterson for pVenus-N1 and to Ting Niu for construction of Venus/YFP-GBF1 plasmids. This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, and by a Chaire d'Excellence from the Agence Nationale de la Recherche, France.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bethune J, Wieland F, Moelleken J (2006) COPI-mediated transport. J Membr Biol 211: 65–79 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J (2003) Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol 4: 409–414 [DOI] [PubMed] [Google Scholar]
- Casanova JE (2007) Regulation of arf activation: the sec7 family of guanine nucleotide exchange factors. Traffic 8: 1476–1485 [DOI] [PubMed] [Google Scholar]
- Cox R, Mason-Gamer RJ, Jackson CL, Segev N (2004) Phylogenetic analysis of Sec7-domain-containing Arf nucleotide exchangers. Mol Biol Cell 15: 1487–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S (2007) The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol 23: 579–611 [DOI] [PubMed] [Google Scholar]
- Hoffman GR, Rahl PB, Collins RN, Cerione RA (2003) Conserved structural motifs in intracellular trafficking pathways: structure of the γCOP appendage domain. Mol Cell 12: 615–625 [DOI] [PubMed] [Google Scholar]
- Lefrancois S, McCormick PJ (2007) The Arf GEF GBF1 is required for GGA recruitment to Golgi membranes. Traffic 8: 1440–1451 [DOI] [PubMed] [Google Scholar]
- Manolea F, Claude A, Chun J, Rosas J, Melancon P (2008) Distinct functions for Arf guanine nucleotide exchange factors at the Golgi complex: GBF1 and BIGs are required for assembly and maintenance of the Golgi stack and trans-Golgi network, respectively. Mol Biol Cell 19: 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E, Corpina RA, Goldberg J (2003) Crystal structure of ARF1*Sec7 complexed with brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell 12: 1403–1411 [DOI] [PubMed] [Google Scholar]
- Mouratou B, Biou V, Joubert A, Cohen J, Shields DJ, Geldner N, Jurgens G, Melancon P, Cherfils J (2005) The domain architecture of large guanine nucleotide exchange factors for the small GTP-binding protein Arf. BMC Genomics 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Boehm M, Boja ES, Vass WC, Bonifacino JS, Fales HM, Randazzo PA (2003) Specific regulation of the adaptor protein complex AP-3 by the Arf GAP AGAP1. Dev Cell 5: 513–521 [DOI] [PubMed] [Google Scholar]
- Niu TK, Pfeifer AC, Lippincott-Schwartz J, Jackson CL (2005) Dynamics of GBF1, a brefeldin a-sensitive arf1 exchange factor at the Golgi. Mol Biol Cell 16: 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Hartnell LM, Jackson CL (2005) Mutations in a highly conserved region of the Arf1p activator GEA2 block anterograde Golgi transport but not COPI recruitment to membranes. Mol Biol Cell 16: 3786–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, Jackson CL (1999) Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell 3: 275–285 [DOI] [PubMed] [Google Scholar]
- Ramaen O et al. (2007) Interactions between conserved domains within homodimers in the BIG1, BIG2, and GBF1 Arf guanine nucleotide exchange factors. J Biol Chem 282: 28834–28842 [DOI] [PubMed] [Google Scholar]
- Renault L, Guibert B, Cherfils J (2003) Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature 426: 525–530 [DOI] [PubMed] [Google Scholar]
- Shin HW, Nakayama K (2004) Guanine nucleotide-exchange factors for arf GTPases: their diverse functions in membrane traffic. J Biochem 136: 761–767 [DOI] [PubMed] [Google Scholar]
- Watson PJ, Frigerio G, Collins BM, Duden R, Owen DJ (2004) γ-COP appendage domain—structure and function. Traffic 5: 79–88 [DOI] [PubMed] [Google Scholar]
- Wegmann D, Hess P, Baier C, Wieland FT, Reinhard C (2004) Novel isotypic γ/ζ subunits reveal three coatomer complexes in mammals. Mol Cell Biol 24: 1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Erickson JW, Lin R, Cerione RA (2000) The γ-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature 405: 800–804 [DOI] [PubMed] [Google Scholar]
- Zhao X, Lasell TK, Melancon P (2002) Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol Biol Cell 13: 119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information