Abstract
We have previously shown that beta-amyloid (Aβ) oligomers induced dynamin 1 and tau cleavage in cultured hippocampal neurons. As a result of this cleavage, dynamin 1 levels decreased and a toxic tau fragment was generated. Aβ-induced cleavage of these proteins was calpain-mediated and impacted both synaptic vesicle recycling and the integrity of neuronal processes (Kelly et al., 2005; Park and Ferreira, 2005; Kelly and Ferreira, 2006, Kelly and Ferreira ,2007). Building on previous reports, these results identified calpain as a potential target for therapeutic intervention in Alzheimer’s disease. In the present study, we tested the ability of A-705253, a novel water-soluble calpain inhibitor with oral availability and enhanced metabolic stability, to prevent Aβ-induced dynamin 1 and tau cleavage in cultured hippocampal neurons. Quantitative Western blot analysis indicated that the incubation of these cells with A-705253 prior to the addition of oligomeric Aβ reduced both dynamin 1 and tau cleavage in a dose-dependent manner. In addition, our results showed that this calpain inhibitor significantly ameliorated the cleavage of these proteins when added simultaneously with oligomeric Aβ. Furthermore, our data indicated that the use of this calpain inhibitor could have some beneficial effects even when added after the cleavage of these proteins have been triggered by Aβ. Collectively, these results suggest that, indeed, specific calpain inhibitors could play an important role in the treatment of Alzheimer’s disease.
Keywords: Alzheimer’s disease, proteolysis, tau, dynamin 1, calpain inhibitors
Two types of pathological structures, senile plaques and dystrophic neurites containing neurofibrillary tangles, are easily detectable in brain areas associated with learning and memory in Alzheimer’s disease (AD) patients. These lesions are the result of the abnormal deposition of beta-amyloid (Aβ) and hyperphosphorylated microtubule-associated protein tau, respectively (Glenner and Wong, 1984; Kosik et al., 1986; Wood et al., 1986; Kondo et al., 1988; Yankner and Mesulam, 1991; Selkoe, 1994; Parihar and Hemnani, 2004; Stern et al., 2004). The coexistence of senile plaques and neurofibrillary tangles in the same brain areas prompted the search for a potential mechanistic link between them. Most of those studies, carried out using cultured neurons as model systems, supported the hypothesis that aggregated Aβ triggered tau phosphorylation followed by the formation of dystrophic neurites in central neurons (Novak et al., 1993; Takashima et al., 1993; Busciglio et al., 1995; Ferreira et al., 1997; Alvarez et al., 1999; Ekinci et al., 1999; Chung et al., 2001). More recently, we have shown that Aβ induced not only tau phosphorylation but also tau cleavage leading to the generation of a 17 kDa toxic fragment in cultured hippocampal neurons (Park and Ferreira, 2005). Furthermore, our data strongly suggested that this tau cleavage was the result of enhanced calpain activity (Park and Ferreira, 2005). The activation of this Ca2+-dependent protease has also been implicated in early stages of AD when functional defects lack associated structural changes. The molecular mechanisms by which calpain could mediate synapse dysfunction underlying early cognitive deficits have not been completely elucidated. However, data obtained recently indicated that Aβ-induced calpain activation resulted in dynamin 1 cleavage (Kelly et al., 2005). Dynamin 1 is a neuron-specific mechanochemical GTPase responsible for vesicle scission from the synaptic terminal membrane (Damke et al., 1994; Clark et al., 1997). As a consequence of Aβ-induced dynamin 1 cleavage, synaptic vesicle recycling and, hence, synaptic transmission were altered in hippocampal neurons (Kelly et al., 2005; Kelly and Ferreira, 2006, Kelly and Ferreira, 2007). The activation of calpain observed in hippocampal neurons cultured in the presence of oligomeric Aβ is in agreement with previous reports showing that AD patients had increased levels of active calpain in the central nervous system (Saito et al., 1993; Tsuji et al., 1998; Veeranna et al., 2004).
Together, these studies identified calpain as a potential target for therapeutic intervention both at early and late stages of the disease. Moreover, initial studies using calpain inhibitors in a mouse model of AD showed an encouraging recovery of cognitive function in transgenic mice that were treated with a calpain inhibitor at an early age (Battaglia et al., 2003). Unfortunately, the potential use of known calpain inhibitors as therapeutic tools in AD is limited due to their low cellular penetration, poor selectivity and kinetics. Recently A-705253, a novel calpain inhibitor with improved pharmacokinetics, has been characterized (Lubisch et al., 2003). This benzoylalanine-derived ketoamide is capable of inhibiting calpain in nanomolar concentrations, has improved oral bioavailability, water solubility, and metabolic stability (Lubisch et al., 2003).
In the present study, we performed a series of experiments to determine whether this novel calpain inhibitor could prevent dynamin 1 and tau cleavage induced by oligomeric Aβ in hippocampal neurons. Our results showed that A-705253 was highly effective in preventing the cleavage of these proteins when added before the Aβ treatment. In addition, the data presented herein indicated that this calpain inhibitor was able to block dynamin 1 and tau cleavage when added simultaneously with Aβ. Furthermore, the data presented suggested that the use of this calpain inhibitor could have some beneficial effects even when added after the Aβ treatment had begun.
EXPERIMENTAL PROCEDURES
Preparation of hippocampal cultures
Embryonic day 18 rat embryos were used to prepare primary hippocampal cultures as previously described (Goslin and Banker, 1991). Briefly, hippocampi were dissected and freed of meninges. The cells were dissociated by trypsinization followed by trituration with a fire-polished Pasteur pipette, and plated at high density (500,000 cells/60-mm dish) in Minimum Essential Medium (MEM) with 10% horse serum. After 2 hrs, the medium was changed to glia-conditioned MEM containing N2 supplements plus ovalbumin (0.1%) and sodium pyruvate (0.1 mM) (N2 medium) (Bottenstein and Sato, 1979). These cultures contain approximately 95% pyramidal neurons and 5% glial cells.
Aβ aggregation and treatment
Synthetic Aβ1–40 (American Peptide, Sunnyvale, CA) was dissolved in N2 medium (0.5 mg/ml) and incubated for 3 days at 37° C to preaggregate the peptide (Rapoport et al., 2002). Preaggregated Aβ was centrifuged to separate the soluble oligomeric (supernatant) from the insoluble □ibrillary (pellet) forms of the peptide as previously described (Kelly and Ferreira, 2006). The oligomeric fraction was added directly to cultured hippocampal neurons at a final concentration of 10 µM, calculated using the initial concentration of the monomeric form of the peptide. Hippocampal neurons were grown in the presence of Aβ for up to 24 hours.
Calpain inhibitor
A stock solution (1mM) of the A-705253 calpain inhibitor (Abbott Laboratories, Abbott Park, IL) was prepared in MilliQ water plus 0.9 % sodium chloride (pH 5.5) and used in 3 experimental paradigms. In the first set of experiments, A-705253 was added to the medium of 3 weeks in culture hippocampal neurons 1 hr prior to, and for the duration of, the oligomeric Aβ treatment (24 hrs) at final concentrations ranging from 1 nM to 10 µM. In the second set of experiments, the calpain inhibitor (final concentration 1µM) and oligomeric Aβ were added simultaneously and the neurons were cultured in the presence of both reagents for 24 hrs. In the last set of experiments, hippocampal neurons were incubated in the presence of oligomeric Aβ for up to 8 hrs before the addition of A-705253 (1 µM). Whole cell extracts were prepared 24 hrs after the addition of Aβ and used for immunoblotting.
Electrophoresis and Immunoblotting
To prepare whole cells extracts, hippocampal neurons kept in culture for 3 weeks were rinsed in PBS, scraped into Laemmli buffer, and homogenized in a boiling water bath for 10 min. Samples were run on 7.5% and 12% sodium-dodecyl sulfate (SDS)-polyacrylamide gels and transferred to Immobilon membranes (Millipore, Billerica, MA) (Laemmli, 1970). Transfer of protein to Immobilon membranes and immunodetection were performed according to Towbin et al. (Towbin et al., 1979) as modified (Ferreira et al., 1989). Immunodetection was performed using the following antibodies: anti-α-tubulin (1:200,000; clone DM1A; Sigma, St. Louis, MO), anti-dynamin 1 (1:5,000; Affinity BioReagents, Golden, CO), anti-tau (clone tau 5, 1:1,000, Invitrogen Corporation, Carlsbad, CA), and anti-spectrin (1:1,000; Chemicon, Temecula, CA). Secondary antibodies conjugated to horseradish peroxidase (Promega, Madison, WI) followed by enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ) were used for the detection of proteins. Immunoreactive bands were imaged using a ChemiDoc XRS system (Bio-Rad, Hercules, CA). Densitometry of these bands was performed using Quantity One software (Bio-Rad) and the values were normalized using tubulin as an internal loading control. At least 3 independent experiments for each experimental condition were used for quantitative Western blot analysis.
Determination of calpain activity
Calpain activity was determined by assessing spectrin cleavage as previously described (Czogalla and Sikorski, 2005). Briefly, whole cell extracts were prepared from hippocampal neurons cultured in the presence or absence of oligomeric Aβ and/or A-705253 and used for quantitative Western blot analysis with a spectrin antibody as described above. Since calpain cleavage produces characteristic spectrin fragments of 145- and 150-kDa (Siman et al., 1984), we determined the 150/240 kDa ratio from treated neurons and compared it to those obtained from untreated controls.
Statistical analysis
The data presented in this study were obtained from at least 3 independent experiments for each experimental condition. Values obtained from untreated controls were considered 100%. Data were expressed as means ± S.E.M. and their statistical significance was analyzed by Student’s t test or one-way ANOVA followed by Fisher’s LSD post-hoc test.
RESULTS
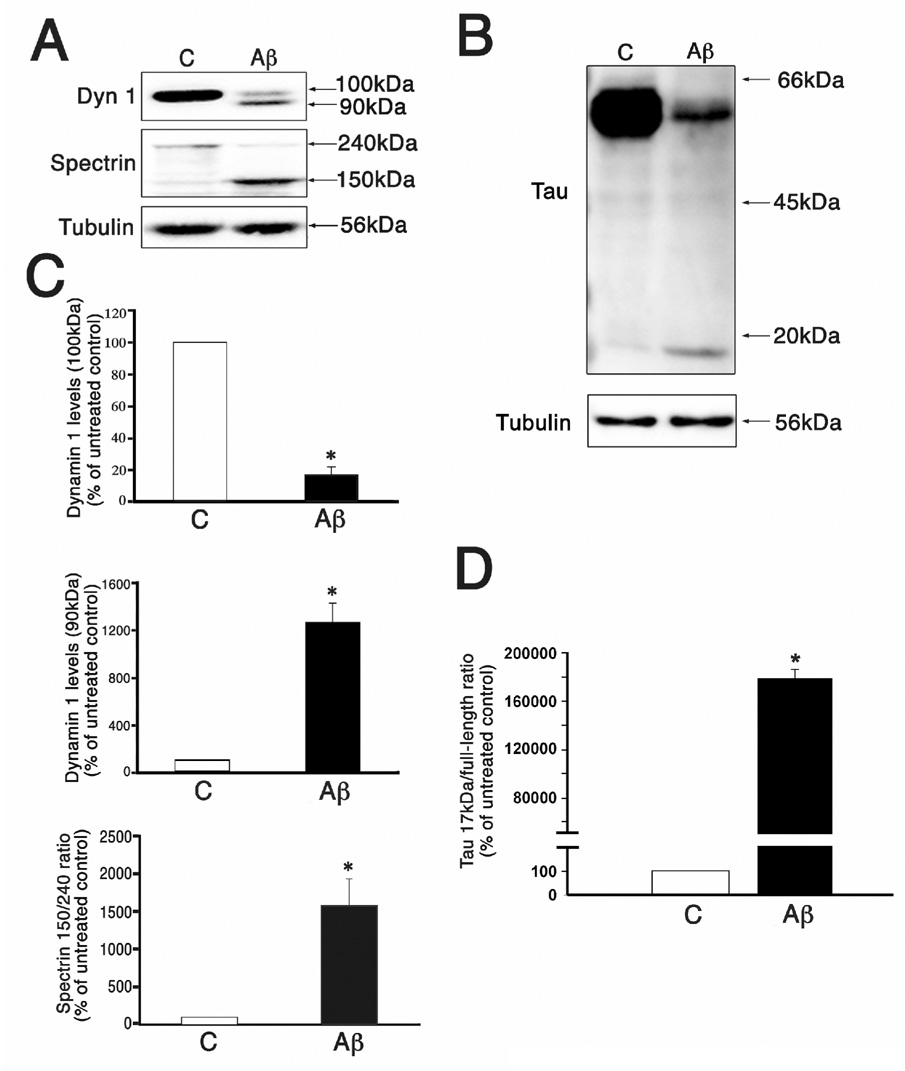
We have previously shown that Aβ induced calpain-mediated cleavage of dynamin 1 and tau in cultured hippocampal neurons (Kelly et al., 2005; Park and Ferreira, 2005; Kelly and Ferreira, 2006, Kelly and Ferreira, 2007). To determine whether this cleavage could be prevented using a novel calpain inhibitor, we first assessed the levels of these cleaved proteins in the presence of oligomeric Aβ. For these experiments, 3 weeks in culture hippocampal neurons were incubated with oligomeric Aβ (at a final concentration of 10 µM) for 24 hrs. Western blot analysis of whole cell extracts obtained from Aβ-treated neurons showed the decrease of full-length dynamin 1 (~100 kDa) and tau (~66 kDa) and the appearance of ~90 kDa and ~17 kDa dynamin 1 and tau immunoreactive bands, respectively (Fig.1A & B). Densitometry of these immunoreactive bands was performed next. In the case of dynamin 1, each band was quantified individually and no ratio was established between them. We have chosen this method of quantification because it has been previously shown that the Aβ-induced decrease in full-length dynamin 1 levels was the result of both calpain-mediated cleavage and the down-regulation of its expression (Kelly et al., 2005). Since calpain inhibitors could only block one of these mechanisms, only a partial recovery of full-length dynamin levels could be expected in A-705253-treated neurons. Establishing a ratio between the two dynamin 1 bands could then mask the effects of a calpain inhibitor. The quantitative analysis of immunoreactive bands showed a significant decrease in full-length dynamin 1 (~100 kDa) and a significant increase in the ~90 kDa dynamin 1 band in Aβ-treated neurons when compared to untreated controls (Fig. 1C). Similar results were obtained when tau immunoreactive bands were analyzed. Thus, a significant decrease in the full-length tau immunoreactive bands (~66 kDa) and a concomitant increase in the 17 kDa tau fragment were easily detectable in Aβ-treated neurons as compared to untreated controls (Fig. 1B & D). These changes resulted in a significant increase in the tau 17 kDa/full-length ratio in Aβ-treated neurons when compared to untreated controls (Fig. 1D). We next determined the extent of calpain activation under these experimental conditions. For these experiments, we assessed calpain activation by determining spectrin cleavage (see also Kelly et al., 2005; Park and Ferreira, 2005; Kelly and Ferreira, 2006). Spectrin degradation is highly sensitive to calpain activation and considered an excellent marker for this protease activity (Czogalla and Sikorski, 2005). Western blot analysis of whole cells extracts reacted with a specific spectrin antibody showed a significant decrease in full-length spectrin (240 kDa) and a concomitant increase in the 150 kDa degradation fragment (Fig. 1C, see also Kelly et al., 2005; Park and Ferreira, 2005; Kelly and Ferreira, 2006). Quantitative analysis of immunoreactive bands showed a significant increase in the spectrin 150/240 kDa ratio in hippocampal neurons treated with oligomeric Aβ when compared to untreated controls (Fig. 1C).
Figure 1. Aβ induced dynamin 1 and tau cleavage in cultured hippocampal neurons.
(A & B) Western blot analysis of whole cell extracts prepared from 3 weeks in culture hippocampal neurons incubated in the absence © or presence of oligomeric Aβ (Aβ) and reacted with dynamin 1 (dyn 1)(A), spectrin (A) and tau (B) antibodies. Strong immunoreactive cleaved dynamin 1 (~90 kDa)(A) and tau (17 kDa)(B) bands were detected in Aβ-treated neurons. Tubulin was used as a loading control. (C & D) Graphs showing the levels of full-length (~100 kDa) and cleaved (~90 kDa) dynamin 1 band, the spectrin 150/240 kDa ratio, and the tau 17 kDa/full-length ratio in control and Aβ-treated hippocampal neurons. Values are expressed as percentage of untreated controls, considering the values obtained in these neurons as 100%. Each number represents the mean ± SEM from 3 different experiments. *Differs from control, p < 0.01.
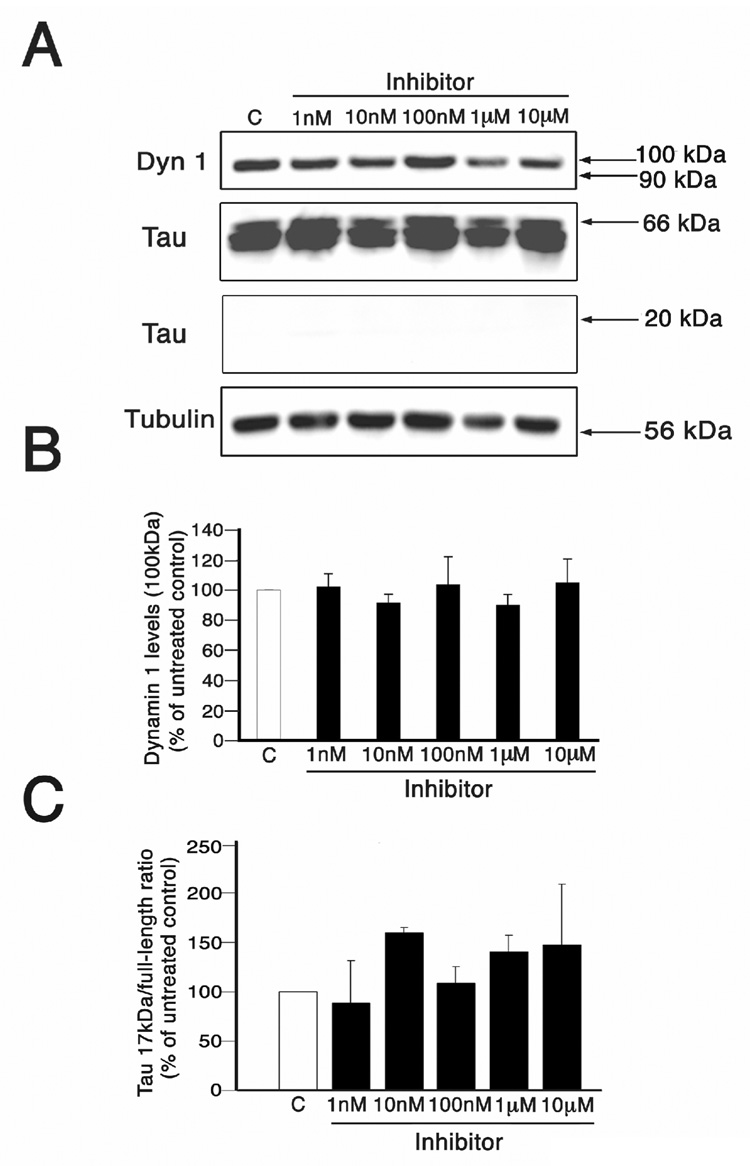
Next, we ruled out potential toxic effects of the calpain inhibitor A-705253 that could lead to cleavage of dynamin 1 and/or tau when added to 3 weeks in cultured hippocampal neurons. For these experiments, Western blot analysis of whole cell extracts prepared from hippocampal neurons cultured in the presence of A-705253 at final concentrations ranging from 1 nM to 10 µM were performed using dynamin 1 and tau antibodies. Neither the dynamin 1-cleaved band nor the 17 kDa tau fragment were detected in hippocampal neurons treated with this inhibitor even at the highest dose used in this study (Fig. 2A–C). In addition, no changes in calpain activity (measured by spectrin cleavage) or morphological alterations were detected in A-705253-treated neurons when compared to untreated controls (data not shown).
Figure 2. A-705253 did not induce changes in dynamin 1 and tau levels in cultured hippocampal neurons.
(A) Western blot analysis of whole cell extracts prepared from 3 weeks in culture hippocampal neurons cultured in the absence (c) or presence of increasing concentrations of A-705253 (inhibitor) and reacted with dynamin 1 (dyn 1) and tau antibodies. No cleaved dynamin 1 or 17 kDa tau bands were detected in treated neurons. Tubulin was used as a loading control. (B & C) Graphs showing the levels of full-length dynamin 1(~100 kDa) and tau (17 kDa/full-length ratio) in control and A-705253-treated hippocampal neurons. Values are expressed as percentage of untreated controls, considering the values obtained in these neurons as 100%. Each number represents the mean ± SEM from 3 different experiments.
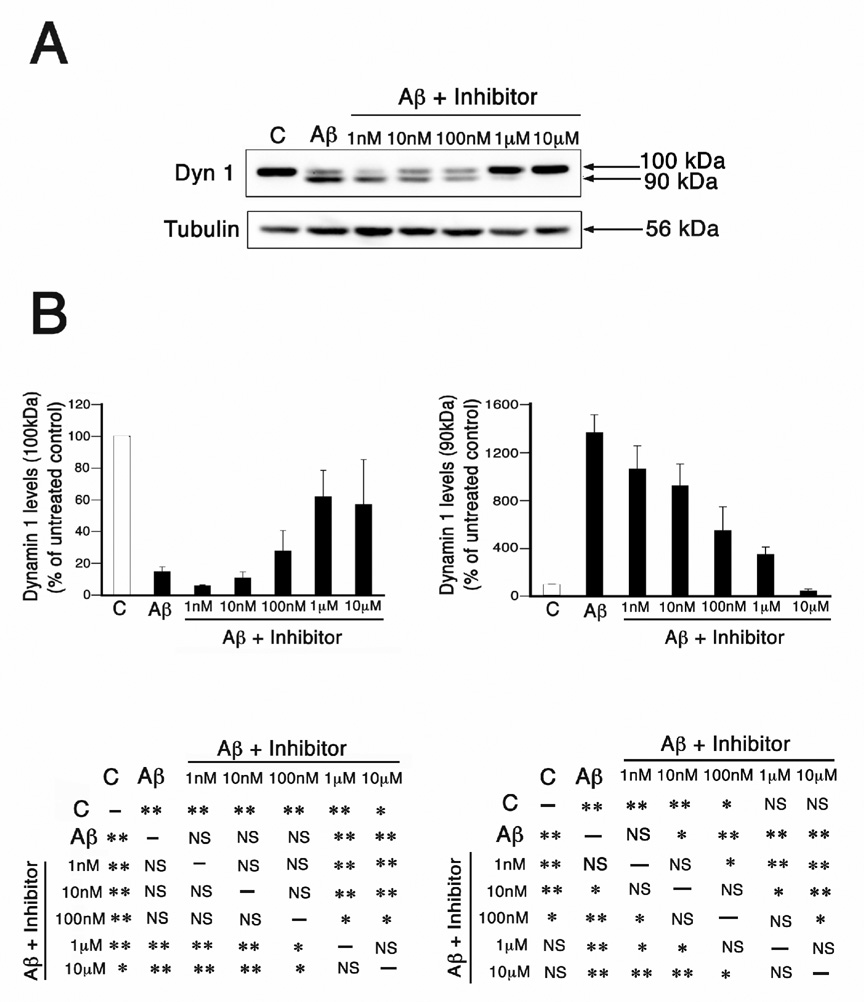
Preincubation with the calpain inhibitor A-705253 prevented dynamin 1 and tau cleavage in Aβ-treated hippocampal neurons
The results described above established the baseline levels of dynamin 1 and tau cleavage, as well as of calpain activity, in Aβ- and A-705253-treated cultured hippocampal neurons. We next assessed the ability of A-705253 to prevent the cleavage of these proteins in Aβ-treated hippocampal neurons using 3 different experimental paradigms. In the first set of experiments, we preincubated 3 weeks in culture hippocampal neurons with increasing concentrations of the calpain inhibitor for 1 hr before the addition of the oligomeric peptide (10 µM). Twenty-four hours later, whole cell extracts were prepared and used for quantitative Western blot analysis. As described above, a significant decrease in the full-length dynamin 1 immunoreactive band and a concomitant increase in the appearance of the cleaved dynamin 1 band were easily detected in Aβ-treated neurons when compared to untreated controls (Fig. 3A, see also Fig. 1A and Kelly et al., 2005). Western blot analysis showed a partial recovery of full-length dynamin 1 and a progressive decrease in the cleaved dynamin 1 band as the concentration of the inhibitor increased (Fig. 3A). Quantitative analysis of immunoreactive bands showed a significant decrease in cleaved dynamin 1 levels in neurons incubated with as little of 10 nM of A-705253 as compared to Aβ-treated controls. Moreover, no significant differences in the levels of cleaved dynamin 1 were detected in hippocampal neurons pretreated with 1 µM calpain inhibitor when compared to untreated controls (Fig. 3B). These results indicated that, when used at this concentration, A-705253 completely reverted the pathological cleavage of dynamin 1 observed in the presence of Aβ.
Figure 3. Preincubation with A-705253 prevented Aβ-induced dynamin 1 cleavage in a dose-dependent manner in cultured hippocampal neurons.
(A) Western blot analysis of whole cell extracts prepared from 3 weeks in culture hippocampal neurons incubated in the absence (c), the presence of oligomeric Aβ (Aβ)or from neurons pretreated with increasing concentrations of A-705253 (inhibitor) for 1 hr before the addition of oligomeric Aβ. The membranes were reacted with dynamin 1 (dyn 1) and tubulin antibodies. Tubulin was used as a loading control. Note the decrease in the immunoreactive band corresponding to cleaved dynamin 1 as the dose of A-705253 increased. (B) Graphs showing the levels of full-length (~100 kDa) and cleaved (~90 kDa) dynamin 1 bands in control, Aβ-treated, and A-705253 plus Aβ-treated hippocampal neurons. Values are expressed as percentage of untreated controls, considering the values obtained in these neurons as 100%. Each number represents the mean ± SEM from 3 different experiments. *Differs from control, p < 0.05. **Differs from control, p < 0.01.
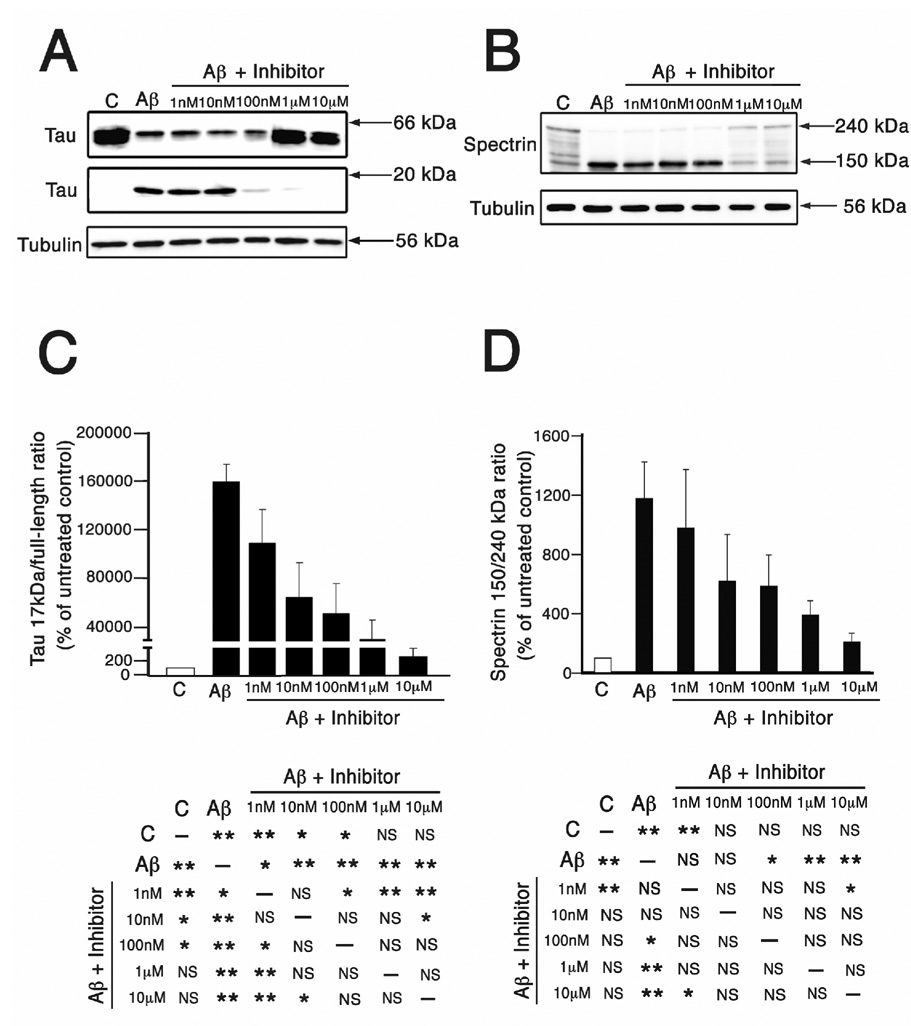
We next determined whether the pretreatment with A-705253 was also effective in preventing the Aβ-induced generation of the 17 kDa tau fragment. Western blot analysis of whole cell extracts prepared under the experimental conditions described above showed a progressive decrease in the 17 kDa tau band and a concomitant increase in full-length tau as the dose of A-705253 increased (Fig. 4A). Densitometry of immunoreactive bands was used to establish the tau 17 kDa/full-length ratio. Statistical analysis of these ratios showed that preincubation with 1 nM of A-705253 was sufficient to significantly decrease tau cleavage when compared to hippocampal neurons treated with Aβ alone (Fig. 4C). As in the case of dynamin 1, 1 µM of A-705253 reversed Aβ-induced tau cleavage. Thus, no statistically significant differences in the tau 17 kDa/full-length ratio were detected when hippocampal neurons preincubated with 1 µM of A-705253 were compared to control neurons cultured in the absence of oligomeric Aβ (Fig. 4C).
Figure 4. Preincubation with A-705253 prevented Aβ-induced tau cleavage and calpain activation in a dose-dependent manner in cultured hippocampal neurons.
(A & B) Western blot analysis of whole cell extracts prepared from 3 weeks in culture hippocampal neurons incubated in the absence (c), the presence of oligomeric Aβ (Aβ), or neurons pretreated with increasing concentrations of A-705253 (inhibitor) for 1 hr before the addition of oligomeric Aβ. The membranes were reacted with tau (A) and spectrin (B) antibodies. Tubulin was used as a loading control. Note the decrease in the immunoreactive bands corresponding to cleaved 17 kDa tau fragment (A) and cleaved 150 kDa spectrin (B) as the dose of A-705253 increased. (C & D) Graphs showing the tau 17 kDa/full-length and spectrin 150/240 kDa ratios in control, Aβ-treated, and A-705253 plus Aβ-treated hippocampal neurons. Values are expressed as percentage of untreated controls, considering the values obtained in these neurons as 100%. Each number represents the mean ± SEM from 3 different experiments. *Differs from control, p < 0.05. **Differs from control, p < 0.01.
A similar pattern was detected when calpain activity was determined by means of spectrin cleavage. Densitometry of immunoreactive bands showed a significant decrease in the spectrin 150/240 kDa ratio in neurons pretreated with at least 100 nM of A-705253 for 1 hr before the addition of the oligomeric peptide when compared to neurons treated with Aβ alone (Fig. 4B). In addition, no statistically significant differences in the spectrin ratio were detected when cells preincubated with A-705253 at final concentrations of 10 nM or higher were compared to untreated controls (Fig. 4D).
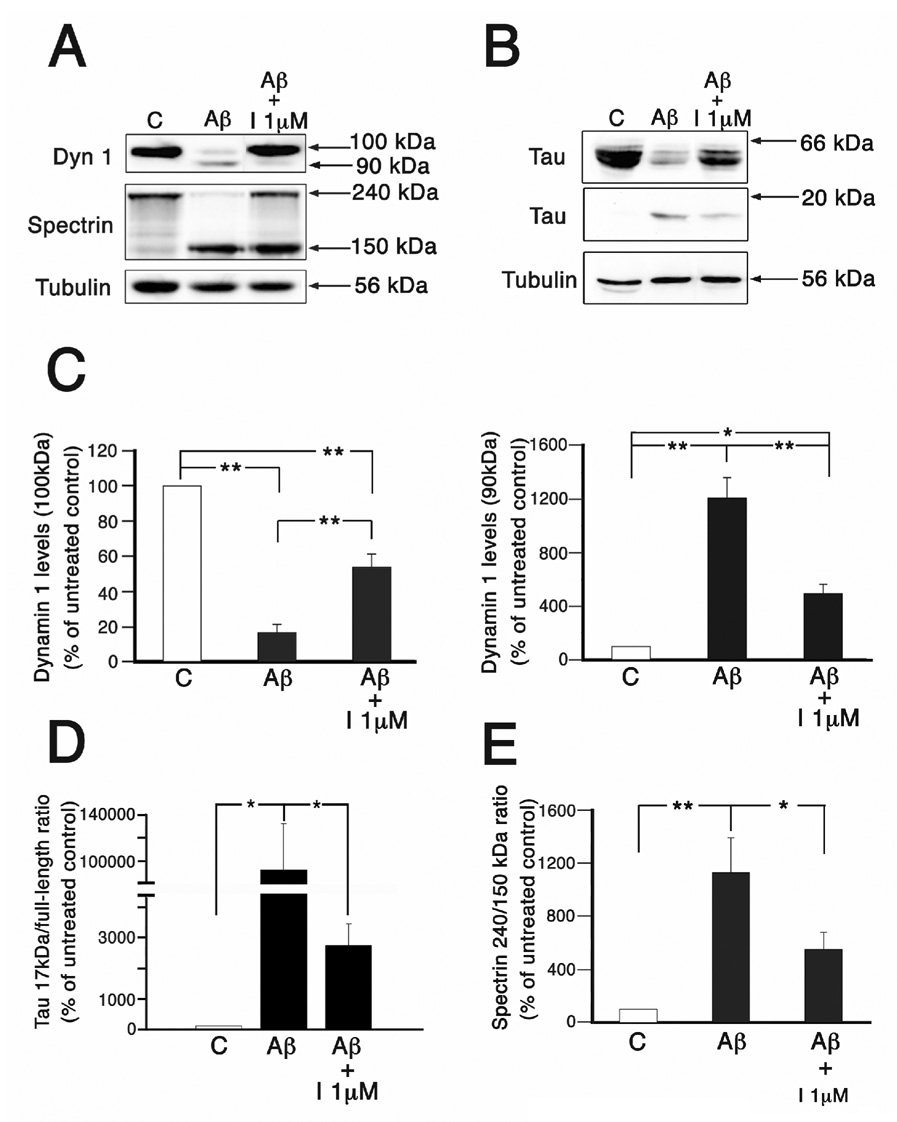
The calpain inhibitor A-705253 prevented dynamin 1 and tau cleavage even when added simultaneously with Aβ in cultured hippocampal neurons
The results described above indicated that the calpain inhibitor A-705253 was effective in preventing dynamin 1 and tau cleavage in a dose-dependent manner when added 1 hr prior to the incubation with oligomeric Aβ. A potential therapeutic use of calpain inhibitors in the context of AD, however, would require compounds capable of preventing or attenuating dynamin 1 and/or tau cleavage at the starting point of the pathological process or even when these molecular mechanisms have been already triggered. Therefore, we investigated whether A-705253 could prevent the cleavage of these proteins when used simultaneously with, or up to 8 hours after the addition of, oligomeric Aβ. Western blot analysis of whole cell extracts prepared 24 hrs after the addition of both A-705253 (1 µM) and Aβ (10 µM) together showed a strong immunoreactive band corresponding to full-length dynamin 1 and a faint cleaved dynamin 1 band (Fig. 5A). This pattern contrasted with the one observed in samples obtained from cultures treated with only Aβ where a prominent dynamin 1-cleaved band could be detected (Fig. 5A). Densitometry of these immunoreactive bands showed a significant recovery of full-length dynamin 1 and a significant decrease of the immunoreactivity of the cleaved band when whole cell extracts obtained from A-705253 plus Aβ-treated neurons were compared to the ones treated only with Aβ (Fig. 5C). A similar pattern was observed when the levels of the 17 kDa tau fragment and calpain activity, as determined by spectrin cleavage, were analyzed (Fig. 5A, B, D & E).
Figure 5. The simultaneous addition of A-705253 and Aβ prevented dynamin 1 and tau cleavage as well as calpain activation in cultured hippocampal neurons.
(A & B) Western blot analysis of whole cell extracts prepared from 3 weeks in culture hippocampal neurons incubated in the absence (c), the presence of both A-705253 (inhibitor) (1 µM) and oligomeric Aβ (Aβ). The membranes were reacted with dynamin 1 (dyn 1) (A), spectrin (A) and tau (B) antibodies. Tubulin was used as a loading control. (C–E) Graphs showing the levels of dynamin 1 full-length (~100 kDa) and cleaved (~90 kDa) bands (C), the tau 17 kDa/full-length ratio (D) and calpain activation determined as the spectrin 150/240 kDa ratio (E) in control, Aβ-treated, and A-705253 plus Aβ-treated hippocampal neurons. Values are expressed as percentage of untreated controls, considering the values obtained in these neurons as 100%. Each number represents the mean ± SEM from 3 different experiments. *Differs from control, p < 0.05. **Differs from control, p < 0.01.
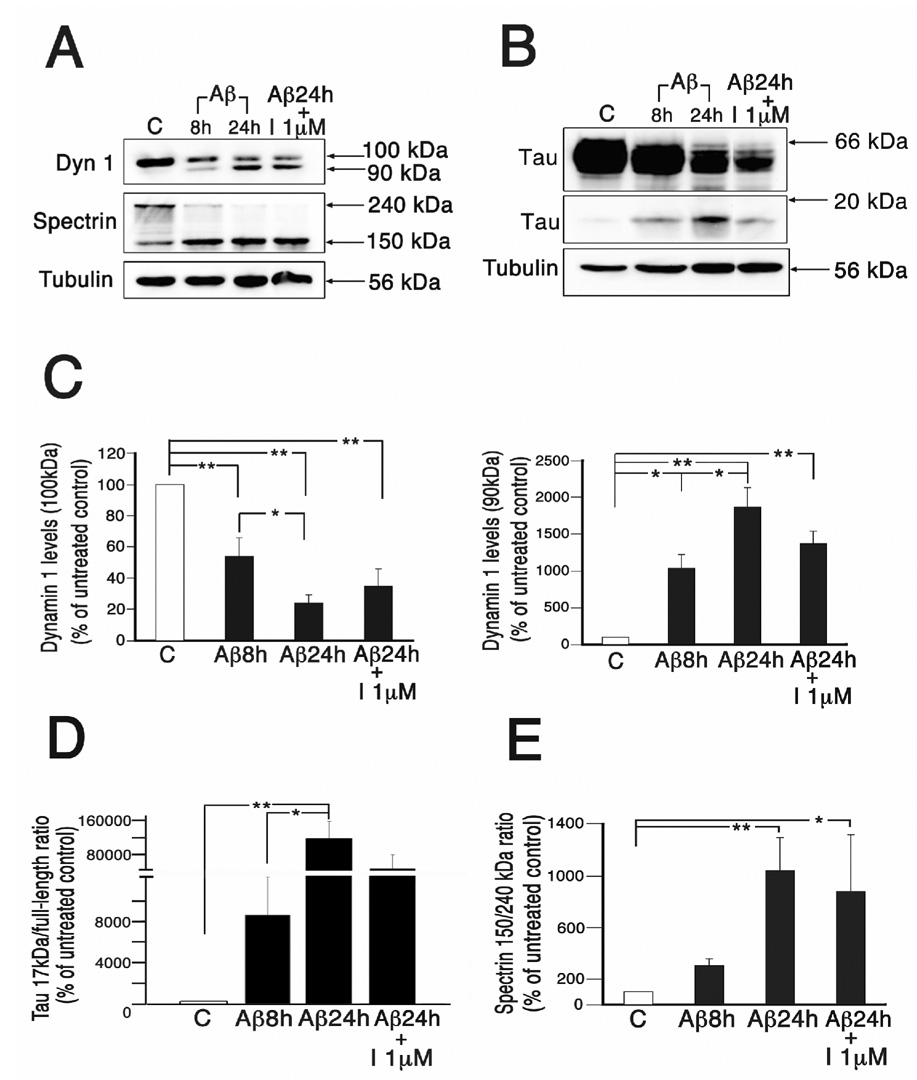
Finally, we assessed whether A-705253 was able to inhibit calpain-mediated dynamin 1 and/or tau cleavage once the pathological process has been initiated by the addition of Aβ. For these experiments, we treated 3 weeks in culture hippocampal neurons for 2, 4, 6, and 8 hours to determine the shortest incubation time that results in a significant dynamin 1 and tau cleavage. Cleaved dynamin 1 and 17 kDa tau levels were assessed by quantitative Western blot analysis. Densitometry of immunoreactive bands confirmed and extended our previous results showing that dynamin 1 and tau cleavage could be detected as early as 8 hrs after the addition of up to 20 µM oligomeric Aβ (Kelly et al., 2005; Park and Ferreira, 2005). Therefore, for this set of experiments we incubated hippocampal neurons for 8 hrs with oligomeric Aβ (10 µM) and then added A-705253 (1 µM). Whole cell extracts were prepared 24 hrs after the addition of Aβ. Quantitative Western blot analysis showed that the addition of this calpain inhibitor after the toxic effects of Aβ had been triggered resulted in a moderate decrease in the cleavage of dynamin 1 and tau when compared with neurons treated with only Aβ for 24 hrs (~27 % and ~61 %, respectively). On the other hand, the levels of these cleaved proteins were higher than the ones obtained from neurons treated with only Aβ for 8 hrs (Fig. 6A & B). However, due to the high variability among samples, these values were not statistically different when compared with either of the Aβ-treated neurons (Fig. 6C & D). A similar pattern was detected when calpain activity was assessed as the spectrin 150/240 kDa ratio (Fig. 6E).
Figure 6. A-705253 was partially effective in blocking Aβ-induced dynamin 1 and tau cleavage and calpain activation when added after the incubation with the oligomeric peptide had begun.
(A & B) Western blot analysis of whole cell extracts prepared from 3 weeks in culture hippocampal neurons incubated in the absence (c), the presence of oligomeric Aβ (Aβ̣ for 8 (8h) (the shortest incubation time resulting in significant dynamin 1 and tau cleavage) and 24 (24 h), or from neurons treated for 8 h with Aβ before the addition of A-705253 (1 µM) and collected 24 h after the addition of Aβ (Aβ 24h + I 1 µM). The membranes were reacted with dynamin 1 (dyn 1) (A), spectrin (A), and tau antibodies (B) Equal amounts of protein were loaded in each lane. The tubulin was used as a loading control. Values obtained from neurons incubated with the calpain inhibitor 8 h after the starting point of the Aβ treatment fell in between the ones obtained from neurons treated with only Aβ (C–E) Graphs show the results of the quantitative analysis of immunoreactive bands. Values are expressed as percentage of untreated controls, considering the values obtained in these neurons as 100%. Each number represents the mean ± SEM from 3 different experiments. *Differs from control, p < 0.05. **Differs from control, p < 0.01.
DISCUSSION
We have previously shown that Aβ-induced calpain-mediated cleavage of dynamin 1 and tau resulted in altered synaptic vesicle recycling and neurite degeneration followed by cell death, respectively (Park and Ferreira, 2005; Kelly and Ferreira, 2006, Kelly and Ferreira, 2007). These data suggest that the functional consequences of Aβ deposition might be diminished by regulating this proteolytic cleavage. In this study, we tested the ability of a novel calpain inhibitor to ameliorate dynamin 1 and tau cleavage in Aβ-treated hippocampal neurons. Our results indicate that the benzoylalanine-derived ketoamide A-705253 is highly effective in preventing the cleavage of these proteins when added prior to the incubation with the oligomeric peptide. In addition, the data obtained showed that this calpain inhibitor is capable of blocking dynamin 1 and tau cleavage also when added simultaneously with Aβ. Furthermore, the data presented suggested that the use of this calpain inhibitor could have some beneficial effects even when added after the cleavage of these proteins have been triggered by Aβ. These results emphasize the potential role of effective calpain inhibitors as therapeutic tools in AD.
In the past decade, a great research effort has been directed to the development of drugs that could slow down the progression or attenuate the symptoms of AD. However, very few drugs have been approved for the treatment of this disease. Drug discovery has now turned to new potential targets implicated in the molecular mechanisms underlying functional and structural deficits associated with this devastating disease (Marks and Berg, 2008). A growing body of evidence strongly suggests that calpain could be one of those targets. Under normal conditions, this Ca2+ -dependent protease induces limited cleavage of a number of substrates leading to the formation of large, usually active, fragments (reviewed by Goll et al., 2003). This type of cleavage suggests a regulatory rather than a digestive function and underscores the importance of maintaining calpain activity in a normal range. Calpastatin, the specific calpain endogenous inhibitor, plays a critical role in modulating the activity of this protease. Indeed, it has been shown that the Aβ-mediated pathological activation of this protease and/or changes in the levels of calpastatin leading to an increased calpain/calpastatin ratio could have deleterious consequences in AD (Vaisid et al., 2007). These calpain-dependent toxic effects of Aβ could be mediated, at least in part, by the cleavage of dynamin 1 and tau (Kelly et al., 2005; Park and Ferreira, 2005; Kelly and Ferreira, 2006; Kelly and Ferreira, 2007, see also this study) as well as by the cleavage of a number of other proteins relevant to AD including the amyloid precursor protein, several signaling proteins (i.e. p35, protein kinase C, and GSK3), and glutamate receptors (both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) and N-methyl-D-aspartic acid (NMDA) receptors) (Bi et al., 1996; Shea et al., 1996; Bi et al., 1998; Lee et al., 2000; Chen and Fernandez, 2005; Fifre et al., 2006; Crespo-Biel et al., 2007; Goni-Oliver et al., 2007; Xu et al., 2007; Wei et al., 2008). The idea that calpain could be a potential therapeutic target in neurodegenerative diseases has been reinforced by studies using the calpain inhibitor MDL 28170. Those studies, whether performed in vitro or in vivo, suggested a protective effect of calpain inhibitors against excitotoxicity (Li et al., 1998; Rami et al., 2000; Kunz et al., 2004; Wu et al., 2004). However, little is known about their protective effects against Aβ toxicity or the molecular mechanisms that could underlie potential protective effects. Our results showing that A-705253 was highly effective in preventing calpain-mediated cleavage of dynamin 1 and tau provide insights into such mechanisms. Using this inhibitor, we detected no significant differences in the levels of the cleaved dynamin 1 and the tau 17 kDa/full-length ratio when hippocampal neurons preincubated with 1 µM of A-705253 for 1 hr before the addition of oligomeric Aβ were compared to untreated controls. On the other hand, the levels of full-length dynamin 1 did not completely recover even when neurons were pretreated with the highest dose of this calpain inhibitor. These results are in agreement with our previous observations showing that Aβ induced the decrease in dynamin 1 levels through two mechanisms in hippocampal neurons (Kelly et al., 2005). These mechanisms involved both the down-regulation of dynamin 1 expression and the calpain-mediated cleavage of this protein (Kelly et al., 2005). The partial recovery of the levels of full-length dynamin 1, in addition to the complete disappearance of the cleaved dynamin 1 band, in A-705253-treated neurons is consistent with the effects of this inhibitor only on the degradation process associated with the decrease in dynamin 1 levels.
The data presented in this study also showed that this calpain inhibitor was more effective in preventing the cleavage of tau than of dynamin 1. While a significant reduction in tau cleavage was detected when neurons preincubated with 1 nM A-705253 were compared to Aβ-treated neurons, dynamin 1 cleavage was only significantly reduced when these neurons were pretreated with at least 10 nM of this calpain inhibitor. This differential sensitivity could be due to the ratio between the protease and specific substrates in hippocampal neurons. Alternatively, it could reflect how accessible the protease was to the inhibitor in different subcellular compartments. Immuno-electron microscopy studies have shown that calpain was highly concentrated in cell bodies and along axons and dendrites (Perlmutter et al., 1988). In the neuritic process, calpain immunoreactive product accumulated along microtubules. This localization overlaps with the cellular distribution of tau (Binder et al., 1985). On the other hand, calpain immunoreactive product was relatively rare in presynaptic terminals, where, localized between synaptic vesicles, dynamin 1 is highly enriched (Kelly et al., 2005). This subcellular localization could restrict the access of the inhibitor to calpain located in the proximity of dynamin 1. Regardless of this apparent difference between the lower doses of A-705253 needed to significantly block the cleavage of tau and dynamin 1, our results showed that this inhibitor is effective in nanomolar concentrations instead of the micromolar concentrations needed for other calpain inhibitors (Battaglia et al., 2003; Park and Ferreira, 2005; Kelly and Ferreira, 2006; McCollum et al., 2006). These differences in efficiency could be due to the improved pharmacokinetics of A-705253. The commonly used calpain inhibitors have poor selectivity, excessive metabolism, and many times required a special route of administration (Fehrentz and Castro, 1983; Bartus et al., 1994). In contrast, the characterization of this inhibitor demonstrated water solubility, metabolic stability, and cell membrane permeability (Lubisch et al., 2003).
Our results also indicated that this inhibitor was highly effective when added together with Aβ and, to a certain extent, capable of attenuating the cleavage of dynamin 1 and tau when added after the pathological process has been triggered. Due to great sample variability, the decrease in cleaved-dynamin 1 (27 %) and 17 kDa tau (61 %) levels observed in the latter experimental condition was not statistically significant. Nevertheless, the trend observed in this experiment raised the possibility that more potent calpain inhibitors could have beneficial effects even at late stages of AD. These results are in agreement with a previous observation showing that A-705253 was capable of blocking neuronal death even when administered up to 2 hrs after inducing the brain injury (Lubisch et al., 2003).
Together, these results suggest that by blocking Aβ-induced calpain activation, A-705253 could prevent the decrease in dynamin 1 levels and the generation of the tau 17 kDa neurotoxic fragment. Through this mechanism, A-705253 could ameliorate the impairment in synaptic vesicle recycling and progressive neurite degeneration associated with the pathological cleavage of these proteins (Park and Ferreira, 2005; Kelly and Ferreira, 2006, Kelly and Ferreira, 2007). Building on previous reports, these data underscore the potential importance of calpain inhibitors as promising therapeutic tools in AD and related neurodegenerative diseases.
Acknowledgments
This study was supported by grants from NIH/NS39080 and Abbott Laboratories to A.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez A, Toro R, Caceres A, Maccioni RB. Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Letters. 1999;459:421–426. doi: 10.1016/s0014-5793(99)01279-x. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Hayward NJ, Elliott PJ, Sawyer SD, Baker KL, Dean RL, Akiyama A, Straub JA, Harbeson SL, Li Z, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- Battaglia F, Trinchese F, Liu S, Walter S, Nixon RA, Arancio O. Calpain inhibitors, a treatment for Alzheimer's disease: position paper. Journal of Molecular Neuroscience. 2003;20:357–362. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- Bi X, Chang V, Molnar E, McIlhinney RA, Baudry M. The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience. 1996;73:903–906. doi: 10.1016/0306-4522(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Bi X, Rong Y, Chen J, Dang S, Wang Z, Baudry M. Calpain-mediated regulation of NMDA receptor structure and function. Brain Research. 1998;790:245–253. doi: 10.1016/s0006-8993(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. Journal of Cell Biology. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, Lorenzo A, Yeh J, Yankner BA. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14:879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Chen M, Fernandez HL. Mu-calpain is functionally required for alpha-processing of Alzheimer's beta-amyloid precursor protein. Biochemical and Biophysical Research Communications. 2005;330:714–721. doi: 10.1016/j.bbrc.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiology of Disease. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- Clark SG, Shurland DL, Meyerowitz EM, Bargmann CI, van der Bliek AM. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Biel N, Canudas AM, Camins A, Pallas M. Kainate induces AKT, ERK and cdk5/GSK3b pathway deregulation, phosphorylates tau protein in mouse hippocampus. Neurochemistry International. 2007;50:435–442. doi: 10.1016/j.neuint.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Czogalla A, Sikorski AF. Spectrin and calpain: a 'target' and a 'sniper' in the pathology of neuronal cells. Cellular and Molecular Life Sciences. 2005;62:1913–1924. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. Journal of Cell Biology. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekinci FJ, Malik KU, Shea TB. Activation of the L voltage-sensitive calcium channel by mitogen-activated protein (MAP) kinase following exposure of neuronal cells to beta-amyloid. MAP kinase mediates beta-amyloid-induced neurodegeneration. Journal of Biological Chemistry. 1999;274:30322–30327. doi: 10.1074/jbc.274.42.30322. [DOI] [PubMed] [Google Scholar]
- Fehrentz JA, Castro B. An efficient synthesis of optically active α–(tert-butoxycarbonylalamino)-aldehydes from aminoacids. Synthesis. 1983:676–678. [Google Scholar]
- Ferreira A, Busciglio J, Caceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Brain Research. Developmental Brain Research. 1989;49:215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Lu Q, Orecchio L, Kosik KS. Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar A beta. Molecular and Cellular Neurosciences. 1997;9:220–234. doi: 10.1006/mcne.1997.0615. [DOI] [PubMed] [Google Scholar]
- Fifre A, Sponne I, Koziel V, Kriem B, Yen Potin FT, Bihain BE, Olivier JL, Oster T, Pillot T. Microtubule-associated protein MAP1A, MAP1B, and MAP2 proteolysis during soluble amyloid beta-peptide-induced neuronal apoptosis. Synergistic involvement of calpain and caspase-3. Journal of Biological Chemistry. 2006;281:229–240. doi: 10.1074/jbc.M507378200. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and Biophysical Research Communications. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiological Reviews. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. Journal of Biological Chemistry. 2007;282:22406–22413. doi: 10.1074/jbc.M702793200. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing nerve cells. Cambridge, Mass: MIT Press; 1991. pp. 251–283. [Google Scholar]
- Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. Journal of Biological Chemistry. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Ferreira A. Beta-amyloid disrupted synaptic vesicle endocytosis in cultured hippocampal neurons. Neuroscience. 2007;147:60–70. doi: 10.1016/j.neuroscience.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BL, Vassar R, Ferreira A. Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. Journal of Biological Chemistry. 2005;280:31746–31753. doi: 10.1074/jbc.M503259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J, Honda T, Mori H, Hamada Y, Miura R, Ogawara M, Ihara Y. The carboxyl third of tau is tightly bound to paired helical filaments. Neuron. 1988;1:827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Niederberger E, Ehnert C, Coste O, Pfenninger A, Kruip J, Wendrich TM, Schmidtko A, Tegeder I, Geisslinger G. The calpain inhibitor MDL 28170 prevents inflammation-induced neurofilament light chain breakdown in the spinal cord and reduces thermal hyperalgesia. Pain. 2004;110:409–418. doi: 10.1016/j.pain.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Li PA, Howlett W, He QP, Miyashita H, Siddiqui M, Shuaib A. Postischemic treatment with calpain inhibitor MDL 28170 ameliorates brain damage in a gerbil model of global ischemia. Neuroscience Letters. 1998;247:17–20. doi: 10.1016/s0304-3940(98)00266-3. [DOI] [PubMed] [Google Scholar]
- Lubisch W, Beckenbach E, Bopp S, Hofmann HP, Kartal A, Kastel C, Lindner T, Metz-Garrecht M, Reeb J, Regner F, Vierling M, Moller A. Benzoylalanine-derived ketoamides carrying vinylbenzyl amino residues: discovery of potent water-soluble calpain inhibitors with oral bioavailability. Journal of Medicinal Chemistry. 2003;46:2404–2412. doi: 10.1021/jm0210717. [DOI] [PubMed] [Google Scholar]
- Marks N, Berg MJ. Neurosecretases provide strategies to treat sporadic and familial Alzheimer disorder. Neurochemistry International. 2008;52:184–215. doi: 10.1016/j.neuint.2007.06.020. [DOI] [PubMed] [Google Scholar]
- McCollum AT, Jafarifar F, Lynn BC, Agu RU, Stinchcomb AL, Wang S, Chen Q, Guttmann RP. Inhibition of calpain-mediated cell death by a novel peptide inhibitor. Experimental Neurology. 2006;202:506–513. doi: 10.1016/j.expneurol.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Novak M, Kabat J, Wischik CM. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer's disease paired helical filament. EMBO Journal. 1993;12:365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MS, Hemnani T. Alzheimer's disease pathogenesis and therapeutic interventions. J Clin Neurosci. 2004;11:456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Park SY, Ferreira A. The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. Journal of Neuroscience. 2005;25:5365–5375. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter LS, Siman R, Gall C, Seubert P, Baudry M, Lynch G. The ultrastructural localization of calcium-activated protease "calpain" in rat brain. Synapse. 1988;2:79–88. doi: 10.1002/syn.890020111. [DOI] [PubMed] [Google Scholar]
- Rami A, Agarwal R, Botez G, Winckler J. mu-Calpain activation, DNA fragmentation, and synergistic effects of caspase and calpain inhibitors in protecting hippocampal neurons from ischemic damage. Brain Research. 2000;866:299–312. doi: 10.1016/s0006-8993(00)02301-5. [DOI] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta -amyloid-induced neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer's disease. Annual Review of Cell Biology. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- Shea TB, Spencer MJ, Beermann ML, Cressman CM, Nixon RA. Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: involvement of calpain-mediated hydrolysis of protein kinase C. Journal of Neurochemistry. 1996;66:1539–1549. doi: 10.1046/j.1471-4159.1996.66041539.x. [DOI] [PubMed] [Google Scholar]
- Siman R, Baudry M, Lynch G. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. Journal of Neuroscience. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Noguchi K, Sato K, Hoshino T, Imahori K. Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7789–7793. doi: 10.1073/pnas.90.16.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer's disease brains. Neuroscience Letters. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- Vaisid T, Kosower NS, Katzav A, Chapman J, Barnoy S. Calpastatin levels affect calapin activation and calpain proteolytic activity in APP transgenic mouse model of Alzheimer's disease. Neurochemistry International. 2007;51:391–397. doi: 10.1016/j.neuint.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Veeranna, Kaji T, Boland B, Odrljin T, Mohan P, Basavarajappa BS, Peterhoff C, Cataldo A, Rudnicki A, Amin N, Li BS, Pant HC, Hungund BL, Arancio O, Nixon RA. Calpain mediates calcium-induced activation of the erk1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer's disease. American Journal of Pathology. 2004;165:795–805. doi: 10.1016/S0002-9440(10)63342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Song MS, MacTavish D, Jhamandas JH, Kar S. Role of calpain and caspase in beta-amyloid-induced cell death in rat primary septal cultured neurons. Neuropharmacology. 2008;54:721–733. doi: 10.1016/j.neuropharm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. Journal of Biological Chemistry. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 2007;53:399–412. doi: 10.1016/j.neuron.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Mesulam MM. Seminars in medicine of the Beth Israel Hospital, Boston. beta-Amyloid and the pathogenesis of Alzheimer's disease. New England Journal of Medicine. 1991;325:1849–1857. doi: 10.1056/NEJM199112263252605. [DOI] [PubMed] [Google Scholar]