Abstract
Purpose
To explore the relationships between interleukin-6 (IL-6) and soluble IL-6 receptor (sIL-6R) levels and disease extent and clinical outcome in childhood neuroblastoma.
Experimental Design
Pre-treatment peripheral blood (PB) (n=53) and bone marrow (BM) (n=18) samples from patients with neuroblastoma were assayed by ELISA for IL-6 and sIL-6R. PB values were compared to healthy pediatric controls (n=28).
Results
PB IL-6 levels were significantly elevated in patients with high risk disease compared to those with low and intermediate risk disease (23.9 pg/mL v. 4.3, p<0.001) and the normal control group (23.9 pg/mL v. 3.3, p<0.001). Similarly, BM IL-6 levels were higher in high risk patients when compared to low and intermediate risk patients (15 pg/mL v. 0, p<0.02). Other factors correlated with higher IL-6 levels were age > 18 months, bony metastases, and unfavorable histology. sIL-6R levels were not significantly correlated with disease stage. Patients with detectable PB IL-6 at diagnosis had significantly lower event free survival (EFS) rates (p<0.008). sIL-6R levels less than 2.5 × 104 pg/mL were also associated with a significantly worse EFS (p = 0.016).
Conclusion
Elevated PB IL-6 levels correlated with features of high risk neuroblastoma and poor prognosis in this population. Decreased PB sIL-6R levels correlated with the presence of metastatic disease. Further study of these markers in children with neuroblastoma appears warranted.
INTRODUCTION
Neuroblastoma, the most common extracranial solid tumor of childhood, accounts for 8−10% of all childhood cancers (1,2). The complex biological and clinical features of neuroblastoma have allowed for the development of a risk-based model for staging and treatment of these patients(3,4). For example, low risk tumors are often well-circumscribed lesions which occur in younger patients, can be treated with surgery alone, and are curable in over 90% of patients (2,5). In contrast, patients with high risk disease are typically older patients who have histologically aggressive tumors that have often spread to the bone and bone marrow at the time of diagnosis. Despite intensive chemotherapy, radiation, and surgery, patients with these tumors have significantly worse survival rates in the range of 30 − 40%(6,7). Low risk and high risk tumor types each make up about 40−45% of neuroblastoma diagnoses. The remaining 10−5% of tumors are classified as intermediate risk. As the name implies, they have a mix of both good and poor prognostic factors. Their survival rates are a little lower than those of low risk tumors at 85−95% overall survival(4,8).
IL-6 is a pleiotropic cytokine with many ascribed effects including stimulation of acute phase reactants, immune regulation, angiogenesis, and osteoclast activation, (9-12). It originates from a multitude of cell types, including mononuclear phagocytes, vascular endothelial cells, fibroblasts, hepatocytes, B-cell lymphomas, and the neoplastic plasma cells of multiple myeloma. It appears to serve as a stimulatory factor in multiple myeloma; produced by both the malignant cells and bone marrow stromal cells(11). In neuroblastoma, some literature indicates production of IL-6 by neuroblastoma cell lines(13,14), however a more recent manuscript asserts IL-6 is produced by mesenchymal stem cells in the marrow microenvironment of neuroblastoma(10).
IL-6 has a dedicated receptor that can be found either on the cell membrane or in a solubilized form (sIL-6R)(9,12). The receptor will bind IL-6 in the circulation as well as at the cellular membrane. The receptor complex binds to the GP-130 transmembrane receptor(9,15). This, then, activates Jak2 which in turn activates multiple pathways, the most described of which is the Stat3 pathway(15,16). Stat3 has been shown to play a role in delaying the initiation of an inflammatory response to cancer as it develops(15,17). Along with IL-6, multiple myeloma cells also produce sIL-6R. sIL-6R antibodies have been studied as targeted therapy in multiple myeloma(18,19).
Both circulating IL-6 and sIL-6R can be detected in the peripheral blood. Increased levels of IL-6 at diagnosis have been associated with advanced disease and poor outcome in multiple neoplasms including prostate cancer(20), non-Hodgkin's lymphoma(21,22), head and neck squamous cell carcinoma(23), breast cancer(24), melanoma(25,26), and multiple myeloma(11). The prognostic value of sIL-6R levels is less well established. It has been investigated as part of biochemical staging in prostate cancer and as a prognostic factor in multiple myeloma(27,28).
Taking together the evidence of circulating IL-6 and sIL-6R levels as markers of poor prognosis in other cancers, as well as the implication of IL-6 activity in the micro-environment of neuroblastoma, we hypothesized that both IL-6 and sIL-6R levels would be elevated in children with neuroblastoma as compared to normal controls. Additionally, we hypothesized that the degree of IL-6 and sIL-6R elevation would be related to the aggressiveness of the neuroblastoma. We therefore investigated the levels of IL-6 and sIL-6R in patients with newly diagnosed neuroblastoma and a healthy pediatric population.
METHODS
Sample Collection
All the work described within this paper was performed with the approval of the Baylor College of Medicine Institutional Review Board. Texas Children's Cancer Center has banked peripheral blood and bone marrow samples from patients with neuroblastoma and ganglioneuroblastoma . For the purpose of this study, we retrospectively selected all samples collected between August 1995 and December 2005 for which the following clinical information was available: age at diagnosis, stage and sites of disease at diagnosis, relapse status, and time to last follow-up. In addition, we collected data regarding risk category (as defined by the Children's Oncology Group risk stratification system (2)) on all patients as well as described prognostic features as available. These features include tumor histology (Shimada favorable or unfavorable staging system (3,4)) and MYCN status (presence or absence of 10 or more copies of gene amplification in the tumor(3,29)), Collection of all samples used occurred at the time of diagnosis, prior to either the start of systemic chemotherapy or complete resection of the tumor. All peripheral blood samples were obtained as whole blood in heparin and separated into either plasma or serum. These were then aliquotted and stored at −80° Celsius. Bone marrow aspiration samples were processed in a similar fashion.
The normal pediatric controls were collected in the following manner. Upon permission from the parent or guardian, whole blood was obtained from otherwise well children undergoing elective surgery such as hernia repair or circumcision. They had no identified infectious process at the time of surgery. The blood was processed in the same manner as described above.
IL-6 Levels
IL-6 levels were determined using a human IL-6 BD OptEIA enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, San Diego, CA). This assay has a range of 4.7 pg/mL – 300 pg/mL. 100 microliters of each sample was prepared and tested as per the manufacturer's instructions. Each sample was tested in duplicate, with the mean used for the final sample value. Mean values <4.7 pg/dL were recorded as 0. Any sample with a value >300 pg/mL was diluted and retested with appropriate adjustment of the final value.
sIL-6R levels
sIL-6R levels were determined using a Quantikine human IL-6 sR ELISA (R&D Systems, Inc Minneapolis, MN). The assay has a range of 31.2 pg/mL – 1000 pg/mL. 10 microliters of each sample was diluted by 100 fold and tested as per the manufacturer's instructions. All samples were tested in duplicate, and the mean was used for the final sample value.
Statistical Considerations
Means of IL-6 and sIL-6R levels were compared based on risk level, age, disease state, histology, MYCN amplification, and presence of metastatic disease in specific sites. Significance of difference in means was obtained using a non-parametric Mann-Whitney comparison. Overall sample sizes were chosen to reach a sample size of greater than 17 in each group whenever possible. This size allowed for detection of at least one standard deviation of difference with a power of 0.8 and an alpha of 0.05. Event free survival curves were generated by Kaplan Meiers analysis curves and compared via a log rank test. All statistics were run using SPSS version 15 software (Chicago, IL). Any sample for which the sorting information was not known was left out of the analysis.
RESULTS
Patient Characteristics
Fifty-three peripheral blood (PB) and 18 bone marrow (BM) samples collected at the time of diagnosis from patients with neuroblastoma were identified for inclusion in the study. This sample set represented 54 patients in total, with both blood and bone marrow coming from 17 patients, blood only coming from 36 patients and bone marrow only coming from one patient. In addition, we utilized 28 pediatric normal control plasma samples. The demographics of the patients and normal controls can be found in Table 1.
Table 1.
Patient and Tumor Characteristics
| Characteristics |
IL-6 |
sIL-6R |
Normal Controls | ||
|---|---|---|---|---|---|
| PB | BM | PB | BM | ||
| Total | 53 | 18 | 35 | 16 | 28 |
| Median Age in months | 21 | 23 | 22 | 29 | 54 |
| (Range) | (1− 165) | (2 − 153) | (2 − 153) | (2 − 153) | (2−204) |
| Gender | |||||
| Male | 26 | 12 | 17 | 11 | 16 |
| Female | 27 | 6 | 18 | 5 | 12 |
| INSS Stage | |||||
| 1 | 8 (15%) | 1 (3%) | 5 (14%) | 1 (6%) | |
| 2 | 8 (15%) | 3 (17%) | 5 (14%) | 2 (13%) | |
| 3 | 6 (11%) | 2 (11%) | 3 (8%) | 2 (13%) | |
| 4 | 28 (53%) | 11 (61%) | 20 (57%) | 10 (63%) | |
| 4S | 3 (6%) | 1 (3%) | 2 (6%) | 1 (6%) | |
| Risk Stratification | |||||
| Low | 18 (34%) | 4 (22%) | 10 (29%) | 3 (19%) | |
| Intermediate | 7 (13%) | 2 (11%) | 5 (14%) | 2 (13%) | |
| High | 28 (53%) | 12 (67%) | 20 (57%) | 11 (69%) | |
| Metastases at Presentation | |||||
| No | 22 (42%) | 6 (33%) | 13 (37%) | 5 (31%) | |
| Yes | 31 (58%) | 12 (67%) | 22 (63%) | 11 (69%) | |
| Bone Metastases | |||||
| No | 27 (51%) | 7 (39%) | 16 (46%) | 6 (38%) | |
| Yes | 26 (49%) | 11 (61%) | 19 (54%) | 10 (63%) | |
| Bone Marrow Metastases | |||||
| No | 30 (57%) | 12 (67%) | 19 (54%) | 11 (69%) | |
| Yes | 23 (43%) | 6 (33%) | 16 (46%) | 5 (31%) | |
| MYCN Amplification | |||||
| Not Amplified | 40 (75%) | 10 (56%) | 25 (71%) | 8 (50%) | |
| Amplified | 11 (21%) | 8 (44%) | 9 (26%) | 8 (50%) | |
| Unknown | 2 (4%) | 0 | 1 (3%) | 0 | |
| Histology | |||||
| Favorable | 9 (17%) | 3 (17%) | 6 (17%) | 6 (38%) | |
| Unfavorable | 12 (23%) | 8 (44%) | 6 (17%) | 3 (19%) | |
| Unknown | 32 (60%) | 7 (39%) | 23 (66%) | 7 (43%) | |
The median age of patients contributing samples for peripheral blood and/or bone marrow was 21 and 23 months respectively. This is above the reported historical median age of patients with neuroblastoma of 18.7 months (2). Although the median age of normal controls is higher than for patients (54 months v. 21 months), the range of ages demonstrates a distribution that overlaps with the patient samples.
The range of characteristics such as stage, sites of disease, and biologic prognostic factors were well distributed amongst the PB samples. High risk patients were more heavily represented in the BM samples (p = 0.021). MYCN amplification status and histology characteristics were not available for some samples as noted in Table 1.
Median follow-up time for patients contributing PB samples was 29 months (range 1 − 128 months). Of these patients, 33% had disease relapse and nearly 20% died of their disease. Median follow-up time for patients contributing BM samples was 10 months (range 3 − 18 months); of these, 3 patients (17%) had relapsed, and 1 patient (6%) had died.
IL-6 Levels and Disease Status
Normal control peripheral blood samples had levels of IL-6 undetectable by our ELISA system in 26 of the 28 children. The remaining two had values of 10.3 pg/mL and 82.3 pg/mL.
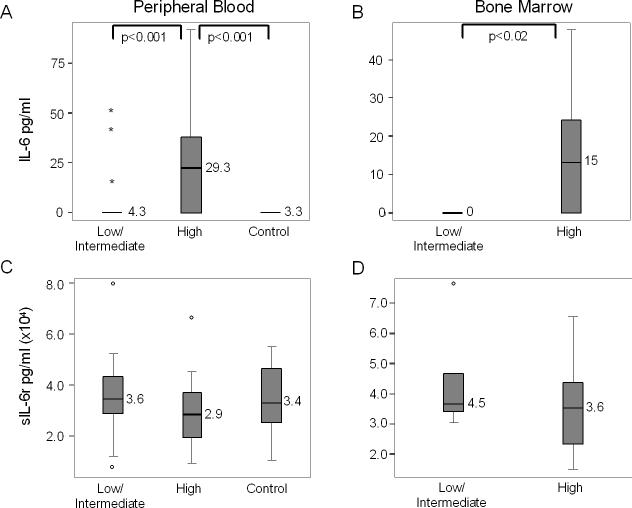
Peripheral blood levels of IL-6 from patients ranged from undetectable to 91.8 pg/mL. As shown in Figure 1, there is a statistically significant difference in the levels of PB IL-6 in patients with high risk disease when compared to those with low and intermediate risk disease (23.9 pg/mL v. 4.3, p<0.001) and to the control group (23.9 pg/mL v. 3.3, p<0.001).
Figure 1.
Levels of IL-6 and sIL-6R in the blood and bone marrow. A) Levels of IL-6 in the peripheral blood of patients with low and intermediate risk neuroblastoma, high risk neuroblastoma, and healthy pediatric controls. * Indicate extreme outliers. B) Levels of IL-6 in the bone marrow of patients with low and intermediate risk neuroblastoma as compared to high risk. C) Levels of sIL-6R in the peripheral blood of patients with low and intermediate risk neuroblastoma, high risk neuroblastoma, and healthy pediatric controls. ° Indicate outlier values. D) Levels of sIL-6R in the bone marrow of patients with low and intermediate risk neuroblastoma as compared to high risk.
Higher IL-6 levels were also present in the bone marrow samples of children with high risk disease when compared to patients with low and intermediate risk disease (15 pg/mL v. undetectable, p < 0.02). (see figure 1b).
Table 2 shows comparisons of peripheral blood and bone marrow IL-6 values in patients when categorized by age, presence or absence of metastatic disease (bone and/or bone marrow), MYCN amplification, and histology. This table illustrates several trends. First, while IL-6 levels in peripheral blood tend to be higher in patients with some poor prognostic features (i.e. age ≥ 18 months, high risk disease, and metastatic disease), it is slightly lower in patients with MYCN amplification as compared to those without it (12.6 pg/mL v. 13.5, p=0.12). In the bone marrow, only risk stratification correlates with a significant difference in IL-6 levels, however metastases at diagnosis and the presence of bone metastases trended towards significance. Surprisingly IL-6 bone marrow levels were almost equivalent in the presence or absence of active bone marrow tumor involvement (9.6 pg/mL v. 10.5, p=0.91).
Table 2.
IL-6 and sIL-6R levels in peripheral blood and bone marrow
|
IL-6 (pg/ml) |
sIL-6R (× 104 pg/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB | BM | PB | BM | |||||||||
| Characteristics | n | Mean ± SEM | p | n | Mean ± SEM | p | n | Mean ± SEM | p | n | Mean ± SEM | p |
| Age | ||||||||||||
| < 18 months | 22 | 4.9 ± 2.3 | 0.012 | 7 | 12 ± 4.7 | 0.426 | 14 | 3.6 ± 0.44 | 0.189 | 6 | 4.4 ± 0.69 | 0.448 |
| ≥ 18 months | 31 | 21.5 ± 4.8 | 11 | 6.9 ± 4.8 | 21 | 2.9 ± 0.32 | 10 | 3.5 ± 0.49 | ||||
| Risk Stratification | ||||||||||||
| Low / Intermediate | 25 | 4.3 ± 2.6 | <0.001 | 6 | 0 ± 0 | 0.014 | 15 | 3.6 ± 0.46 | 0.142 | 5 | 4.5 ± 0.82 | 0.533 |
| High | 28 | 23.9 ± 4.9 | 12 | 15 ± 4.4 | 20 | 2.9 ± 0.31 | 11 | 3.6 ± 0.45 | ||||
| Metastases at Presentation | ||||||||||||
| No | 22 | 7.9 ± 3.4 | 0.049 | 6 | 2.6 ± 2.6 | 0.111 | 13 | 3.9 ± 0.47 | 0.046 | 5 | 4.6 ± 0.78 | 0.336 |
| Yes | 31 | 19.4 ± 4.6 | 12 | 13.7 ± 4.6 | 22 | 2.8 ± 0.30 | 11 | 3.5 ± 0.45 | ||||
| Bone Metastases | ||||||||||||
| No | 27 | 9.2 ± 3.3 | 0.062 | 7 | 2.2 ± 2.2 | 0.053 | 16 | 3.7 ± 0,41 | 0.085 | 6 | 4.3 ± 0.68 | 0.588 |
| Yes | 26 | 20.3 ± 5.3 | 11 | 15 ± 4.8 | 19 | 2.8 ± 0.33 | 10 | 3.6 ± 0.49 | ||||
| Bone Marrow Metastases | ||||||||||||
| No | 30 | 7.8 ± 2.7 | 0.001 | 11 | 9.6 ± 4.4 | 0.91 | 19 | 3.9 ± 0.37 | 0.002 | 10 | 4.1 ± 0.59 | 0.903 |
| Yes | 23 | 23.5 ± 5.9 | 6 | 10.5 ± 6.7 | 16 | 2.4 ± 0.27 | 5 | 3.7 ± 0.42 | ||||
| MYCN Amplification | ||||||||||||
| Not Amplified | 40 | 13.5 ± 3.5 | 0.119 | 10 | 6.3 ± 4.2 | 0.079 | 25 | 3.1 ± 0.43 | 0.27 | 8 | 4.4 ± 0.51 | 0.172 |
| Amplified | 11 | 12.6 ± 4.3 | 8 | 14.7 ± 5.3 | 9 | 3.6 ± 0.56 | 8 | 3.3 ± 0.59 | ||||
| Histology | ||||||||||||
| Favorable | 9 | 6 ± 4.4 | 0.047 | 3 | 0 ± 0 | 0.095 | 6 | 4.2 ± 0.79 | 0.522 | 3 | 4.9 ± 0.13 | 0.197 |
| Unfavorable | 12 | 16.4 ± 8 | 8 | 15.3 ± 6.1 | 6 | 3.8 ± 0.61 | 6 | 3.4 ± 0.77 | ||||
IL6 Receptor Levels and Disease Status
sIL-6R levels did not significantly correlate with disease status. In peripheral blood there was a small trend towards higher sIL-6R levels in patients with low or intermediate risk disease when compared to patients with high risk disease (3.6 × 104 pg/mL v. 2.9 × 104, p=0.14), however this trend was not apparent in the bone marrow (4.5 × 104 pg/mL v. 3.6 × 104, p=0.53) (Figure 1c and d). As with the IL-6 levels, the PB normal controls were similar to the low/intermediate risk disease group with an average level of 3.4 × 104 pg/mL.
Table 2 shows comparisons of peripheral blood and bone marrow sIL-6R values in patients when stratified by age, presence or absence of metastatic disease (bone and/or bone marrow), MYCN amplification, and histology. This table illustrates that metastatic disease at diagnosis was associated with a significantly lower peripheral blood sIL-6R level (2.8 × 104 pg/mL v. 3.9 × 104; p = 0.046). The significant difference remains when bone marrow disease is the metastatic site (2.4 × 104 pg/mL v. 3.9 × 104; p = 0.002) and trends towards significance with bone metastases (2.8 × 104 pg/mL v. 3.7 × 104; p = 0.085). Other categories show patients with poorer prognostic features having lower sIL-6R levels, but not with a significant difference. No significant differences were seen in the bone marrow, although sIL-6R levels were consistently lower in the patients with poor prognostic features.
Levels and Survival
We assessed IL-6 and sIL-6R peripheral blood levels in patients with NB at diagnosis and their relationship to event free survival (EFS). Patients with a peripheral blood IL-6 level of >0 pg/mL at the time of diagnosis had a significantly lower EFS rate (log rank p<0.008) (figure 2a). When analyzing high risk patients only, the difference in EFS is lost (figure 2b). For sIL-6R levels, there is a significant difference in EFS between the patients with sIL-6R levels lower than 2.5 × 104 pg/mL as compared with those higher (p = 0.016) (figure 2c). The level 2.5 ×104 pg/mL represents the cutoff for the lowest third of sIL-6R values obtained. Again, this significance is lost when only the high risk patients are included (figure 2d).
Figure 2.
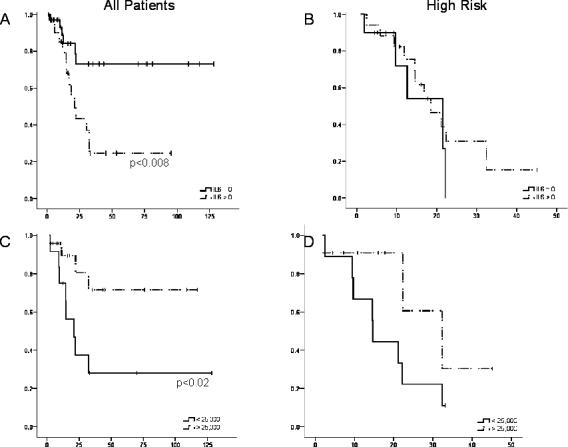
Event Free Survival in correlation with IL-6 and sIL-6R levels. All patients were included in this analysis. Patients were censored at the time of their last follow-up visit. A) Event free survival (EFS) of all patients with neuroblastoma based on the presence or absence of IL-6 in the peripheral blood at diagnosis. B) EFS of patients with high risk neuroblastoma only based on the presence or absence of IL-6 in the peripheral blood at diagnosis. C) EFS of all patients with neuroblastoma based on the level of sIL-6R present in the peripheral blood at diagnosis. D) EFS of patients with high risk neuroblastoma only based on the level of sIL-6R present in the peripheral blood at diagnosis.
DISCUSSION
Our data show that patients with neuroblastoma who have elevated peripheral blood levels of IL-6 at diagnosis tend to have high risk disease and generally poor outcomes. Also, IL-6 bone marrow levels were significantly increased in high risk patients. In addition, sIL-6R levels followed an inverse relationship with disease such that lower levels were present in the peripheral blood of patients with high risk disease, although this trend was not statistically significant. Peripheral blood sIL-6R levels were significantly lower in patients presenting with metastatic disease as compared to those with only local disease. Finally, we presented peripheral blood IL-6 and sIL-6R levels for healthy pediatric patients.
Multiple studies have shown a role for elevated IL-6 levels at diagnosis as a marker of poor prognosis in various cancers including multiple myeloma(11), malignant melanoma(25,26), non-Hodgkin's lymphoma(21,22), prostate cancer(20), squamous cell carcinoma of the head and neck(23), and various sarcomas(30,31). In this manuscript we have demonstrated that patients with neuroblastoma who have elevated levels of IL-6 at diagnosis also have features of high risk disease, including unfavorable histology, metastatic disease in general and specifically in the presence of bone marrow disease. Additionally, patients with elevated IL-6 have decreased event free survival when compared to those without. Patients with MYCN amplification do not have significantly different IL-6 levels at presentation than those without, suggesting elevated levels are independent of this known risk factor. It is not, however, independent of the current overall risk stratification system as patients classified as high risk have significantly higher IL-6 levels at diagnosis.
When we investigated bone marrow as a potential microenvironment source for the IL-6, the marrow levels were again significantly increased when samples were stratified by the patient's risk category. BM IL-6 levels in patients with metastatic disease and specifically those with cortical bone lesions approached significance but did not achieve it. One potential cause for the lack of significance may be the number of bone marrow samples available for study. For this reason, we are unable to draw conclusions from this work as to whether the bone marrow microenvironment is a source of IL-6.
It is unclear where the source of the IL-6 lies in neuroblastoma, though it is likely that there is more than one source at play. Given its pleiotropic nature, it is possible that the PB IL-6 levels are indicative of a systemic response to the tumor. The consistency of IL-6 level elevations in the blood more than the bone marrow would support this. Still, the IL-6 may be coming from the tumor or the tumor microenvironment. As mentioned in the introduction, some cell line work has supported constitutive production of IL-6 by NB cell lines (14,32). More recently, though, it has been published that PCR analysis of five NB cell lines failed to demonstrate IL-6 mRNA, suggesting that the tumor cells themselves are not the source of the IL-6(10). In the same work, it was proposed that the IL-6 present in the bone marrow may come from the bony microenvironment. The presence of neuroblastoma cells was shown to induce the bone marrow mesenchymal cells to secrete IL-6 which in turn caused osteoclast activation. This same group has added to the picture by proposing that IL-6 stimulates proliferation and survival of neuroblastoma cells in the bone marrow microenvironment(33). This would suggest that the elevated levels seen in the presence of metastatic disease are part of a neuroblastoma stimulated pro-proliferation loop similar to the one seen in multiple myeloma.
In comparison to IL-6 levels, sIL-6R levels followed an inverse relationship with disease such that lower levels were present in the peripheral blood of patients with metastatic disease and with bone marrow disease. This finding was in contrast to previously published work in prostate cancer which showed an increase in sIL-6R levels was a poor prognostic factor(27). We were not able to correlate IL-6 levels with sIL-6R levels either directly or inversely in individual patients (data not shown). While sIL-6R levels varied in the bone marrow samples, we found no correlation with disease state. As with IL-6, two potential roles for sIL-6R emerge from this data. The first is as a systemic consumption response to aggressive disease as evidenced by the decreased circulating levels. In this model, the sIL-6R levels are decreased in the presence of metastatic disease. The second potential role is as part of the bone marrow microenvironment as evidenced by the persistent presence of sIL-6R in the bone marrow. These observations add to the argument that the IL-6 pathway functions in multiple ways in neuroblastoma.
We postulate that the significance of elevated circulating levels of IL-6 and decreased circulating levels of sIL-6R in neuroblastoma is two-fold. First, IL-6 and its receptor may have roles as peripheral blood markers of active, aggressive disease neuroblastoma. While IL-6 and sIL-6R levels do not stratify patients at diagnosis independent of the already established risk categorization, they may prove beneficial as indicators of active disease which could be used to monitor patients for relapse. Many techniques have been described as markers for residual or returning disease in patients with neuroblastoma including immunocytology, RT-PCR, and flow cytometry(34,35). These techniques, however, are dependent on the circulation of neuroblastoma cells for successful detection. As a circulating cytokine which is a reaction to tumor growth, IL-6 and its receptor may represent useful alternatives to these techniques. As we have shown, they are readily detectable in the peripheral blood through a commercially available assay. This would follow the model of a marker such as ferritin, previously described as a prognostic marker in neuroblastoma (36,37). The significant contrast between peripheral blood levels in the presence of high risk disease as compared to those in patients with low risk disease or healthy pediatric controls along with the ease of the assay recommend further investigation into the utility of IL-6 and sIL-6R levels to monitor for tumor recurrence. To address this, a longitudinal study measuring IL-6 levels in a group of patients at diagnosis and then throughout treatment, remission, and relapse is indicated.
The second potential significance of IL-6 in neuroblastoma is as a new therapeutic pathway. If, as the literature suggests, IL-6 is mediating the aggression and growth of neuroblastoma, elevated circulating levels of IL-6 and its receptor may identify patients for whom the IL-6 complex is a therapeutic target. Anti-IL-6 and anti-IL-6 receptor agents have been studied in both pre-clinical and clinical trials with moderate success in multiple myeloma, where IL-6 is known to be a stimulator of growth in a positive feed back loop fashion(18,19,38,39). Additionally, tocilizumab, a humanized anti-IL-6 receptor antibody has been trialed successfully in both adults and children with rheumatologic conditions, where IL-6 is known to play a role (40,41). One potential downside to the use of IL-6 antagonists is brought up by the work of Hatzi, et al. which suggested that IL-6 actually inhibits neuroblastoma angiogenesis and therefore acts to limit tumor size (14). Because of this potentially protective effect of IL-6, anti-IL-6 and anti- IL-6R agents will need to be investigated in closely in pre-clinical models prior to human use.
In addition to its discussion of IL-6 and sIL-6R in neuroblastoma patients, this paper also presents normal pediatric peripheral blood IL-6 and sIL-6R levels. Although adult baseline values were recently published(42), this is, to our knowledge, the first time pediatric normal values have appeared in the literature. The range and mean values were similar to those seen in adult study.
To conclude, peripheral blood levels of IL-6 and sIL-6R may have a role as active disease markers in high risk neuroblastoma. Further study of their roles in a larger cohort of patients with neuroblastoma is warranted.
Acknowledgments
Grant Support: National Cancer Institute grant NIH-NCI 1 K12 CA90433-04
Footnotes
Statement of Translational Relevance:
The following paper describes levels of IL-6 and soluble IL-6 receptor (sIL-6R) in the peripheral blood and bone marrow of patients with neuroblastoma at diagnosis. The paper finds that IL-6 and, to a lesser extent, sIL-6R, can differentiate patients with poor risk factors e.g. high risk disease and/or metastatic disease, from other neuroblastoma patients. The clinical relevance of these findings are two-fold. First, IL-6 and sIL6R may have roles as peripheral blood markers of active, aggressive disease neuroblastoma. Second, IL-6 and sIL-6R may have use in neuroblastoma as a new therapeutic pathway. If, as the literature suggests, IL-6 is mediating the aggression and growth of neuroblastoma, elevated circulating levels of IL-6 and its receptor may identify patients for whom the IL-6 complex is a therapeutic target. Additionally, this paper provides normal pediatric values for peripheral blood IL-6 and sIL-6R levels which can be used for reference for future studies.
Reference List
- 1.Goodman MT, Gurney JG, Smith M, Olsham A. Sympathetic Nervous System Tumors. In: Ries L, Smith MA, Gurney JG, et al., editors. SEER: Pediatric Monograph. National Cancer Institute; Bethesda: 1999. pp. 65–72. [Google Scholar]
- 2.Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo P, Poplack D, editors. Principles and practice of pediatric oncology. Lippincott Williams and Wilkins; Philadelphia: 2006. pp. 993–70. [Google Scholar]
- 3.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 4.Matthay KK, Perez C, Seeger RC, et al. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children's Cancer Group study. J Clin Oncol. 1998;16:1256–64. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Perez CA, Matthay KK, Atkinson JB, et al. Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: a children's cancer group study. J Clin Oncol. 2000;18:18–26. doi: 10.1200/JCO.2000.18.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 7.Lau L, Tai D, Weitzman S, Grant R, Baruchel S, Malkin D. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol Oncol. 2004;26:227–32. doi: 10.1097/00043426-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005;17:7–13. doi: 10.1097/01.mop.0000150631.60571.89. [DOI] [PubMed] [Google Scholar]
- 9.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 10.Sohara Y, Shimada H, Minkin C, Erdreich-Epstein A, Nolta JA, DeClerck YA. Bone marrow mesenchymal stem cells provide an alternate pathway of osteoclast activation and bone destruction by cancer cells. Cancer Res. 2005;65:1129–35. doi: 10.1158/0008-5472.CAN-04-2853. [DOI] [PubMed] [Google Scholar]
- 11.Lauta VM. A review of the cytokine network in multiple myeloma: diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97:2440–52. doi: 10.1002/cncr.11072. [DOI] [PubMed] [Google Scholar]
- 12.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer. 2007;110:1911–28. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 13.Candi E, Knight RA, Spinedi A, Guerrieri P, Melino G. A possible growth factor role of IL-6 in neuroectodermal tumours. J Neurooncol. 1997;31:115–22. doi: 10.1023/a:1005706019048. [DOI] [PubMed] [Google Scholar]
- 14.Hatzi E, Murphy C, Zoephel A, et al. N-myc oncogene overexpression down-regulates IL-6; evidence that IL-6 inhibits angiogenesis and suppresses neuroblastoma tumor growth. Oncogene. 2002;21:3552–61. doi: 10.1038/sj.onc.1205440. [DOI] [PubMed] [Google Scholar]
- 15.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Akira S. IL-6-regulated transcription factors. Int J Biochem Cell Biol. 1997;29:1401–18. doi: 10.1016/s1357-2725(97)00063-0. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006;97:439–47. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honemann D, Chatterjee M, Savino R, et al. The IL-6 receptor antagonist SANT-7 overcomes bone marrow stromal cell-mediated drug resistance of multiple myeloma cells. Int J Cancer. 2001;93:674–80. doi: 10.1002/ijc.1388. [DOI] [PubMed] [Google Scholar]
- 19.Tassone P, Neri P, Burger R, et al. Combination therapy with interleukin-6 receptor superantagonist Sant7 and dexamethasone induces antitumor effects in a novel SCID-hu In vivo model of human multiple myeloma. Clin Cancer Res. 2005;11:4251–8. doi: 10.1158/1078-0432.CCR-04-2611. [DOI] [PubMed] [Google Scholar]
- 20.George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res. 2005;11:1815–20. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen LM, Klausen TW, Davidsen UH, Johnsen HE. Early changes in serum IL-6 and VEGF levels predict clinical outcome following first-line therapy in aggressive non-Hodgkin's lymphoma. Ann Hematol. 2005;84:510–6. doi: 10.1007/s00277-005-1020-x. [DOI] [PubMed] [Google Scholar]
- 22.el-Far M, Fouda M, Yahya R, el-Baz H. Serum IL-10 and IL-6 levels at diagnosis as independent predictors of outcome in non-Hodgkin's lymphoma. J Physiol Biochem. 2004;60:253–8. doi: 10.1007/BF03167070. [DOI] [PubMed] [Google Scholar]
- 23.Riedel F, Zaiss I, Herzog D, Gotte K, Naim R, Hormann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25:2761–5. [PubMed] [Google Scholar]
- 24.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007;102:129–35. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 25.Mouawad R, Rixe O, Meric JB, Khayat D, Soubrane C. Serum interleukin-6 concentrations as predictive factor of time to progression in metastatic malignant melanoma patients treated by biochemotherapy: a retrospective study. Cytokines Cell Mol Ther. 2002;7:151–6. doi: 10.1080/13684730210002328. [DOI] [PubMed] [Google Scholar]
- 26.Soubrane C, Rixe O, Meric JB, Khayat D, Mouawad R. Pretreatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with biochemotherapy: a retrospective study. Melanoma Res. 2005;15:199–204. doi: 10.1097/00008390-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kattan MW, Shariat SF, Andrews B, et al. The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol. 2003;21:3573–9. doi: 10.1200/JCO.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Kyrtsonis MC, Dedoussis G, Zervas C, et al. Soluble interleukin-6 receptor (sIL-6R), a new prognostic factor in multiple myeloma. Br J Haematol. 1996;93:398–400. doi: 10.1046/j.1365-2141.1996.4721018.x. [DOI] [PubMed] [Google Scholar]
- 29.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–4. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 30.Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol. 2003;84:151–9. doi: 10.1002/jso.10305. [DOI] [PubMed] [Google Scholar]
- 31.Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J. Cytokine serum levels in soft tissue sarcoma patients: correlations with clinico-pathological features and prognosis. Int J Cancer. 2002;100:463–71. doi: 10.1002/ijc.10496. [DOI] [PubMed] [Google Scholar]
- 32.Knezevic-Cuca J, Stansberry KB, Johnston G, et al. Neurotrophic role of interleukin-6 and soluble interleukin-6 receptors in N1E-115 neuroblastoma cells. J Neuroimmunol. 2000;102:8–16. doi: 10.1016/s0165-5728(99)00151-4. [DOI] [PubMed] [Google Scholar]
- 33.Ara T, Seeger RC, DeClerck YA. Abstract # 160: Bone marrow stromal-derived interleukin-6 stimulates proliferation and survival of neuroblastoma cells in the bone marrow microenvironment. Advances in Neuroblastoma Research. 12th Annual Conference; May 17−20 2006. [Google Scholar]
- 34.Corrias MV, Faulkner LB, Pistorio A, et al. Detection of neuroblastoma cells in bone marrow and peripheral blood by different techniques: accuracy and relationship with clinical features of patients. Clin Cancer Res. 2004;10:7978–85. doi: 10.1158/1078-0432.CCR-04-0815. [DOI] [PubMed] [Google Scholar]
- 35.Beiske K, Ambros PF, Burchill SA, Cheung IY, Swerts K. Detecting minimal residual disease in neuroblastoma patients-the present state of the art. Cancer Lett. 2005;228:229–40. doi: 10.1016/j.canlet.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 36.Hann HW, Levy HM, Evans AE. Serum ferritin as a guide to therapy in neuroblastoma. Cancer Res. 1980;40:1411–3. [PubMed] [Google Scholar]
- 37.Hann HW, Evans AE, Siegel SE, et al. Prognostic importance of serum ferritin in patients with Stages III and IV neuroblastoma: the Childrens Cancer Study Group experience. Cancer Res. 1985;45:2843–8. [PubMed] [Google Scholar]
- 38.Rossi JF, Fegueux N, Lu ZY, et al. Optimizing the use of anti-interleukin-6 monoclonal antibody with dexamethasone and 140 mg/m2 of melphalan in multiple myeloma: results of a pilot study including biological aspects. Bone Marrow Transplant. 2005;36:771–9. doi: 10.1038/sj.bmt.1705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau P, Harousseau JL, Wijdenes J, Morineau N, Milpied N, Bataille R. A combination of anti-interleukin 6 murine monoclonal antibody with dexamethasone and high-dose melphalan induces high complete response rates in advanced multiple myeloma. Br J Haematol. 2000;109:661–4. doi: 10.1046/j.1365-2141.2000.02093.x. [DOI] [PubMed] [Google Scholar]
- 40.Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–29. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 41.Woo P, Wilkinson N, Prieur AM, et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–R1288. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klabusay M, Kohutova V, Coupek P, Nenickova M, Tesarvoa E. Simultaneous analysis of cytokines and co-stimulatory molecules concentrations by ELISA technique andof probabilities of measurable concentrations of interleukins IL-2, IL-4, IL-5, IL-6, CXCL8 (IL-8), IL-10, IL-13 occurring in plasma of healthy blood donors. Mediators of Inflammation. 2006;2006:1–7. doi: 10.1155/MI/2006/65237. [DOI] [PMC free article] [PubMed] [Google Scholar]