1. Introduction
The ability to detect small changes in frequency is an important factor in speech understanding. Age-related differences in the ability to detect these changes may be associated with poorer speech recognition (Buss et al., 2004). Several behavioral studies have reported an age-related decline in frequency difference limen, either as reduced frequency discrimination (He et al., 1998; Humes, 1996; Konig, 1957) or frequency modulation detection (He et al., 2007). However, substantial variability has been observed both within and across studies (see Jesteadt and Sims 1975, for a review). This variability may reflect differences in tasks and procedures, such as the standard frequency examined, and the subject’s hearing sensitivity, level of attention, or ability to perform the task (He et al., 1998; Turner et al., 1982). For instance, He et al (1998) examined frequency discrimination in younger and older adults with normal hearing and observed a frequency-dependent aging effect, such that age-related differences were larger at lower frequencies than at higher frequencies. Similarly, frequency modulation detection was poorer in older than younger subjects and age-related differences were larger at 500 Hz than at 4000 Hz (He et al., 2007). Age-related changes in cognitive functioning, including selective attention, may also negatively affect performance on auditory processing tasks, such as frequency discrimination (Hallgren et al., 2001; Humes, 1996; Humes, 2005; Humes et al., 1991; Humes et al., 2005; Humes et al., 1996; Humes et al., 2006; Humes et al., 1994). Therefore, it is likely that age-related changes in the peripheral, central auditory and cognitive systems contribute to age-related declines in the ability to detect small changes in frequency. However, the relative contributions of, and interactions among, these factors remain unclear.
The effect of aging on auditory physiologic processes associated with frequency discrimination has been measured using endogenous cortical potentials including the mismatch negativity (MMN), an early preattentive potential, and the P300, a later cognitive attention-related potential (Bertoli et al., 2005). Despite similar frequency discrimination performance on behavioral measures, pronounced effects of age were observed in the physiologic responses, such that older adults were less sensitive to the smallest frequency change, as indicated by an absent MMN to the smallest deviant stimuli. Older adults also had significantly delayed latencies and reduced amplitudes of the P300 component as compared to younger adults. These results suggest a decreased sensitivity to frequency change at preattentive levels of auditory processing as well as changes in attentional and cognitive processing of the stimuli.
The effect of aging on the neurophysiological representation of small changes in frequency has not been reported using the obligatory sensory-perceptual potential, the P1-N1-P2. When collected using a passive listening paradigm, the P1-N1-P2 provides a means to study age-related changes in auditory processing while minimizing the contribution of cognitive factors, such as attention. The P1-N1-P2 is thought to reflect synchronous neuronal activation of structures in the thalamic-cortical segment of the central auditory system (Naatanen et al., 1987; Wolpaw et al., 1975; Woods, 1995). In general, the P1-N1-P2 response is considered an onset response and can be elicited by a variety of stimuli. However, several studies have examined the P1-N1-P2 not to the onset of the stimulus but to a change that occurs during an ongoing stimulus, often referred to as the Acoustic Change Complex (ACC) (Martin et al., 1999). This paradigm provides a means to examine the neural response to the acoustic change (i.e., change in frequency) with minimal interference from the P1-N1-P2 onset response. The paradigm has been used successfully in electrophysiologic studies to elicit the P1-N1-P2 in response to frequency shifts during an ongoing sound in young adults (McCandless et al., 1970; Wunderlich et al., 2001). Results from these studies have shown that the P1-N1-P2 response to changes in frequency is related to the magnitude of the frequency change, such that small changes in frequency, approaching the just noticeable difference measured behaviorally, elicit smaller response amplitudes and prolonged latencies, as compared to larger changes in frequency. Although these studies did not examine frequency discrimination directly, the presence of the P1-N1-P2 response reflects the physiologic detection of the frequency change at the level of the auditory cortex, which in turn may be related to frequency discrimination abilities.
In the current study, small changes in frequency in an otherwise continuous pure-tone were used to elicit the P1-N1-P2 in older and younger adults with normal hearing. Several factors have been shown to affect frequency difference limens measured behaviorally, including subject age, stimulus paradigm (frequency discrimination vs frequency modulation), and carrier frequency. In general, regardless of stimulus paradigm, age-related effects on frequency difference limens are larger at lower carrier frequencies than at higher carrier frequencies (He et al., 1998; 2007). In order to assess the effects of frequency, P1-N1-P2 responses in the current study were recorded in response to small changes in frequency in 500 Hz and 3000 Hz pure tones. Based on the frequency-dependent aging effect reported for frequency discrimination and frequency modulation detection measured behaviorally (He et al., 1998; 2007), we predicted that older adults would be less sensitive to frequency changes than younger adults, with larger age-related differences observed at lower frequencies (500 Hz) than at higher frequencies (3000 Hz).
Pronounced effects of aging have been reported for P1-N1-P2 response latencies and amplitudes (Anderer et al., 1996; Boutros et al., 2000; Harkrider et al., 2005; Laffont et al., 1989; Pfefferbaum et al., 1980; Schroeder et al., 1995; Tremblay et al., 2004; Woods, 1992). However, less is known about age-related effects on response amplitudes and latencies when the P1-N1-P2 response is used to measure acoustic changes contained within a signal. Using a similar ACC paradigm, Harris et al. (2007) reported decreased sensitivity to intensity changes and significantly delayed response latencies in older subjects as compared to younger subjects, with greater differences at lower than higher frequencies. These changes in response latency have been associated with a general slowing of neuronal processing, as well as decreased neuronal synchrony or temporal jitter within the central auditory nervous system. Therefore, in addition to decreased sensitivity to changes in frequency, older subjects were predicted to exhibit changes in brain activity, including delayed latencies.
2. Methods
2.1 Subjects
Two groups of human subjects were included: younger (18–30 years, n = 10) and older (65–80 years, n =10). Each older subject completed the Short Portable Mental Status Questionnaire and had two or fewer errors, signifying intact intellectual functioning (Pfeiffer, 1975). Several authors have hypothesized a link between N1-P2 amplitudes and tinnitus (Attias et al., 1993; Hoke et al., 1989; Kadner et al., 2002). Therefore, each subject completed a tinnitus questionnaire; only one older subject reported a significant history of tinnitus but did not report having tinnitus at the time of testing. Prior to electrophysiologic testing, conventional pure-tone thresholds were measured with a Madsen OB922 clinical audiometer calibrated to appropriate ANSI standards (ANSI S3.6,1996) and equipped with TDH-39 headphones. Thresholds were measured in 5-dB steps.
2.2 Stimuli
A brief and constant increase in the frequency of an otherwise continuous pure tone was used to elicit the P1-N1-P2. The continuous tone was presented at a signal frequency of either 500 or 3000 Hz and returned to that frequency after each change (Figure 1). Although there is no common consensus as to the appropriate stimulus scale for frequency discrimination, several behavioral studies have suggested using a percentage of Δf/f. When plotted logarithmically, psychometric functions can be fit by a logistic function with a constant slope. Differences such as hearing loss or stimulus frequency result in a horizontal shift of the function but not a change in slope (He et al., 1998; Nelson et al., 1986). Therefore, in the current study the change in frequency is reported as a % of Δf/f. Each frequency increment (i.e., 10 Hz for a 2% change from the 500-Hz signal) was presented for 150 ms (10 ms rise/fall time, 130 ms plateau) in duration. To obtain a precise measure of P1-N1-P2 threshold, responses were measured to frequency changes of 1-Hz increments. Additional measures were obtained using five fixed frequency-change values, including 0% (no change), 2%, 4%, 6%, and 8%. The frequency change was measured electrically at the input to the earphones (Instek oscilloscope Model GOS-620) and acoustically calibrated with the earphone placed in a NBS-9A coupler using a Larson-Davis sound level meter (Larson Davis 800B) equipped with a 1 in pressure microphone (Model 2575). The interval between each frequency change was 3 sec, selected to minimize age-related rate effects (Tremblay et al., 2004). The signals were generated using Tucker-Davis Technologies (TDT) System 3 modules (sampling rate 50 kHz). The system incorporated a programmable attenuator and a head phone buffer/interface for impedance matching. The signal was presented monaurally to the right ear at 70 dB SPL through TDH-39 headphones.
Figure 1.
A simulated segment of the stimulus sequence showing a change in frequency in an otherwise continuous pure tone. Frequency changes were presented every 3 seconds. Averaging was triggered 100 ms (baseline) prior to the frequency change.
All subjects had normal hearing (defined as thresholds ≤ 25 dB HL at 250, 500, 1000, 2000, 3000 and 4000 Hz) and normal immittance. Mean pure-tone audiometric thresholds (±1 S.E.M.) are shown in Figure 2. Mean group differences in thresholds ranged from 1 to 7 dB from 250 through 4000 Hz. At 8000 Hz, mean threshold differences were 33.5 dB, as some older subjects had thresholds greater than 25 dB HL. Differences in audiometric thresholds between older and younger subjects at each frequency were assessed using independent sample t-tests. There were no significant differences between younger and older subjects from 250–3000 Hz (p>.05). However, older subjects had significantly higher thresholds than younger subjects at 4000 and 8000 Hz [t(18)= −2.207, p=0.041 and t(18)= −6.882, p<.0001 for 4000 and 8000 Hz, respectively].
Figure 2.
Mean pure-tone thresholds (dB HL) and standard errors (±1 S.E.M) for the test ear of younger subjects (closed) and older subjects (open) plotted as a function of frequency (Hz). Thresholds for younger and older subjects were significantly different only at 4000 and 8000 Hz.
2.3 EEG recordings
P1, N1 and P2 cortical potentials were recorded using a single channel recording configuration. Surface electrodes were placed at the vertex (Cz) and on each mastoid. The noninverting electrode was the vertex, the inverting electrode was the ipsilateral (stimulated) ear, and the common electrode was the contralateral (non-stimulated) ear. The average inter-electrode impedance was < 5 kΩ. The responses from the electrodes were amplified 100,000x (Grass Model 12 Neurodata Acquisition System), filtered from 3 Hz (high-pass filter, 6 dB/octave) to 30 Hz (low-pass filter, 6 dB/octave), and routed to a 24-bit A/D converter. Averaging was triggered by the change in frequency in the continuous pure tone (Figure 1). Data were collected and stored for analysis using a TDT BioSig system.
2.4 Procedures
Testing was conducted in a sound-treated room. Subjects sat in a reclining chair and read quietly during electrophysiologic testing. Testing was conducted over two, two hour sessions. A break (no auditory stimulation) was provided every 20 minutes or as requested by the subject. Initially, each subject’s sensitivity (threshold) to the frequency change was assessed by varying the frequency change in 1-Hz steps. The presence of the P1-N1-P2 response reflects the physiological representation of the frequency change at the level of the auditory cortex, an important factor in frequency discrimination abilities. Additionally, thresholds measured electrophysiologically can closely approximate behavioral thresholds. Therefore, “P1-N1-P2 threshold” is used here to refer to the smallest frequency change required to elicit a repeatable P1-N1-P2 response, and serves as an index of the sensitivity at the level of the auditory cortex to the frequency change. P1-N1-P2 thresholds were collected on the first visit. In a second visit, frequency changes of 0, 2, 4, 6, and 8% (Δf/f) were presented in homogenous blocks of 100 stimuli each at 500 and 3000 Hz. Responses were averaged across a 1000 msec time window, which included a 100-ms pre-stimulus period and 900-ms post-stimulus period (after onset of frequency change). Epochs with artifact measuring in excess of ± 50 µV were excluded from the average waveform. Rejection rates were never greater than 10% per run for any subject. Excluding sweeps with excess artifact, 100 sweeps were averaged per individual waveform. Each averaged waveform was then baseline corrected for the prestimulus period. Two replications were obtained for each frequency change and averaged. Frequency (500 or 3000 Hz) was counterbalanced and Δf/f was randomized across subjects.
P1, N1 and P2 peak latencies and amplitudes were determined for all conditions. The group average waveforms (Figure 5) were used to determine the latency windows for identification of P1, N1 and P2, and were consistent with previously reported literature (e.g. Harkrider et al., 2006; Martin et al., 1999; Tremblay et al., 2004). The response window for N1 was 80–200 ms; P2 was identified as the greatest positive amplitude following N1. Latencies were determined from the onset of the change in frequency and then peak amplitudes for P1, N1 and P2 were measured.
Figure 5.
Group averaged waveforms for younger subjects (solid) and older subjects (dashed), recorded from electrode Cz, in response to frequency changes of 0, 2, 4, 6 and 8% (Δf/f) at 500 Hz (A) and 3000 Hz (B).
Differences between subject groups, signal frequency (500 and 3000 Hz), and frequency change (Δf/f) were assessed using t-tests and repeated measures analyses of variance (ANOVA); p values of <0.05 were considered statistically significant.
3. Results
3.1 Sensitivity to frequency change: effects of age and frequency
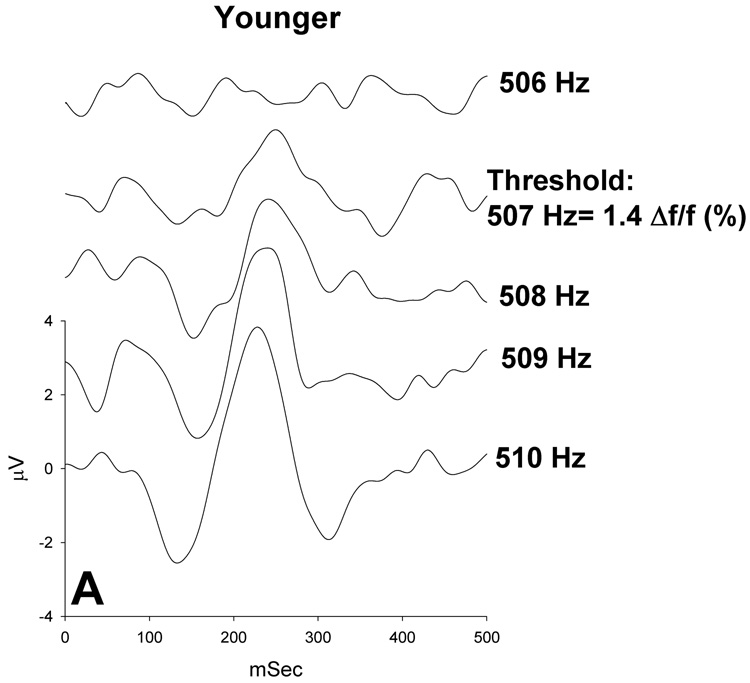
P1-N1-P2 thresholds were assessed in 1-Hz steps and converted to Δf/f % at 500 and 3000 Hz. Figure 3 shows a typical series of P1-N1-P2 responses from a younger subject (A) and an older subject (B) as a function of Δf/f %; threshold has been labeled (Δf/f=1.4%). Figure 4 illustrates the smallest Δf/f % that elicited a P1-N1-P2 response at 500 Hz (A) and 3000 Hz (B) for younger subjects (black bars) and older subjects (gray bars). P1-N1-P2 thresholds ranged from 0.8% to 1.77% in younger subjects and from 1.2% to 3.4% in older subjects. As seen in Figure 4, within-group variances at 500 Hz were smaller for the younger subjects than the older subjects and thresholds were separated across age groups. P1-N1-P2 thresholds of younger subjects are grouped to the left of the graph (lower thresholds), whereas older subjects show larger thresholds. Only one older subject had a threshold within the range of the younger subjects. At 3000 Hz, P1-N1-P2 thresholds show greater overlap between younger and older subjects, but three older subjects have substantially higher thresholds. A repeated measures ANOVA, with standard frequency (500 and 3000 Hz) as a repeated measure and age as a grouping factor, revealed a significant main effect of age [F(1,18)= 55.321, p<.001], no significant main effect of frequency [F(1,18)= .001, p=.979] and a significant frequency by age interaction [F(1,18) =10.810, p=.004]. That is, age-related differences P1-N1-P2 thresholds were larger at 500 Hz than at 3000 Hz. These findings are consistent with previously reported behavioral results showing a frequency-dependent effect on age-related changes in frequency difference limens (He et al., 1998; 2007).
Figure 3.
Representative waveforms recorded from one younger subject (A) and one older subject (B) in response to a range of frequency changes at 500 Hz. Threshold was defined as the smallest frequency change that elicited a P1-N1-P2 response. The younger subject’s threshold was determined to be 507 Hz or 1.4% Δf/f and the older subject’s threshold was determined to be 510 or 2.0 % Δf/f (as labeled).
Figure 4.
Distribution of P1-N1-P2 thresholds (in Δf/f %) for 500 Hz (A) and 3000 Hz (B) for younger subjects (n=10) and older subjects (n=10). Older subjects had significantly higher thresholds at 500 Hz and 3000 Hz and age-related differences were significantly larger at 500 Hz than at 3000 Hz.
3.2 P1, N1 and P2 waveforms
Figure 5 depicts the group average P1-N1-P2 waveforms (Cz) for younger subjects (solid) and older subjects (dashed), recorded from electrode Cz, in response to frequency changes of 0, 2, 4, 6, and 8% (Δf/f) at 500 Hz (A) and 3000 Hz (B). Both groups showed decreases in latency and increases in amplitude with increases in Δf/f. At 500 Hz, despite significantly higher P1-N1-P2 thresholds, response amplitudes appear similar between younger and older subjects and N1 latencies appear prolonged. At 3000 Hz, response amplitudes appear smaller and latencies appear prolonged in older than younger subjects.
3.3 P1, N1 and P2 response latencies: Effects of age, signal frequency, and Δf
Peak latency values and standard errors (± 1 S.E.M.) of P1, N1 and P2 for each age group are plotted in Figure 6 for younger subjects (closed) and older subjects (open) as a function of Δf/f (%) at 500 Hz (Panels A,C, E) and 3000 Hz (Panels B, D, F). Examination of P1, N1 and P2 latencies were performed separately using a three-way repeated measures ANOVA with subject age as a grouping factor, and signal frequency (500 Hz, 3000 Hz) and Δf as repeated measures. Group analyses were not performed on response characteristics to the 2% signal change condition due to the large number of missing values for older subjects, which was likely a result of their elevated P1-N1-P2 thresholds.
Figure 6.
Mean latencies and standard errors (± 1 S.E.M.) for younger subjects (closed) and older subjects (open) as a function of Δf/f (%) at 500 Hz (Panels A,C,E) and 3000 Hz (Panels B, D, F). Older subjects had significantly delayed N1 latencies at 500 Hz as compared to younger subjects. At 3000 Hz, P2 latencies were significantly delayed in older subjects as compared to younger subjects.
For P1 latencies, there was a significant main effect of frequency (p=.005), such that P1 latencies were longer at 500 Hz than at 3000 Hz. There were no significant main effects of subject age (p=.091) or significant interactions (p>.05). Additionally, there was a significant main effect of Δf (p<.001). Post-hoc t-tests were used to examine the main effect of Δf on P1 latency. P1 latencies significantly decreased with increasing Δf from 2% to 4% (p<.001) and 4% to 6% (p<.001). Although P1 latencies continued to decrease, differences in P1 latencies from 6% to 8% did not reach statistical significance (p=.093).
For N1 latencies, there was a significant main effect of subject age (p=.026), frequency (p<.001), and Δf (p<.001). There were no significant interactions (p>.05). N1 latencies were significantly prolonged in older than younger subjects. Similar to P1, N1 latencies were longer at 500 Hz as compared to 3000 Hz and N1 latencies significantly decreased with increasing Δf (p<.05).
For P2 latencies, there was a significant main effect of age (p=.047) and Δf (p<.001). P2 latencies did not significantly differ across frequency (p=.116) and there were no significant interactions (p>.05). P2 latencies were significantly prolonged in older than younger subjects. Similar to P1, P2 latencies significantly decreased with increasing Δf from 2% to 4% (p<.001) and 4% to 6% (p=.012), but did not significantly increase from 6% to 8% (p=.274). The degree of this effect was similar for both younger and older subjects as there were no significant interactions of Δf and subject age.
3.4 P1, N1 and P2 response amplitudes: Effects of age, signal frequency, and Δf
Figure 7 shows mean amplitudes (relative to baseline) and standard errors (± 1 S.E.M) for younger subjects (closed) and older subjects (open) as a function of Δf/f (%) at 500 Hz (Panels A, C, E) and 3000 Hz (Panels B, D, F). P1, N1 and P2 amplitude values were analyzed separately using three, three-way repeated measures ANOVAs with subject age as the grouping factor, and signal frequency (500 Hz, 3000 Hz) and Δf as repeated measures.
Figure 7.
Mean amplitudes (relative to baseline) and standard errors (±1 S.E.M.) for younger subjects (closed) and older subjects (open) as function of Δf/f (%) at 500 Hz (Panels A,C, E) and 3000 Hz (Panels B, D, F). N1 amplitudes at 3000 Hz were significantly smaller in older subjects than younger subjects.
As shown in Panels A and B, P1 amplitudes appear larger in older than in younger subjects at 500 Hz and similar to younger subjects at 3000 Hz, however, the interaction between frequency and age did not reach significance (p=.09). Furthermore, there were no significant main effects of age (p=.233), frequency (p=.368), or Δf (p=.421).
For N1 amplitudes, there was a significant frequency by age interaction (p=.007) and a significant main effect of Δf (p<.001). There were no significant main effects of age (p=.518) or frequency (p=.239). Due to the significant frequency by age interaction, two repeated measure ANOVAs with age as the grouping factor and Δf as the repeated measure were performed at 500 and 3000 Hz. Although the largest N1 amplitudes were found in older subjects at 500 Hz, differences in N1 amplitude between younger and older subjects did not reach statistical significance (p=.519). In contrast, N1 amplitudes at 3000 Hz were significantly smaller in older subjects than younger subjects (p=.03), consistent with higher P1-N1-P2 thresholds. With increasing Δf, N1 amplitudes increased from 2% to 4% (p<.001) and from 4% to 6% (p=.002), but differences were not significant from 6% to 8% (p=.205). Similar to latency changes, the degree of this effect was similar for younger and older subjects, as there were no significant Δf by age interactions.
For P2 amplitudes, there was a significant main effect of Δf (p<.001), similar to N1 amplitudes. There were no significant main effects of age (p=.540), frequency (p=.351), or significant interactions. Post hoc t-tests showed that P2 amplitudes increased with increasing Δf from 2% to 4% (p=.001) and from 4% to 6% (p=.02), but increases in response amplitude did not reach statistical significance from 6% to 8% (p=.185).
3.5 Associations among cortical potentials, audiometric thresholds, and auditory brainstem potentials
Pearson correlation coefficients were calculated to determine the extent to which P1-N1-P2 thresholds and P1-N1-P2 response characteristics (amplitudes and latencies) varied with audiometric thresholds (250–8000 Hz) in older subjects. Correlations were small to modest (and not statistically significant) between older subjects’ audiometric thresholds and P1-N1-P2 thresholds, and P1, N1, and P2 latencies and amplitudes (Pearson correlation coefficients ranged from −0.472 to 0.387; p>0.05). Furthermore, as part of the protocol for an ongoing study of age-related hearing loss being conducted within our laboratory, auditory brainstem responses (ABR) were measured in the older subjects. ABR amplitude and latency measures were collected with 80 dB SPL click stimuli (21.1 clicks/sec). Wave V latencies ranged from 5.29 ms to 6.4 ms (average 5.78 ms) and wave V amplitudes ranged from .13 µV to .39 µV (average .295 µV). There were no significant correlations between ABR wave V response latencies or amplitudes and P1-N1-P2 thresholds, response latencies or amplitudes (p>.05) (Pearson correlation coefficients ranged from −0.547 to 0.540).
4. Discussion
4.1 Main findings
A goal of the present study was to investigate the extent to which aging affects the neural representation of and/or sensitivity to small changes in frequency in adults with normal hearing. Consistent with previous behavioral studies of age-related changes in frequency discrimination and frequency modulation detection, older subjects were significantly less sensitive than younger subjects to changes in frequency as assessed with the P1-N1-P2 response. Larger age-related differences in P1-N1-P2 thresholds were observed at lower frequencies (500 Hz) than at higher frequencies (3000 Hz). In addition to decreased sensitivity to changes in frequency, age-related differences in response latencies and amplitudes of the P1-N1-P2 response were also evident.
4.2 P1-N1-P2 thresholds; comparison to previous studies
A P1-N1-P2 threshold was defined as the smallest change in frequency at 500 and 3000 Hz required to elicit a response. P1-N1-P2 thresholds ranged from 0.8% to 1.77% in younger subjects and from 1.2% to 3.4% in older subjects. Previous electrophysiologic studies have shown that the P1-N1-P2 can be elicited by similar small changes in frequency in younger adults (McCandless et al., 1970; Yingling et al., 1983). Limited data are available regarding the effects of age on P1-N1-P2 thresholds when elicited by small changes in frequency. However, Bertoli et al (2005) examined the MMN in older and younger subjects in response to frequency contrasts at 1000 Hz and reported that larger frequency contrasts were required to elicit the MMN in older subjects than younger subjects.
4.3 Comparison to previous behavioral measures of frequency difference limens
It is well established that in addition to subject age, frequency difference limens may be affected by psychophysical method and stimulus paradigm (Hartmann, 1997; He et al., 1998; He et al., 2007). Although frequency difference limens were not measured behaviorally in the current study, a majority of the older adults (8 of 10) who participated in electrophysiologic testing also participated in a recent study of frequency modulation detection conducted in our laboratory (He et al., 2007). In the study by He et al. (2007) detection of frequency modulation was measured with 500- and 4000-Hz carriers using a constant stimuli, yes-no method. Pearson correlation coefficients were calculated to determine the association between P1-N1-P2 thresholds measured electrophysiologically and frequency modulation detection thresholds measured behaviorally in older subjects. Despite differences in stimuli and procedures, significant positive correlations (p< 0.01) were observed between older subjects’ P1-N1-P2 thresholds at 500 and 3000 Hz and frequency modulation detection thresholds at 500 and 4000 Hz (Figure 8). These results are consistent with previous studies that have shown a close relationship between the smallest change required to elicit a significant FMFR and the smallest change which was detectable behaviorally in the same young subjects (Picton et al., 1987).
Figure 8.
P1-N1-P2 thresholds plotted as a function of frequency modulation detection thresholds for 8 of 10 older subjects. Frequency modulation detection thresholds were measured with carrier frequencies of 500 (A) and 4000 Hz (B) (see He et al., 2007).
A frequency-dependent aging effect was observed in the current study, such that older subjects were significantly less sensitive to frequency change (higher P1-N1-P2 thresholds) than younger subjects, with significantly larger age-related differences at 500 Hz than at 3000 Hz. This result is consistent with those observed previously for frequency discrimination and frequency modulation detection. Moreover, similar frequency-dependent aging effects were observed on behavioral (He et al., 1998) and electrophysiologic (Harris et al., 2007) measures of intensity discrimination. As first suggested by He et al. (1998), consistent frequency-dependent aging effects across these measures may suggest a common underlying mechanism for both discrimination tasks. Based on a spectrotemporal model of auditory processing, frequency and intensity discrimination at lower frequencies is based primarily on temporal information and may be related to phase-locking properties of the neural fiber response, whereas spectral cues dominate performance at higher frequencies where phase-locking cues are diminished. Therefore, similar to behavioral measures, the age-related increase in P1-N1-P2 thresholds in response to small changes in frequency and intensity at lower but not higher frequencies for older adults may be associated with age-related declines in temporal processing.
Age-related changes observed in a passive listening paradigm, as used in the current study, suggest that changes in discrimination abilities may be related in part to age-related changes in the underlying generators of the P1-N1-P2 response, within the central auditory system. Although, differences in the P1-N1-P2 suggest age-related changes in auditory processing, changes in attention and cognition can not be ruled out. Bertoli et al (2005) examined age-related changes in behavioral measures of frequency discrimination thresholds for a 1000 Hz tone as well as several electrophysiologic responses elicited by small frequency contrasts (presented as two separate tones in an odd-ball paradigm) including the P1-N1-P2 (elicited by the onset of the standard stimuli, not to a change in frequency), the MMN and the P300. In contrast to He et al. (1998) and others (Humes et al., 1996; Konig, 1957), no significant differences in behavioral discrimination thresholds were reported. However, larger frequency contrasts were required to elicit the MMN in older subjects and significant effects of aging were reported for P1, N1, and P2 responses as well as the N2b and P3b (elicited in response to the frequency contrast stimulus when the difference was consciously perceived). Bertoli et al. (2005) hypothesized that age-related changes in the electrophysiologic potentials reflect a decrease in inhibitory control, decreased sensitivity in preattentive stimulus discrimination, and a more effortful delayed stimulus evaluation.
4.4 Effect of frequency on P1, N1, and P2 response latencies and amplitudes
Several previous electrophysiologic studies have shown a frequency effect on the amplitude and latency of N1 and P2. In general, both amplitude (Antinoro et al., 1969; Jacobson et al., 1992; Wunderlich et al., 2001) and latency (Alain et al., 1997; Jacobson et al., 1992; Wunderlich et al., 2001) decreased as frequency increased. In the current study, P1 and N1 (but not P2) latencies varied significantly with signal frequency, such that P1 and N1 latencies were larger at 500 Hz than at 3000 Hz. These results are similar to findings reported by Wunderlich and Cone-Wesson (2000) that N1 latencies changed with signal frequency (400, 1500, and 3000 Hz pure tones), P2 latencies did not. Furthermore, although the absolute range of frequencies (Δf) tested was 240 Hz (8%) for the 3000 Hz signal but only 40 Hz (8%) for the 500 Hz signal, response amplitudes were not significantly affected by signal frequency (500 Hz, 3000 Hz). This was in contrast to previous electrophysiologic studies which have assessed the effects of signal frequency on response amplitudes of the P1-N1-P2 response. Differences between these previous studies and the current study may be attributed to differences in procedures. In the current study, averaging was triggered by a change in frequency imbedded in a continuous pure tone and not by the onset of a pure tone. Therefore, response amplitudes were more likely to be related to frequency discrimination abilities at a particular frequency, as described below, and are consistent with results from Yingling and Nethercut (1983) using similar stimuli.
4.5 Effect of Δf on P1, N1, and P2 response latencies and amplitudes
In addition to the effects of signal frequency, response latencies and amplitudes of the P1, N1 and P2 were significantly affected by changes in Δf, such that amplitudes increased and latencies decreased as Δf increased. Increases in response latency and amplitude tapered off at the largest signal changes (6% to 8%). These results are consistent with previous electrophysiologic studies, in which response amplitudes and latencies appeared to be related to the magnitude of the frequency change, such that amplitudes increased and latencies decreased as Δf increased (Yingling and Nethercut, 1983; McCandless and Rose, 1970). Similar results were reported for the frequency modulated following response (FMFR), a steady-state evoked response thought to be related to frequency discrimination. Boettcher et al. (2002) reported that FMFR response amplitudes increased as a function of modulation depth over depths of 0–40%, then reached a plateau (Boettcher et al., 2002), whereas Picton et al. (1987) reported a relatively linear increase in response amplitude for modulation depths of 10–70%, with increases in amplitude tapering off at higher modulation depths (Picton et al., 1987). Additionally, increases in response amplitudes and latencies elicited by a change in frequency were comparable to changes in response amplitudes and latencies elicited by increases in intensity, such that responses to small changes in intensity have longer latencies and smaller amplitudes than responses to larger changes in intensity (Harris et al., 2007).
4.6 Effect of age on P1, N1, and P2 response latencies and amplitudes
In addition to a decline in sensitivity to the frequency change, as evident by higher P1-N1-P2 thresholds, age-related changes in auditory processing were also present in the characteristics of the P1-N1-P2 response of older adults. N1 and P2 latencies were prolonged for older subjects as compared to younger subjects. Prolonged latencies have been associated with a general slowing of neuronal processing, as well as decreased neuronal synchrony or temporal jitter within the central auditory nervous system. Moreover, Tremblay et al. (2004) reported that N1 latencies were prolonged in older subjects in response to changes in the temporal cues of speech stimuli, but not to the onset of a simple pure-tone stimulus, and hypothesized that age-related changes in N1 may be due to reduced neural synchrony and not simply a result of age-related delays in neural conduction times. Similarly, Harkrider et al. (2005) observed that P2 latencies were delayed in older subjects in response to changes in the spectral cues of speech stimuli and suggested that prolonged latencies in older listeners may be due to reduced neural synchrony in response to changes in spectral cues. Bertoli et al (2005) observed similar increases in P2 latencies in older normal-hearing and hearing-impaired adults as compared to younger adults.
Similar to changes in P1-N1-P2 thresholds, age-related changes in response amplitudes occurred in a frequency-dependent manner. The N1 generally increases in amplitude with increasing stimulus audibility. Therefore, increased P1-N1-P2 thresholds should result in a decrease in response amplitudes. In contrast, although P1-N1-P2 thresholds were significantly larger at 500 Hz for older than younger subjects, P1 and N1 amplitudes appeared larger (not smaller) in older than in younger subjects, although differences did not reach statistical significance. Several studies have reported age-related increases in P1 amplitudes (Tremblay et al., 2004) and N1 amplitudes (Harkrider et al., 2005; Harris et al., 2007). Additionally, Boettcher et al (2002) reported increased amplitudes of the FMFR in older than younger subjects using a frequency-modulated sinusoid with a 500-Hz carrier frequency. Similar increases in response amplitudes of the middle latency response have also been reported (Chambers et al., 1991; Woods et al., 1986). Findings of enhanced amplitudes but prolonged latencies (as seen with the 500-Hz carrier) in some older subjects are consistent with changes in neuronal synchrony and/or changes in inhibitory control. In contrast, at 3000 Hz, N1 response amplitudes were significantly smaller in older than younger subjects. Reduced N1 amplitudes at 3000 Hz in the current study are consistent with the decreased sensitivity to frequency changes at 3000 Hz observed in older subjects, such that those subjects with the smallest N1 amplitudes also had the highest thresholds. Similar decreases in response amplitude with age have been reported previously (Picton et al., 1984; Tremblay et al., 2004).
For the current study, latency and amplitude measurements were analyzed from electrode Cz. In general, P1-N1-P2 responses are larger at frontal midline electrode sites, including Cz. Additionally, several studies examining aging effects have reported results from these midline electrode sites (Anderer et al., 1996; Harkrider et al., 2005; McCandless et al., 1970; Tremblay et al., 2004; Yingling et al., 1983), allowing for comparisons with the current study. However, age-related changes in the scalp topography of the P1-N1-P2 have also been reported (Anderer et al., 1996; Smith et al., 1980). Therefore, future studies would benefit from the inclusion of additional electrode sites to examine possible age-related changes in scalp topography when the P1-N1-P2 is elicited in response to small changes in frequency.
4.7 Associations among cortical potentials, audiometric thresholds, and auditory brainstem potentials
Several behavioral studies have shown that hearing loss can negatively affect frequency discrimination (Simon et al., 1993; Turner et al., 1982). Similarly, hearing loss can affect N1 and P2 response latencies and amplitudes (Oates et al., 2002; Tremblay et al., 2003). In the current study, audiometric thresholds for all subjects were within the normal range through 4000 Hz and were closely matched for younger and older subjects at 500 Hz and 3000 Hz. However, audiometric thresholds at 4000 and 8000 Hz were significantly higher for older than younger subjects. Therefore, it was important to determine whether those subjects with higher audiometric thresholds were also the subjects with higher P1-N1-P2 thresholds, and/or increased response latencies and amplitudes. As discussed earlier, there were no significant correlations between audiometric thresholds (250 Hz–8000 Hz) and P1-N1-P2 thresholds, response amplitudes, or response latencies.
The ABR is thought to reflect synchronized neuronal activity from the auditory nerve to the brainstem. Several studies have shown decreased amplitudes and prolonged latencies of wave V in older adults with and without hearing loss (see review in Boettcher et al., 2002). Therefore, age-related differences in the P1-N1-P2 response may simply reflect changes generated at these earlier levels within the central auditory system. We examined whether P1-N1-P2 thresholds, and response latencies and amplitudes were correlated with ABR measures in older subjects. Similar to audiometric thresholds, no significant correlations were observed among ABR wave V response latencies or amplitudes and P1-N1-P2 thresholds, response latencies or amplitudes. The lack of significant correlations with both audiometric thresholds and ABR measures suggests that the age-related differences in sensitivity to frequency change assessed with cortical evoked potentials, as well as abnormal response latencies and amplitudes, do not simply reflect age-related changes in the auditory periphery, but may be due to changes that occur later in the central auditory system, such as the auditory cortex. However, although these findings suggest that age-related changes in the auditory periphery were not primary contributors to the observed age-related differences in the P1-N1-P2, altered central activity may still result from more subtle changes in the auditory periphery not identified by standard pure tone audiograms or click ABR measurements.
4.5 Conclusions
Older subjects had significantly decreased sensitivity to small changes in frequency (higher P1-N1-P2 thresholds), with larger age-related differences at lower than at higher frequencies. In addition to increased P1-N1-P2 thresholds, age-related changes in the neurophysiologic representation of the frequency change were also evident. These age-related changes indicate that the processing of small changes in frequency as indexed by the P1-N1-P2 response is impaired in older adults and may be the result of changes in the central auditory system. As noted in the introduction, several factors may contribute to age-related declines in frequency discrimination, including changes in both auditory and cognitive processing. In the present study, a passive paradigm was employed to elicit the P1-N1-P2, thereby reducing age-related confounds such as attention and memory. Therefore, age-related differences in P1-N1-P2 thresholds and response latencies and amplitudes may reflect an age-related decline in preattentive stimulus discrimination abilities of older subjects. However, this hypothesis needs to be tested directly in future studies, given that cortical potentials, such as the P1-N1-P2, are contingent on the resolution of the auditory periphery through the auditory cortex. In addition, although attention was directed away from the stimulus (by silent reading), subjects’ attention to the auditory stimulus cannot be ruled out, which may have affected P1-N1-P2 responses.
Acknowledgements
This investigation was supported by NIH/NIDCD P50 DC00422 and conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR-14516 from the National Center for Research Resources, National Institutes of Health. Helpful contributions from Fu-Shing Lee are gratefully acknowledged. Our gratitude is also given to the editor Jos Eggermont, Bob Burkard and an anonymous reviewer for their constructive comments that greatly improved this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American National Standards Institute. American National Standard specification for audiometers. ANSI S3.6-1996. New York: American National Standards Institute, Inc.; 1996. [Google Scholar]
- Alain C, Woods DL, Covarrubias D. Activation of duration-sensitive auditory cortical fields in humans. Electroencephalogr Clin Neurophysiol. 1997;104:531–539. doi: 10.1016/s0168-5597(97)00057-9. [DOI] [PubMed] [Google Scholar]
- Anderer P, Semlitsch HV, Saletu B. Multichannel auditory event-related brain potentials: effects of normal aging on the scalp distribution of N1, P2, N2 and P300 latencies and amplitudes. Electroencephalogr Clin Neurophysiol. 1996;99:458–472. doi: 10.1016/s0013-4694(96)96518-9. [DOI] [PubMed] [Google Scholar]
- Antinoro F, Skinner PH, Jones JJ. Relation between sound intensity and amplitude of the AER at different stimulus frequencies. J Acoust Soc Am. 1969;46:1433–1436. doi: 10.1121/1.1911881. [DOI] [PubMed] [Google Scholar]
- Attias J, Urbach D, Gold S, Shemesh Z. Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear Res. 1993;71:106–113. doi: 10.1016/0378-5955(93)90026-w. [DOI] [PubMed] [Google Scholar]
- Bertoli S, Smurzynski J, Probst R. Effects of age, age-related hearing loss, and contralateral cafeteria noise on the discrimination of small frequency changes: psychoacoustic and electrophysiological measures. J Assoc Res Otolaryngol. 2005;6:207–222. doi: 10.1007/s10162-005-5029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA, Madhotra D, Poth EA, Mills JH. The frequency-modulation following response in young and aged human subjects. Hear Res. 2002;165:10–18. doi: 10.1016/s0378-5955(01)00398-7. [DOI] [PubMed] [Google Scholar]
- Boettcher FA. Presbyacusis and the auditory brainstem response. J Speech Lang Hear Res. 2002;45:1249–1261. doi: 10.1044/1092-4388(2002/100). [DOI] [PubMed] [Google Scholar]
- Boutros NN, Reid MC, Petrakis I, Campbell D, Torello M, Krystal J. Similarities in the disturbances in cortical information processing in alcoholism and aging: a pilot evoked potential study. Int Psychogeriatr. 2000;12:513–525. doi: 10.1017/s1041610200006621. [DOI] [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH. Spectral integration of synchronous and asynchronous cues to consonant identification. J Acoust Soc Am. 2004;115:2278–2285. doi: 10.1121/1.1691035. [DOI] [PubMed] [Google Scholar]
- Chambers RD, Griffiths SK. Effects of age on the adult auditory middle latency response. Hear Res. 1991;51:1–10. doi: 10.1016/0378-5955(91)90002-q. [DOI] [PubMed] [Google Scholar]
- Hallgren M, Larsby B, Lyxell B, Arlinger S. Cognitive effects in dichotic speech testing in elderly persons. Ear Hear. 2001;22:120–129. doi: 10.1097/00003446-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Harkrider AW, Plyler PN, Hedrick MS. Effects of age and spectral shaping on perception and neural representation of stop consonant stimuli. Clin Neurophysiol. 2005;116:2153–2164. doi: 10.1016/j.clinph.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Harris KC, Mills JH, Dubno JR. Electrophysiologic correlates of intensity discrimination in cortical evoked potentials of younger and older adults. Hear Res. 2007;228:58–68. doi: 10.1016/j.heares.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann WM. Signals, Sound, and Sensation. New York: Springer; 1997. pp. 431–448. [Google Scholar]
- He N, Dubno JR, Mills JH. Frequency and intensity discrimination measured in a maximum-likelihood procedure from young and aged normal-hearing subjects. J Acoust Soc Am. 1998;103:553–565. doi: 10.1121/1.421127. [DOI] [PubMed] [Google Scholar]
- He NJ, Mills JH, Dubno JR. Frequency modulation detection: effects of age, psychophysical method, and modulation waveform. J Acoust Soc Am. 2007;122:467–477. doi: 10.1121/1.2741208. [DOI] [PubMed] [Google Scholar]
- Hoke M, Feldmann H, Pantev C, Lutkenhoner B, Lehnertz K. Objective evidence of tinnitus in auditory evoked magnetic fields. Hear Res. 1989;37:281–286. doi: 10.1016/0378-5955(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Humes LE. Speech understanding in the elderly. J Am Acad Audiol. 1996;7:161–167. [PubMed] [Google Scholar]
- Humes LE. Do 'auditory processing' tests measure auditory processing in the elderly? Ear Hear. 2005;26:109–119. doi: 10.1097/00003446-200504000-00001. [DOI] [PubMed] [Google Scholar]
- Humes LE, Christopherson L. Speech identification difficulties of hearing-impaired elderly persons: the contributions of auditory processing deficits. J Speech Hear Res. 1991;34:686–693. doi: 10.1044/jshr.3403.686. [DOI] [PubMed] [Google Scholar]
- Humes LE, Floyd SS. Measures of working memory, sequence learning, and speech recognition in the elderly. J Speech Lang Hear Res. 2005;48:224–235. doi: 10.1044/1092-4388(2005/016). [DOI] [PubMed] [Google Scholar]
- Humes LE, Coughlin M, Talley L. Evaluation of the use of a new compact disc for auditory perceptual assessment in the elderly. J Am Acad Audiol. 1996;7:419–427. [PubMed] [Google Scholar]
- Humes LE, Lee JH, Coughlin MP. Auditory measures of selective and divided attention in young and older adults using single-talker competition. J Acoust Soc Am. 2006;120:2926–2937. doi: 10.1121/1.2354070. [DOI] [PubMed] [Google Scholar]
- Humes LE, Watson BU, Christensen LA, Cokely CG, Halling DC, Lee L. Factors associated with individual differences in clinical measures of speech recognition among the elderly. J Speech Hear Res. 1994;37:465–474. doi: 10.1044/jshr.3702.465. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Lombardi DM, Gibbens ND, Ahmad BK, Newman CW. The effects of stimulus frequency and recording site on the amplitude and latency of multichannel cortical auditory evoked potential (CAEP) component N1. Ear Hear. 1992;13:300–306. doi: 10.1097/00003446-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Jesteadt W, Sims SL. "Decision processes in frequency discirmiantion, ". J. Acoust. Soc. Am. 1975;57:1161–1168. doi: 10.1121/1.380574. [DOI] [PubMed] [Google Scholar]
- Kadner A, Viirre E, Wester DC, Walsh SF, Hestenes J, Vankov A, Pineda JA. Lateral inhibition in the auditory cortex: an EEG index of tinnitus? Neuroreport. 2002;13:443–446. doi: 10.1097/00001756-200203250-00016. [DOI] [PubMed] [Google Scholar]
- Konig E. Pitch discrimination and age. Acta Otolaryngol. 1957;48:475–489. doi: 10.3109/00016485709126909. [DOI] [PubMed] [Google Scholar]
- Laffont F, Bruneau N, Roux S, Agar N, Minz M, Cathala HP. Effect of age on auditory evoked responses (AER) and augmenting-reducing. Neurophysiol Clin. 1989;19:15–23. doi: 10.1016/s0987-7053(89)80081-4. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory, event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear Hear. 1999;20:33–44. doi: 10.1097/00003446-199902000-00004. [DOI] [PubMed] [Google Scholar]
- McCandless GA, Rose DE. Evoked cortical responses to stimulus change. J Speech Hear Res. 1970;13:624–634. doi: 10.1044/jshr.1303.624. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Freyman RL. Psychometric functions for frequency discrimination from listeners with sensorineural hearing loss. J Acoust Soc Am. 1986;79:799–805. doi: 10.1121/1.393470. [DOI] [PubMed] [Google Scholar]
- Oates PA, Kurtzberg D, Stapells DR. Effects of sensorineural hearing loss on cortical event-related potential and behavioral measures of speech-sound processing. Ear Hear. 2002;23:399–415. doi: 10.1097/00003446-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Roth WT, Kopell BS. Age-related changes in auditory event-related potentials. Electroencephalogr Clin Neurophysiol. 1980;49:266–276. doi: 10.1016/0013-4694(80)90221-7. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Champagne SC, Nelson RF. The effects of age on human event-related potentials. Psychophysiology. 1984;21:312–325. doi: 10.1111/j.1469-8986.1984.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Skinner CR, Champagne SC, Kellett AJ, Maiste AC. Potentials evoked by the sinusoidal modulation of the amplitude or frequency of a tone. J Acoust Soc Am. 1987;82:165–178. doi: 10.1121/1.395560. [DOI] [PubMed] [Google Scholar]
- Schroeder MM, Ritter W, Vaughan HG., Jr The mismatch negativity to novel stimuli reflects cognitive decline. Ann N Y Acad Sci. 1995;769:399–401. doi: 10.1111/j.1749-6632.1995.tb38155.x. [DOI] [PubMed] [Google Scholar]
- Simon HJ, Yund EW. Frequency discrimination in listeners with sensorineural hearing loss. Ear Hear. 1993;14:190–201. doi: 10.1097/00003446-199306000-00006. [DOI] [PubMed] [Google Scholar]
- Smith DB, Michalewski HJ, Brent GA, Thompson LW. Auditory averaged evoked potentials and aging: factors of stimulus, task and topography. Biol Psychol. 1980;11:135–151. doi: 10.1016/0301-0511(80)90048-4. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Billings C, Rohila N. Speech evoked cortical potentials: effects of age and stimulus presentation rate. J Am Acad Audiol. 2004;15:226–237. doi: 10.3766/jaaa.15.3.5. quiz 264. [DOI] [PubMed] [Google Scholar]
- Turner CW, Nelson DA. Frequency discrimination in regions of normal and impaired sensitivity. J Speech Hear Res. 1982;25:34–41. doi: 10.1044/jshr.2501.34. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Penry JK. A temporal component of the auditory evoked response. Electroencephalogr Clin Neurophysiol. 1975;39:609–620. doi: 10.1016/0013-4694(75)90073-5. [DOI] [PubMed] [Google Scholar]
- Woods DL. Auditory selective attention in middle-aged and elderly subjects: an event-related brain potential study. Electroencephalogr Clin Neurophysiol. 1992;84:456–468. doi: 10.1016/0168-5597(92)90033-8. [DOI] [PubMed] [Google Scholar]
- Woods DL. The component structure of the N1 wave of the human auditory evoked potential. Electroencephalogr Clin Neurophysiol Suppl. 1995;44:102–109. [PubMed] [Google Scholar]
- Woods DL, Clayworth CC. Age-related changes in human middle latency auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1986;65:297–303. doi: 10.1016/0168-5597(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Effects of stimulus frequency and complexity on the mismatch negativity and other components of the cortical auditory-evoked potential. J Acoust Soc Am. 2001;109:1526–1537. doi: 10.1121/1.1349184. [DOI] [PubMed] [Google Scholar]
- Yingling CD, Nethercut GE. Evoked responses to frequency shifted tones: tonotopic and contextual determinants. Int J Neurosci. 1983;22:107–118. doi: 10.3109/00207459308987389. [DOI] [PubMed] [Google Scholar]