Abstract
Aeromonas sobria infection often advances to sepsis, in which interaction of bacterial components with plasma proteins possibly causes various disorders. This bacterium releases a serine protease (ASP), a putative virulence factor, and binds to fibrinogen. To study the ASP effect on fibrinogen, we incubated fibrinogen or plasma with ASP and investigated their clotting elicited by thrombin, which converts fibrinogen to a fibrin clot. Enzymatically active ASP retarded plasma clotting in a dose-dependent manner starting at an ASP concentration of 10 nM. ASP also retarded fibrinogen clotting at 3 nM and above, which appeared to correspond to ASP cleavage of fibrinogen at the Aα-chain. Consistent with containing serine protease activity for an ASP-specific substrate, the culture supernatant of an ASP gene-introduced strain retarded plasma and fibrinogen clotting more than that of the wild-type strain. The culture supernatant of an ASP gene-disrupted strain that releases negligible serine protease activity for the ASP-specific substrate did not affect plasma clotting. These results indicate that ASP is the main fibrinogenolytic protease released from A. sobria. Impaired plasma clottability induction through fibrinogen degradation is a new virulence activity of ASP and may contribute to hemorrhagic tendencies in sepsis caused by infection with this bacterium.
Keywords: Aeromonas sobria, sepsis, serine protease, fibrinogen, plasma clotting, bleeding tendency
Introduction
Aeromonas species are facultative anaerobic Gram-negative rods that are widely distributed in aquatic environments (Jones & Wilcox, 1995) and commonly implicated as causative agents of gastroenteritis (Janda & Brenden, 1987; Janda & Abbott, 1998). Aeromonas infections, either through wounds or via the digestive tract, often develop into sepsis, particularly in immunocompromised individuals (Janda & Abbott, 1998). Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae are three major Aeromonas subspecies, and A. sobria, frequently isolated from patients' blood (Janda et al., 1984), is markedly more virulent than other Aeromonas species (Janda & Brenden, 1987).
Aeromonas species release several putative virulence factors, including hemolysins, enterotoxins, and proteases (Janda, 1991). As a putative virulence factor, we purified a 65-kDa serine protease, referred to as Aeromonas serine protease (ASP), from the culture supernatant of A. sobria (Yokoyama et al., 2002). ASP is a member of the kexin family of subtilisin-like proteases (Siezen & Leunissen, 1997) and preferentially cleaves peptide bonds at the carboxy-terminal side of paired basic residues (Kobayashi et al., 2006). Recently, we found that ASP caused vascular leakage and lowered blood pressure by cleaving components of the plasma kallikrein/kinin system (Imamura et al., 2006), suggesting a contribution of ASP to septic shock in A. sobria infections. Thus, interaction of ASP with plasma proteins may be associated with the pathophysiology of the infectious disease caused by this pathogen, especially in sepsis.
Fibrinogen, a plasma protein, is converted to a fibrin clot by thrombin, protecting the host from blood loss by bleeding (Mann & Lundblad, 1987). Degradation of fibrinogen and fibrin leads to loss of plasma clottability and rebleeding, respectively, causing hemorrhagic tendencies that are a prominent symptom in disseminated intravascular coagulation (DIC), a common and potentially deadly complication in sepsis patients (Levi, 2001). The ability of Aeromonas species to bind fibrinogen (Ascencio et al., 1990) suggests that ASP released from A. sobria may degrade fibrinogen and abrogate plasma clottability. To examine this virulence activity, we investigated the fibrinogenolytic activity of ASP, and to study the contribution of ASP to fibrinogen degradation by A. sobria, we measured the fibrinogenolytic activity of the culture supernatants from an ASP gene-disrupted or -introduced strain.
Materials and methods
Materials
Human fibrinogen and bovine thrombin were purchased from Sigma-Aldrich Corp. (St. Louis, MO), and t-butyloxycarbonyl–l-glutamyl–l-lysyl–l-lysine–4-methylcoumaryl-7-amide (Boc–Glu–Lys–Lys–MCA) was obtained from the Peptide Institute (Minoh, Japan). Other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan). Normal human plasma was prepared by centrifugation of a mixture of 9 vol of freshly drawn blood from healthy volunteers and 1 vol of 3.8% (w/v) sodium citrate. The bacterial strains, plasmids, and their sources used in this study are listed in Table 1. Both Eschrichia coli and A. sobria were grown at 37 °C in Luria–Bertani (LB) broth and agar plates. A wild-type strain 288 was isolated clinically (Fujii et al., 1998).
Table 1.
Strains and plasmids
| Properties | Sources or references | |
|---|---|---|
| Strains | ||
| A. sobria strains | ||
| 288 | Wild-type strain producing ASP and metalloprotease | Fujii et al. (1998) |
| 288 (Δasp) | 288 strain disrupted both asp and orf2 | Khan et al. (2007) |
| T94 | Atypical strain not producing ASP and metalloprotease | Khan et al. (2007) |
| E. coli | ||
| HB101 | Cloning host, supE44, Δ(mcrC-mrr), recA13, ara-14, proA2, lacY1, galK2, rpsL20, xyl-5, mtl-1, leuB6, thi-1 | TaKaRa Co. |
| SY327λpir | Δ(lac-pro) argE(Am) recA56 nalA Rfr (λpir) | Nishibuchi et al. (1991) |
| SM10λpir | Thi-1 thr leu tonA lacy supE recA::RP4-2Tc::Mu λpir R6K, Kmr | Nishibuchi et al. (1991) |
| Plasmids | ||
| Cloning vectors | ||
| pUC119 | General cloning vector, Apr | TaKaRa Co. |
| pSA19CP | Shuttle vector replicable in E. coli and Aeromonas, Cmr | Nomura et al. (2002) |
| pXAC623 | Suicide vector, Cmr, pEX100T and pKTN701 derivative with R6K ori and mob RP4 | Schweizer & Hoang (1995), Nishibuchi et al. (1991) |
| Constructed plasmids | ||
| pUC119-5528 | pUC119 derivative carrying 5528-bp gene fragment containing asp and orf2 | Okamoto et al. (2000) |
| pUC119-5528 (Δasp) | pUC119-5528 defect of the gene fragment from 1776 to 3408 nt | Khan et al. (2007) |
| pXAC-5528 (Δasp) | pXAC623 derivative defect of the gene fragment from 1776 to 3408 nt in 5528-bp gene fragment | Khan et al. (2007) |
| PSA19-5528 | pSA19CP derivative carrying 5528-bp gene fragment containing asp and orf2 | Okamoto et al. (2000) |
Preparation of asp-disrupted or -introduced strain
Fragments of strain 288 chromosomal DNA digested with the EcoRI were inserted into the EcoRI site of pUC119 (Okamoto et al., 2000) and introduced into E. coli HB101 (TaKaRa Co., Kyoto, Japan). Protease-positive clones were selected using the LB agar medium containing 3% skim milk (Nakarai Co., Kyoto, Japan). Sequence analysis of the cloned insert DNA (5528 bp) in pUC119-5528 (Nomura et al., 2002) identified an operon composed of two structural genes, asp and orf2, which codes for a specific chaperone required for ASP maturation (GenBank accession no. AF253471).
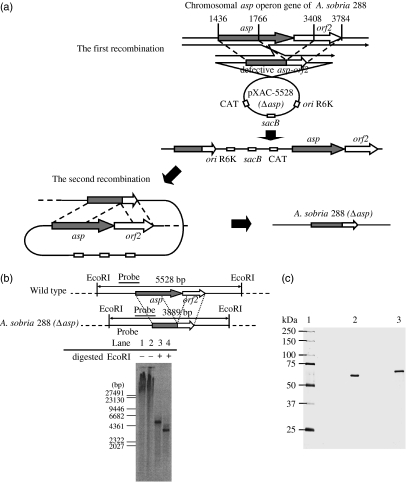
Disruption of asp and orf2 was carried out according to the homologous recombination technique (Kuroda et al., 2005) using a suicide vector pXAC623 (Nishibuchi et al., 1991; Schweizer & Hoang, 1995). To cleave off the regions from 1766 to 3408, pUC119-5528 was digested with NcoI and BglII and treated with Klenow fragment (TaKaRa Co.). The defective gene fragment was circularized by T4 DNA ligase (TaKaRa Co.) [pUC119-5528 (Δasp)] (Khan et al., 2007) and amplified by PCR (2 min at 94 °C, followed by 25 cycles of 10 s at 98 °C, 30 s at 50 °C, and 5 min at 72 °C) using two primers: 5′-TTTCGTTCTAGAGCCGGGCCACGTTCA-3′ and 5′-CGACCCTCTAGAGGGGGCGCCCGGCGG-3′. These primers cover the upstream and downstream regions of the defective gene, respectively, and contain a XbaI site (underlined). The amplified gene fragment was digested using XbaI and inserted into a unique XbaI site of the suicide vector pXAC623. The plasmid, designated as pXAC-5528 (Δasp) (Fig. 1a), was introduced into E. coli SM10λpir. The SM10λpir was cultured with strain 288 until the exponential phase and was harvested on a membrane filter (0.2-μm pore size, Advantec Co., Tokyo, Japan) by filtration. This membrane was placed on an LB-broth agar plate and incubated at 37 °C for 3 h to deliver pXAC-5528 (Δasp) from SM10λpir to strain 288 by conjugation. The bacterial cells on the membrane were resuspended in LB broth and cultured at 37 °C for 1 h. The first homologous recombination produced a 288 strain possessing both the wild and defective asp-orf2 genes, and CAT and sacB genes from the pXAC623 vector (Fig. 1a). The cell suspension spread onto an LB-broth agar plate containing 5 μg mL−1 chloramphenicol and 50 μg mL−1 ampicillin was incubated at 30 °C overnight. Only the recombinant strain with the CAT gene grew, and was cultured in LB broth without antibiotics. During this procedure, the second homologous recombination occurred between the full-length and the defective asp-orf2 genes (Fig. 1a). To exclude the strain that did not have the second recombination, therefore retaining the sacB gene, the bacterial culture spread onto an LB-broth agar plate containing 10% sucrose was incubated at 25 °C. The asp-orf2-disrupted 288 strain created, designated as 288 (Δasp), was confirmed by Southern-hybridization analysis (Fig. 1b, lane 4).
Fig. 1.
(a) Disruption of asp-orf2 using suicide vector pXAC-5528 (Δasp). The first homologous recombination produced a mutant 288 possessing asp-orf2 and the defective gene on pXAC-5528 (Δasp), and CAT and sacB genes. The second homologous recombination occurred between both types of genes located in tandem and produced the asp-orf2-disrupted strain. (b) Southern-hybridization analysis of asp-orf2. The asp-orf2 detection DNA probe (b, horizontal bar) that had EcoRI digestion sites at both sides was amplified using two primers AP-20 (5′-CATCGGCGGCAACCGCGGAA-3′) and AP-25 (5′-ATGCCGCTCTCCTTGCCGGT-3′), and labeled digoxigenin DNA Labeling Kit. Total DNAs extracted from both 288 (lanes 1 and 3) and 288 (Δasp) (lanes 2 and 4) using Qiagen Genomic-tips were digested with EcoRI and separated on 0.7% agarose gel. Southern-hybridization reaction with the digoxigenin-labeled probe was performed and hybridized fragments were detected with the digoxigenin luminescent detection kit. Numbers along the left side indicate DNA sizes in base pairs (bp). (C) SDS-PAGE of ASP. ASP (0.6 μg) was analyzed using a SDS-polyacrylamide gel (10%) in the presence (lane 3) or absence of 2-ME (lane 2), and the gel was silver-stained. Lane 1, molecular size markers.
To prepare a high ASP secretion strain, strain T94 was introduced with pSA19-5528 containing asp-orf2 and CAT genes (T94/pSA19-5528), and selected using LB-broth agar plates containing 5 μg mL−1 chloramphenicol. Strain T94 introduced the vector alone (T94/pSA19CP) was used as a control.
CFU count
Two hundred microliters of the culture of a strain at the stationary phase was added to 100 mL of LB-broth medium. The medium was incubated at 37 °C with shaking (160 r.p.m.) and 100 μL of the culture sample was taken every 3 h. The sample was serially diluted, plated onto LB-agar plates, and cultured for bacterial colony count.
Measurement of the protease activity of culture supernatants
The bacterial cell culture supernatant, after a 12-h incubation at 37 °C was centrifuged at 12 000 g for 15 min. The culture supernatant (35 μL) treated with or without a serine protease inhibitor di-isopropyl fluorophosphate (DFP) (final 1 mM) was added to 65 μL of 1% azocasein in 10 mM Tris-HCl buffer (pH 7.5) and incubated at 37 °C for 1 h, followed by addition of 100 μL of 10% trichloroacetic acid. After centrifugation at 13 000 g, 100 μL of the supernatant was mixed with an equal volume of 1 N NaOH and the A450 nm was measured. The culture supernatant (133 μL) treated with or without DFP (final 1 mM) was added to 12 μL of 1.1 mM Boc–Glu–Lys–Lys–MCA in 11 mM sodium phosphate buffer (pH 7.5) and incubated at 37 °C for 30 min, followed by addition 150 μL of acetic acid (130 mM). The AMC released was measured fluorophotometrically at λex=380 nm and λem=440 nm using a fluorescence spectrophotometer (Model 650-40, Hitachi Co., Japan).
Purification of ASP
ASP was purified from the culture supernatant of T94/pSA19-5528 (Okamoto et al., 2000) by successive column chromatography. The ASP sample was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide gel) and found to be homogenous, showing a single band under reducing and nonreducing conditions, with a molecular mass of 65 kDa (Fig. 1c). To abolish enzymatic activity, ASP and culture supernatants were treated with 1 mM DFP and dialyzed against 10 mM Tris–HCl (pH 7.3) containing 150 mM NaCl (TBS).
Thrombin time (TT) assay
Conversion of fibrinogen to fibrin by thrombin leads to clotting; thus, fibrinogen degradation results in impaired clotting. To examine the fibrinogenolytic activity of ASP, the thrombin-induced clotting time prolongation of fibrinogen or plasma incubated with ASP was measured with KC4 (Trinity Biotech, Bray, Ireland). For the TT assay, 67.5 μL of citrated human plasma or fibrinogen (3 mg mL−1) was incubated with 7.5 μL of ASP in a plastic cell at 37 °C for 3 min, followed by addition of 75 μL of bovine thrombin (5 U mL−1) to initiate clotting. As a control, TBS, used for ASP dilution, was added instead of ASP.
SDS-PAGE
ASP (15 μL, 300 nM) was incubated with 135 μL of human fibrinogen (3 mg mL−1 in 50 mM Tris–HCl, pH 7.4, containing 0.1 M NaCl) at 37 °C. At various time periods, 15 μL of the mixture was withdrawn, followed by the addition of 1 μL of DFP (10 mM) to terminate the reaction. Samples were analyzed by SDS-PAGE under reducing conditions using a 10% polyacrylamide gel. Coomassie Brilliant Blue R-250 was used for protein staining.
Results
Reduction of fibrinogen and plasma clottability by ASP
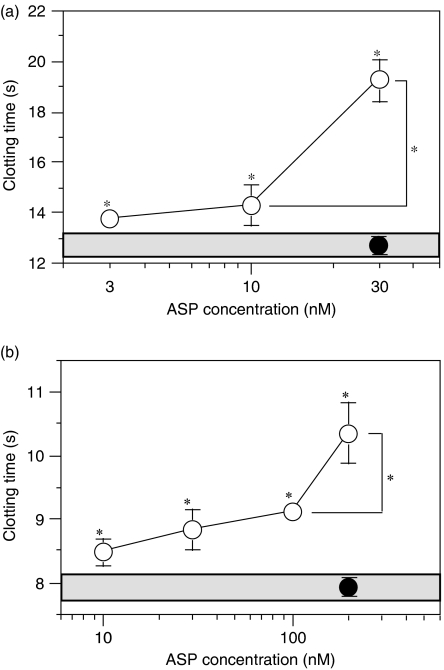
To determine whether ASP could affect fibrinogen clottability, we measured TT after incubating ASP with this clotting factor at the normal plasma concentration (3 mg mL−1) (Halkier, 1991). ASP prolonged the fibrinogen TT in a dose-dependent manner, starting at an ASP concentration of 3 nM (Fig. 2a). To examine the ASP effect on fibrinogen under semi-physiological conditions, we measured TT after incubating plasma with ASP. ASP prolonged plasma TT in a dose-dependent manner, starting at an ASP concentration of 10 nM (Fig. 2b), indicating that ASP can reduce the clottability of fibrinogen in plasma. Because DFP-inactivated ASP did not affect TT (Fig. 2a and b), the TT prolongation effect of ASP occurred through its proteolytic cleavage of fibrinogen. Because ASP did not activate plasminogen (data not shown), the involvement of plasmin-mediated fibrinogen degradation in this ASP prolongation effect on thrombin-induced plasma clotting is unlikely. That the clottability reduction of plasma required more ASP than that of fibrinogen (Fig. 2a and b) suggests interference of plasma proteins, including possibly protease inhibitors, in the reaction. Overall, these results indicated that ASP impaired plasma clottability through fibrinogen cleavage.
Fig. 2.
TT prolongation by ASP. For the TT assay, 67.5 μL of fibrinogen (3 mg mL−1) (a) or citrated human plasma (b) was incubated with 7.5 μL of ASP in a plastic cell at 37°C for 3 min, followed by addition of 75 μL of bovine thrombin (5 U mL−1) to initiate clotting. As a control, TBS was added instead of ASP. ASP concentrations in plasma or fibrinogen solution are shown. Values are expressed as means±SD (n=4). ○, nontreated ASP; •, DFP-inactivated ASP. The gray zone indicates the range of TT assayed using TBS instead of ASP. *P<0.01 vs. the control value and between indicated values.
Cleavage of human fibrinogen by ASP
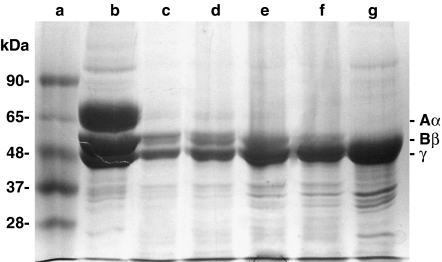
To study the mechanism of the fibrinogen clottability reduction by ASP, fibrinogen was analyzed by SDS-PAGE after incubation with the protease (Fig. 3). ASP degraded fibrinogen in an incubation time-dependent manner and the Aα-chain (66 kDa) disappeared completely at 2 min, followed by Bβ-chain (54 kDa) degradation. Because no clear band, except for the Bβ- and γ-chains, was seen after the Aα-chain disappeared, it seems likely that the Aα-chain was quickly degraded to small peptides. The decrease in the Bβ-chain was coincident with an increase in a band with a molecular weight similar to that of the γ-chain and the appearance of bands smaller than the γ-chain.
Fig. 3.
SDS-PAGE analysis of fibrinogen incubated with ASP. Human fibrinogen (3 mg mL−1) was incubated with ASP (30 nM) for various time periods and 15 μL of the mixture was withdrawn, followed by addition of 1 μL of DFP (10 mM). Samples were analyzed by SDS-PAGE under reducing conditions using a 10% polyacrylamide gel. Coomassie Brilliant Blue R-250 was used for protein staining. a, markers; b–g, incubated for 0, 2, 4, 8, 16, and 32 min, respectively.
Effect of the culture supernatants of the ASP gene-disrupted or -inserted strain on fibrinogen clottability
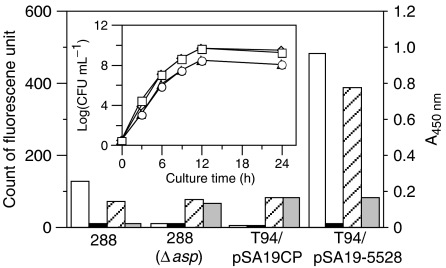
To investigate the role of ASP in fibrinogen degradation by A. sobria, the culture supernatants of the asp-disrupted or the asp-introduced strains were examined for their effects on TT. The asp-disrupted strain and the wild-type strain grew at almost the same rate, and growth of the T94 strains introduced pSA19-5528 or the vector was slightly lower (Fig. 4, inset). To confirm the effect of asp gene modification, we measured the protease activity of culture supernatants using substrates Boc–Glu–Lys–Lys–MCA highly specific for ASP (Kobayashi et al., 2006), and azocasein for a broad range of specificities. The supernatant of the asp-disrupted strain contained negligible DFP-sensitive protease activity for both substrates, whereas DFP-sensitive protease activity in the supernatant of the asp-introduced strain was about fourfold higher than that in the supernatant of the wild strain (Fig. 4). DFP inactivated most of the supernatant protease activity of the wild strain for both substrates and of the asp-introduced strain for Boc–Glu–Lys–Lys–MCA, but these supernatants, except for the wild strain supernatant, contained DFP-resistant azocaseinolytic activity at similar levels (Fig. 4). These results indicated that ASP accounted for almost all of the serine protease activity released from the wild strain and was absent in the culture supernatant of the asp-disrupted strain.
Fig. 4.
Protease activity of culture supernatants for azocasein (right two bars) or Boc–Glu–Lys–Lys–MCA (10 μL, 10 mM) (left two bars). Open and closed bars indicate the activity of the supernatant treated without or with DFP, respectively. 288, wild strain; 288 (Δasp), asp-disrupted strain; T4/pSA19CP, vector-introduced strain; T4/pSA19-5528, asp gene-introduced strain. Inset, the time course of growth of strains. The growth of each strain in the culture medium was measured by CFU. □, 288; ⋄, 288 (Δasp); ○, T4/pSA19CP; ▵, T4/pSA19-5528.
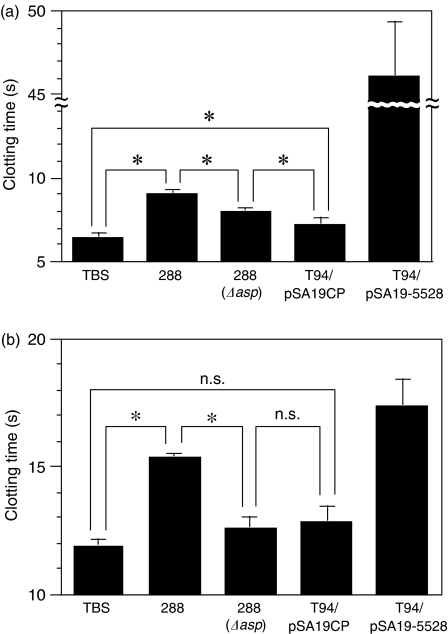
The culture supernatants prolonged fibrinogen TT; in terms of this effect, the wild-type strain was much lower than the asp-introduced strain, but higher than the asp-disrupted strain (Fig. 5a). Although the asp-disrupted strain did not secrete ASP (Fig. 4), its culture supernatant prolonged fibrinogen TT significantly (Fig. 5a), apparently indicating the effect of non-serine-type fibrinogenolytic protease(s) in the supernatant (Fig. 4). The supernatant of the vector-introduced T94 strain, secreting neither ASP nor metalloprotease (Table 1), also had a significant fibrinogenolytic activity lower than that of the wild strain (Fig. 5a). The culture supernatant of the asp-introduced strain prolonged human plasma TT the most, followed by that of the wild-type strain; culture supernatants of the asp-disrupted and the vector-introduced strains exerted no significant effect on plasma TT (Fig. 5b), consistent with the order of the serine protease activity for the ASP-specific substrate in the supernatants (Fig. 4). Based on the DFP-sensitive serine protease activity for the ASP-specific substrate, the wild-strain culture supernatant was estimated to contain about 170 nM ASP, corresponding to 17 nM in the TT assay. The TT-prolongation effect of the wild-type strain supernatant (Fig. 5a and b) appeared to be close to that of 17 nM ASP (Fig. 2a and b), and slightly higher, probably because of the effect of other proteases in the supernatant. Taken together, ASP likely accounted for most of the fibrinogen clottability-abrogating activity secreted from the wild A. sobria and caused impaired plasma clotting.
Fig. 5.
TT prolongation by culture supernatants. For the TT assay, 67.5 μL of human fibrinogen (4 mg mL−1) (a) or 52.5 μL of citrated plasma (b) was incubated with 7.5 or 22.5 μL, respectively, of the supernatants in a plastic cell at 37°C for 2 min, followed by addition of 75 μL of bovine thrombin (5 U mL−1) to initiate coagulation. Values are expressed as means±SD (n=4). *P<0.01; NS, not significant.
Discussion
Invading bacteria interact with host components, causing infectious diseases. Plasma proteins leak from the blood stream into infected sites through vascular permeability, enhanced by inflammatory responses against infections, and in sepsis, bacteria flow in the plasma. It is relevant that the interaction of plasma proteins with bacterial components is involved in the pathophysiology of infectious disease. In fact, the interaction of ASP with human fibrinogen resulted in rapid degradation (Fig. 3) and clottability reduction (Fig. 2a and b) of this clotting factor. The asp-disrupted strain culture supernatant, deficient in ASP (Fig. 4), lacked most of the TT prolongation activity, whereas that of the wild-type or the ASP gene-introduced strain exerted this activity in a serine protease-dependent manner (Fig. 5a and b), indicating a major contribution of ASP to the fibrinogenolytic activity released from A. sobria. Furthermore, the finding that the culture supernatant of the A. sobria wild-type strain impaired plasma clottability (Fig. 5b) suggests the induction of hemorrhagic tendencies due to fibrinogen degradation by ASP secreted from this pathogen in sepsis. A subtilisin-like serine protease from group B Streptococcus has been reported to cleave human fibrinogen and the fact that the protease-gene-null mutant was 10 times less virulent in a neonatal rat sepsis model of group B Streptococcus infection indicates that this fibrinogen-cleaving protease is an important virulence factor of this pathogen (Harris et al., 2003). Fibrinogenolysis by ASP is thought to be a new virulence activity of A. sobria, in addition to the kinin-releasing activity associated with shock induction (Imamura et al., 2006).
Estimating from the previous results (Imamura et al., 1995), the result that the fibrinogen TT was prolonged by ASP at 30 nM (Fig. 2b) indicated that more than 80% of the fibrinogen lost clottability after a 3-min incubation with ASP, corresponding to the disappearance of the Aα-chain by 2 min (Fig. 3). The rapid cleavage of the fibrinogen Aα-chain by ASP (Fig. 3), like the physiological fibrinogenolytic protease plasmin (Pizzo et al., 1972), indicates ASP's preference for this chain as a substrate. ASP is a subtilisin-like protease that prefers paired Lys and/or Arg residues and cleaves the peptide bonds at the carboxy-terminal side of the paired basic amino acid residues (Kobayashi et al., 2006). That such paired residues are fewer in the γ-chain (two pairs) than in the Aα- and the Bβ-chains (seven pairs) (Halkier, 1991) may be associated with the γ-chain being relatively resistant to ASP, but is not consistent with ASP degrading the Aα-chain faster than the Bβ-chain (Fig. 3). The ability of ASP to cleave the peptide bonds at the carboxy-terminal side of Arg residues (Imamura et al., 2006; Kobayashi et al., 2006) and differences in the three-dimensional structure between the Aα- and Bβ-chains may explain the ASP cleavage preference for the Aα-chain (Fig. 3). Although ASP is the major fibrinogenolytic protease in the culture supernatant of the wild-type A. sobria strain, significant fibrinogenolytic activity remains in the asp-disrupted strain culture supernatant (Fig. 5a), even though it contains negligible serine protease activity (Fig. 4). Aeromonas species produce metalloproteases (Toma et al., 1999), and the culture supernatant of the vector-introduced T94 strain, which does not produce ASP and metalloprotease (Khan et al., 2007), also contained DFP-resistant caseinolytic activity comparable to that of the asp-disrupted strain (Fig. 4). Nonserine proteases are likely involved in the residual fibrinogenolytic activity in the culture supernatant of the asp-disrupted strain. However, the result that culture supernatants of the asp-disrupted or the vector-introduced T94 strains exerted no significant effect on plasma TT (Fig. 5b) suggested a marginal effect of other proteases on the plasma clottability. Taken together, fibrinogen cleavage at the Aα-chain by ASP appears to be primarily responsible for plasma clottability abrogation by A. sobria.
This study demonstrates that ASP, secreted by A. sobria at the infection sites or in the circulation, can cause fibrinogen degradation as a major executioner, possibly contributing to induction of hemorrhagic tendencies in sepsis. Accordingly, ASP is considered to be an important virulence factor; thus, it may be a therapeutic target for A. sobria infection diseases and ASP-specific inhibitors may be useful as drugs. Aeromonas species other than A. sobria also secrete subtilisin-type serine proteases (64 kDa) (Coleman & Whitby, 1993), and their infections also cause sepsis (Janda & Brenden, 1987; Janda et al., 1994). Thus, such inhibitors may be widely beneficial in the treatment of diseases caused by Aeromonas infections.
Acknowledgments
This study was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science for Scientific Research (B) (Grant No. 18390125 to T.I.). The authors have no conflicting financial interests.
Statement
Reuse of this article is permitted in accordance with the Creative Commons Deed, Attribution 2.5, which does not permit commercial exploitation.
References
- Ascencio F, Aleljung P, Olusanya O, Wadström T. Type I and IV collagen and fibrinogen binding to Aeromonas species isolated from various infections. Zentralbl Bakteriol. 1990;273:186–194. doi: 10.1016/s0934-8840(11)80248-3. [DOI] [PubMed] [Google Scholar]
- Coleman G, Whitby PW. A comparison of amino acid sequence of the serine protease of the fish pathogen Aeromonas salmonicida with those of other subtilisin-type enzymes relative to their substrate-binding sites. J Gen Microbiol. 1993;139:245–249. doi: 10.1099/00221287-139-2-245. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Nomura T, Kanzawa H, Kameyama M, Yamanaka H, Akita M, Setsu K, Okamoto K. Purification and characterization of enterotoxin produced by Aeromonas sobria. Microbiol Immunol. 1998;42:703–714. doi: 10.1111/j.1348-0421.1998.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Halkier T. Mechanisms in Blood Coagulation, Fibrinolysis and the Complement System. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- Harris TO, Shelver DW, Bohnsack JF, Rubens CE. A novel streptococcal surface protease promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen. J Clin Invest. 2003;111:61–70. doi: 10.1172/JCI16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Potempa J, Pike RN, Travis J. Effect of free and vesicle-bound cysteine proteinases of Porphyromonas gingivalis on plasma clot formation: implications for bleeding tendency at periodontitis sites. Infect Immun. 1995;63:4877–4882. doi: 10.1128/iai.63.12.4877-4882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Kobayashi H, Khan R, Nitta H, Okamoto K. Induction of vascular leakage and blood pressure lowering through kinin release by a serine proteinase from Aeromonas sobria. J Immunol. 2006;177:8723–8729. doi: 10.4049/jimmunol.177.12.8723. [DOI] [PubMed] [Google Scholar]
- Janda JM. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin Microbiol Rev. 1991;4:397–410. doi: 10.1128/cmr.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. 1998;27:332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- Janda JM, Brenden R. Importance of Aeromonas sobria in Aeromonas bacteremia. J Infect Dis. 1987;155:589–591. doi: 10.1093/infdis/155.3.589. [DOI] [PubMed] [Google Scholar]
- Janda JM, Reitano M, Bottone EJ. Biotyping of Aeromonas isolates as a correlate to delineating a species-associated disease spectrum. J Clin Microbiol. 1984;19:44–47. doi: 10.1128/jcm.19.1.44-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda JM, Guthertz LS, Kokka RP, Shimada T. Aeromonas species in septicemia: laboratory characteristics and clinical observations. Clin Infect Dis. 1994;19:77–83. doi: 10.1093/clinids/19.1.77. [DOI] [PubMed] [Google Scholar]
- Jones BL, Wilcox MH. Aeromonas infection and their treatment. J Antimicrob Chemother. 1995;35:453–561. doi: 10.1093/jac/35.4.453. [DOI] [PubMed] [Google Scholar]
- Khan R, Takahashi E, Ramamurthy T, Takeda Y, Okamoto K. Salt in surroundings influences the production of serine protease into milieu by Aeromonas sobria. Microbiol Immunol. 2007;51:963–976. doi: 10.1111/j.1348-0421.2007.tb03993.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Takahashi E, Oguma K, Fujii Y, Yamanaka H, Negishi T, Kobayashi SA, Tsuji T, Okamoto K. Cleavage specificity of the serine protease of Aeromonas sobria, a member of the kexin family of subtilases. FEMS Microbiol Lett. 2006;256:165–170. doi: 10.1111/j.1574-6968.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Mizushima T, Tsuchiya T. Physiological roles of three Na+/H+ antiporters in the halophilic bacterium Vibrio parahaemolyticus. Microbiol Immunol. 2005;49:711–719. doi: 10.1111/j.1348-0421.2005.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Levi M. Pathogenesis and treatment of disseminated intravascular coagulation in the septic patient. J Crit Care. 2001;16:167–177. doi: 10.1053/jcrc.2001.30666. [DOI] [PubMed] [Google Scholar]
- Mann KGR, Lundblad L. Biochemistry of thrombin. In: Colman RW, Hirsh J, Marder VJ, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 2nd edn. Philadelphia, PA: JB Lippincott; 1987. pp. 148–161. [Google Scholar]
- Nishibuchi M, Kumagai K, Kaper JB. Contribution of the tdh1 gene of Kanagawa phenomenon-positive Vibrio parahaemolyticus to production of extracellular thermostable direct hemolysin. Microb Pathog. 1991;11:453–460. doi: 10.1016/0882-4010(91)90042-9. [DOI] [PubMed] [Google Scholar]
- Nomura T, Fujii Y, Yamanaka H, Kobayashi H, Okamoto K. The protein encoded at the 3′ end of the serine protease gene of Aeromonas sobria functions as a chaperone in the production of the protease. J Bacteriol. 2002;184:7058–7061. doi: 10.1128/JB.184.24.7058-7061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nomura T, Hamada M, Fukuda T, Noguchi Y, Fujii Y. Production of serine protease of Aeromonas sobria is controlled by the protein encoded by the gene lying adjacent to the 3′ end of the protease gene. Microbial Immunol. 2000;44:787–798. doi: 10.1111/j.1348-0421.2000.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Pizzo SV, Schwartz ML, Hill RL, McKee PA. The effect of plasmin on the subunit structure of human fibrinogen. J Biol Chem. 1972;247:636–645. [PubMed] [Google Scholar]
- Schweizer HP, Hoang TT. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Siezen RJ, Leunissen JAM. Subtilase: the superfamily of subtilisin-like serine proteases. Protein Sci. 1997;6:501–523. doi: 10.1002/pro.5560060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C, Ichinose Y, Iwanaga M. Purification and characterization of an Aeromonas caviae metalloprotease that is related to the Vibrio cholerae hemagglutinin/protease. FEMS Microbiol Lett. 1999;170:237–242. doi: 10.1111/j.1574-6968.1999.tb13379.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Fujii Y, Noguchi Y, Nomura T, Akita M, Setsu K, Yamamoto S, Okamoto K. Physicochemical and biological properties of an extracellular serine protease of Aeromonas sobria. Microbiol Immunol. 2002;46:383–390. doi: 10.1111/j.1348-0421.2002.tb02710.x. [DOI] [PubMed] [Google Scholar]