Endocytosis is the process by which cells internalize portions of their cell membrane along with embedded proteins. These membrane proteins can be recycled back to the plasma membrane from the endosomal compartments to be reused or, alternatively, follow a degradative pathway to the lysosomes. Seven transmembrane receptors (7TMRs) are a well-studied class of resident plasma-membrane proteins that have been extensively characterized in terms of their downregulation and removal from the cell surface. The cytoplasmic tails of activated receptors can be phosphorylated, thereby creating binding sites for the arrestin proteins—so-named because they physically preclude the interaction of receptors with G-proteins, thereby arresting further signalling from these receptors. Arrestin proteins also promote the internalization of receptors by acting as adaptors for the endocytic proteins clathrin and AP2 (Lefkowitz et al, 2006). Humans have been presumed to have four ‘true' arrestins, two photoreceptor-specific visual arrestins and two ubiquitous β-arrestins, so-called because they were discovered as β2-adrenergic-receptor-binding proteins. The arrestin family of adaptor proteins has recently expanded, and the new arrestin-related proteins are no longer limited to the 7TMRs, but are now known to couple to other transmembrane proteins for internalization.
Membrane proteins are often observed to be ubiquitinated during arrestin-mediated internalization. Monoubiquitination of yeast cell-surface receptors is now recognized as an internalization signal, whereas ubiquitination of mammalian 7TMRs and growth factor receptors has been shown to function as a lysosomal sorting signal (Haglund et al, 2003). Interestingly, β-arrestins have been shown to function as adaptors in the process of receptor ubiquitination—as in the case of the β2-adrenergic receptor—and are themselves ubiquitinated. Ubiquitination of arrestin is important for its stable binding to activated receptors; thus, slower deubiquitination of arrestin correlates with stable receptor association (Shenoy, 2007). Two studies have recently identified a family of arrestin-like proteins that have a similar role in the endocytosis of several yeast membrane receptors (Nikko et al, 2008; Lin et al, 2008).
Many proteins involved in the process of endocytosis have ubiquitin-interacting motifs. Ubiquitin-interacting motifs typically show low affinity (Kd ∼ 10–500 μM) for ubiquitin but often occur in tandem. This provides an opportunity for the clustering of high-specificity low-affinity interactions that characterize networks that can undergo rapid assembly and disassembly, and have a built-in ‘dynamic instability' (Praefcke et al, 2004). These interactions are analogous to those of DxF motifs (that occur in proteins such as EPS15) with AP2 adaptor proteins, which lead to AP2 clustering. At the plasma membrane, ubiquitinated adaptors and/or cargo might interact with the ubiquitin-binding proteins EPS15 and epsin (Fig 1). Interactions with members of the endosomal sorting complex required for transport, ESCRT-I and ESCRT-II, become important in the late stages of endocytosis, when ubiquitinated cargo proteins might need to be sorted away from a recycling pathway into the luminal vesicles of multivesicular bodies for targeting to the lysosomes.
Figure 1.
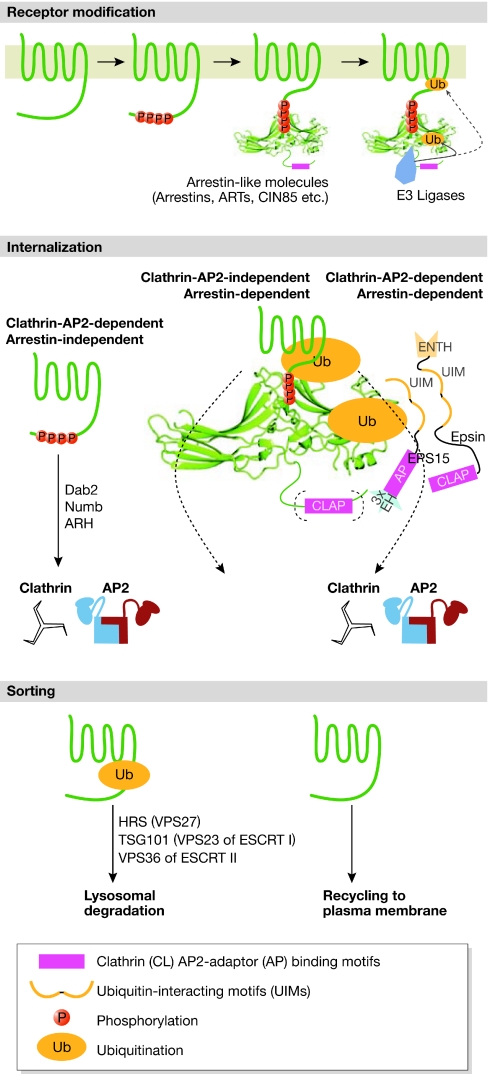
Role of ubiquitin and arrestin-like adaptor proteins in receptor internalization and transport. Plasma-membrane proteins such as transporters, receptors (for example, the 7TMR shown here) and channels when active or ready to be internalized, are modified at the plasma membrane by kinases and/or ubiquitin ligases. There are many internalization pathways, probably determined by the adaptor proteins—such as arrestin, ART, Dab2, Numb, ARH, CIN85 and epsin—that bind to the cytoplasmic tail of the transmembrane protein. A common pathway uses AP2 adaptor proteins as a link to clathrin-coated vesicles. The internalization of 7TMRs requires an interaction with ubiquitinated arrestin adaptors, which can mediate either clathrin-dependent or clathrin-independent internalization. However, it is clear that ‘receptor' ubiquitination is not always required for internalization and it might be that phosphorylation is also not essential. Numerous weak affinities are a characteristic of these endocytic events, as has been noted in the text for AP2 adaptor interactions. In the case of arrestins, the attached ubiquitin can interact with accessory proteins that have many ubiquitin-interacting motifs (UIMs; shown here in epsin and EPS15) and these could cluster several arrestin-bound receptors. In addition, the CLAP motifs (that bind to clathrin and AP2 adaptors) found in the β-arrestins and in the accessory proteins also tend to cluster and solidify the accumulation of ‘receptor-cargo' into pits. Once receptors are internalized (by any of the pathways shown) and the endocytic machinery has dissociated, the presence or absence of direct receptor ubiquitination determines whether it is sorted to the lysosomes for degradation—by interacting with components of the ESCRT machinery on endosomes—or to the recycling pathway for re-exposure on the plasma membrane. ARH, autosomal recessive hypercholesterolemia protein; ART, arrestin-related trafficking adaptor; CIN85, Cbl interacting protein of 85 kDa; Dab2, Disabled 2 protein; ENTH, epsin amino-terminal homology domain; ESCRT, endosomal sorting complex required for transport; HRS, hepatocyte growth factor-regulated tyrosine kinase substrate; TSG101, protein product of the tumour susceptibility gene 101; VPS, vacuolar protein sorting; 7TMR, seven transmembrane receptors.
Ubiquitination of plasma-membrane proteins in yeast acts as the signal for endocytosis, as well as for entry into the vacuole—which is equivalent to the lysosome of mammalian cells—and is carried out by the E3 ubiquitin ligase Rsp5. Rsp5 is the only member of the Nedd4 family of ubiquitin E3 ligases present in yeast (Belgareh-Touze et al, 2008) and has a modular structure with an amino-terminal C2 domain, three WW domains that recognize PY motifs (X1-Pro-X2-Tyr, where X1 is often a proline), and a carboxy-terminal catalytic HECT domain. Rsp5-mediated ubiquitination and internalization is a general characteristic of most plasma-membrane proteins in yeast; however, these plasma-membrane substrates do not contain PY motifs and thus there is a need for adaptor proteins. Although proteins with PY motifs have been identified in yeast, they do not seem to be required for the endocytosis of cell-surface receptors.
In a recent study, Nikko et al (2008) examined the stress-induced downregulation of the Saccharomyces cerevisiae manganese transporter Smf1 using the toxic agent cadmium. This report established that ubiquitination of Lys 33 and Lys 34 on Smf1 prevented its stress-induced endocytosis. Drawing on the analogy of arrestin-mediated downregulation and ubiquitination of 7TMRs in mammalian cells, and recent homology searches that expanded the family of arrestins (Alvarez, 2008), Pelham and co-workers identified several candidates in Saccharomyces that are homologues of arrestins. They initially focused on two arrestin-like protein candidates, Ecm21 and Csr2, which contain PY motifs that have previously been shown to bind directly to, and be ubiquitinated by, Rsp5. Deletion of the genes for these two arrestin-related proteins markedly reduced the ubiquitination of Smf1 and its endocytic delivery to the yeast vacuole. Deletion of all seven arrestin-related molecules containing PY motifs resulted in a more complete reduction of Smf1 ubiquitination and internalization. Thus, arrestin-related molecules are the adaptors that mediate the Rsp5-dependent ubiquitination of the plasma-membrane protein Smf1. As seen for mammalian arrestins, the Ecm21 adaptor binds directly to phosphorylated Smf1, Ecm21 is itself ubiquitinated and this is necessary for Smf1 transporter endocytosis. PY motifs in Ecm21 recruit the WW-domain-containing E3 ligase Rsp5, leading to the ubiquitination of both Ecm21 and Smf1, and subsequent internalization from the plasma membrane. In contrast to mammalian 7TMRs, it must be noted that phosphorylation of Smf1, although essential, is not a trigger for its endocytosis—there is no evidence that cadmium induces the phosphorylation of Smf1. Conversely, it seems that Smf1 is probably phosphorylated by the resident plasma-membrane kinases Yck1/2, which constitutes a signal that would act to target the arrestin-related adaptors to the plasma-membrane-resident Smf1 requiring rapid removal, rather than targeting the intracellular pool of Smf1.
In another recent report by Lin et al (2008), arrestin-related transport adaptors were also found to target specific plasma-membrane proteins for endocytic downregulation by recruiting the ubiquitin E3 ligase Rsp5. In this study, Emr and co-workers screened the collection of 4,652 S. cerevisiae knockout strains for increased sensitivity to canavanine, a toxic arginine analogue that enters the cell through the arginine transporter Can1. Yeast strains impaired in the endocytic removal of plasma-membrane proteins accumulated Can1 at the plasma membrane, resulting in excess transporter activity (uptake of toxic canavanine) and impaired growth. The authors chose to focus on an uncharacterized canavanine-supersensitive strain (cvs7) that showed a strong defect in Can1 ubiquitination and endocytosis. The Cvs7 protein (later renamed Art1 by Emr and co-workers) was also found to be required for endocytosis of the methionine transporter Mup1, but not for the internalization of other plasma-membrane proteins such as the uracil transporter Fur4, the iron transporter Ftr1, the multidrug transporter Pdr5 and the α-factor receptor Ste2. Thus, Cvs7 acts as a cargo-specific endocytic adaptor.
Two adjacent PY motifs were observed in Cvs7 and found to engage the WW domains of the ubiquitin ligase Rsp5. Fusion of the Cvs7 PY motifs to the arginine transporter Can1 at the plasma membrane resulted in its constitutive transport to the vacuole. The N-terminus of Cvs7 has homology to mammalian arrestins and the PY motifs are located after this homology domain. The functional homology of Cvs7 to mammalian arrestins was established by verifying that the mutation of conserved residues within the arrestin motif ablated Cvs7 function. This led to the identification of the yeast protein family of arrestin-related trafficking adaptors (ARTs)—which has nine members that have conserved arrestin and PY motifs, seven of which are predicted to have the arrestin fold—and thus Cvs7 was renamed Art1. That these ART proteins could act as adaptors for the Rsp5 ubiquitin ligase was confirmed by showing the ability of immobilized WW domains (from Rsp5) to pull down the ART proteins from yeast lysates. ART proteins therefore link the cytoplasmic tails of specific cargoes to the Rsp5 ubiquitin ligase in response to distinct endocytic transport signals.
Thus, an appreciation of the interactions of WW domains with PY motifs led to the identification of the ART family. It is interesting in this regard that a ‘true' mammalian arrestin, β-arrestin 2, has recently been shown to act as an adaptor for the WW-domain-containing E3 ubiquitin ligase NEDD4 to promote agonist-stimulated ubiquitination of the β2-adrenergic receptor (Shenoy et al, 2008). β-arrestin 2 does not contain any genuine PY motifs, but was nevertheless shown to bind to NEDD4.
In addition to ARTs, Vps26—a component of the five-subunit retromer complex involved in retrograde transport from endosomes to the trans-Golgi network—has been shown to present the same overall fold as the arrestins (Shi et al, 2006). The Vps26 domain is present in all eukaryotes—which is good news for plants because they were hitherto thought to completely lack arrestins. This diversity of adaptors (see Fig 1) allows various cargoes to be linked independently to many endocytic routes, providing robustness to the process of internalization, such that even if one portal is compromised others are available for entry. The studies discussed here show the specificity of the ART family members for various plasma-membrane proteins and, if the affinities for cargo and the concentrations of ART family members are different (for example, depending on the environment or the stage of the life cycle), this would allow the kinetics of internalization to be fine-tuned for each cargo.
The arrestin clan now comprises ARTs, β-arrestins, visual-arrestins and the Vps26 families in eukaryotes, and the Spo0M family in archaea and bacteria (Alvarez, 2008). Thus, the mammalian arrestins have expanded from 4 initial members—two visual and two β-arrestins—to 14 members by the addition of 6 α-arrestins (ARTs) and 4 VPS26 members (which share higher sequence similarity with α-arrestins). α- and β-arrestins are substantially different: the tails of β-arrestins contain clathrin and AP2-interacting motifs, whereas those of α-arrestins contain PY motifs; β-arrestins are generally cytoplasmic in unstimulated cells, whereas α-arrestins are associated with membranes; β-arrestins have an amphipathic helix (helix 1) that is sequestered into the inactive conformation of the N-terminal domain and is presumably released on activation by receptor engagement, whereas α-arrestins do not. Future studies will need to decipher the function of ARTs in mammalian cells and their relationships to known endocytic pathways but, without a doubt, the yeast studies of the Pelham and Emr groups have given us the essential clues.

References
- Alvarez CE (2008) On the origins of arrestin and rhodopsin. BMC Evol Biol 8: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgareh-Touze N, Leon S, Erpapazoglou Z, Stawiecka-Mirota M, Urban-Grimal D, Haguenauer-Tsapis R (2008) Versatile role of the yeast ubiquitin ligase rsp5p in intracellular trafficking. Biochem Soc Trans 36: 791–796 [DOI] [PubMed] [Google Scholar]
- Haglund K, Di Fiore PP, Dikic I (2003) Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci 28: 598–603 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ (2006) New roles for β-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 24: 643–652 [DOI] [PubMed] [Google Scholar]
- Lin CH, Macgurn JA, Chu T, Stefan CJ, Emr SD (2008) Arrestin-related ubiquitin ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135: 714–725 [DOI] [PubMed] [Google Scholar]
- Nikko E, Sullivan JA, Pelham HR (2008) Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep 9: 1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke GJ, Ford MG, Schmid EM, Olesen LE, Gallop JL, Peak-Chew SY, Vallis Y, Babu MM, Mills IG, McMahon HT (2004) Evolving nature of the ap2 α-appendage hub during clathrin-coated vesicle endocytosis. EMBO J 23: 4371–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK (2007) Seven-transmembrane receptors and ubiquitination. Circ Res 100: 1142–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM (2008) Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the β2-adrenergic receptor. J Biol Chem 283: 22166–22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Rojas R, Bonifacino JS, Hurley JH (2006) The retromer subunit vps26 has an arrestin fold and binds vps35 through its C-terminal domain. Nat Struct Mol Biol 13: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]