Abstract
Two key issues must be addressed in the discussion of targeting leukemic stem cells: (1) can the leukemic stem cell be targeted in vivo, and (2) how to assess whether the leukemic stem cell is actually being targeted. Currently several small molecule and antibody- or ligand-based agents have shown activity in selectively targeting the leukemic stem cell. However, there is debate about how to use these targeted agents and how to identify and quantitate the leukemic stem cell to determine whether or not it is being targeted. Parameters are suggested here to help identify and quantitate leukemic stem cells in the clinical context.
Keywords: leukemic stem cells, dimethylaminoparthenolide, DMAPT, target, functional assay, differentiation
INTRODUCTION
It has been 14 years since John Dick’s seminal paper in Nature provided the first phenotypic handle on primary human leukemia stem cells.1 A few years ago, the concept of targeting leukemia stem cells was just an interesting biological concept. However, there is now a certain degree of impatience in the research community surrounding this concept and how it can be put into practice. One challenge is to determine whether the agents that can target leukemic stem cells are effectively working in patients. The second challenge is to determine how to assess whether the leukemic stem cell is being targeted in vivo.
SELECTIVELY TARGETING LEUKEMIC STEM CELLS
Small molecules
A variety of small-molecule regimens selectively target the leukemic stem cell. MG-132, a reagent-grade proteasome inhibitor that has similar activities to bortezomib, has significant selective activity for leukemia stem cells, both alone2 and in combination with idarubicin,3 in both in vitro and xenograft model systems. ABT-737, a BCL-2 inhibitor and BH3 mimetic, has been shown to have activity against phenotypically described stem cells.4
TDZD-8 (4-benzyl, 2-methyl, 1,2,4-thiadiazolidine, 3,5 dione) is a very different class of molecule.5 It has selective activity for leukemia stem cells, but the molecular mechanism behind it is different from some of the other small molecule compounds. TDZD-8 appears to selectively impair membrane integrity, specifically in leukemia cells. Parthenolide, a naturally occurring molecule found in the medicinal plant feverfew, induces apoptosis in acute myeloid leukemia (AML) stem cells.6 Celastrol and 4-hydroxy-2-nonenal, which eradicate AML cells at the bulk, progenitor, and stem cell level, were discovered using high through-put screens that utilized the gene expression pattern evoked by parthenolide as a template.7
Parthenolide analogs
DMAPT (dimethylaminoparthenolide), a parthenolide derivative (Figure 1), is a compound that has a fairly significant preclinical rationale for going forward.8 DMAPT is readily water soluble and is 70% orally bioavailable. Pharmacologic studies in rodents and dogs have shown the drug to be tolerable well beyond the level at which in vitro activity is observed, without any known associated acute toxicity. However, the drug has a short half-life in rodents, making it challenging to set up xenograft models to test biological efficacy.
Figure 1.
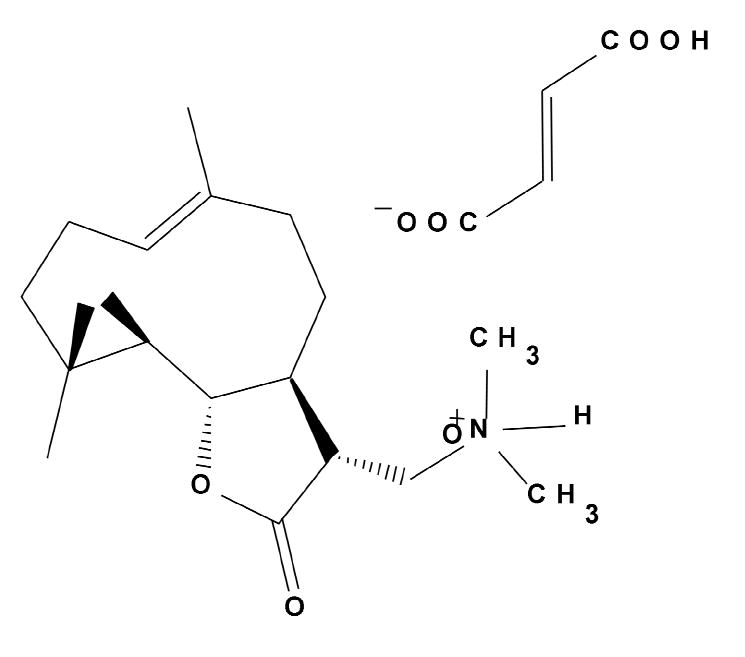
Chemical structure of dimethylaminoparthenolide fumarate (DMAPT), a parthenolide analog
Preclinically, DMAPT shows the preferred selective activity in an agent of this nature: it is selectively cytotoxic to CD34-selected bulk and primitive AML populations, and it does not cause death in the normal cell population. The IC50 is approximately 10 times higher for normal cells. In immune-deficient mice, cells treated with DMAPT were significantly impaired in their engraftment ability, while the control specimens did not show a significant effect. Although it is not known which patient populations will benefit most from this drug, it is clear that there is some broad activity across the AML patient population.
When canines with severe acute leukemia were treated with 50 mg/kg daily DMAPT, the levels of CD34-positive cells were largely reduced. The reduction was not likely due to overt cytotoxicity, since the overall white blood cell counts were elevated at the beginning and remained elevated. DMAPT probably induces a biological change in the tumor population that is consistent with differentiation, as evidenced histologically by increased levels of maturing myeloid cells. A differentiation agent is an ideal way to treat a self-renewing stem cell because it inhibits the cell’s self-renewal and pushes differentiation. Furthermore, research has shown that NF-κB signaling is a very common characteristic of leukemias, and therefore, inhibiting that pathway is potentially important. In vivo, DMAPT treated cells show increased cytoplasmic relocalization of the p65 subunit, which is consistent with NF-κB inhibition.
To determine whether or not leukemic stem cells were being targeted by DMAPT, NOD/SCID mice were transplanted with primary canine specimens. After several weeks’ allowance for engraftment, the marrow of the mice was examined by flow cytometry to distinguish murine from canine cells. Before treatment, 2 of 3 canine leukemia cell samples achieved engraftment of the NOD/SCID mice, which was determined using CD45-positive cells as a marker. After 12 days of treatment with DMAPT, there was significant inhibition of the overall engraftment ability of the primary canine cells. The marrow from the primary cohort was then retransplanted into a secondary cohort to further evaluate the engraftment levels. The results indicated that it is possible to impair the biologic potential of the leukemia stem cell compartment, at least in the context of a large animal with a spontaneous leukemia. Based on the preclinical findings, the drug is proceeding towards clinical trials.
Antibody- or ligand-based agents
In parallel with the development of targeted small molecules, development of antibody- or ligand-based agents has progressed. A diphtheria toxin-interleukin-3 fusion protein [DT(388)IL3] was shown to have activity in animal models9 and is now being evaluated in the clinic.10 CXCR4, which mediates the SDF-1 interaction, had promising activity in murine models.11 Antibodies against CD4412 and CD123, which targets the IL-3 receptor alpha chain,13 are in various stages of development. Agents of this nature have the interesting potential to impede interactions with the microenvironment and/or directly target leukemia cells. These agents in combination with some of the small molecules might make some interesting therapies.
ASSESSING WHETHER LEUKEMIC STEM CELLS ARE BEING TARGETED
It is still unclear how to effectively assess whether leukemic stem cells are actually being targeted. As new targeted agents enter clinical trials, the solution to this problem becomes more pressing. In addition, candidate agents may be more or less effective at targeting leukemia stem cells based on the patient population: whether patients have achieved remission and the targeted agent is used as maintenance therapy, or whether patients are relapsed or refractory (Figure 2).
Figure 2.
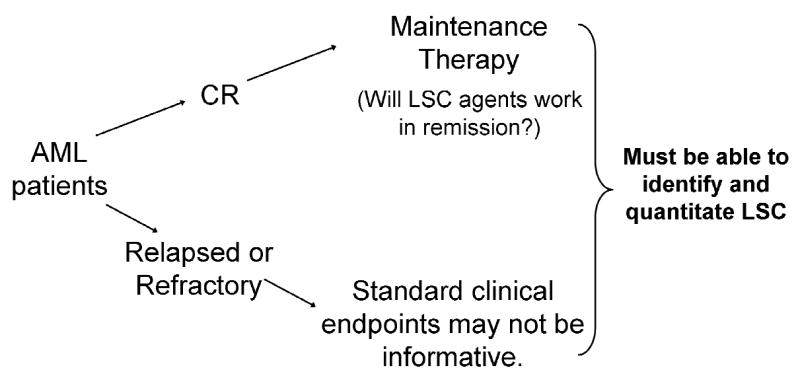
Potential use of leukemia stem cell (LSC)-targeted agents
A targeted agent could be used as part of a maintenance regimen to destroy the residual leukemic stem cells in patients in remission. It is an attractive concept to think that a small residual amount of disease contains the stem cell compartment. The concept is particularly appealing if a targeted agent can be used in a maintenance regimen to eliminate the last bit of disease, especially if the targeted agent has a relatively low toxicity and can be used for a long period of time. However, there is no evidence that any of the agents currently available can target leukemia stem cells in a remission patient. The biology of cells in minimal residual disease conditions appears to be very different from that of cells found in a de novo and heavy tumor-burden context. This difference can impact whether the drugs work or not. As clinical trials progress, the targeted agents may fail because of the physiology of the tumor cells, not because the drug is ineffective with all leukemic stem cells.
Cancers are heterogeneous, and this is exceptionally true of leukemic stem cells. From patient to patient, molecular markers for stem cells differ greatly. In some patients, the leukemic stem cell may have a particular pattern of phenotypic markers such as CD38 and CD34, but other patients can have very different expression levels and frequencies of the same antigens. This heterogeneity makes determining the frequency of the leukemic stem cell in an individual patient extremely difficult. Similarly, during treatment, the phenotype and the genetics of the leukemic stem cell are also likely to be highly unstable. During chemotherapeutic challenge, patients can experience a dramatic change in the phenotype of their leukemic stem cell. The candidate leukemic stem cell population in a de novo patient at presentation may or may not be present after treatment. This phenotypic change suggests that the genetics of these stem cells are also probably changing. What we define as a leukemic stem cell is a highly dynamic and highly unstable entity in the context of any patient population and probably varies as a consequence of therapeutic regimen. This makes the overall monitoring and analysis of these populations quite challenging.
Parameters for analysis of leukemic stem cell in clinical trials
Current standard clinical endpoints may not be informative to evaluate the tumor in patients with relapsed or refractory disease. A reduction in total tumor burden may or may not be indicative of what is happening at the stem-cell level. Consequently, investigators must be able to identify and quantitate leukemic stem cells in any kind of clinical context.
To do so, a number of steps can be taken. Clinical trials must be temporal and patient-specific. Specimens must be gathered before, during, and after treatment. The phenotype of each patient’s leukemic stem cell population must be defined up front and verified by a functional assay to help quantitate it. In the course of treatment, the population must be continuously monitored, as has been done successfully by Gerrit Jan Schuurhuis and colleagues.14
The panel of antibodies must be customized for each patient because each patient may have a different leukemic stem cell phenotype. Some antibodies that appear to be useful in terms of defining leukemia stem cells are CD34; CD38; and CD123, the IL-3-receptor alpha chain.15 CD9616 and CD47 (I. Weissman, unpublished) have also been reported to be upregulated on leukemia stem cells.
Ideally, a molecular marker will be present. Molecular markers, like NPM and FLT3, are present in a large percentage of AML patients. For many patients, these molecular markers can be used in residual disease to determine overall tumor burden.
Though a very well-refined phenotypic panel that uses multiple markers is a good surrogate, a functional assay is the only rigorous way to define the stem cell population. Therefore, employing a quantitative functional analysis will be a very important and very difficult practical aspect of determining whether the leukemic stem cells are being targeted. During treatment, the ability for progenitors to form colonies in vitro or for leukemic stem cells to engraft NOD/SCID mice in vivo will be a critical element of determining whether or not the markers are correctly identifying stem cells. This will certainly be challenging, especially in the context of residual disease patients in remission, but it must be attempted.
CONCLUSION
Just as cancers are heterogeneous, so too are leukemic stem cells, and the ability to target and quantitate leukemic stem cells is complicated by this heterogeneity. As research expands our understanding of leukemic stem cell biology and physiology, investigators must incorporate that knowledge into their strategies for targeting and analyses for quantifying leukemic stem cells. They must also determine where agents that target leukemic stem cells will be of most use: as maintenance therapy that targets minimal residual disease in remission patients, or as treatment for relapsed or refractory patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 3.Guzman ML, Swiderski CF, Howard DS, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci U S A. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Guzman ML, Li X, Corbett CA, et al. Rapid and selective death of leukemia stem and progenitor cells induced by the compound 4-benzyl, 2-methyl, 1,2,4-thiadiazolidine, 3,5 dione (TDZD-8) Blood. 2007;110:4436–4444. doi: 10.1182/blood-2007-05-088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman ML, Rossi RM, Karnischky L, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassane DC, Guzman ML, Corbett C, et al. Discovery of agents that eradicate leukemia stem cells using an in silico screen of public gene expression data. Blood. 2008 doi: 10.1182/blood-2007-11-126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman ML, Rossi RM, Neelakantan S, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feuring-Buske M, Frankel AE, Alexander RL, Gerhard B, Hogge DE. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors. Cancer Res. 2002;62:1730–1736. [PubMed] [Google Scholar]
- 10.No authors listed. DT388IL3 fusion protein in treating patients with acute myeloid leukemia or accelerated phase or blastic phase chronic myeloid leukemia . http://clinicaltrials.gov/ct2/show/NCT00397579. 1-10-2008. 4-29-0008.
- 11.Tavor S, Petit I, Porozov S, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64:2817–2824. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 12.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 13.Lock RB, Jin L, Lee EM, et al. CD123 (IL-3 receptor {alpha} chain) neutralization by a monoclonal antibody selectively eliminates human acute myeloid leukemic stem cells. Blood. 2007;110:55a. doi: 10.1016/j.stem.2009.04.018. abstr 161. [DOI] [PubMed] [Google Scholar]
- 14.van Rhenen A, Moshaver B, Kelder A, et al. Aberrant marker expression patterns on the CD34+CD38- stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 2007;21:1700–1707. doi: 10.1038/sj.leu.2404754. [DOI] [PubMed] [Google Scholar]
- 15.Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 16.Hosen N, Park CY, Tatsumi N, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A. 2007;104:11008–11013. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]


