Abstract
Background
Entamoeba histolytica, the cause of invasive amebiasis, phagocytoses apoptotic host cells during tissue invasion. In mammals, collectin family members (e.g., mannose binding lectin (MBL)) and the structurally related protein C1q bind to apoptotic cells, and stimulate macrophage phagocytosis via a conserved collagenous tail domain. The collectins also bind to bacteria, E. histolytica's usual source of nutrients.
Methods
To test the possibility that the collectins are ligands that stimulate E. histolytica phagocytosis, we used a flow-cytometry-based assay for amebic phagocytosis, a method for making single-ligand particles to delineate a given ligand's ability to initiate phagocytosis, and purified human C1q, MBL, and collagenous collectin tails.
Results
Apoptotic lymphocytes opsonized with serum or human C1q were phagocytosed more efficiently than control cells, an effect that was dependent on ligand density. C1q and the collectins alone were adequate to trigger amebic phagocytosis, since single-ligand particles coated with C1q, MBL, or purified collectin tails were phagocytosed more efficiently than control particles. Furthermore, C1q, MBL, and C1q's tail domain were all chemoattractants for E. histolytica.
Conclusions
C1q and MBL can serve as opsonins on apoptotic cells that stimulate E. histolytica phagocytosis, an effect mediated at least in part by the collagenous collectin tail domain.
Keywords: Entamoeba histolytica, phagocytosis, C1q, mannose binding lectin, collectin
Introduction
Entamoeba histolytica, an enteric ameba that causes dysentery and liver abscesses, may cause up to 100,000 deaths annually [1]. However, approximately 90% of E. histolytica infections are asymptomatic, with the amebic trophozoites dwelling in the colonic lumen and acquiring nutrients by phagocytosis of bacteria [2–7]. Amebic phagocytosis of host cells is a prominent histopathologic finding in amebiasis [8]. In fact, visualization of amebas with phagocytosed erythrocytes is the only characteristic that enables distinction of E. histolytica from the commensal parasite Entamoeba dispar by light microscopy [9].
Despite the importance of phagocytosis in E. histolytica biology, little is known about the amebic receptors and the ligands they bind. Entamoeba histolytica induces caspase-dependent host cell apoptosis and host cell apoptosis precedes phagocytosis by amebae [10, 11]. Furthermore, D-galactose, which inhibits a D-galactose/N-acetyl-D-galactosamine (Gal/GalNAc) specific E. histolytica surface lectin and blocks adherence to and killing of healthy cells, inhibits phagocytosis of apoptotic cells poorly [11]. Calcium ionophore treatment induces erythrocyte membrane changes resembling changes on apoptotic cells [12, 13]. Not surprisingly, therefore, Ca2+ ionophore-induced erythrocyte membrane changes stimulate amebic phagocytosis [14]. Collectively, these data suggest a sequential process of cell killing followed by exposure of new ligands on the dying cell and recruitment of phagocytosis receptors in addition to the Gal/GalNAc specific lectin.
Since tissue invasion and phagocytosis of host cells has no clear evolutionary benefit for E. histolytica and acquisition of nutrients by phagocytosis of bacteria is a major evolutionary pressure, it is logical that E. histolytica may recognize ligands shared by apoptotic cells and bacteria. That is, though phagocytosis of apoptotic cells by E. histolytica may be essential for virulence (this is unknown), it may be the result of coincidental evolution, and E. histolytica may preferentially phagocytose apoptotic cells simply because they have key surface similarities to bacteria. Consistent with this, Ghosh and Samuelson showed that several proteins required for virulence are used by the parasite to kill and phagocytose bacteria [15].
The collectins, which are pattern recognition molecules of the innate immune system, are ligands recognized by macrophages that are common to apoptotic cells and bacteria [16–23]. In humans, collectin family members include the mannose binding lectin (MBL), and surfactant proteins A and D (SP-A and SP-D) [23]. Though SP-A and SP-D were originally identified in lung secretions, it is clear that collectins are broadly distributed at mucosal surfaces including in intestinal secretions [24–32]. Structurally, they share a conserved N-terminal collagenous tail and each has a C-terminal C-type lectin domain that binds to apoptotic cells and bacteria [23]. C1q has no lectin domain, but shares the collagenous tail [19]. C1q binds directly to apoptotic cells and facilitates macrophage phagocytosis [19]. Recognition of the collectins and C1q by macrophages is thought to be mediated by cell surface calreticulin (also called cC1qR), which binds to the collectin tail [19, 33–36].
Calreticulin is abundant in purified E. histolytica phagosomes [37, 38]. Since calreticulin is the receptor for the collagenous tail of C1q and the collectins opsonize both apoptotic cells and bacteria, we hypothesized that C1q and the collectins are ligands for an E. histolytica phagocytosis receptor. Here, we present data demonstrating that C1q and the collectins are strong stimulants of E. histolytica phagocytosis, and that E. histolytica trophozoites migrate towards C1q and the collectins, an effect mediated in part by their collagenous tail domain.
Materials and Methods
Reagents
The streptavidin-Alexa Fluor 488 conjugate and 5 (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) were purchased from Invitrogen. Sulfo-NHS-LC-biotin was purchased from Pierce Biotechnology. Streptavidin-coated 2 µm green fluorescent latex beads were purchased from Polysciences, Inc. Human C1q was purchased from Quidel, Corp. Human mannose binding lectin (MBL) was purchased from USBiological. The collagenous tail of SP-A was a gift from Peter Henson (National Jewish Medical Center, Denver, CO). The collagenous C1q tail was purified essentially as previously described [39]. Briefly, human C1q was dialyzed against 0.15 M NaCl, 0.1 M NaAcetate, pH 4.5, incubated for 24 h at 37°C in 10 µg/ml pepsin, and applied to a Superdex 200 10/300GL column (GE Healthcare). Proteins were eluted with 5 mM CaCl2 in Tris buffered saline. Purity was >90% as assessed by SDS-PAGE and silver staining.
Cell lines and tissue culture
Entamoeba histolytica trophozoites (strain HM-1:IMSS) were grown axenically in TYI-S-33 (trypticase-yeast extract-iron serum) medium supplemented with 100 U of penicillin/ml and 100 µg of streptomycin sulfate/ml at 37°C [40]. Trophozoites were used during mid-log-phase growth. All experiments were performed in either phosphate buffered saline (PBS) or medium 199 (Gibco BRL) supplemented with 5.7 mM cysteine, 25 mM HEPES, and 0.5% bovine serum albumin (BSA) at pH 6.8.
The human leukemia T-cell line Jurkat (clone E6-1, American Type Culture Collection) was grown in RPMI 1640 medium (Gibco BRL) supplemented with 10% fetal bovine serum, 100 U of penicillin/ml and 100 µg/ml of streptomycin sulfate [41]. For experiments using C1q, Jurkat cells were cultured under serum free conditions using AIM V + GlutaMAX-I medium (Gibco) to avoid confounding effects due to serum collectins [21]. Jurkat cell apoptosis was induced by exposure to ultraviolet light for 10 min followed by 3 h incubation at 37°C, or by incubation overnight (37°C) with 40 µM etoposide. CFSE labeling was done by incubating cells for 20 min at 37°C in PBS containing 11.2 µM CFSE. Unbound dye was quenched by incubation with an equal volume of 20% BSA (20 min, 37°C). Cells were washed twice prior to use.
Flow cytometric measurement of collectin binding
We used biotinylated C1q and flow cytometry to measure C1q binding to lymphocytes. Human C1q and Ovalbumin were biotinylated using the amine-reactive reagent Sulfo-NHS-LC-biotin according to the manufacturer's instructions, and then dialyzed against PBS. Jurkat lymphocytes were fixed with 3% paraformaldehyde, blocked with 10% BSA in PBS (15 min, room temperature (RT)) followed by a streptavidin/biotin blocking reagent (Vector Laboratories), incubated with the indicated biotinylated protein (30 µg/ml, 1 h, RT), washed, and bound biotinylated proteins were labeled by incubation with a streptavidin-Alexa 488 conjugate (1 h, RT). Fluorescence was measured using a Coulter EPICS XL-MCL flow cytometer and analyzed using WinMDI software (version 2.8).
Confocal microscopy
To assess C1q-biotin binding to lymphocytes by microscopy, Jurkat lymphocytes were stained with C1q-biotin in cytometry tubes as above, and then pipetted onto glass slides. Slides were examined using a BioRad MRC 1024ES confocal microscope. Where indicated, a Zeiss 510 META confocal microscope was used to verify phagocytosis of latex beads.
Preparation of single-ligand fluorescent particles
To prepare fluorescent latex particles coated with a single ligand, protein ligands were biotinylated using Sulfo-NHS-LC-biotin, dialyzed against PBS, and bound to streptavidin coated 2 µm fluorescent latex particles. Both the biotin-coupling reaction and bead coating were performed according to protocols supplied by the respective reagent's manufacturer.
Phagocytosis assays
All phagocytosis experiments were performed in the presence of 110 mM D-galactose to inhibit the amebic D-Gal/GalNAc specific lectin. The flow cytometry phagocytosis assay has been previously described [11]. Entamoeba histolytica trophozoites were mixed with either CFSE-labeled Jurkat cells or 2 µm single-ligand fluorescent latex beads. The mixture was centrifuged and incubated at 37°C for the indicated time. Adherent beads and cells were washed away with an ice-cold solution of 110 mM D-galactose in PBS, amebas were fixed with 3% paraformaldehyde, and then analyzed using a Coulter EPICS XL-MCL flow cytometer and WinMDI software (version 2.8). Amebas were distinguished from non-phagocytosed beads and host cells based on forward and side scatter characteristics. Amebas with fluorescence above background levels were considered to be phagocytic, and both the percent of positive amebas and mean fluorescence of positive amebas were measured. Data are presented as the phagocytic index, calculated as the percent amebae positive × the mean fluorescence of positive amebae. The phagocytic index has been used to quantify macrophage phagocytosis [42].
In some cases, phagocytosis was quantified by microscopy. For this, fluorescent beads were centrifuged onto trophozoites adherent to glass coverslips (200 × g, 5 min, room temperature), followed by incubation for 45 min at 37°C. The coverslips were washed three times with 110 mM D-galactose in PBS to remove adherent but unengulfed beads, and inverted onto GelMount. Phagocytosis was quantified by a blinded observer using an epifluorescent microscope by scoring at least 100 parasites as positive or negative for phagocytosis, and, if positive, counting the number of beads phagocytosed. A phagocytic index was again calculated, though here defined as the percent of amebas positive for phagocytosis × the mean number of beads phagocytosed per positive ameba.
Chemotaxis assay
E. histolytica chemotaxis was measured using a transwell migration assay with 6.5 mm transwell plates (Costar). This assay was adapted from one described previously [43], and measured migration of E. histolytica trophozoites through a polycarbonate membrane with 8.0 µm pores from an upper compartment filled with PBS to a lower compartment filled with either PBS, TYI-S-33 medium (positive control), or PBS containing BSA (negative control) or the indicated collectin. Entamoeba histolytica trophozoites (1 × 104 in PBS) were added to the upper compartment, the plates were incubated for 2 h at 37°C followed by 15 min at 4°C, the fluid was aspirated from the lower compartment and used to gently wash adherent amebas from the bottom of the membrane. The trophozoites were then centrifuged, resuspended in 100 µl, and the number in 10 µl were counted using a hemacytometer.
Statistics
All quantitative data were expressed as the mean and standard deviation (SD) of the mean. Significance was determined using the unpaired Student's t test.
Results
Serum components stimulate E. histolytica phagocytosis
The collectins and C1q bind to apoptotic cells and bacteria, and facilitate macrophage phagocytosis [16–23]. To begin assessing the possibility that collectins stimulate amebic phagocytosis, we examined the effect serum-treatment of apoptotic lymphocytes had on E. histolytica phagocytosis using a flow cytometry-based phagocytosis assay. For this, fluorescently-labeled apoptotic Jurkat lymphocytes were treated with either heat inactivated fetal bovine serum or BSA (negative control) prior to interaction with E. histolytica trophozoites. Approximately 20% more E. histolytica trophozoites engulfed the serum treated apoptotic cells than control cells (Figure 1, M1 gate, 120 ± 3.2 vs. 100 ± 1.3 percent positive (serum vs. BSA treated, mean and SD normalized to BSA control), n=3, p=.0006, Student's t test). We concluded that serum components may bind to apoptotic cells and stimulate amebic phagocytosis.
Figure 1. Effect of serum on E. histolytica phagocytosis of apoptotic lymphocytes.
Apoptotic T lymphocytes were CFSE-labeled, treated with heat inactivated fetal bovine serum or BSA (negative control), and incubated with E. histolytica trophozoites (30 min, 37°C, ameba:cell=1:4) in the presence of 100 mM D-galactose to inhibit the amebic Gal/GalNAc lectin. Phagocytosis was assayed using flow cytometry. Representative FACS histograms of amebic fluorescence are shown. M1 gate indicates phagocytic amebae.
Human C1q stimulates E. histolytica phagocytosis of apoptotic cells
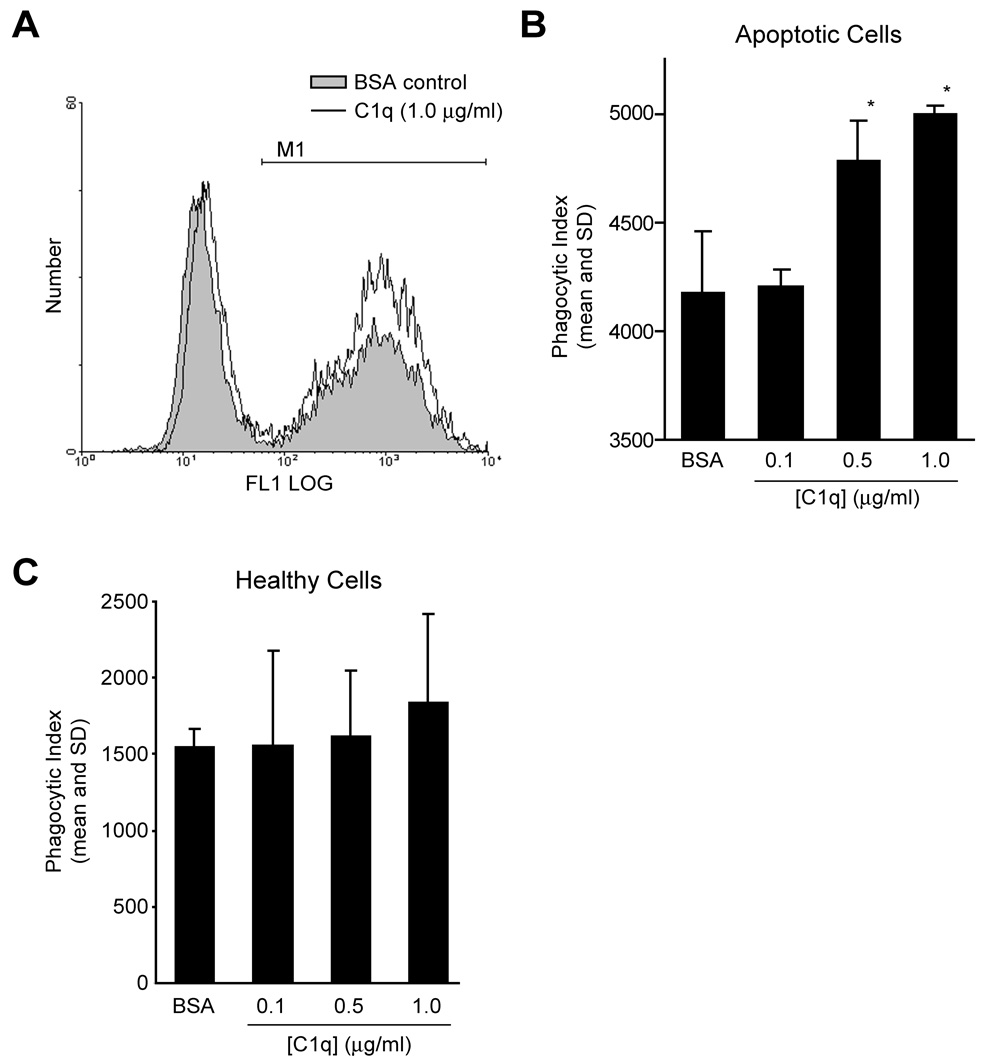
To directly test whether human C1q enhances E. histolytica phagocytosis, we compared phagocytosis of apoptotic lymphocytes pre-treated with human C1q to phagocytosis of apoptotic cells pre-treated with BSA (negative control). Significantly more E. histolytica trophozoites engulfed apoptotic lymphocytes pre-treated with C1q than control cells, and the effect was dose dependent (Figure 2A and B). We next examined if the effect of opsonization with C1q was specific for phagocytosis of apoptotic compared to viable lymphocytes. As we reported previously, phagocytosis of healthy cells by E. histolytica was poor in the presence of D-galactose, suggesting that novel ligands for E. histolytica phagocytosis receptors become exposed on or bound to dying host cells [11]. Consistent with the possibility that C1q is one such ligand, pre-treatment of healthy lymphocytes with C1q had no significant effect on phagocytosis (Figure 2C).
Figure 2. C1q facilitates E. histolytica phagocytosis of apoptotic but not healthy lymphocytes.
Apoptotic or healthy lymphocytes were fluorescently-labeled, treated with purified human C1q or BSA (negative control)(30 min, 37°C), and incubated with amebic trophozoites (30 min, 37°C, ameba:host cell=1:4) in the presence of 100 mM D-galactose. (A) Representative FACS histograms of amebic fluorescence following incubation with apoptotic lymphocytes are shown. M1 gate indicates phagocytic amebae. (B and C) Dose effect of C1q on E. histolytica phagocytosis of apoptotic (B) or healthy (C) lymphocytes (mean phagocytic index and SD, n=3, * indicates p < .05, Student's t test).
To examine the difference between dying and healthy cells in greater detail, we used flow cytometry to assess binding of C1q-biotin to apoptotic and viable cells. Ovalbumin-biotin was used as a negative control, and binding was detected using a streptavidin-Alexa Fluor 488 conjugate. Biotinylated-C1q bound to both viable and apoptotic lymphocytes equally, as reported previously (Figure 3A and B) [19]. No binding of ovalbumin-biotin was detected, so the C1q-biotin staining was specific. We compared C1q-biotin binding to healthy and apoptotic cells using confocal microscopy to determine if the binding pattern was different. Interestingly, C1q bound diffusely to the surface of viable lymphocytes, but localized to blebs on the surface of apoptotic cells (Figure 3C). Together, these data suggest that C1q is a ligand that stimulates E. histolytica phagocytosis of apoptotic lymphocytes, and that higher localized ligand density on apoptotic blebs may play an important role in initiating phagocytosis.
Figure 3. C1q-biotin binds to the surface of both viable and apoptotic lymphocytes, but with different patterns.
Apoptotic or healthy Jurkat T lymphocytes were fixed and stained with either C1q-biotin or Ovalbumin (Ova)-biotin (negative control) followed by streptavidin-Alexa 488. (A and B) FACS assessment of C1q-biotin binding to healthy (A) and apoptotic (B) lymphocytes demonstrated equal binding to both. (C) Confocal microscopy demonstrated diffuse surface distribution of C1q on healthy lymphocytes (photo on left), with localization to apparent surface blebs on apoptotic lymphocytes (three photos on right). Original magnification ×600. 10 µm scale bars are shown.
C1q alone is adequate to stimulate phagocytosis
It was important to determine if C1q alone triggers E. histolytica phagocytosis and to develop a method to examine the specificity of the effect of C1q. For this, we used fluorescent beads coupled to C1q or BSA (negative control). These "single-ligand" particles were prepared using biotinylated C1q and BSA, and streptavidin-coated 2 µm latex beads. As assayed using flow cytometry, the C1q-coated particles were phagocytosed approximately ten times more efficiently than the control particles (Figure 4A and Table 1). Confocal microscopy was used to verify uptake of the particles by E. histolytica, ensuring that the increased amebic fluorescence observed by flow cytometry was not due to non-phagocytosed beads adherent to the amebic surface (Figure 4B). The confocal section shown was taken roughly midway through several E. histolytica trophozoites. As a confirmatory test, C1q-bead engulfment was assayed independently using an epifluorescent microscope. Though this assay was more subjective due to difficulty determining if beads were adherent to or within amebae, the results were similar (phagocytic index of 15.5 ± 7.5 vs. 4.9 ± 2.7, mean and SD, n=5, p=.02 vs. BSA control, Student's t test). We concluded that human C1q alone is adequate to trigger E. histolytica phagocytosis.
Figure 4. Phagocytosis of C1q-coated single-ligand particles by E. histolytica trophozoites.
Streptavidin-modified 2 µm fluorescent latex beads were coated with biotinylated C1q or BSA (negative control). (A) Representative FACS histograms of amebic fluorescence following incubation with coated beads (45 min, 37°C, ameba:bead=1:10) in 75 mM D-galactose. M1 gate indicates phagocytic amebae. (B) Confocal microscopy verifying uptake of C1q-coated particles by E. histolytica trophozoites. Shown is a section thru the middle of several trophozoites following incubation with C1q-coated particles as above. Original magnification ×1000. A 20 µm scale bar is shown. (C) Dependence of amebic phagocytosis on C1q-density. C1q-coated particles with varying ligand density were prepared by incubating a fixed number of streptavidin-coated beads with increasing concentrations of C1q-biotin. Phagocytosis was assayed as above, except using a 90 min time point. Graph shows the mean phagocytic index and SD versus the concentration of C1q-biotin used for bead preparation (n=3).
TABLE 1.
Phagocytosis of C1q- and collectin-coated latex beads by Entamoeba histolytica trophozoites.
| Ligand | % of E. histolytica trophozoites positive for phagocytosisa | Fluorescence of phagocytic trophozoitesa | Phagocytic Indexa |
|---|---|---|---|
| BSA control | 100 | 1 | 100 |
| C1q | 527.3 ± 4.7b | 1.9 ± .1b | 996.8 ± 39.6b |
| MBL | 181.5 ± 4.0b | 1.8 ± .02b | 321.1 ± 11.6b |
| C1q tail | 245.8 ± 31.3b | 1.15 ± .07 | 282.7 ± 51.8b |
| SP-A tail | 124.1 ± 3.9c | .86 ± .00 | 107.3 ± 3.3 |
The percent of trophozoites positive for phagocytosis and the mean fluorescence of phagocytic trophozoites following incubation with C1q- or collectin-coated streptavidin fluorescent latex beads was measured using flow cytometry. Data were normalized to results obtained with BSA-coated beads. The phagocytic index is the % of phagocytic trophozoites × the mean fluorescence of phagocytic trophozoites. Values are means ± SD (three samples per group).
Significantly different from the corresponding value for the BSA control as calculated by the Student's t test (p ≤ .005 for b; p ≤ .05 for c).
Given the different effects C1q opsonization had on uptake of apoptotic and healthy cells, we next examined the effect of ligand density by varying the concentration of C1q-biotin used to prepare the single-ligand particles while holding the number of particles fixed. We were unable to measure the quantity of ligand bound, but the particles were expected to become saturated as the available streptavidin binding sites became occupied. As shown in Figure 4C, the phagocytic index was directly related to the amount of C1q on the beads. This result suggested that increased ligand density on apoptotic blebs may account for the different effects C1q opsonization had on phagocytosis of apoptotic versus viable lymphocytes.
Mannose binding lectin and the collagenous collectin tail both stimulate E. histolytica phagocytosis
Macrophages phagocytose apoptotic cells opsonized with C1q and other collectin proteins via receptor recognition of the collagenous tail shared by members of this protein family [19, 33–36]. To determine if E. histolytica recognized C1q-coated apoptotic cells and particles via the collectin tail, we prepared single-ligand beads with mannose binding lectin, and the collagenous collectin tails from SP-A and C1q. As summarized in Table 1, these ligands all significantly stimulated E. histolytica phagocytosis compared to BSA-coated control beads. Though the largest effect was observed with intact C1q, it is impossible to directly compare the results for the different particles, since we were unable to determine the absolute quantity of ligand on the beads or to use sufficient quantities of these proteins to prepare the beads at saturating concentrations. We concluded that E. histolytica phagocytosis of the C1q- and MBL-coated beads is mediated at least in part by recognition of the collagenous tail these proteins share, but cannot exclude that the globular head of C1q also contributes.
C1q, mannose binding lectin, and the collectin tail are chemoattractants for E. histolytica
Macrophages migrate towards C1q [44]. We used a transwell migration assay to assess whether E. histolytica trophozoites migrate towards these molecules. For this, trophozoites in reference media (PBS) were placed in the upper chamber of transwells, separated from a lower chamber containing the reference media with and without the potential chemoattractants by a polycarbonate membrane with 8.0 µm pores. We counted the number of amebae that migrated to the lower chamber after 90 minutes incubation. TYI-S-33 medium (a known chemoattractant for E. histolytica) was used as a positive control. Bovine serum albumin, which like the C1q and MBL was purified from serum, served as a negative control. C1q was a strong stimulant of E. histolytica migration, an effect which was specific and dose dependent (Table 2). The amebae also migrated towards MBL and the C1q tail. We concluded that in addition to being specific ligands for E. histolytica phagocytosis, MBL and C1q are chemoattractants for amebae. Furthermore, their chemoattractant effect is mediated at least in part by the collagenous collectin tail.
TABLE 2.
Transwell migration of E. histolytica trophozoites in response to soluble C1q and MBL.
| Attractant | Number of trophozoites migrating to the lower chamber |
|---|---|
| Mean ± SD | |
| PBS | 1.5 ± .7 |
| TYI-S-33 | 1000 ± 0a |
| BSA (100 µg/ml) | 6.8 ± 2.5 |
| C1q (5.8 µg/ml) | 239 ± 24a |
| C1q (100 µg/ml) | 319.5 ± 43.1b |
| MBL (5.8 µg/ml) | 70 ± 1.4a |
| C1q tail (5.8 µg/ml) | 53 ± 4.2a |
Significantly different from the BSA control as calculated by the Student's t test (n = 2, p ≤ .005 for a and p ≤ .01 for b).
Discussion
The major conclusion of this work is that host collectins and C1q can serve as opsonins on apoptotic cells that stimulate E. histolytica phagocytosis. Serum and C1q treatment of apoptotic cells increased phagocytosis by amebae, and, since single-ligand C1q beads were aggressively phagocytosed, C1q alone is adequate to stimulate E. histolytica phagocytosis. Since E. histolytica phagocytosis was directly related to the quantity of C1q present, the density of C1q appears to be important for stimulating particle uptake. This is consistent with the different binding patterns of C1q to healthy and apoptotic cells, and failure of C1q opsonization to increase amebic uptake of healthy cells at the concentrations used.
C1q adheres to the Fc domain of IgG via its globular head and the collectins adhere to many protein glycoconjugates via their C-type lectin domain [22, 23]. This raises the possibility that C1q and/or MBL facilitate E. histolytica phagocytosis simply by tethering the opsonized cells or particles to the amebic surface. Though it was not possible to compare directly phagocytosis of particles coated with intact C1q, MBL, and the purified C1q tail, intact C1q was the most potent chemoattractant; the purification process may partially inactivate the C1q tail, but this result suggests an important contribution by the globular head domain which future studies are needed to delineate. Nevertheless, two lines of evidence suggest that specific receptors participate in uptake of these particles. First, the collagenous collectin tails from both C1q and SP-A stimulated E. histolytica phagocytosis. Unlike the globular head domain, the collagenous tail is believed to interact only with the C1q receptor on macrophages making non-specific tethering of collagenous tail-coated beads to the amebic surface unlikely. Second, chemotaxis in eukaryotes is characteristically receptor mediated [45], and C1q, MBL, and the C1q tail were all chemoattractants for E. histolytica. Therefore, migration of E. histolytica towards these opsonins strongly suggests at least one specific E. histolytica receptor. These data are consistent with a model of E. histolytica phagocytosis in which C1q or collectins bound to the surface of apoptotic cells trigger phagocytosis via interaction with a receptor that binds the collagenous collectin tail domain. With the exception of C1q, the collectins bind to bacteria and are present in mucosal secretions [22–25, 27–29, 31, 32]. We have not directly tested the effect collectins have on phagocytosis of bacteria, but it is likely that the collectins also facilitate this process.
The identity of the E. histolytica receptor that recognizes the collagenous collectin tail remains an important unanswered question and the focus of our current work. The endoplasmic reticulum chaperone calreticulin has been found on the surface of many cell types, and is believed to be the mammalian receptor for the collagenous tail of C1q and the collectins [33–36]. As noted above, calreticulin is abundant in purified E. histolytica phagosomes, making it a leading candidate [37, 38]. In preliminary work, human C1q co-immunoprecipitated with epitope-tagged calreticulin expressed in E. histolytica trophozoites, and recombinant E. histolytica calreticulin interacted directly with C1q in vitro (Teixeira, Heron, and Huston, unpublished data). However, we currently have no direct evidence that calreticulin functions as an E. histolytica phagocytosis receptor. Regardless of a role for calreticulin, it should be possible to identify the relevant E. histolytica receptor using biochemical methods and the ligands identified in this study.
The collectin tail stimulates both phagocytosis and chemotaxis by E. histolytica, but phagocytosis of untreated apoptotic cells was dramatic, even when grown in the absence of serum. This suggests that the collectins are one of many ligands on apoptotic cells that stimulate E. histolytica phagocytosis, and highlights the likelihood that multiple amebic receptors contribute. Phosphatidylserine, which is exposed during apoptosis and following Ca2+ ionophore-treatment, appears to be one additional ligand [11, 14]. Receptors that have been identified include the Gal/GalNAc lectin that is implicated in adherence and cell killing, and the serine-rich E. histolytica protein [46, 47]. The experiments presented here were performed in the presence of D-galactose, excluding the possibility that the D-Gal/GalNAc specific lectin is responsible for amebic recognition of the collagenous collectin tail; a role for the serine-rich E. histolytica protein, however, remains possible.
Though phagocytosis is central to E. histolytica biology, the contribution of phagocytosis to pathogenesis remains undefined. Experimental mutants deficient in phagocytosis have reduced virulence in an animal model [48, 49]. Every mutant yet described has been defective in multiple virulence traits, however, making concrete conclusions about the role of phagocytosis in disease impossible. A better understanding of the ligands and receptors required for E. histolytica phagocytosis of apoptotic cells and bacteria should ultimately provide a means to generate amebae with a defined defect in phagocytosis, the tool required to discern the contribution of phagocytosis to invasive amebiasis. Given known collectin polymorphisms and participation of host collectins in amebic uptake of apoptotic cells and possibly of colonic bacteria, it is possible to envisage scenarios in which differences in the quantity and/or quality of opsonins could contribute to the outcome of E. histolytica infection [50].
Acknowledgements
We are grateful to Scott Tighe at the UVM Cancer Center's Flow Cytometry Facility for expert advice.
Financial support: This work was supported by National Institutes of Health grants K08 AI053678 and P20 RR021905 to C.D.H., and by a Young Investigator's Award from the American Society for Clinical Investigation to C.D.H.
Footnotes
Potential conflicts of interest: none.
Presented in part: XV Seminario Sobre Amibiasis Oaxaca, Mexico. 2006. (Oral presentation), and Woods Hole Molecular Parasitology Meeting XVIII. 2007. (Abstract #234C).
References
- 1.WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28–29 January, 1997. WHO Epidemiol Bull. 1997;18:13–14. [PubMed] [Google Scholar]
- 2.Blessmann J, Ali IKM, Ton Nu PA, et al. Longitudinal study of intestinal Entamoeba histolytica infections in asymptomatic adult carriers. J Clin Microbiol. 2003;41:4745–4750. doi: 10.1128/JCM.41.10.4745-4750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque R, Ali IM, Petri WA., Jr Prevalence and immune response to Entamoeba histolytica infection in preschool children in Bangladesh. Am J Trop Med Hyg. 1999;60:1031–1034. doi: 10.4269/ajtmh.1999.60.1031. [DOI] [PubMed] [Google Scholar]
- 4.Stauffer W, Abd-Alla M, Ravdin JI. Prevalence and incidence of Entamoeba histolytica infection in South Africa and Egypt. Arch Med Res. 2006;37:266–269. doi: 10.1016/j.arcmed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs L. The elimination of viable bacteria from cultures of Endamoeba histolytica and the subsequent maintenance of such cultures. Am J Hyg. 1947;46:172–176. doi: 10.1093/oxfordjournals.aje.a119160. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M. Nutrition and physiology of Endamoeba histolytica. Bacteriology Reviews. 1953;17:189–212. doi: 10.1128/br.17.3.189-212.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rees CW, Reardon LV, Jacobs L. The cultivation of the parasitic protozoa without bacteria. Am J Trop Med. 1941;21:695–716. [Google Scholar]
- 8.Griffin JL. Human amebic dysentery: Electron microscopy of Entamoeba histolytica contacting, ingesting, and digesting inflammatory cells. Am J Trop Med Hyg. 1972;21:895–906. [PubMed] [Google Scholar]
- 9.Gonzalez-Ruiz A, Haque R, Aguirre A, et al. Value of microscopy in the diagnosis of dysentery associated with invasive Entamoeba histolytica. J Clin Pathol. 1994;47:236–239. doi: 10.1136/jcp.47.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huston CD, Houpt ER, Mann BJ, Hahn CS, Petri WA. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol. 2000;2:617–625. doi: 10.1046/j.1462-5822.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 11.Huston CD, Boettner DR, Miller-Sims V, Petri WA. Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect Immun. 2003;71:964–972. doi: 10.1128/IAI.71.2.964-972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEvoy L, Williamson P, Schlegel RA. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Nat Acad Sci USA. 1986;83:3311–3315. doi: 10.1073/pnas.83.10.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratosin D, Estaquier J, Petit F, et al. Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ. 2001;8:1143–1156. doi: 10.1038/sj.cdd.4400946. [DOI] [PubMed] [Google Scholar]
- 14.Boettner DR, Huston CD, Sullivan JA, Petri WA. Entamoeba histolytica and Entamoeba dispar utilize externalized phosphatidylserine for recognition and phagocytosis of erythrocytes. Infect Immun. 2005;73:3422–3430. doi: 10.1128/IAI.73.6.3422-3430.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh SK, Samuelson J. Involvement of p21racA, phosphoinositide 3-kinase, and vacuolar ATPase in phagocytosis of bacteria and erythrocytes by Entamoeba histolytica: suggestive evidence for coincidental evolution of amebic invasiveness. Infect Immun. 1997;65:4243–4249. doi: 10.1128/iai.65.10.4243-4249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nepomuceno RR, Ruiz S, Park M, Tenner AJ. C1qRP is a heavily O-glycosylated cell surface protein involved in the regulation of phagocytic activity. J Immunol. 1999;162:3583–3589. [PubMed] [Google Scholar]
- 17.Ghiran I, Barbashov SF, Klickstein LB, Tas SW, Jensenius JC, Nicholson-Weller A. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J Exp Med. 2000;192:1797–1808. doi: 10.1084/jem.192.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CA, deCathelineau A, Hoffman PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandivier RW, Ogden CA, Fadok VA, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: Calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 21.Nauta AJ, Raaschou-Jenson N, Roos A, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–2863. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 22.Cambi A, Koopman M, Figdor CG. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 23.van de Wetering JK, van Golde LMG, Batenburg JJ. Collectins: players of the innate immune system. Eur J Biochem. 2004;271:1229–1249. doi: 10.1111/j.1432-1033.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama J, Hoffman A, Brown C, et al. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002;50:993–996. doi: 10.1177/002215540205000713. [DOI] [PubMed] [Google Scholar]
- 25.Madsen J, Kliem A, Tornoe I, Skjodt K, Kock C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 26.Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SMJ. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005;73:2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paananen R, Glumoff V, Hallman M. Surfactant protein A and D expression in the porcine Eustachian tube. FEBS Lett. 1999;452:141–144. doi: 10.1016/s0014-5793(99)00602-x. [DOI] [PubMed] [Google Scholar]
- 28.MacNeill C, Umstead TM, Phelps DS, et al. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology. 2004;111:91–99. doi: 10.1111/j.1365-2567.2003.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uemura K, Saka M, Nakagawa T, et al. L-MBP is expressed in epithelial cells of mouse small intestine. J Immunol. 2002;169:6945–6950. doi: 10.4049/jimmunol.169.12.6945. [DOI] [PubMed] [Google Scholar]
- 30.Lin Z, deMello D, Phelps DS, Koltun WA, Page M, Floros J. Both human SP-A1 and SP-A2 genes are expressed in small and large intestine. Pediatr Pathol Mol Med. 2001;20:367–386. [PubMed] [Google Scholar]
- 31.Murray E, Khamri W, Walker MM, et al. Expression of surfactant protein D in the human gastric mucosa and during Helicobacter pylori infection. Infect Immun. 2002;70:1481–1487. doi: 10.1128/IAI.70.3.1481-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubio S, Lacaze-Masmonteil T, Chailley-Heu B, Kahn A, Bourbon JR, Ducroc R. Pulmonary surfactant protein A (SP-A) is expressed by epithelial cells of the small and large intestine. J Biol Chem. 1995;270:12162–12169. doi: 10.1074/jbc.270.20.12162. [DOI] [PubMed] [Google Scholar]
- 33.White TK, Zhu Q, Tanzer ML. Cell surface calreticulin is a putative mannoside lectin which triggers mouse melanoma spreading. J Biol Chem. 1995;270:15926–15929. doi: 10.1074/jbc.270.27.15926. [DOI] [PubMed] [Google Scholar]
- 34.Arosa FA, de Jesus O, Porto G, Carmo AM, de Sousa M. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex Class I molecules. J Biol Chem. 1999;274:16917–16922. doi: 10.1074/jbc.274.24.16917. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra R, Thiel S, Reid KBM, Sim RB. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J Exp Med. 1990;172:955–959. doi: 10.1084/jem.172.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuart GR, Lynch NJ, Day AJ, Schwaeble WJ, Sim RB. The C1q and collectin binding site within C1q receptor. Immunopharmacology. 1997;38:73–80. doi: 10.1016/s0162-3109(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 37.Boettner DR, Huston CD, Linford AS, et al. Entamoeba histolytica phagocytosis of human erythrocytes involves PATMK, a member of the transmembrane kinase family. PLoS Pathog. 2008;4:e8. doi: 10.1371/journal.ppat.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marion S, Laurent C, Guillen N. Signalization and cytoskeleton activity through myosin IB during the early steps of phagocytosis in Entamoeba histolytica: a proteomic approach. Cell Microbiol. 2005;7:1504–1518. doi: 10.1111/j.1462-5822.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 39.Reid KB. Isolation, by partial pepsin digestion, of the three collagen-like regions present in subcomponent C1q of the first component of human complement. Biochemical J. 1976;155:5–17. doi: 10.1042/bj1550005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond LS, Harlow DR, Cunnick C. A new medium for axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 41.Weiss A, Wiskocil RL, Stobo JD. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 productions reflects events occurring at a pre-translational level. J Immunol. 1984;133:123–128. [PubMed] [Google Scholar]
- 42.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. Journal of Immunology. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 43.Bailey GB, Leitch GJ, Day DB. Chemotaxis by Entamoeba histolytica. J Protozool. 1985;32:341–346. doi: 10.1111/j.1550-7408.1985.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 44.Vegh Z, Kew RR, Gruber BL, Ghebrehiwet B. Chemotaxis of human monocyte-derived dendritic cells to complement component C1q is mediated by the receptors gC1qR and cC1qR. Mol Immunol. 2006;43:1402–1407. doi: 10.1016/j.molimm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Jin T, Hereld D. Moving toward understanding eukaryotic chemotaxis. Eur J Cell Biol. 2006;85:905–913. doi: 10.1016/j.ejcb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Petri WA, Jr, Smith RD, Schlesinger PH, Murphy CF, Ravdin JI. Isolation of the galactose binding lectin of Entamoeba histolytica. J Clin Invest. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teixeira JE, Huston CD. Participation of the serine-rich Entamoeba histolytica protein in amebic phagocytosis of apoptotic host cells. Infect Immun. 2008;76:959–966. doi: 10.1128/IAI.01455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orozco E, Guarneros G, Martinez-Palomo A. Entamoeba histolytica: Phagocytosis as a virulence factor. J Exp Med. 1983;158:1511–1521. doi: 10.1084/jem.158.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez MA, Orozco E. Isolation and characterization of phagocytosis- and virulence-deficient mutants of Entamoeba histolytica. J Infect Dis. 1986;154:27–32. doi: 10.1093/infdis/154.1.27. [DOI] [PubMed] [Google Scholar]
- 50.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]