Abstract
Aims
To investigate the importance of a maternal and paternal family history of Type 2 diabetes and their combined association with plasma leptin and adiponectin levels in overweight Latino children with a family history of Type 2 diabetes (T2DM).
Methods
This cross-sectional study investigated the combined association of a maternal and paternal family history of T2DM with leptin and adiponectin in 175 overweight Latino children (age 11.1 ± 1.7 years). All subjects had a family history of T2DM. Plasma adiponectin and leptin levels, body fat measured by dual-energy X-ray absorptiometry, Tanner stage, age and insulin sensitivity were assessed.
Results
After adjustment for age, gestational diabetes, insulin sensitivity and body fat, a combined maternal and paternal family history of T2DM was associated with higher leptin concentrations (P = 0.004) compared with a maternal or paternal family history alone. This association was most pronounced at Tanner stage 1 (P for interaction family history × tanner stage = 0.022). The presence of a combined maternal and paternal family history of T2DM accounted for 4% (P = 0.003) of the variation in leptin concentrations. No such combined association was observed for adiponectin levels.
Conclusions
Maternal and paternal family history of T2DM may have an additive impact on leptin, but not on adiponectin levels independent of adiposity and insulin sensitivity in overweight Latino children. This may contribute to a further clinically relevant deterioration of metabolic health in this population.
Diabet. Med. 25, 1043–1048 (2008)
Keywords: adiponectin, leptin, diabetes
Introduction
Adipose tissue has characteristics similar to endocrine organs. It secretes hormones affecting glucose metabolism and insulin sensitivity such as leptin and adiponectin [1]. Leptin acts to reduce food intake and increase energy expenditure [2]. In adults, levels of circulating leptin are directly proportional to total fat mass [3] and are negatively associated with insulin sensitivity [4,5]. Conversely, adiponectin decreases insulin resistance by stimulating glucose uptake and fatty acid oxidation in skeletal muscle [6,7]. We recently showed that leptin and adiponectin were independently associated with insulin sensitivity in overweight Hispanic adolescents [8].
Leptin levels increase before puberty and trigger the onset of puberty in humans [9]. In children approaching puberty, leptin levels are closely related to luteinizing hormone and follicle-stimulating hormone, and leptin is therefore an important facilitator in the early phases of human puberty [9,10].
In adults, a family history of Type 2 diabetes (T2DM) is associated with higher concentrations of circulating leptin [11–13] and lower concentrations of circulating adiponectin [14–18]. In most studies, this association was independent of adiposity. In offspring of parents with T2DM, future risk of diabetes is higher in those with maternal history compared with those with paternal history [19]. However, to our knowledge no information is currently available on the association of maternal vs. paternal family history of T2DM with leptin and adiponectin levels, nor has any study addressed this question in an adolescent population.
Therefore, the aim of the present study was to investigate the importance of a maternal and paternal family history of T2DM and their combined association with plasma leptin and adiponectin levels in overweight Latino children with a family history of T2DM. The hypothesis was that children with both a maternal and paternal family history of T2DM have higher leptin and lower adiponectin levels than children with a family history of T2DM in one parent's family, either maternal or paternal. We also hypothesized that a maternal family history of T2DM may be more important than a paternal family history.
Participants and methods
Study design
For the present study, a cross-sectional subgroup of 175 subjects was used based on the availability of leptin and adiponectin data measured at entry into the Study of Latino Adolescents at Risk (SOLAR) Diabetes Project. Detailed study descriptions have been published previously [20]. Participants were recruited from the East and Central Los Angeles County. Participants were included with an age of 8–13 years, a body mass index ≥ 85th percentile for age and sex according to the Centers for Disease Control and Prevention, a Latino ancestry (all four grandparents Latino by parental self-report) and a family history of T2DM in at least one parent, sibling or grandparent (parental self-report); and absence of diabetes, determined by an oral glucose tolerance test using a dose of 1.75 g of glucose per kg body weight (to maximum 75 g). Participants were excluded if they were taking medications known to affect body composition, known to have any condition which affects body composition or fat distribution, or had had any major illness. The Institutional Review Board of the University of Southern California (USC) approved the study protocol. Written informed consent from parents and assent from children were obtained.
Biochemical measures
For this study, data were collected over two separate clinical visits in the first annual visit. Children were admitted to the USC General Clinical Research Center (GCRC) at approximately 07.30 h after an overnight fast, no food or caloric beverages after 20.00 h. A licensed paediatric healthcare provider conducted a detailed medical history, including parental interview with detailed assessment of family history of diabetes; Tanner staging based on breast stage in girls and pubic hair stage in boys was assessed. Children then completed a 2-h oral glucose tolerance test on the same day. At the second clinical visit, after an overnight fast, a frequently sampled intravenous glucose tolerance test (FSIVGTT) was conducted and body composition was assessed. The evening before, the children were served dinner and an evening snack, and only water and non-caloric and non-caffeinated beverages were permitted after 20.00 h.
The methods of the study have been previously reported in detail elsewhere [14]. Briefly, total body fat was assessed by a whole body scan using dual energy X-ray absorptiometry (Hologic QDR 4500W; Hologic, Bedford, MA, USA). Central fat distribution was measured directly by magnetic resonance imaging at the Los Angeles County/USC Imaging Science Center. A single-slice axial TR 400/16 view of the abdomen at the level of the umbilicus was analysed for cross-sectional area of adipose tissue using a General Electric 1.5 Sigma LX-Echospeed device with a General Electric 1.5-T magnet (Waukesha, WI, USA).
After an overnight fast, the FSIVGTT was performed to determine insulin dynamics. A topical anaesthetic (EMLA cream; AstraZeneca, Wilmington, DE, USA) was applied to the antecubital area of both arms and an hour later a flexible intravenous catheter was inserted into both arms. Two fasting blood samples, at −15 and −5 min, were pooled for determination of basal glucose and insulin values. At time zero, glucose (25% dextrose; 0.3 g/kg of body weight) was administered intravenously. Blood samples were then collected at the following time points: 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, 120 and 180 min [21,22]. Insulin (0.02 U/kg of body weight; Humulin R—regular unmodified insulin; Eli Lilly and Co., Indianapolis, IN, USA) was injected intravenously at 20 min. Plasma was analysed for glucose and insulin, and values were entered into the Minmod Millennium 2003 computer program (version 5.16; Richard N. Bergman, University of Southern California, Los Angeles, CA, USA) for determination of insulin sensitivity (SI), the acute insulin response (AIR = insulin area under the curve above basal for the first 10 min of the FSIVGTT) and the disposition index (DI = product of AIR and SI as a measure of pancreatic β-cell function) [22]. Blood samples from the FSIVGTT were centrifuged immediately to obtain plasma, and aliquots were frozen at −70°C until assayed. Glucose was assayed in duplicate on a Yellow Springs Instrument 2700 Analyzer (Yellow Springs Instrument, Yellow Springs, OH, USA) using the glucose oxidase method. Insulin was assayed in duplicate using a specific human insulin enzyme-linked immunosorbent assay kit from Linco Research (St Charles, MO, USA). Plasma adiponectin was measured using radioimmunoassay kits (Linco Research) with an intra-assay coefficient of variation (CV) of 3.9% and an interassay CV of 8.5%. For plasma leptin, radioimmunoassay kits were used (Linco Research) with an intra-assay CV of 3.9% and an interassay CV of 8.5%.
Statistical analysis
All data are reported as mean ± se. Statistical analyses were performed using spss 11 (SPSS Inc., Chicago, IL, USA). Baseline characteristics of boys and girls were compared using Student's t-test or χ2 test. Variables not normally distributed (adiponectin, leptin, SI, DI, AIR, total fat mass and lean body mass) were log transformed before performing statistical analyses.
ancova models were used to estimate the cumulative association of family history of T2DM on plasma adiponectin and leptin levels as dependent variables. Family history of T2DM was modelled in two ways: first, a model was encoded including (i) one parent's family, either maternal or paternal family history, or (ii) both, maternal and paternal family history. Next, to test whether maternal and paternal family history of T2DM had differential associations with plasma adiponectin and leptin, another model was performed encoding (i) maternal family history, (ii) paternal family history, and (iii) both maternal and paternal family history. Maternal family history of T2DM is defined as the presence of T2DM in a member of the mother's family (siblings and parents of the mother, or the mother herself). Consistently, paternal family history of T2DM is defined as presence of T2DM in the father's family (siblings and parents of the father, or the father himself). The same models were performed for maternal or paternal diabetes without considering other family members. All models were adjusted for gender, Tanner stage, gestational diabetes of the mother while carrying the participant, age, and percentage body fat and an interaction term for Tanner stage and gender. Analyses were repeated with and without inclusion of family history. R2 change is given for the inclusion of family history. For leptin, models were performed stratified by gender and adjusted as mentioned above. In figures, estimated geometrical means are given stratified by gender and Tanner stage after adjustment for gestational diabetes, age, and percentage body fat.
Results
General characteristics of the study population are shown in Table 1. Due to the inclusion criteria, all subjects had a family history of T2DM. A maternal family history was reported by 47%, a paternal family history by 24% and a combination of maternal and paternal family history by 29% of subjects. Neither plasma leptin nor plasma adiponectin concentrations were associated with the occurrence of gestational diabetes.
Table 1.
Characteristics of the study cohort
| Characteristics* | Boys (n = 101) | Girls (n = 74) | P-value† |
|---|---|---|---|
| Age (years) | 11.2 ± 0.2 | 11.1 ± 0.2 | 0.704 |
| Body mass index (kg/m2) | 28.2 ± 0.5 | 28.7 ± 0.6 | 0.435 |
| Body mass index percentile | 97.1 ± 0.3 | 97.4 ± 0.3 | 0.449 |
| Total body fat (%) | 37.6 ± 0.7 | 40.6 ± 0.6 | 0.002 |
| Subcutaneous fat (cm2) | 330 ± 15 | 357 ± 16 | 0.239 |
| Visceral fat (cm2) | 47 ± 2 | 50 ± 2 | 0.457 |
| SI (×10−4/min/(µU/ml))‡ | 2.17 ± 0.14 | 1.88 ± 0.15 | 0.104 |
| Plasma leptin (µg/l) | 23.2 ± 1.1 | 31.1 ± 1.5 | 0.533 |
| Plasma adiponectin (mg/l) | 10.2 ± 0.3 | 10.0 ± 0.4 | 0.220 |
| Gestational diabetes (%) | 19 | 23 | 0.570 |
| Family history of Type 2 diabetes | 0.375 | ||
| Maternal only (%) | 43 | 53 | |
| Paternal only (%) | 24 | 23 | |
| Both (%) | 33 | 24 |
Data are mean ± se, and %.
Students t-tests and χ2 test used to compare differences between male and female patients.
SI, insulin sensitivity.
Leptin
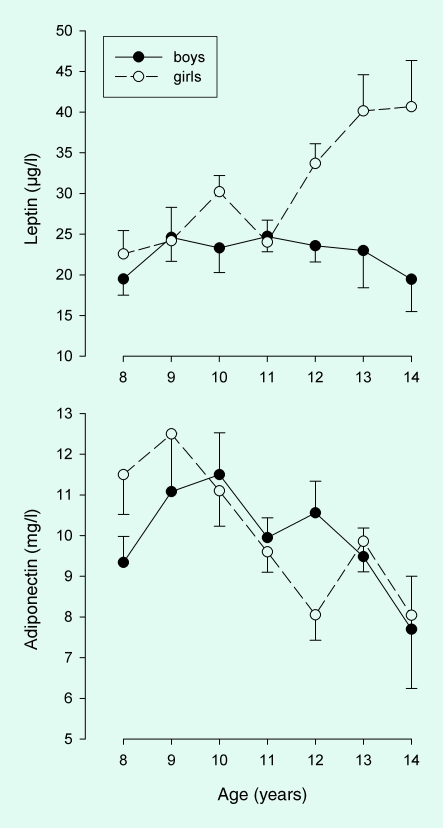
Plasma leptin during puberty increased with age in girls but not in boys (age × gender interaction, P = 0.005, Fig. 1), but no significant interaction for family history × gender was observed.
FIGURE 1.
Serum leptin and adiponectin by age in overweight Hispanic boys (n = 101) and girls (n = 74).
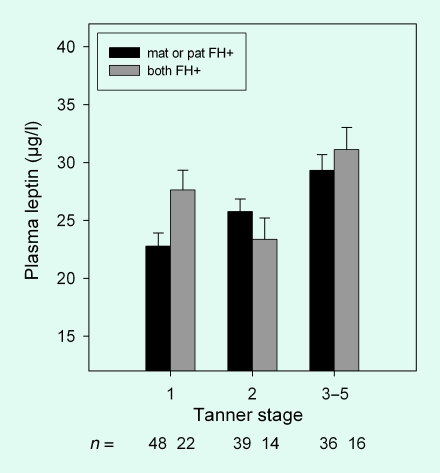
In analyses adjusted for age, gender, gestational diabetes and SI, a combined maternal and paternal family history of T2DM was associated with higher plasma leptin (P = 0.044) compared with a history of T2DM in only one parent's family. After additional adjustment for body fat, the association between a combined maternal and paternal family history of T2DM and plasma leptin was limited to Tanner stage 1 (P = 0.004; P for interaction family history × tanner stage = 0.022; Fig. 2). No significant differences were observed between maternal only and paternal only family history of T2DM. The combination of maternal and paternal family history of T2DM accounted for 4% (P = 0.003) of the variation in leptin concentrations. The association between leptin concentrations and combined family history of T2DM was not affected by additional adjustment for visceral and subcutaneous fat. If only the history of T2DM for first-degree relatives (mother and father together) was included in the model, no association with combined maternal and paternal family history was observed, suggesting that general family history of T2DM is important for the strength of this association compared with family history in first-degree relatives alone.
FIGURE 2.
Plasma leptin in overweight Hispanic children (n = 175) with maternal (mat) or paternal (pat) family history (FH) of Type 2 diabetes. Data are estimate marginal means ± se adjusted for age, body fat, gestational diabetes and insulin sensitivity. The interaction term for type of family history × Tanner stage, P < 0.022.
Adiponectin
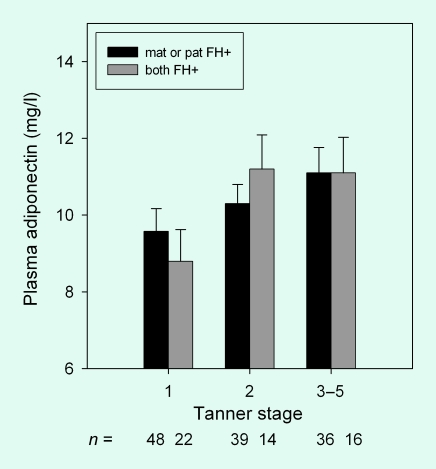
Adiponectin decreased with age in boys and girls (r = −0.231, P = 0.002, Fig. 1). Maternal and paternal family history of T2DM did not have a significant association with plasma adiponectin levels (Fig. 3). Combined maternal and paternal history of T2DM for first-degree relatives was also not associated with plasma adiponectin levels. No gender or Tanner stage interactions were observed.
FIGURE 3.
Plasma adiponectin in overweight Hispanic children (n = 175) with maternal (mat) or paternal (pat) family history (FH) of Type 2 diabetes. Data are estimate marginal means ± se adjusted for age, body fat, gestational diabetes and insulin sensitivity. The interaction term for type of family history × Tanner stage, P < 0.427.
Discussion
The major finding of the present study is that in overweight Hispanic children a combined maternal and paternal family history of T2DM is associated with higher leptin levels compared with a family history of diabetes in the maternal or paternal side alone. This association was independent of adiposity. No such association was observed for plasma adiponectin levels.
Several studies have shown that a family history of T2DM is associated with higher circulating leptin levels [11–13]. Recent data from the Quebec family study suggest that genetic variation at the fat/fat mass and obesity associated (FTO) locus contributes to the aetiology of obesity, insulin resistance and increased plasma leptin levels [23]. Several polymorphisms in the leptin gene affect the receptor binding activity of the protein [24] and secretion by adipose tissue. For example, a polymorphism in the promoter region of the human leptin gene has been associated with T2DM or impaired glucose metabolism [25]. This polymorphism increases leptin protein expression and secretion [26]. Another polymorphism, located in an exonic region of the leptin gene, has been associated with glucose homeostasis in response to exercise [27] and circulating leptin levels [28]. However, the association between the polymorphism and leptin levels was not consistent in different studies [29,30]. The association with a combined maternal and paternal family history of T2DM supports the hypothesis of a hereditary link between leptin and diabetes risk.
Other mechanisms explaining the association with a family history of diabetes could be related to epigenetic effects that are associated with both family history of T2DM and leptin [31]. Fetal programming by maternal diabetes is unlikely to be the cause of the association, because all results were adjusted for gestational diabetes.
Hormonal changes during puberty result in gender-specific differences in levels of leptin levels; oestrogen is known to stimulate and testosterone to suppress leptin secretion [32]. Jansson et al. have shown that leptin levels were only higher in male subjects with a family history of diabetes, but not in female subjects, suggesting a pronounced gender difference in the association between family history of diabetes and leptin levels [11]. In our study, however, no significant interaction was observed between gender and family history of T2DM, suggesting that gender differences in leptin levels did not affect the association between family history and leptin.
In the present study, a combined maternal and paternal history of T2DM was associated with leptin independent of the adjustment for adiposity. In analyses additionally adjusted for adiposity, the association was stronger in participants of Tanner stage 1 than in those in other Tanner stages. This observation suggests that with progress of puberty other adiposity-related changes outweigh the association between family history of T2DM and leptin. In contrast, studies in adults suggest that a family history of T2DM is associated with higher concentrations of circulating leptin [11–13] independent of adiposity.
Several studies have also suggested an association between a family history of T2DM and adiponectin levels resulting in lower adiponectin levels in adult offspring of diabetic patients compared with control subjects [14–18]. Several polymorphisms in the adiponectin gene have been associated with an increased risk of diabetes. In the present study, we did not observe any cumulative association between maternal and paternal family history of T2DM and adiponectin levels as observed for leptin. However, in the present study only subjects with a family history of diabetes were included, and no comparison between presence and absence of family history could be made.
The present study is limited by its cross-sectional design. Therefore, we could not address the association of leptin and adiponectin with the combined maternal and paternal family history of diabetes on longitudinal changes in adiponectin and leptin that may occur during puberty. We have recently shown in a longitudinal study of the same cohort that the association between a maternal family history of T2DM and insulin dynamics becomes more pronounced during growth [33]. Longitudinal studies on the association between family history and leptin and adiponectin levels during growth will be necessary to demonstrate whether this is similar for leptin and adiponectin.
In conclusion, a combination of a maternal and paternal family history of T2DM is associated with higher leptin levels independent of adiposity and SI in overweight Latino children compared with a maternal or paternal family history alone. No such association was observed for adiponectin.
Acknowledgments
This study was supported by the NIH Grant R01 DK 59211, the General Clinic Research Center (GCRC) and the National Center for Research Resources Grant M01 RR 00043 as well as an American Diabetes Association, Mentor-Based Postdoctoral Fellowship 2005 (awarded to M.I.G.). C.K. was supported by a training grant of the USC Center for Transdisciplinary Research on Energetics (NCI Grant 1U54CA116848-01), L.A.K. and J.N.D. by American Diabetes Association (Mentor-Based Postdoctoral Fellowship) grant under the direction of M.I.G., C.K.R. by a National Institute on Aging Training Award (5 T32 AG000093-24). C.M.T-C. was supported by a minority supplement grant under the direction of M.I.G. (3R01DK59211–06S1). We are grateful to the project managers, Quintilia Avila and Christina Ayala, and to the nurses and nutrition staff at the USC-GCRC.
Abbreviations
- AIR
acute insulin response
- CV
coefficient of variation
- DI
disposition index
- FSIVGTT
frequently sampled intravenous glucose tolerance test
- GCRC
General Clinical Research Center
- SI
insulin sensitivity
- T2DM
Type 2 diabetes
- USC
University of Southern California
Competing interests
Nothing to declare.
References
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249–256. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 4.Donahue RP, Prineas RJ, Donahue RD, Zimmet P, Bean JA, De Court M, et al. Is fasting leptin associated with insulin resistance among nondiabetic individuals? The Miami Community Health Study. Diabetes Care. 1999;22:1092–1096. doi: 10.2337/diacare.22.7.1092. [DOI] [PubMed] [Google Scholar]
- 5.Wauters M, Considine RV, Yudkin JS, Peiffer F, De Leeuw I, Van Gaal LF. Leptin levels in Type 2 diabetes: associations with measures of insulin resistance and insulin secretion. Horm Metab Res. 2003;35:92–96. doi: 10.1055/s-2003-39054. [DOI] [PubMed] [Google Scholar]
- 6.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Thamer C, Haap M, Heller E, Joel L, Braun S, Tschritter O, et al. Beta cell function, insulin resistance and plasma adiponectin concentrations are predictors for the change of postprandial glucose in non-diabetic subjects at risk for Type 2 diabetes. Horm Metab Res. 2006;38:178–182. doi: 10.1055/s-2006-925204. [DOI] [PubMed] [Google Scholar]
- 8.Koebnick C, Roberts CK, Shaibi GQ, Kelly LA, Lane CL, Toledo-Corral C, et al. Adiponectin and leptin are independently associated with insulin sensitivity but not insulin secretion or beta-cell function in overweight Hispanic adolescents. Horm Metab Res. 2008 doi: 10.1055/s-2008-1077097. in press. Epub 8 June 2008, PMID 18563679. [DOI] [PubMed] [Google Scholar]
- 9.Bluher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes. 2007;14:458–464. doi: 10.1097/MED.0b013e3282f1cfdc. [DOI] [PubMed] [Google Scholar]
- 10.Maqsood AR, Trueman JA, Whatmore AJ, Westwood M, Price DA, Hall CM, et al. The relationship between nocturnal urinary leptin and gonadotrophins as children progress towards puberty. Horm Res. 2007;68:225–230. doi: 10.1159/000101335. [DOI] [PubMed] [Google Scholar]
- 11.Jansson PA, Eliasson B, Lindmark S, Eriksson JW. Endocrine abnormalities in healthy first-degree relatives of Type 2 diabetes patients—potential role of steroid hormones and leptin in the development of insulin resistance. Eur J Clin Invest. 2002;32:172–178. doi: 10.1046/j.1365-2362.2002.00963.x. [DOI] [PubMed] [Google Scholar]
- 12.Nyholm B, Fisker S, Lund S, Moller N, Schmitz O. Increased circulating leptin concentrations in insulin-resistant first-degree relatives of patients with non-insulin-dependent diabetes mellitus: relationship to body composition and insulin sensitivity but not to family history of non-insulin-dependent diabetes mellitus. Eur J Endocrinol. 1997;136:173–179. doi: 10.1530/eje.0.1360173. [DOI] [PubMed] [Google Scholar]
- 13.Vauhkonen I, Niskanen L, Haffner S, Kainulainen S, Uusitupa M, Laakso M. Insulin resistant phenotype is associated with high serum leptin levels in offspring of patients with non-insulin-dependent diabetes mellitus. Eur J Endocrinol. 1998;139:598–604. doi: 10.1530/eje.0.1390598. [DOI] [PubMed] [Google Scholar]
- 14.Behre CJ, Brohall G, Hulthe J, Fagerberg B. Serum adiponectin in a population sample of 64-year-old women in relation to glucose tolerance, family history of diabetes, autoimmunity, insulin sensitivity, C-peptide, and inflammation. Metabolism. 2006;55:188–194. doi: 10.1016/j.metabol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, Kashyap S, et al. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia. 2004;47:816–820. doi: 10.1007/s00125-004-1359-x. [DOI] [PubMed] [Google Scholar]
- 16.Furuhashi M, Ura N, Higashiura K, Miyazaki Y, Murakami H, Hyakukoku M, et al. Low adiponectin level in young normotensive men with a family history of essential hypertension. Hypertens Res. 2005;28:141–146. doi: 10.1291/hypres.28.141. [DOI] [PubMed] [Google Scholar]
- 17.Kowalska I, Straczkowski M, Nikolajuk A, Krukowska A, Kinalska I, Gorska M. Plasma adiponectin concentration and tumor necrosis factor-alpha system activity in lean non-diabetic offspring of Type 2 diabetic subjects. Eur J Endocrinol. 2006;154:319–324. doi: 10.1530/eje.1.02084. [DOI] [PubMed] [Google Scholar]
- 18.Pellme F, Smith U, Funahashi T, Matsuzawa Y, Brekke H, Wiklund O, et al. Circulating adiponectin levels are reduced in nonobese but insulin-resistant first-degree relatives of Type 2 diabetic patients. Diabetes. 2003;52:1182–1186. doi: 10.2337/diabetes.52.5.1182. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell BD, Valdez R, Hazuda HP, Haffner SM, Monterrosa A, Stern MP. Differences in the prevalence of diabetes and impaired glucose tolerance according to maternal or paternal history of diabetes. Diabetes Care. 1993;16:1262–1267. doi: 10.2337/diacare.16.9.1262. [DOI] [PubMed] [Google Scholar]
- 20.Goran MI, Bergman RN, Avila Q, Watkins M, Ball GD, Shaibi GQ, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for Type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 21.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 23.Do R, Bailey SD, Desbiens K, Belisle A, Montpetit A, Bouchard C, et al. Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes. 2008;57:1147–1150. doi: 10.2337/db07-1267. [DOI] [PubMed] [Google Scholar]
- 24.Imagawa K, Numata Y, Katsuura G, Sakaguchi I, Morita A, Kikuoka S, et al. Structure–function studies of human leptin. J Biol Chem. 1998;273:35245–35249. doi: 10.1074/jbc.273.52.35245. [DOI] [PubMed] [Google Scholar]
- 25.Ren W, Zhang SH, Wu J, Ni YX. Polymorphism of the leptin gene promoter in pedigrees of Type 2 diabetes mellitus in Chongqing, China. Chin Med J (Engl) 2004;117:558–561. [PubMed] [Google Scholar]
- 26.Hoffstedt J, Eriksson P, Mottagui-Tabar S, Arner P. A polymorphism in the leptin promoter region (−2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34:355–359. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- 27.Hager J, Clement K, Francke S, Dina C, Raison J, Lahlou N, et al. A polymorphism in the 5′ untranslated region of the human ob gene is associated with low leptin levels. Int J Obes Relat Metab Disord. 1998;22:200–205. doi: 10.1038/sj.ijo.0800567. [DOI] [PubMed] [Google Scholar]
- 28.Lakka TA, Rankinen T, Weisnagel SJ, Chagnon YC, Lakka HM, Ukkola O, et al. Leptin and leptin receptor gene polymorphisms and changes in glucose homeostasis in response to regular exercise in nondiabetic individuals: the HERITAGE family study. Diabetes. 2004;53:1603–1608. doi: 10.2337/diabetes.53.6.1603. [DOI] [PubMed] [Google Scholar]
- 29.Lucantoni R, Ponti E, Berselli ME, Savia G, Minocci A, Calo G, et al. The A19G polymorphism in the 5′ untranslated region of the human obese gene does not affect leptin levels in severely obese patients. J Clin Endocrinol Metab. 2000;85:3589–3591. doi: 10.1210/jcem.85.10.6860. [DOI] [PubMed] [Google Scholar]
- 30.Mammes O, Betoulle D, Aubert R, Giraud V, Tuzet S, Petiet A, et al. Novel polymorphisms in the 5′ region of the LEP gene: association with leptin levels and response to low-calorie diet in human obesity. Diabetes. 1998;47:487–489. doi: 10.2337/diabetes.47.3.487. [DOI] [PubMed] [Google Scholar]
- 31.Stoger R. The thrifty epigenotype: an acquired and heritable predisposition for obesity and diabetes? Bioessays. 2008;30:156–166. doi: 10.1002/bies.20700. [DOI] [PubMed] [Google Scholar]
- 32.Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, et al. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab. 2000;85:2509–2518. doi: 10.1210/jcem.85.7.6689. [DOI] [PubMed] [Google Scholar]
- 33.Kelly LA, Lane CJ, Weigensberg MJ, Koebnick C, Roberts CK, Davis JN, et al. Parental history and risk of Type 2 diabetes in overweight Latino adolescents: a longitudinal analysis. Diabetes Care. 2007;30:2700–2705. doi: 10.2337/dc07-0050. [DOI] [PubMed] [Google Scholar]