Abstract
Pathogen entry into host tissue is a critical first step in causing infection. For foliar bacterial plant pathogens, natural surface openings, such as stomata, are important entry sites. Historically, these surface openings have been considered as passive portals of entry for plant pathogenic bacteria. However, recent studies have shown that stomata can play an active role in limiting bacterial invasion as part of the plant innate immune system. As counter-defense, the plant pathogen Pseudomonas syringae pv. tomato DC3000 uses the virulence factor coronatine to actively open stomata. In nature, many foliar bacterial disease outbreaks require high humidity, rain, or storms, which could promote stomatal opening and/or bypass stomatal defense by creating wounds as alternative entry sites. Further studies on microbial and environmental regulation of stomatal closure and opening could fill gaps in our understanding of bacterial pathogenesis, disease epidemiology, and microbiology of the phyllosphere.
Keywords: Coronatine, plant hormones, guard cell, plant defense, innate immunity, Pseudomonas syringae
INTRODUCTION
The phyllosphere of terrestrial plants provides one of the most important niches for microbial inhabitation (15, 72). The phyllosphere comprises both the surface and interior of a leaf. The leaf interior is composed mostly of mesophyll cells, in which the bulk of photosynthate is made; some vascular tissues (veins); and a large, air-filled intercellular space (apoplast) between mesophyll cells (Figure 1). Because of its close proximity to mesophyll cells, the leaf apoplast is thought to contain abundant nutrients, whereas nutrients on the leaf surfaces are much more limited and spatially heterogeneous, mostly leaked out from the apoplast through natural surface openings and wounds (72). Numerous microbes, both pathogenic and saprophytic, can survive and even proliferate to various degrees as epiphytes on the plant surface without causing disease. Strict epiphytes complete their life cycles on the surface of plants and generally do not (or are unable to) colonize the leaf apoplast (6, 7). In contrast, foliar pathogens, such as Pseudomonas syringae, can multiply aggressively in the apoplast as pathogenic endophytes, ultimately leading to disease production under favorable conditions (47). Entry into plant tissue is likely a critical first step in the establishment of foliar infection. To gain access to the intercellular spaces and internal leaf tissues, pathogens must cross the surface cuticle and epidermis. Many fungal plant pathogens have the ability to directly penetrate the epidermis using cuticle- and cell wall-degrading enzymes, mechanical force, or both (78). Bacteria, however, cannot directly penetrate the leaf epidermis and must enter leaf tissues by alternative means.
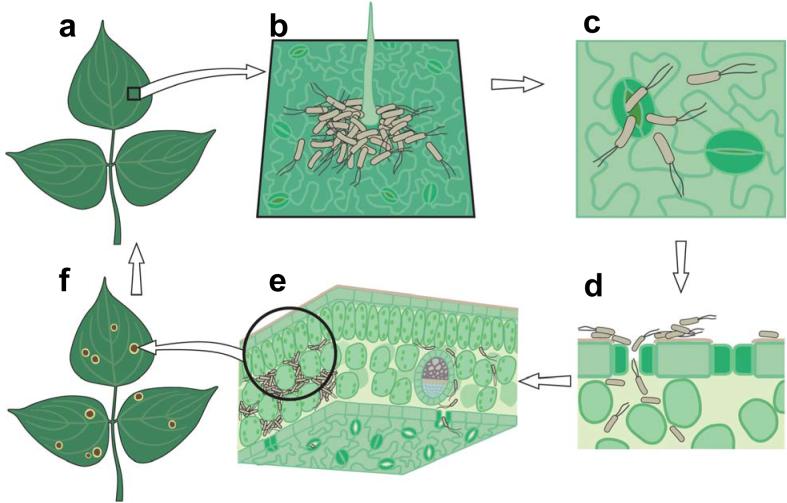
Figure 1.
A simplified diagram of the infection cycle of Pseudomonas syringae. (a) A diagram of healthy plant leaves. (b) Bacterial cells on a leaf surface, illustrating aggregation of some bacteria near a trichome. (c) Bacteria penetrating open stomate. (d) Cross section of a leaf showing bacteria colonizing the plant apoplast. (e) Extensive multiplication of bacteria in the apoplast of a leaf. (f) Visible disease-associated necrosis and chlorosis.
There are a number of natural surface openings through which bacterial pathogens have been observed to enter the plant including: a) hydathodes: water-exuding pores at the edges of leaves (e.g. Xanthomonas campestris pv. campestris), b) stomata: openings in the aerial part of plants that control gas exchange and water transpiration between the plant interior and the environment (e.g. Pseudomonas syringae), c) nectarthodes: nectar-secreting pores at the point of emergence of the styles and stamens (e.g. Erwinia amylovora), and d) lenticels: respiration pores in stems and roots (E. carotovora var. Atroseptica). Among natural openings, stomata dominate in number in the aerial part of the plant and therefore represent one of the most important routes for the entry of foliar bacterial pathogens. In addition to natural openings, bacteria can also reach the plant interior through surface wounds caused by insects, mechanical or frost damage, hail, wind-blown sand, rain storm, or damage caused by lateral root formation, breakage of trichomes, or abscission of leaves (leaf scars).
It has been widely assumed that natural surface openings and wounds are passive ports for bacterial entry, where bacteria lack active mechanisms for gaining entry, and plants similarly lack active mechanisms for preventing entry. However, recent evidence suggests that entry of bacteria into leaf tissue through stomata is more complex and dynamic than the simple act of swimming into the leaf through passive openings (77). In this review, we discuss the recent finding on this topic, focusing mainly on the foliar pathogen Pseudomonas syringae. We will begin with a brief overview of the lifestyle of P. syringae before presenting in-depth discussion of the role of stomata in bacterial infection and host defense.
PSEUDOMONAS SYRINGAE—A MODEL FOLIAR PATHOGEN
Basic Infection Cycle
The species P. syringae is composed of strains that collectively infect hundreds of plant species and cause disease symptoms ranging from leaf spots to stem cankers. Different strains of P. syringae are known for their diverse and host-specific interactions with plants. A specific strain may be assigned to one of about 50 pathovars based on its limited host range at the plant species level (35), and then further assigned to a race based on differential interactions among cultivars of a plant species. The infection cycle of P. syringae is typical for a foliar pathogenic bacterium. In the field, there are multiple sources of P. syringae inocula, including infected seeds, diseased plants, and/or asymptomatic alternative hosts or even nonhost plants (47). In a successful disease cycle, P. syringae strains generally live two lifestyles that are spatially and temporally interconnected: an initial, largely epiphytic phase upon the arrival on the surface of a healthy plant and a subsequent, largely endophytic phase in the apoplast (6; see Figure 1 for a conceptual illustration). Under favorable environmental conditions (e.g., heavy rain, high humidity, moderate temperature), P. syringae can multiply very aggressively in a susceptible host plant, resulting in the appearance, within several days, of disease symptoms (e.g., water-soaking in the apoplast of infected tissues, localized tissue necrosis, and various tissue discolorations). It has been suggested that some strains of P. syringae may be primarily epiphytes and cause disease only accidentally (46, 47).
In terms of the mode of pathogenesis, P. syringae may be best described as a hemi-biotrophic pathogen, because the most aggressive phase of population increase occurs in the absence of apparent host cell death. However, at the late stage of pathogenesis (often after bacteria have almost reached the peak population in the apoplast), host cells die and infected tissues show extensive necrosis. This pathogenesis mode seems distinct from that of strictly biotrophic pathogens, which obtain nutrients from living host cells without causing host cell death, or of strictly necrotrophic pathogens, which kill host cells as the main strategy of obtaining nutrients, causing extensive host cell death during early stages of infection.
Epiphytic vs. Endophytic Lifestyles
The fact that P. syringae has evolved two distinct lifestyles (epiphytic vs. endophytic) begs the question of how P. syringae (and other foliar bacterial pathogens) makes the transition from the epiphytic phase to the endophytic phase during a successful infection cycle. This is clearly one of the most outstanding questions in bacterial disease epidemiology, yet we still have little understanding of the process. The plant surface is generally thought to be a hostile environment for microorganisms with limited nutrients, drastic and rapid variations in temperature and humidity even during a single day, direct exposure to damaging UV irradiation, and competition and/or antagonism from other epiphytes (6, 72, 101). Studies with molecular biosensors have provided insights on the real, micro-scale environment that bacteria live in as epiphytes (72). It seems that the phyllosphere is a very heterogeneous space in which a few nutrient-rich spots are present. Preferred niches are found along the veins, trichomes, and stomata (47, 82). Bacteria that land on less favorable areas of the leaf have to evolve mechanisms to cope with the harsh environment. Some bacteria avoid the stress in the leaf surface by creating micro-environments to protect themselves. For instance, some Pseudomonas strains alter the leaf surface properties by producing surfactants that increase the wetness of the leaf surface (19, 50). In addition, epiphytes frequently live in large, heterogeneous aggregates and biofilms on the leaf surface, presumably to better resist environmental stresses such as desiccation (25, 81, 82).
Because P. syringae and other foliar pathogens can multiply very aggressively in the leaf apoplast, one might ask why P. syringae does not just quickly enter the plant and multiply as endophytes, instead of evolving a separate epiphytic lifestyle. The plant interior would presumably shield bacteria from many of the surface-associated stresses. As this question is intimately related to the discussion of the entry process, we would like to consider several possible evolutionary forces that may have driven the development and maintenance of a distinct epiphytic lifestyle in foliar pathogenic bacteria in spite of their high multiplication potential in the apoplast.
First, the plant interior may present another harsh environment for bacteria. It is known that many saprophytic and human pathogenic bacteria could multiply significantly on the plant surface 6, 15); however, they generally do not seem to be able to multiply inside the leaf apoplast (57; W. Underwood and S.Y. He, unpublished data). It is likely that the apoplast contains higher levels of defense compounds and/or unique defense mechanisms not encountered on the leaf surface, because the surface cuticle and epidermis provide formidable physical barriers that would reduce diffusion of chemicals to the plant surface. In support of this notion, several recent studies show that the leaf surface of cutin-defective mutant Arabidopsis plants seem to contain higher concentrations of anti-fungal compounds (10, 20, 106). Nevertheless, it is now well documented that virulent P. syringae and presumably other foliar pathogens have evolved virulence effectors to evade or suppress host defenses in the apoplast (23, 53, 88). Therefore, although the apoplast may be a hostile environment and inhabitable for saprophytic and other nonphytopathogenic bacteria, it seems unlikely that host defense in the apoplast would be the primary reason why P. syringae and other adapted foliar pathogens need to evolve and maintain an epiphytic lifestyle.
The second possible reason for maintaining an epiphytic lifestyle could be that the notion of nutrient abundance in the plant apoplast may be incorrect. Theoretical calculations by Hancock & Huisman (42) suggest that there is a sufficient amount of nutrition in the apoplast to support high-density growth of bacteria without active transport of sugars from host cells. Consistent with such calculations, P. syringae pv. tomato (Pst) DC3000 can grow to very high numbers in the wash fluid of the Arabidopsis leaf apoplast, almost approaching those in rich media (Figure 2). However, as preparation of apoplast wash fluids involves infiltration of water into the apoplast followed by low-speed centrifugation, it is possible that these retrievable apoplast nutrients are normally sequestered in locations not accessible by bacteria. Interestingly, one of the early disease symptoms commonly associated with foliar infections by pseudomonads and xanthomonads is water-soaking in the apoplast of infected tissues (30, 52), although it is not yet clear whether apoplast water-soaking is a bacterial strategy for retrieving nutrients or simply a side effect of host cell damage caused by infection. In any case, even if the bulk of apoplast nutrients is not accessible to nonphytopathogenic bacteria, it seems likely that P. syringae and other adapted foliar pathogens must have evolved effective virulence mechanisms to obtain nutrients from the host apoplast.
Figure 2.
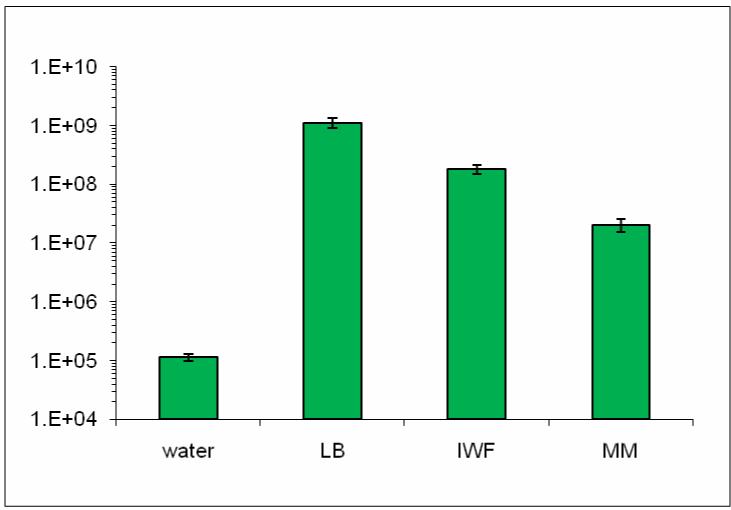
Pst DC3000 populations 8 h after transferring 1.2×106 CFU/ml bacteria in water, Luria-Bertani broth (LB), intercellular wash fluid (IWF), and hrp-inducing minimal medium (MM). Data points are means and standard deviations of three replicate cultures.
If neither host defense nor nutrient availability could provide a definitive explanation for the need of foliar pathogens to evolve and maintain an extended epiphytic lifestyle, a third possible reason could be that entry sites on the leaf surface are limited. How limited are the potential entry sites on the plant surface? In leaves, as mentioned above, stomata dominate in number and represent an important surface opening for bacterial pathogens to enter the plant. The densities of stomata vary among plant species and are regulated developmentally and in response to environmental changes (64, 116). There may be up to 300 stomata per mm2 on the leaf surfaces, and the stomatal pores may occupy up to 2% of the total leaf surface area (64, 87, 116). Therefore, despite their abundance, stomata account for only a minor portion of the leaf surface; the chance of a bacterial immigrant arriving directly on top of a natural opening, such as a stomate, is small. The density of stomata on the upper leaf (where bacteria are most likely to land) is generally significantly lower than on the lower surface of the leaf. Furthermore, stomata open only during the day, to uptake CO2 for photosynthesis; they close at night to reduce water transpiration. Thus, during periods of a disease cycle, the only niche available for many individuals of a P. syringae population might be the surface of a plant, even though the plant apoplast may ultimately provide a better niche for P. syringae. The limited entry sites would require a successful bacterial pathogen to adhere and move toward natural openings or wounds. Indeed, adherence and motility have been shown to be important for surface infection by P. syringae and other foliar pathogens (5, 43, 41, 90, 95). Furthermore, movement toward natural openings and wounds would probably occur only if the leaf surface is wet, which may be one of the reasons why foliar disease outbreaks often follow periods of rain and high humidity (47).
STOMATAL RESPONSE TO BACTERIA
Stomata are composed of a pair of specialized epidermal cells referred to as guard cells (Figure 3). Stomata regulate gas exchange between the plant and environment and control of water loss by changing the size of the stomatal pore. This stomatal movement is affected by several environmental stimuli, such as relative humidity, CO2 concentration, and light intensity. The plant hormone abscisic acid (ABA) plays a central role in guard cell signaling leading to stomatal closure during drought stress. The ABA signal in guard cells is transduced through a complex network of signaling events, including the production of compounds such as nitric oxide (NO) and H2O2, signaling intermediaries such as the guard cell-specific OST1 kinase, changes in cytosolic Ca2+ levels, and Ca2+ oscillations (31, 51, 84, 96, 97). The signaling events triggered by ABA ultimately lead to regulation of ion channels, such as the guard cell outwardly rectifying potassium channel GORK1, which modulates ion efflux from the guard cells (48). Ion efflux from the guard cells also drives the efflux of water and results in a change in guard cell turgor that causes closure of the stomatal pore. For a more comprehensive overview of guard cell signaling networks, the reader is directed to two recent reviews (31, 51).
Figure 3.
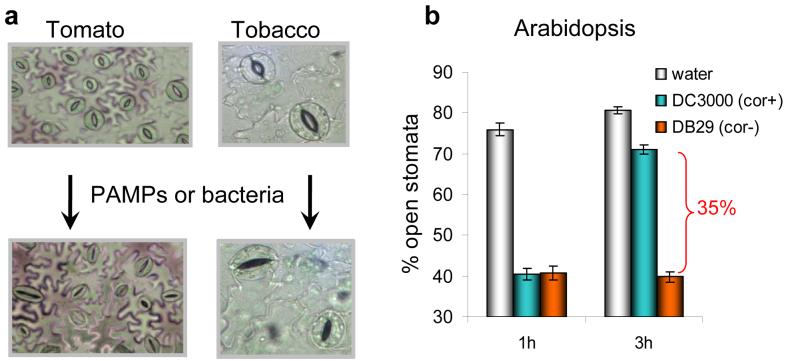
(a) Light-conditioned leaf epidermis (top pictures) showing mostly open stomata and the same leaf epidermis after 1 h exposure to purified PAMP or live bacteria (bottom pictures). Note that most stomatal pores are closed after exposure to bacteria or PAMPs. (b) Stomatal response in light-exposed epidermal peels of Arabidopsis leaves exposed to water, DC3000, or DB29. DB29 is a coronatine-biosynthesis-defective (cor) mutant (17). Stomatal response to this cor mutant is similar to response to DC3118, another cor mutant (74, 77).
Until recently, it was thought that stomata were a strictly passive port of entry, suggesting that bacteria could freely invade through open stomata. However, Melotto and colleagues (77) found that Arabidopsis stomata close in response to live bacteria and purified pathogen/microbe-associated molecular patterns (PAMP/MAMP). Bacterium-induced stomatal closure requires PAMP signaling and homeostasis of the defense hormone salicylic acid (SA), and it is connected to signaling regulated by ABA in the guard cell.
Basic Observations
Melotto et al. (77) used light-adapted Arabidopsis plants, in which 70-80% of stomata in the leaves are open, for their experiments. When leaves or epidermal peels were exposed to a suspension of Pst DC3000, a virulent pathogen of tomato and Arabidopsis (54, 114), a marked reduction in the number of open stomata (to circa 30%) was observed within 1 to 2 h of incubation (Figure 3). In contrast, the number of open stomata remained virtually the same in leaves incubated with water. Interestingly, the Pst DC3000-induced closing of stomata was transient. After 3 h of incubation, most stomata (Figure 3b) reverted to the open state.
Because the phyllosphere is also colonized by other microbes, including human pathogenic bacteria (11, 72, 85), Melotto and colleagues (77) conducted experiments with Escherichia coli O157:H7, a human pathogenic bacterium commonly associated with vegetable-based food poisoning (91). Again, stomatal closure was observed within 2 h of incubation. However, in this case, stomata closure persisted for the duration of the experiment (4 to 8 h). These results demonstrate that stomata actively close as an initial response to both plant and human pathogenic bacteria, and Pst DC3000 has evolved a mechanism (or mechanisms) to re-open stomata 3 h after incubation with plant leaves or epidermal peels.
The ability of both human and plant pathogenic bacteria to induce stomatal closure within the first hour of contact with plant tissue suggests that guard cells can sense conserved bacterial molecules. PAMPs are such molecules, and they are best known for their ability to stimulate innate immunity in plants and animals (37, 102). Indeed, flg22 (a biologically active peptide derived from flagellin; 3, 123) and LPS (120) cause stomatal closure in the wild-type Col-0 plant (77), suggesting that stomatal closure is part of the PAMP-triggered innate immune response.
As mentioned above, one difference between stomatal response to the human pathogen E. coli O157:H7 and to the plant pathogen Pst DC3000 is that stomata re-open after approximately 3 hr of incubation with Pst DC3000, but not with E. coli O157:H7. Melotto et al. (77) suspected that Pst DC3000, but not E. coli O157:H7, may have evolved a natural virulence mechanism that can counter PAMP-induced stomatal closure. Pst DC3000 contains two well-characterized virulence factors: the hrc/hrp gene-encoded type III secretion system (TTSS), which delivers numerous effector proteins into the host cell (1, 38, 45, 70), and the phytotoxin coronatine (COR) (9). It was found that a COR-deficient mutant could not re-open closed stomata, whereas a hrc mutant was not affected in the ability to re-open stomata (77). Furthermore, green fluorescent protein (GFP)-labelled Pst DC3000 bacteria were able to reach the upper surface of Arabidopsis epidermal peels when placed underneath, whereas fewer GFP-labelled cor- bacteria were found on the upper surface of the epidermal peels (77). Taken together, these results show that one of the virulence factors involved in suppressing stomatal defense in this bacterium is coronatine.
These initial observations indicate that stomata are not merely passive ports for bacterial entry. Guard cells could perceive live bacteria including P. syringae and E. coli and close a significant percentage of stomata, suggesting that plants have evolved mechanisms to reduce the entry of bacteria as an integral part of the plant immune system. Because not all stomata are closed in response to bacteria or PAMPs (77), the apparently non-responsive and/or dead open stomata would provide a route for bacterial entry at a basal level. In the case of the virulent plant pathogen Pst DC3000, bacteria produce the diffusible virulence factor COR to re-open closed stomata, thereby increasing the number of sites for bacterial invasion (Figure 4).
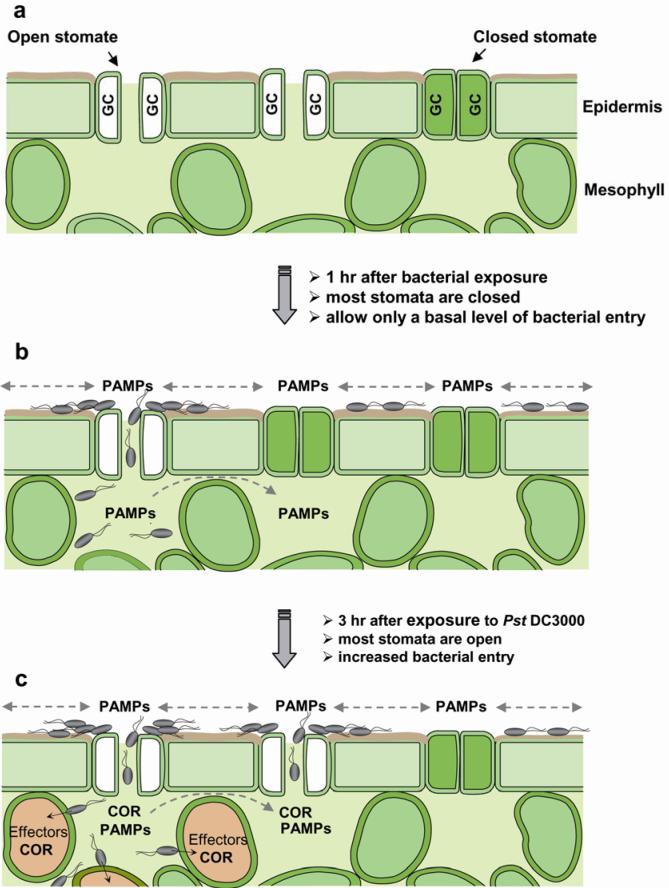
Figure 4.
A diagram depicting stomata as entry sites for bacterial invasion. (a) A cross-sectional view of leaf epidermis and mesophyll cells showing that stomata, formed by pairs of guard cells (GC), in light-adapted Arabidopsis leaves are mostly fully open. (b) Upon exposure to bacteria, guard cells perceive PAMPs and many stomata close within 1 h. Because not all stomata are closed, those ‘non-responsive’ open stomata (e.g., stomate on the left) may provide a route for bacterial entry at a basal level. Dashed arrows indicate diffusion of PAMPs on the epidermal surface or in the intercellular space (apoplast) between mesophyll cells, which can also perceive PAMPs and activate additional host defenses. (c) In the case of the virulent plant pathogen Pst DC3000, 3 h after infection bacteria produce diffusible COR in the apoplast and/or on the plant surface to re-open closed stomata (e.g., middle stomate), thereby increasing the number of sites for bacterial invasion. Pst DC3000 bacteria also inject TTSS effectors into mesophyll cells to suppress host defenses and to release nutrients. COR and TTSS effectors have overlapping functions in the suppression of host defenses in the apoplast and in the promotion of disease symptoms (see text). COR also induces disease susceptibility systemically in leaves (27; see text). This figure is adapted from Underwood et al. (110) with permission of the Publisher.
Signaling Components during Stomatal Response to Bacteria and PAMPs
How do stomatal guard cells sense bacteria and PAMPs? Currently our understanding of this process is at an infant stage. Plants sense PAMPs via leucine-rich repeat (LRR) receptors (4, 9, 44), such as the flagellin receptor FLS2 (21, 37). In addition to flagellin, other highly conserved bacterial molecules such as RNA-binding motif RNP-1, elongation factor Tu, and LPS, also elicit hallmark responses of PAMP-induced innate immune response (33, 63, 120, 122). Melotto and colleagues (77) found that the flg22 PAMP failed to induce the closure of stomata in epidermal peels of the Arabidopsis fls2 flagellin receptor mutant (37), suggesting that guard-cell perception of flg22 requires the cognate PAMP receptor. Both flg22 and LPS induced the production of nitric oxide (NO) in guard cells of wild-type stomata within 10 min. In addition, Nω-nitro-L-arginine (L-NNA), an inhibitor of nitric oxide synthase, effectively prevented stomatal closure caused by flg22, LPS, and E. coli O157:H7, suggesting that NO is also required for PAMPs and bacteria to close stomata.
In addition to the PAMP signal transduction pathway, SA and ABA were found to be required for stomatal response to bacteria or PAMPs. Neither flg22 nor LPS induce stomatal closure in the ABA biosynthetic mutant aba3-1 (68) or ABA signaling mutant ost1-2 (84). Similarly, stomata in SA-deficient Arabidopsis nahG (27) or eds16 (115) plants do not respond to PAMPs (77). The defect in PAMP/bacterium-triggered stomatal closure in SA-deficient plants is interesting, as previous studies have shown that SA is a potent inducer of stomatal closure (66, 75, 83). Taken together, these results suggest mechanistic connections between PAMP signaling, SA homeostasis, and ABA homeostasis and signaling in guard cell response to bacteria.
It should be pointed out that the requirement of SA and ABA for PAMP/bacterium-triggered stomatal closure does not mean necessarily that the SA or ABA signaling network lies downstream of the PAMP signaling network, with the PAMP receptors at the top of a signaling cascade. It is equally possible that PAMP, SA, and ABA signaling networks function in parallel, but are interconnected through specific branches, in the guard cell. A defect in the SA or ABA signaling network may cause pathway perturbation in the guard cell that indirectly compromises PAMP signaling. In addition, it also remains to be determined whether perception of PAMPs by immune receptors such as FLS2 leads to increased biosynthesis of SA and ABA or, simply, PAMP signaling in the guard cell requires a basal level of SA and ABA. Clearly, a more comprehensive analysis using additional signaling mutants is necessary to understand the connections between various branches of SA, ABA, and PAMP signaling networks in the guard cell. It is also worth mentioning that current studies of plant defenses are mostly based on mixed cell types (i.e., whole leaves). It is possible that stomatal guard cells have unique signaling properties that may require cell type-specific analyses.
COR-Mediated Suppression of Stomata-Based Defense
The discovery that COR is required for re-opening stomata by Pst DC3000 represents the first identification of a bacterial virulence factor that suppresses stomatal closure (77). COR is a non-host-specific polyketide toxin consisting of two distinct structural components, coronafacic acid (CFA) and coronamic acid (CMA); it is produced by at least five different pathovars of P. syringae: pvs. tomato, glycinea, atropurpurea, maculicola, and morsprunorum (8). The leaves of many plants develop chlorosis when treated with purified COR (8). In addition, COR is required for full virulence of COR-producing P. syringae strains on Arabidopsis, tomato, and soybean, as it promotes bacterial growth and contributes to symptom development (Figure 5; 16, 17, 18, 80, 121). Although COR is one of the most extensively studied bacterial phytotoxins, the precise molecular mechanisms through which COR acts to promote bacterial virulence and disease development are not yet clear. The possibility that COR could suppress an early defense response during Pst DC3000 infection of Arabidopsis and tomato was suggested more than a decade ago. Mittal and Davis (80) first noticed that cor- mutant bacteria achieved population levels similar to those of wild-type Pst DC3000 in Arabidopsis leaves inoculated by infiltrating bacterial suspension (1×106 CFU.ml-1) directly into the leaf intercellular spaces, but caused fewer disease symptoms, including less chlorosis and necrosis compared to inoculation with wild type. However, when bacteria (1×108 CFU.ml-1) were dip-inoculated onto the surface of plant leaves, cor- mutant bacteria achieved significantly lower population levels than wild-type Pst DC3000 and were unable to cause disease symptoms.
Figure 5.

Tomato leaves 5 days after dip-inoculation with 1×107 CFU/ml suspension of the wild-type bacterium Pst DC3000 (left) and the coronatine-defective mutant Pst DC3118 (right). Whereas Pst DC3000 caused necrotic lesions with diffuse chlorosis, the coronatine-defective mutant bacteria caused only some lesions and no chlorosis.
A similar study was conducted with P. syringae pv. glycinea (Psg), a cool-weather pathogen of soybean. In this pathogen, production of COR is temperature-dependent. COR production peaks at a temperature of 18°C, whereas at 28°C no COR production can be detected (18, 89). Interestingly, when spray-inoculated onto soybean plants, bacteria derived from cultures grown at 18°C achieved significantly higher population levels and caused more severe disease symptoms than bacteria cultured at 28°C (18). However, when bacteria were artificially infiltrated into the leaf intercellular spaces, no effect of the pre-inoculation temperature of bacterial cultures was observed. Additionally, COR-deficient mutants of Psg PG4180 were severely reduced in multiplication (∼10,000-fold less than wild type) when inoculated by spraying. When inoculated by infiltration, however, the COR-deficient mutant bacteria achieved population levels only 5- to 10-fold lower than wild type. These results again suggest that COR plays an important role early in the infection process, likely by promoting entry of bacteria into leaf tissues.
The demonstrated ability of COR to suppress stomatal closure therefore offers one possible explanation for these earlier observations. COR also plays roles in the late stages of the infection process, including suppression of local and systemic host defenses and promotion of disease symptoms (24, 62, 110, 112). As disease progresses, Pst DC3000 heavily colonizes the sub-stomatal cavity (14) and it is possible that COR-induced stomatal opening is also important for the release of bacteria to the leaf surface to facilitate subsequent spread to nearby healthy plants.
How might COR Suppress Bacterium/PAMP-Triggered Stomatal Closure?
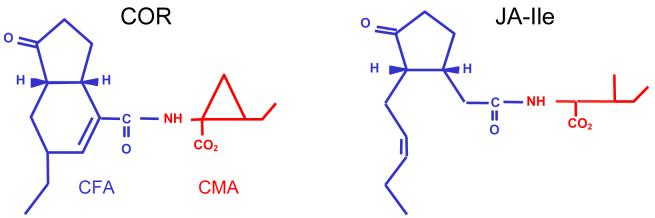
COR has long been hypothesized to be a structural mimic of jasmonates (JA), possibly by binding to the same or similar receptors. In particular, COR is most similar to JA conjugated to the amino acid isoleucine (JA-Ile; Figure 6) (61, 99). COR also seems to have functional similarities to JA (34, 39, 60, 113). COR induces a number of responses that are also induced by treatment with JA, including induction of protease inhibitors and phytoalexins and regulation of JA-responsive transcripts (89, 104, 107, 111, 113, 121). Identification of the Arabidopsis JA/COR perception mutant coi1, which is insensitive to both JA and COR, further suggests a similar mode of action (58, 117). The COI1 gene encodes the F-box subunit of the SCFCOI1 ubiquitin ligase, which is involved in the ubiquitin-conjugation pathway of protein degradation (28, 109, 119). The coi1 and jai1 mutants of Arabidopsis and tomato show enhanced resistance to Pst DC3000 (34, 58, 121) and the ability of COR to inhibit stomatal closure is dependent on the COI1 gene (77). However, the reduction in bacterial multiplication in coi1 and jai1 plants cannot be fully explained by a lack of COR function. This reduction is much greater than that observed after inoculation with cor- mutant bacteria, suggesting that other virulence factors produced by Pst DC3000 also target COI1/JAI1-dependent pathways to promote susceptibility. Consistent with this hypothesis, several studies suggest that COR and TTSS effector proteins have overlapping functions and act synergistically to induce JA signaling (107, 121). Thus, although COR has a unique role in suppressing stomatal closure (77), its contribution in later stages of infection could be potentially masked by the functions of some TTSS effectors under certain conditions, as hrp genes and TTSS effectors are also important for bacterial multiplication and symptom production (26, 71).
Figure 6.
The chemical structures of JA-Ile and coronatine are similar.
Although COR likely mimics JA-Ile in exerting its virulence function, Suhita and colleagues (100) showed that exogenous application of JA or methyl JA (MeJA) alone caused stomatal closure. However, the authors did not examine a possible effect of JA or MeJA on ABA-induced stomatal closure. In our own experiments we found that high concentrations (>20 μM) of JA and MeJA (Sigma Co.) caused significant stomatal closure. However, lower concentrations (e.g., 1 to 10 μM) of JA and MeJA did not; instead, like COR, they effectively inhibited ABA from closing stomata (M. Melotto and S.Y. He, unpublished data). These results suggest a potentially bimodal effect of exogenous JA and MeJA on stomatal response, depending on concentrations used.
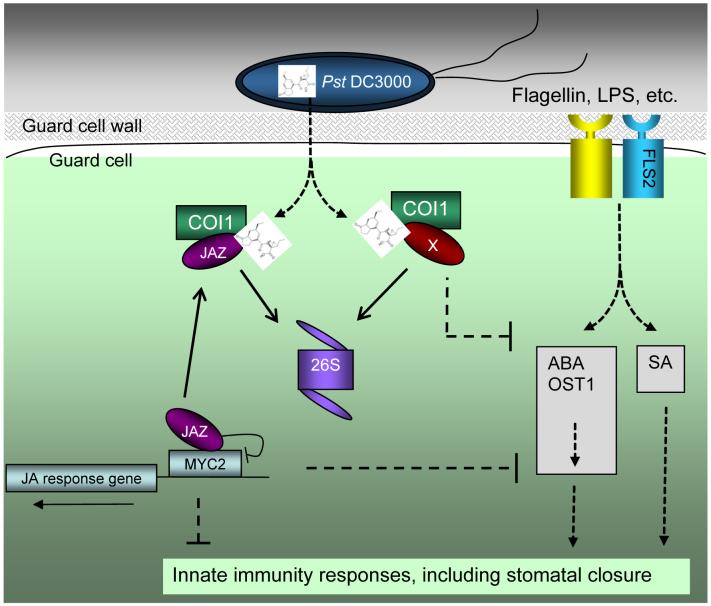
Recent studies have begun to shed some light on how COR and JA might act through SCFCOI1 to perform their functions in the plant cell (22, 108). The COI1 protein shares a high sequence similarity with TIR1, which is the F box subunit of the SCFTIR1 ubiquitin ligase involved in the ubiquitination and degradation of AUX/IAA repressor proteins during plant hormone auxin signaling (55). TIR1 and AUX/IAA proteins interact with each other and this interaction does not require any other plant protein, but is facilitated by auxin, which directly binds to the TIR1 receptor protein (29, 105). This exciting demonstration raises the possibility that F-box proteins or E3 ubiquitin ligase-substrate complexes may have a general role in serving as receptors of other plant hormones and related ligands. It was shown recently that COI1 interacts with JAZ (jasmonate ZIM-domain) proteins in Arabidopsis (22, 108). JAZ proteins are repressors of the JA signaling pathway and they are degraded by the 26S proteasome in a SCFCOI1-dependent manner (22, 108). Remarkably, COI1 and JAZ proteins interact in yeast cells (i.e., in the absence of any other plant protein), but only in the presence of JA-Ile (108) or COR (M. Melotto and S.Y. He, unpublished data). This observation implicates COI1-JAZ complexes as receptors of JA-Ile and COR in plants (Figure 7).
Figure 7.
A model of COR action in the plant cell (possibly all cell types). COR (structure shown) is secreted by Pst DC3000 into the plant cell and increases the affinity of the COI1 protein (as part of the SCFCOI1-ubiquitin ligase complex; not shown here) toward JAZ repressor and possibly other host proteins (denoted by “X”). The SCFCOI1 complex catalyzes ubiquitination of JAZ and other host proteins, which are then degraded through the 26S proteasome (denoted as “26S”). JAZ proteins normally function as repressors by physically binding to transcriptional activators (such as MYC2) of jasmonate response genes. In the stomatal guard cell, PAMPs (e.g., flagellin and LPS) are perceived by cognate immune receptors (e.g., flagellin receptor FLS2). Perception of PAMPs triggers stomatal closure, which requires SA, ABA, and OST1 kinase. COR-mediated inhibition of PAMP-triggered stomatal closure and other innate immune responses could be mediated by JAZ and/or “X” proteins.
It is not yet clear how the SCFCOI1-dependent action of COR translates into inhibition of stomatal closure. Several studies have shown strong antagonistic interactions between JA signaling and SA- or ABA-mediated signaling (2, 16, 36, 65, 69, 73, 93, 98). For instance, transcripts associated with SA-mediated defenses are upregulated in coi1 and jai1 mutants and the reduced susceptibility of coi1 to Pst DC3000 is dependent on SA accumulation (58, 121). Additionally, the multiplication of cor- mutant bacteria is significantly enhanced in plants defective in SA accumulation, such as those expressing the salicylate hydroxylase encoded by NahG, and plants defective in SA biosynthesis, such as the Arabidopsis sid2 (eds16-1) mutants (13, 16, 77). Similarly, the Arabidopsis ABA biosynthesis mutant aba3-1 and the guard cell signaling mutant ost1, allow enhanced multiplication of cor- mutant bacteria after surface inoculation (77). Therefore, an attractive hypothesis is that COR exploits the endogenous antagonistic interactions between JA, SA, and ABA hormone signaling pathways in plants to affect stomatal response. Future research should determine whether COR antagonizes both SA and ABA signaling pathways or inhibits only SA or ABA signaling, which in turn affects the other pathway through various network connections. Currently the entire signaling network in the guard cell is not well understood and a systems biology approach will ultimately be necessary to fully understand the extremely dynamic nature that characterizes guard cell signaling (69).
POSSIBLE IMPLICATIONS IN OTHER PATHOSYSTEMS
The discovery of bacterium/PAMP-induced stomatal closure in Arabidopsis as part of the plant innate immune response against bacterial invasion prompts an important question: Are stomata regulated in other pathosystems? To begin to answer this question, Melotto and colleagues (77) investigated the response of tomato stomata to Pst DC3000 and LPS. They found that stomatal movement in tomato was affected similarly to that in Arabidopsis. However, only a few pathovars of P. syringae produce COR. Therefore, if stomatal regulation is widespread in plants, it is likely that other phytopathogenic bacteria employ distinct virulence factors or lifestyles to overcome or circumvent stomatal closure. P. syringae pv. tabaci, which does not produce COR, induced closure of stomata initially, but was able to re-open stomata at later time points, similar to Pst DC3000 (77). These results suggest that there may be other virulence factors used by some bacteria to overcome stomatal defense.
It is possible that the ability to overcome stomatal defense, or lack thereof, may influence pathogenesis strategies and the lifestyle of foliar bacteria. Some foliar bacteria may not produce virulence factors that suppress stomatal closure and therefore may not be able to actively overcome PAMP-induced stomatal closure. These bacteria would probably have to wait for favorable environmental conditions under which stomatal defense is compromised, or rely on wounds, or evolve alternative mechanisms to exploit other natural openings. Interestingly, histochemical detection of a β-glucuronidase (GUS)-expressing strain of the vascular pathogen Xanthomonas campestris pv. campestris (Xcc) demonstrated that Xcc enters Arabidopsis leaves primarily through hydathodes, whereas the closely related X. campestris pv. armoraciae enters leaves primarily through stomata (49), even though Xcc can multiply and cause disease if pressure-infiltrated through stomata (92). While the molecular mechanisms underlying this portal of entry preference are not yet known, it is tempting to speculate that Xcc may lack the ability to overcome stomatal defense and, instead, has evolved the ability to enter leaves through hydathodes.
The inability to efficiently enter leaf tissues through stomata may also be a factor in promoting the evolution of an enhanced ability to live epiphytically on leaf surfaces. On the other hand, Pst DC3000 can actively overcome stomatal defense and appears to be highly invasive and a great endophyte, but is a relatively poor epiphytic colonizer of tomato leaf surfaces (14). In the future, it will be interesting to determine whether there is a correlation between lack of ability to overcome stomatal defense and enhanced epiphytic fitness. Comparison of the complete genome sequences of Pst DC3000 and P. s. pv. syringae (Pss) B728a, a bean pathogen with a well-known epiphytic phase in the field, reveals several intriguing differences that may be related to adaptation to specific lifestyles (32). As mentioned above, UV irradiation and water deficiency are common stresses on the leaf surface and adapted epiphytes presumably have evolved mechanisms to cope with such stresses (6, 72, 101). Indeed, Pss 728a carries several copies of the rulAB operon involved in mutagenic DNA repair and UV resistance, whereas Pst DC3000 carries only one rulB gene (32). Furthermore, the regulatory sequence of the rulB gene in Pst DC3000 seems distinct from that of rulAB operons in Pss B728a, suggesting possibly different regulation of UV-tolerance-related genes in these two strains. Adapted epiphytes respond to water deficiency on the leaf surface often by accumulating certain solutes to increase osmotolerance. Pss B728a seems to contain more genes involved in the production of osmotolerance-associated solutes than Pst DC3000 (32).
In addition to plant-bacterial interactions, stomatal regulation has also been observed in some plant-fungal interactions. For example, the fungal toxin fusicoccin has long been known to promote stomatal opening and to antagonize ABA-induced stomatal closure through activation of a plasma membrane H+ ATPase; it has been widely used as a tool for the study of many aspects of plant biology (92). Oligogalacturonic acid (OGA), an elicitor derived from the degradation of the plant cell wall by fungal cell wall-degrading enzymes, and chitosan, a component of the fungal cell wall, were both shown to affect stomatal movements in tomato (67). Both OGA and chitosan elicited H2O2 production in guard cells and inhibited light-induced opening of closed stomata. Additionally, OGA accelerated midday stomatal closure in tomato and in Commelina communis promoted closure and inhibited opening of stomata. Chitosan and yeast elicitor derived from Saccharomyces cerevisiae were both found to induce stomatal closure and activate Ca2+ influx currents in a manner similar to ABA in Arabidopsis guard cells (59). Oxalic acid is a virulence factor of several phytopathogenic fungi, including Sclerotinia sclerotiorum. Guimaraes & Stotz (40) made the interesting observation that stomatal pores of Vicia faba leaves infected with an oxalate-deficient mutant of S. sclerotiorum were partially closed, whereas wild-type fungus causes stomatal opening. Furthermore, exogenous application of oxalic acid induces stomatal opening. Open stomata seem to be the exit sites of many fungal hyphae from infected leaves. Finally, stomata have also been implicated in the response to resistance (R) gene-mediated recognition of a fungal defense elicitor, Avr9. Recognition of the Avr9 protein from the fungus Cladosporium fulvum by transgenic Nicotiana tabacum plants expressing the Cf-9 R protein from tomato led to the activation of current through outward-rectifying K+ channels and the inactivation of current through inward-rectifying K+ channels (12). This pattern of regulation of cation channels would be predicted to promote stomatal closure. Although in general the biological relevance of the reported responses of stomata to fungal-derived compounds with respect to plant defense or fungal invasion is not yet clear, it is possible that stomate-based defense and counter defense also occur in some plant-fungal interactions.
CONCLUDING REMARKS
Stomata have been historically regarded as passive ports of bacterial entry into plant leaves. The discovery of stomatal defense and counter-defense in Arabidopsis plants is likely to open up several new areas for research. Some of the questions of significant importance that remain to be answered are highlighted in the FUTURE ISSUES box.
Understanding the effects of varying environmental conditions on the effectiveness of stomatal defense would be a particularly important area to enhance our understanding of outbreaks of foliar bacterial disease in the field. Stomata are required to respond to a number of stimuli, including light, humidity, CO2 concentration, microbes, and the circadian clock. How these inputs are prioritized by guard cells and the mechanisms of prioritization are unknown but are interesting and important questions for future study. It is interesting to note that severe outbreaks of bacterial disease in crop plants are often associated with periods of heavy rain or high humidity. These conditions could create wounds and leaf surface wetness which would be favorable for bacterial movement. It is also possible that, under such environmental conditions, stomatal defense is partially compromised because high humidity promotes stomatal opening (86, 94, 103, 118), therefore allowing more bacteria to enter the leaf tissue through stomata to promote infection. To promote infection in the laboratory, researchers commonly use surfactants to reduce surface tension and expose plants to very high humidity for an extended period after surface inoculation. Is stomatal defense partially compromised under such conditions? Also, the surface structures (e.g. cuticle, trichomes, stomata) in different plant species may differ in sensitivity to surfactant, humidity, and/or mechanical pressure. It would be important to determine how stomata in different plant species are affected by surfactants, humidity, and/or pressure spray in early stages of infection.
Stomata open during the day and close at night. In the absence of extensive wounding, foliar bacteria may have to infect leaves primarily during the day. However, COR was previously found to induce opening of dark-closed stomata in broad bean and Italian ryegrass, demonstrating that COR not only blocks closing of stomata, but also can promote opening of closed stomata (79). The ability of COR to promote opening of dark-closed stomata may provide a mechanism for bacterial entry into plant leaves at night when most stomata are closed, which may have significant epidemiological implications.
Because 108 cfu/ml bacteria are necessary to reproducibly and uniformly induce disease in Arabidopsis leaves by the surface inoculation method (17, 54, 123), Melotto et al. (77) used this inoculation dose in most of their experiments. In nature, however, epiphytic populations of bacteria are expected to be highly variable, even on the same leaf, due to tremendous spatial heterogeneity of nutrient availability and/or leaf surface topology (72). The total epiphytic bacterial population on the leaf surface can be very high, up to 108 cfu/g fresh weight (56, 72). Even when the total number of bacteria is low on a leaf, bacterial concentrations at specific sites can be high, especially in aggregates (72), which could contribute to the observed discrete infection sites/lesions on the same leaf in the field (in contrast to the uniform infection of entire leaves in the laboratory). It will be interesting to determine how the sizes and locations of these bacterial communities affect stomatal defense and whether aggregation of bacteria near stomata promotes stomatal movement by exposing stomata to high concentrations of compounds that promote opening or closure. Clearly much is to be learned and hopefully future research will provide answers to some of these important questions and contribute to advances and discoveries in the study of bacterial pathogenesis, guard cell signaling, and microbial ecology.
SUMMARY POINTS
Numerous bacteria can survive and multiply as epiphytes on a plant surface without causing disease. Saprophytes complete their life cycles on the surface of plants; foliar pathogens eventually enter the plant and multiply in the apoplast as pathogenic endophytes. Bacterial entry into plant tissue is a key step for pathogenesis. Unlike fungal pathogens, bacteria cannot directly penetrate the plant epidermis and must rely on wound and natural openings to colonize their host.
Historically, natural surface openings and wounds have been regarded as passive ports of bacterial entry into plant leaves. However, a recent study provides evidence for an active role of stomata in limiting bacterial infection. Stomatal guard cells have the ability to perceive live bacteria and close the stomatal pore within 1 h of exposure to bacterial suspension.
Bacterium-induced stomatal closure appears to be part of the plant innate immune system and can be activated by bacterial PAMPs such as the flagellin peptide flg22. Stomatal closure requires PAMP signaling and SA and ABA homeostasis; it is connected to signaling regulated by ABA in the guard cell.
The foliar pathogenic bacterium P. syringae pv. tomato DC3000 produces the virulence factor COR to suppress stomatal closure and promote bacterial entry through stomata. COR, a molecular mimic of JA-Ile, likely modulates the JA signaling pathway in the plant to antagonize the SA and/or ABA signaling that promotes stomatal closure.
The discovery of stomatal defense and counter-defense in Arabidopsis plants is likely to open up new areas for research. Such research should contribute to advances and discoveries in the study of bacterial pathogenesis, guard cell signaling, and microbial ecology.
FUTURE ISSUES
Disease epidemiology Do the environmental conditions that modulate stomatal closure and opening (e.g., humidity, light, temperature, circadian rhythm) influence the effectiveness of stomatal defense? If so, does failure of stomatal defense under certain environmental conditions (especially rain and high humidity) contribute to disease outbreaks in the field? The sizes of stomata opening vary greatly among plant species. Do the different stomatal sizes impact the effectiveness of stomatal defense?
Pathogen lifestyles Different strains of pathogenic bacteria seem to have different durations of the epiphytic phase before initiating endophytic infection. Is there a correlation between a long epiphytic phase and a lack of anti-stomate virulence factors? In other words, do pathogenic bacteria prefer to colonize the surface of the plant or are they forced to stay there until environmental conditions allow massive invasion?
Stomatal defense across plant species Do all plants have stomatal defense mechanisms? Melotto et al. (77) reported that stomatal movement in response to LPS and Pst DC3000 in tomato are similar to that in Arabidopsis. In the future, it would be important to examine this response in other plants and other bacterial and/or fungal diseases.
Phyllosphere ecology How do the sizes of microbial communities in the phyllosphere influence stomatal defense? How does stomatal defense impact the distribution of microbial communities on the plant surface and inside the plant?
Stomate-targeted virulence factors How many bacterial and fungal virulence factors target stomatal defense? COR is produced by only a few P. syringae pathovars and if stomatal defense is wide-spread among plant species, foliar bacterial pathogens could have evolved other virulence factors or developed alternative lifestyles (e.g., extended epiphytic phase) or infection modes (e.g., through other types of natural openings) to overcome or circumvent stomatal defenses. What are the mechanisms by which other virulence factors inactivate stomatal defense?
ACKNOWLEDGEMENTS
We thank K. Bird for assistance in preparation of this paper, and Wiley-Blackwell Publishing Ltd. for permission to use the content of the reference Underwood et al. (110). Supported by grants from the U.S. Department of Energy and National Institutes of Health.
ABBREVIATIONS/ACRONYMS
- ABA
abscisic acid
- CFA
coronafacic acid
- CMA
coronamic acid
- COI1
coronatine insensitive 1
- COR
coronatine
- hrp
hypersensitive response and pathogenicity
- JA
jasmonic acid
- JAZ
jasmonate ZIM domain protein
- LPS
lipopolysaccharide
- MAMP
microbe-associated molecular pattern
- MeJA
methyl jasmonic acid
- NO
nitric oxide
- OGA
oligogalacturonic acid
- PAMP
pathogen-associated molecular pattern
- Pst
Pseudomonas syringae pv. tomato
- SA
salicylic acid
- TIR1
transport inhibitor response 1
- TTSS
type III secretion system
Footnotes
KEY TERMS/DEFINITIONS
Coronatine: A phytotoxin produced by several pathovars of Pseudomonas syringae consisting of coronafacic acid (CFA), an analog of methyl jasmonic acid (MeJA), and coronamic acid (CMA), which resembles 1-aminocyclopropane-1-carboxylic acid (ACC), a precursor to ethylene.
Endophytes: Organisms that grows inside a living plant.
Epiphytes: Organisms that grows on the surface of a living plant.
Jasmonates: A group of lipid-derived plant hormones involved in plant sexual reproduction and defense against insects and some pathogens; synthesized from linolenic acid by the octadecanoid pathway.
Pathogen-associated molecular pattern (PAMP): Sometimes called microbe-associated molecular pattern (MAMP), referring to host immune response-eliciting molecules common to many classes of microbes, such as bacterial flagellin.
Phyllosphere: Leaf surfaces or total above-ground surfaces of a plant. In this review, phyllosphere also includes the leaf interior.
Plant innate immune system: A complex defense network consisting of two branches; the first recognizes molecules common to many classes of microbes, such as bacterial flagellin, and the second responds to specific pathogen virulence factors.
SCF ubiquitin ligase: A type of E3 ubiquitin ligase consisting of SKP1, CULLIN1, and F-box proteins. E3 ubiquitin ligases catalyze conjugation of ubiquitin to target proteins, which is a key step in initiating protein degradation through the 26S proteasome.
Stomata: Microscopic pores in the epidermis of aerial parts of a plant essential for gas exchange and water transpiration.
Type III secretion system: A specialized protein secretion system used by Gram-negative bacteria to inject effector proteins into plant or animal host cells; composed of approximately 20 proteins located in the bacterial or host cell wall/membrane.
LITERATURE CITED
- 1.Alfano JR, Collmer A. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 2004;42:385–414. doi: 10.1146/annurev.phyto.42.040103.110731. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, et al. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–79. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, et al. MAP kinase signaling in Arabidopsis innate immunity. Nature. 2002;415:977–83. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel FM. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 2005;6:973–79. doi: 10.1038/ni1253. [DOI] [PubMed] [Google Scholar]
- 5.Bayot RG, Ries SM. Role of motility in apple blossom infection by Erwinia amylovora and studies of fire blight control with attractant and repellent compounds. Phytopathology. 1986;76:41–45. [Google Scholar]
- 6.Beattie GA, Lindow SE. The secret life of foliar bacterial pathogens on leaves. Annu. Rev. Phytopathol. 1995;33:145–72. doi: 10.1146/annurev.py.33.090195.001045. [DOI] [PubMed] [Google Scholar]
- 7.Beattie GA, Lindow SE. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology. 1999;89:353–59. doi: 10.1094/PHYTO.1999.89.5.353. [DOI] [PubMed] [Google Scholar]
- 8.Bender CL, Alarcon-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999;63:266–92. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A comprehensive review on major bacterial toxins: lipodepsipeptides, coronatine, phaseolotoxin, and tabtoxin.
- 9.Bent AF, Mackey D. Elicitors, effectors, and R genes: The new paradigm and a life supply of questions. Annu. Rev. Phytopathol. 2007;45:399–436. doi: 10.1146/annurev.phyto.45.062806.094427. [DOI] [PubMed] [Google Scholar]
- 10.Bessire M, Chassot C, Jacquat A-C, Humphry M, Borel S, et al. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 2007;26:2158–68. doi: 10.1038/sj.emboj.7601658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beuchat LR. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002;4:413–23. doi: 10.1016/s1286-4579(02)01555-1. [DOI] [PubMed] [Google Scholar]
- 12.Blatt MR, Grabov A, Brearley J, Hammond-Kosack K, Jones JDG. K+ channels of Cf-9 transgenic tobacco guard cells as targets for Cladosporium fulvum Avr9 elicitor-dependent signal transduction. Plant J. 1999;19:453–62. doi: 10.1046/j.1365-313x.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 13.Block A, Schmelz E, Jones JB, Klee HJ. Coronatine and salicylic acid: the battle between Arabidopsis and Pseudomonas for phytohormone control. Mol. Plant Pathol. 2005;6:79–83. doi: 10.1111/j.1364-3703.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- 14.Boureau T, Routtu J, Roine E, Taira S, Romantschuk M. Localization of hrpA-induced Pseudomonas syringae pv. tomato DC3000 in infected tomato leaves. Mol. Plant Pathol. 2002;3:451–60. doi: 10.1046/j.1364-3703.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 15.Brandl M. Fitness of human pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 2006;44:367–92. doi: 10.1146/annurev.phyto.44.070505.143359. [DOI] [PubMed] [Google Scholar]
- 16.Brooks DM, Bender CL, Kunkel BN. The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol. Plant Pathol. 2005;6:629–39. doi: 10.1111/j.1364-3703.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DM, Hernández-Guzmán G, Kloek AP, Alarcón-Chaidez F, Sreedharan A, et al. Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol. Plant-Microbe Interact. 2004;17:162–74. doi: 10.1094/MPMI.2004.17.2.162. [DOI] [PubMed] [Google Scholar]
- 18.Budde IP, Ullrich MS. Interactions of Pseudomonas syringae pv. glycinea with host and nonhost plants in relation to temperature and phytotoxin synthesis. Mol. Plant-Microbe Interact. 2000;9:951–61. doi: 10.1094/MPMI.2000.13.9.951. [DOI] [PubMed] [Google Scholar]
- 19.Bunster L, Fokkema NJ, Schippers B. Effect of surface-active Pseudomonas spp. on leaf wettability. Appl. Environ. Microbiol. 1989;55:1340–45. doi: 10.1128/aem.55.6.1340-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chassot C, Nawrath C, Métraux JP. Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 2007;49:972–80. doi: 10.1111/j.1365-313X.2006.03017.x. [DOI] [PubMed] [Google Scholar]
- 21.Chinchilla D, Boller T, Robatzek S. Flagellin signalling in plant immunity. Adv. Exp. Med. Biol. 2007;598:358–71. doi: 10.1007/978-0-387-71767-8_25. [DOI] [PubMed] [Google Scholar]
- 22.Chini A, Fonseca S, Fernández G, Adie B, Chico JM, et al. The JAZ family of repressors is the missing link in jasmonate signaling. Nature. 2007;448:666–71. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- The authors identified JAZ repressor proteins, the targets of the SCFCOI1 E3 ubiquitin ligase in Arabidopsis. Furthermore, they show that JAZ3 interacts with COI1 and AtMYC2, a transcription factor involved in jasmonate signaling, illustrating a signaling cascade in which SCFCOI1-mediated degradation of JAZ proteins de-represses the activity of AtMYC2 and possibly other transcription factors.
- 23.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–14. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, et al. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. USA. 2005;102:1791–96. doi: 10.1073/pnas.0409450102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The authors show intriguing evidence that coronatine is required for P. syringae to induce systemic susceptibility to secondary infections.
- 25.Danhorn T, Fuqua C. Biofilm formation by plant associated bacteria. Annu. Rev. Microbiol. 2007;61:401–22. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- 26.DebRoy S, Thilmony R, Kwack YB, Nomura K, He SY. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2004;101:9927–32. doi: 10.1073/pnas.0401601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–50. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 28.Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, et al. COI1 links jasmonate signaling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J. 2002;32:457–66. doi: 10.1046/j.1365-313x.2002.01432.x. [DOI] [PubMed] [Google Scholar]
- 29.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–45. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 30.El-Banoby FE, Rudolph K. Induction of water-soaking in plant leaves by extracellular polysaccharides from phytopathogenic pseudomonads and xanthomonads. Physiol. Plant Pathol. 1979;15:341–49. [Google Scholar]
- 31.Fan LM, Zhao Z, Assmann SM. Guard cells: a dynamic signaling model. Curr. Opin. Plant Biol. 2004;7:537–46. doi: 10.1016/j.pbi.2004.07.009. [DOI] [PubMed] [Google Scholar]
- A salient description of stomatal biology during abiotic stress, focusing on the mechanisms and molecular players involved in the movement of guard cells.
- 32.Feil H, Feil WS, Chain P, Larimer F, DiBartolo G, et al. Comparison of the complete genome sequences of Pseudomonas syringae pv. syringae B728a and pv. tomato DC3000. Proc. Natl. Acad. Sci. USA. 2005;102:11064–69. doi: 10.1073/pnas.0504930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This paper describes an interesting genome-wide comparison between Pseudomonas syringae pv. syringae strain B728a, which has a prominent epiphytic phase, and P. syringae pv. tomato strain DC3000, which has a poor epiphytic phase. Several genes (operons) were identified as candidates for lifestyle adaptations.
- 33.Felix GT, Boller T. Molecular sensing of bacteria in plants. The highly conserved RNA-binding motif RNP-1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J. Biol. Chem. 2003;278:6201–08. doi: 10.1074/jbc.M209880200. [DOI] [PubMed] [Google Scholar]
- 34.Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–59. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardan L, Shafik H, Belouin S, Broch R, Grimont F, Grimont PAD. DNA relatedness among the pathovars of Pseudomonas syringae and description of Pseudomonas tremae sp. nov. and Pseudomonas cannabina sp. nov. [ex Sutic and Dowson 1959] Int. J. Syst. Bacteriol. 1999;49:469–78. doi: 10.1099/00207713-49-2-469. [DOI] [PubMed] [Google Scholar]
- 36.Glazebrook J. Contrasting mechanisms of defense against biotrophic and nectrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–27. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Gómez L, Boller T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell. 2000;5:1003–11. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 38.Grant SR, Fisher EJ, Chang JH, Mole BM, Dangl JL. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 2006;60:425–49. doi: 10.1146/annurev.micro.60.080805.142251. [DOI] [PubMed] [Google Scholar]
- 39.Greulich F, Yoshihara T, Ichihara A. Coronatine, a bacterial phytotoxin, acts as a stereospecific analog of jasmonate type signals in tomato cells and potato tissues. J. Plant Physiol. 1995;147:359–66. [Google Scholar]
- 40.Guimaraes RL, Stotz HU. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 2004;136:3703–11. doi: 10.1104/pp.104.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haefele DM, Lindow SE. Flagellar motility confers epiphytic fitness advantages to Pseudomonas syringae. Appl. Environ. Microbiol. 1987;53:2528–33. doi: 10.1128/aem.53.10.2528-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hancock JG, Huisman OC. Nutrient movement in host-pathogen systems. Annu. Rev. Phytopathol. 1981;19:309–31. [Google Scholar]
- 43.Hattermann DR, Ries SM. Motility of Pseudomonas syringae pv. glycinea and its role in infection. Phytopathology. 1989;79:284–89. [Google Scholar]
- 44.He P, Shan L, Sheen J. Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell. Microbiol. 2007;9:1385–96. doi: 10.1111/j.1462-5822.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 45.He SY, Nomura K, Whittam TS. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim. Biophys. Acta. 2004;1694:181–206. doi: 10.1016/j.bbamcr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Hirano SS, Upper CD. Population biology and epidemiology of Pseudomonas syringae. Annu. Rev. Phytopathol. 1990;28:155–77. [Google Scholar]
- 47.Hirano SS, Upper CD. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae - a pathogen, ice nucleus, and epiphyte. Microb. Mol. Biol. Rev. 2000;64:624–53. doi: 10.1128/mmbr.64.3.624-653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A review integrating studies conducted in the field and laboratory on Pseudomonas syringae epidemiology and biology in the phyllosphere.
- 48.Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA. 2003;100:5549–54. doi: 10.1073/pnas.0733970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hugouvieux V, Barber CE, Daniels MJ. Entry of Xanthomonas campestris pv. campestris into hydathodes of Arabidopsis thaliana leaves: A system for studying early infection events in bacterial pathogenesis. Mol. Plant-Microbe Interact. 1998;11:537–43. doi: 10.1094/MPMI.1998.11.6.537. [DOI] [PubMed] [Google Scholar]
- 50.Hutchison ML, Johnstone K. Evidence for the involvement of the surface active properties of the extracellular toxin tolaasin in the manifestation of brown blotch disease symptoms by Pseudomonas tolaasii on Agaricus bisporus. Physiol. Mol. Plant Path. 1993;42:373–84. [Google Scholar]
- 51.Israelsson M, Seigel RS, Young J, Hashimoto M, Iba K, Schroeder JI. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr. Opin. Plant Biol. 2006;9:654–63. doi: 10.1016/j.pbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Janse JD. Phytobacteriology: Principles and Practice. CABI Publishing; Cambridge, MA: 2005. p. 352. [Google Scholar]
- 53.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–29. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 54.Katagiri F, Thilmony R, He SY. The Arabidopsis thaliana-Pseudomonas syringae interaction. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis Book. American Society of Plant Biologists; Rockville, MD: 2002. doi: 10.1199/tab.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kepinski S, Leyser O. Auxin-induced SCFTIR1-Aux/IAA interaction involves stable modification of the SCFTIR1 complex. Proc. Natl. Acad. Sci. USA. 2004;101:12381–86. doi: 10.1073/pnas.0402868101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinkel LL, Wilson M, Lindow SE. Plant species and plant incubation conditions influence variability in epiphytic bacterial population size. Microb. Ecol. 2000;39:1–11. doi: 10.1007/s002489900182. [DOI] [PubMed] [Google Scholar]
- 57.Klement Z. Hypersensitivity. In: Mount MS, Lacy GH, editors. Phytopathogenic Prokaryotes. Vol. 2. Academic Press; New York: 1982. pp. 149–177. [Google Scholar]
- 58.Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, et al. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J. 2001;26:509–22. doi: 10.1046/j.1365-313x.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- 59.Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, et al. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 2002;130:2152–63. doi: 10.1104/pp.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koda Y, Kikuta Y, Kitahara T, Nishi T, Mori K. Comparisons of various biological activities of stereoisomers of methyl jasmonate. Phytochemistry. 1992;31:1111–14. [Google Scholar]
- 61.Krumm T, Bandemer K, Boland W. Induction of volatile biosynthesis in the lima bean (Phaseolus lunatus) by leucine- and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: Evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signaling pathway. FEBS Lett. 1995;377:523–29. doi: 10.1016/0014-5793(95)01398-9. [DOI] [PubMed] [Google Scholar]
- 62.Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant. Biol. 2002;5:325–31. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- This review highlights the molecular interplay between three plant hormones, salicylic acid, jasmonic acid, and ethylene during plant defense against diverse pathogens.
- 63.Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 2004;16:3496–507. doi: 10.1105/tpc.104.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lake JA, Quick WP, Beerling DJ, Woodward FI. Signals from mature to new leaves. Nature. 2001;411:154. doi: 10.1038/35075660. [DOI] [PubMed] [Google Scholar]
- 65.Laurie-Berry N, Joardar V, Street IH, Kunkel BN. The Arabidopsis thaliana JASMONATE INSENSITIVE 1 gene is required for suppression of salicylic acid-dependent defenses during infection by Pseudomonas syringae. Mol. Plant-Microbe Interact. 2006;19:789–800. doi: 10.1094/MPMI-19-0789. [DOI] [PubMed] [Google Scholar]
- 66.Lee J-S. The mechanism of stomatal closing by salicylic acid in Commelina communis L. J. Plant Biol. 1998;41:97–102. [Google Scholar]
- 67.Lee S, Choi H, Suh S, Doo I-S, Oh K-Y, et al. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina comunis. Plant Physiol. 1999;121:147–52. doi: 10.1104/pp.121.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, et al. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two loci. Plant J. 1996;10:655–61. doi: 10.1046/j.1365-313x.1996.10040655.x. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Brader G, Karioli T, Palva ET. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006;46:477–91. doi: 10.1111/j.1365-313X.2006.02712.x. [DOI] [PubMed] [Google Scholar]
- 70.Lindgren PB. The role of hrp genes during plant-bacterial interactions. Annu. Rev. Phytopathol. 1997;35:129–52. doi: 10.1146/annurev.phyto.35.1.129. [DOI] [PubMed] [Google Scholar]
- 71.Lindgren PB, Peet RC, Panopoulos NJ. Gene cluster of Pseudomonas syringae pv. ‘phaseolicola’ controls pathogenicity of bean plants and hypersensitivity on nonhost plants. J. Bacteriol. 1986;168:512–22. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 2003;69:1875–83. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An insightful review highlighting unique and intriguing features of microbial biology in the phyllosphere and the need to probe real micro-environmental conditions to which colonizers of the leaf surface are exposed.
- 73.Lorenzo O, Solano R. Molecular players regulating the jasmonate signalling network. Curr. Opin. Plant Biol. 2005;8:532–40. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Ma SW, Morris VL, Cuppels DA. Characterization of a DNA region required for production of the phytotoxin coronatine by Pseudomonas syringae pv. tomato. Mol. Plant-Microbe Interact. 1991;4:69–77. [Google Scholar]
- 75.Manthe B, Schulz M, Schnable H. Effects of salicylic acid on growth and stomatal movements on Vicia faba L.: evidence for salicylic acid metabolism. J. Chem. Ecol. 1992;18:1525–39. doi: 10.1007/BF00993226. [DOI] [PubMed] [Google Scholar]
- 76.Marré E. Fusicoccin: A tool in plant physiology. Annu. Rev. Plant Physiol. 1979;30:273–288. [Google Scholar]
- 77.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–80. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- The authors provide evidence that stomatal guard cells respond to live bacteria and PAMPs and that coronatine prevents stomata from bacterium/PAMP-induced closure.
- 78.Mendgen K, Hahn M, Deising H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996;34:367–86. doi: 10.1146/annurev.phyto.34.1.367. [DOI] [PubMed] [Google Scholar]
- 79.Mino Y, Matsuchita Y, Sakai R. Effect of coronatine on stomatal opening in leaves of broadbean and Italian ryegrass. Ann. Phytopath. Soc. Japan. 1987;53:53–55. [Google Scholar]
- 80.Mittal SM, Davis KR. Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Mol. Plant-Microbe Interact. 1995;8:165–71. doi: 10.1094/mpmi-8-0165. [DOI] [PubMed] [Google Scholar]
- 81.Monier JM, Lindow SE. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc. Natl. Acad. Sci. USA. 2003;100:15977–82. doi: 10.1073/pnas.2436560100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monier JM, Lindow SE. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl. Environ. Microbiol. 2004;70:346–55. doi: 10.1128/AEM.70.1.346-355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mori IC, Pinontoan R, Kawano T, Muto S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 2001;42:1383–88. doi: 10.1093/pcp/pce176. [DOI] [PubMed] [Google Scholar]
- 84.Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;12:3089–99. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naimi JS, Wicklund JH, Olsen SJ, Krause G, Wells JG, et al. Concurrent outbreaks of Shigella sonnei and enterotoxigenic Escherichia coli infections associated with parsley: Implications for surveillance and control of foodborne illness. J. Food Prot. 2003;66:535–41. doi: 10.4315/0362-028x-66.4.535. [DOI] [PubMed] [Google Scholar]
- 86.Nejad AR, van Meeteren U. The role of abscisic acid in disturbed stomatal response characteristics of Tradescantia virginiana during growth at high relative air humidity. J. Exp. Bot. 2007;58:627–36. doi: 10.1093/jxb/erl234. [DOI] [PubMed] [Google Scholar]
- 87.Noble PS. Introduction to Biophysical Plant Physiology. Freeman; San Francisco: 1974. p. 488. [Google Scholar]
- 88.Nomura K, Melotto M, He SY. Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr. Opin. Plant Biol. 2005;8:361–68. doi: 10.1016/j.pbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Palmer DA, Bender CL. Ultrastructure of tomato leaf tissue treated with the pseudomonad phytotoxin coronatine and comparison with methyl jasmonate. Mol. Plant-Microbe Interact. 1995;8:683–92. [Google Scholar]
- 90.Panopoulos HI, Schroth MN. Role of flagellar motility in the invasion of bean leaves by Pseudomonas phaseolicola. Phytopathology. 1974;64:1389–97. [Google Scholar]
- 91.Park S, Worobo RW, Durst RA. Escherichia coli O157:H7 as an emerging foodborne pathogen: a literature review. Crit. Rev. Biotechnol. 2001;21:27–48. doi: 10.1080/20013891081674. [DOI] [PubMed] [Google Scholar]
- 92.Parker JE, Barber CE, Fan MJ, Daniels MJ. Interaction of Xanthomonas campestris with Arabidopsis thaliana: characterization of a gene from X. c. pv. raphani that confers avirulence to most A. thaliana accessions. Mol. Plant-Microbe Interact. 1993;6:216–24. doi: 10.1094/mpmi-6-216. [DOI] [PubMed] [Google Scholar]
- 93.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/s0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 94.Roelfsema MR, Hedrich R. In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol. 2005;167:665–91. doi: 10.1111/j.1469-8137.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 95.Romantschuk M. Attachment of plant pathogenic bacteria to plant surfaces. Annu. Rev. Phytopathol. 1992;30:225–43. doi: 10.1146/annurev.py.30.090192.001301. [DOI] [PubMed] [Google Scholar]
- 96.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:627–58. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 97.Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature. 2001;410:327–30. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- 98.Spoel SP, Koornneef A, Claessens SMC, Korzelius JP, van Pelt JA, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–70. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–27. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol. 2004;134:1536–45. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sundin GW. Ultraviolet radiation on leaves: its influence on microbial communities and their adaptations. In: Lindow SE, Hecht-Poinar EI, Elliott VJ, editors. Microbiology of the Phyllosphere. APS Press; St Paul, MN: 2002. pp. 27–41. [Google Scholar]
- 102.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 103.Talbott LD, Rahveh E, Zeiger E. Relative humidity is a key factor in the acclimation of the stomatal response to CO2. J. Exp. Bot. 2003;54:2141–47. doi: 10.1093/jxb/erg215. [DOI] [PubMed] [Google Scholar]
- 104.Tamogami S, Kodama O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry. 2000;54:689–94. doi: 10.1016/s0031-9422(00)00190-4. [DOI] [PubMed] [Google Scholar]
- 105.Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–45. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 106.Tang D, Simonich MT, Innes RW. Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol. 2007;144:1093–103. doi: 10.1104/pp.106.094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thilmony R, Underwood W, He SY. Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006;46:34–53. doi: 10.1111/j.1365-313X.2006.02725.x. [DOI] [PubMed] [Google Scholar]
- 108.Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, et al. JAZ1 is a target of the SCFCOI1 ubiquitin ligase during jasmonate signaling. Nature. 2007;448:661–65. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- The authors identified JAZ repressor proteins, the targets of the SCFCOI1 E3 ubiquitin ligase in Arabidopsis. Furthermore, they show that JAZ proteins physically interact with the COI1 protein in the presence of jasmonoyl isoleucine (JA-Ile) in yeast cells (i.e., in the absence of other plant proteins), strongly implicating COI1 or JAZ protein as a receptor for JA-Ile, which is structurally similar to coronatine.
- 109.Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:S153–S164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Underwood W, Melotto M, He SY. Role of stomata in bacterial invasion. Cell. Microbiol. 2007;9:1621–29. doi: 10.1111/j.1462-5822.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- 111.Uppalapati SR, Ayoubi P, Weng H, Palmer DA, Mitchell RE, et al. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 2005;42:201–17. doi: 10.1111/j.1365-313X.2005.02366.x. [DOI] [PubMed] [Google Scholar]
- 112.Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, et al. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant-Microbe Interact. 2007;20:955–65. doi: 10.1094/MPMI-20-8-0955. [DOI] [PubMed] [Google Scholar]
- 113.Weiler E, Kutchan WTM, Gorba T, Brodschelm W, Neisel U, Bublitz F. The Pseudomonas phytotoxin coronatine mimics octadecanoid signaling molecules of higher plants. FEBS Lett. 1994;345:9–13. doi: 10.1016/0014-5793(94)00411-0. [DOI] [PubMed] [Google Scholar]
- 114.Whalen MC, Innes RW, Bent AF, Staskawicz BJ. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial gene determining avirulence on both Arabidopsis and soybean. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–65. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 116.Woodward FI. Stomatal numbers are sensitive to increases in CO2 concentration from the pre-industrial levels. Nature. 1987;327:617–18. [Google Scholar]
- 117.Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–94. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 118.Xie X, Wang Y, Williamson L, Holroyd GH, Tagliavia C, et al. The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr. Biol. 2006;16:882–87. doi: 10.1016/j.cub.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 119.Xu LH, Liu FQ, Lechner E, Genschik P, Crosby WL, et al. The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell. 2002;14:1919–35. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, et al. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA. 2004;101:15811–816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 2003;36:485–99. doi: 10.1046/j.1365-313x.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 122.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–60. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 123.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–67. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]