Abstract
BACKGROUND CONTEXT
The intervertebral disc is a common source of low back pain. Prospective studies suggest that treatments that intermittently distract the disc might be beneficial for chronic low back pain. Although the potential exists for distraction therapies to affect the disc biomechanically their effect on intradiscal stress is debated.
PURPOSE
To determine if distraction alone, distraction combined with flexion or distraction combined with extension can reduce nucleus pulposus pressure and posterior anulus compressive stress in cadaveric lumbar discs compared to simulated standing or lying.
STUDY DESIGN
Laboratory study using single cadaveric motion segments.
OUTCOME MEASURES
Strain gauge measures of nucleus pulposus pressure and compressive stress in the anterior and posterior annulus fibrosus
METHODS
Intradiscal stress profilometry was performed on 15 motion segments during 5 simulated conditions: standing, lying, and 3 distracted conditions. Disc degeneration was graded by inspection from 1 (normal) to 4 (severe degeneration).
RESULTS
All distraction conditions markedly reduced nucleus pressure compared to either simulated standing or lying. There was no difference between distraction with flexion and distraction with extension in regard to posterior annulus compressive stress. Discs with little or no degeneration appeared to distributed compressive stress differently than those with moderate or severe degeneration.
CONCLUSIONS
Distraction appears to predictably reduce nucleus pulposus pressure. The effect of distraction therapy on the distribution of compressive stress may be dependent in part on the health of the disc.
Keywords: Lumbar Spine, Spinal Manipulation, Intervertebral Disk, Biomechanics, Stress Profilometry
Introduction
Low back pain (LBP) is a ubiquitous problem in developed countries. The cost of LBP to the United States economy is estimated to be more than 100 billion dollars annually [1, 2]. The relationship between disc degeneration and back pain is incompletely understood. Disc degeneration is a progressive process that results in biomechanical compromise of the motion segment. Nucleus pulposus pressure decreases in proportion to the degree of degeneration in persons with chronic LBP [3]. The tensile modulus and Poisson’s ratio of the anulus fibrosus are likewise reduced [4]. As a result, anulus fibrosus fibers fail at lower loads leading to further degeneration [5] and abnormal spinal motion [6-8]. Although the course of disc degeneration cannot be predictably altered many investigators are seeking ways to enhance disc physiology and retard or reverse degeneration.
Many treatments using traction (axial distraction) have been devised in an attempt to relieve LBP by affecting the disc and nerve roots. A meta-analysis of the traction literature concluded that, as a group, there was no evidence that traction therapies were beneficial for LBP [9]. Nonetheless, some randomized trials have suggested that chronic LBP might be relieved by traction methods [10-13] and these treatments continue to be used in practice. The most commonly used methods are intermittent axial traction (which includes proprietary devices such as VAX-D, and DRX9000) and distraction-manipulation. Distraction manipulation combines axial distraction with intermittent off-axis moments, usually flexion or extension. It is different than typical spinal manipulative therapy which uses a high velocity impulse during treatment. It is commonly used by chiropractors [14] as well as physical therapists and osteopathic and medical physicians.
Several mechanisms have been proposed to explain how distraction therapies might affect the disc. These include reducing nucleus pulposus pressure, changing the position of the nucleus relative to the posterior anulus, reducing posterior anulus stress, and changing the disc-nerve interface. [15, 16] [17] Although both axial distraction and distraction-manipulation may temporarily reduce nucleus pulposus pressure, [18, 19] their effect on the distribution of stress in the disc is unknown.
The objective of this study was to determine the effect of distraction therapies (axial distraction, distraction with flexion, and distraction with extension) on vertical (compressive) and horizontal stress in anterior anulus, posterior anulus, and nucleus pulposus regions of the disc. We used the technique of intradiscal stress profilometry to estimate the stress in human cadaver motion segments under 5 conditions [20, 21]. We hypothesized that all 3 forms of distraction would significantly reduce nucleus pulposus stress compared to axial loads simulating standing or sitting. We also hypothesized that distraction with flexion would reduce posterior disc stress more than axial distraction or distraction with extension. Finally, we sought to determine if degenerative discs were affected by distraction differently than relatively healthy ones.
Materials and Methods
Specimens
Ten fresh, frozen (-20 degrees C) cadaveric lumbar spines (L1-S1) with mean age of 66.4 years (SD 13.8 years, range 40 to 82) were chosen for testing. These spines were screened for HIV/AIDS, Hepatitis B and C, tuberculosis, and Creutzfeldt - Jakob disease. Prospective specimens were imaged with anterior-posterior and lateral radiographs; those with severe osteoporosis, post-traumatic deformity, bone pathology or significant anatomical anomaly were excluded. Spines with diffuse (multi-level), severe degenerative changes that might make stress profilometry testing difficult were also excluded.
Cadavers were thawed overnight in a refrigerator and L1 through S3 was removed en bloc. The iliotransverse ligaments were sacrificed in this process. Non-ligamentous soft tissues were then removed leaving intact the lumbar vertebral bodies and all ligamentous structures including anterior longitudinal ligament, posterior longitudinal ligament, interspinous ligaments, intertransverse ligaments and facet joint capsules. Each specimen was divided into either 2 or 3 separate vertebra-disc-vertebra units, yielding 25 motion segments. K-wires were placed in the vertebral bodies and facet joints and each motion segment was potted in circular acrylic fixtures using polymethylmethacrylate. A custom jig kept the superior and inferior fixtures parallel to each other and to the plane of the disc. Specimens were kept moist with saline soaked toweling during preparation and testing.
Preliminary testing
The degree of distraction force and flexion-extension moments necessary to simulate these treatments in isolated motion segments was unknown. Studies have reported the amount of vertebral displacement occurring during traction [22-24] and distraction-manipulation [16] in vivo or in cadavers. Therefore, we conducted preliminary tests with 4 randomly chosen lumbar motion segments to estimate the forces needed to produce similar displacements. Distraction of 90 N produced an average increase in posterior disc height of 1.8 mm. Adding a pure moment of 5 Nm to the distracted motion segments produced an average angular displacement of 4.3 degrees in flexion and 4.5 degrees in extension. These displacements were similar to those previously reported to occur during distraction. An axial load of 500 N was used to simulate the load on the lumbar spine during quiet standing and 300 N to simulate lying (non-weight bearing) [25].
The transducer (Model OrthoAR, Medical Measurements, Inc., Hackensack, NJ, USA) was previously shown to accurately measure positive hydrostatic pressure up to 2 MPa [26] but the linearity of measurements in the negative range had not been reported. Because negative values might be encountered during distraction, negative pressure values (from a custom calibration chamber) were plotted against the transducer output. The response was linear to -30 kPa (-225 mmHg) with R2 = 0.9996. This range includes the negative pressures reported to occur during distraction therapies [18, 19].
Intradiscal stress profilometry technique
The technique of intradiscal stress profilometry was performed as described by McNally et al [20]. Measurements were obtained with a high-pressure strain gauge transducer mounted on a blunt 1.3 mm × 15 cm needle. By pulling the transducer through the disc at a constant rate a ‘stress profile’ is produced. Stress in the normal nucleus pulposus is isotropic (equal in all directions) but stress in the anulus is typically anisotropic. Therefore the transducer was oriented to measure both the vertical and horizontal stress values in each specimen. The transducer output from the anulus (oriented to detect vertical or compressive stress) has been shown to be proportional to the compressive stress perpendicular to the transducer sensing surface [21].
Biomechanical testing set-up
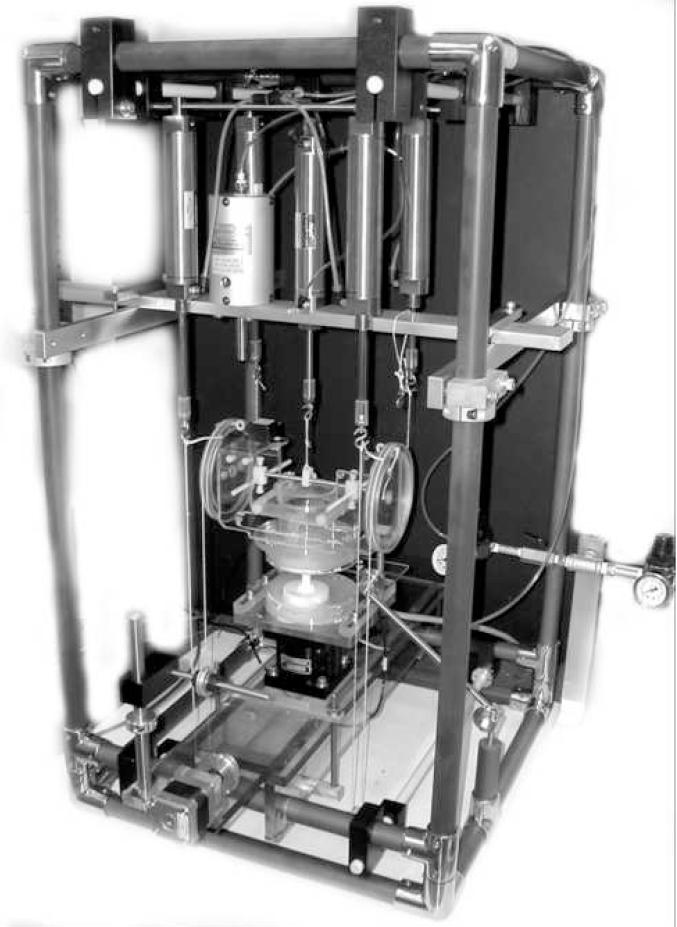
The potted motion segments were attached to a custom testing device that could apply pure bending moments and axial compression or distraction simultaneously (Figure 1). The lower vertebra was centered on a 6 degree-of-freedom load cell (JR3, Woodland, CA, USA) and maintained in a neutral (0 moment) position with respect to the global coordinate system. Compression and distraction loads were applied to the upper vertebra using pneumatic actuators. Pure moments in flexion or extension were applied with a pulley apparatus fixed to the upper acrylic fixture with force supplied by pneumatic actuators. Angular displacement of the upper and lower fixtures (relative to the transverse or X axis) was measured with miniature tilt sensors with an accuracy of 0.03 degrees RMS over their 20 degree range (Model CXTLA02, Crossbow Technology Inc., San Jose, CA, USA). The transducer was extracted by a stepper motor/pulley system that pulled a cable attached to the needle hub at 2mm/second. LabVIEW software (National Instruments Inc., Austin, TX, USA) was used for data acquisition and to control transducer extraction. Data was collected at 30 Hz. The transducer was calibrated using a custom pressure chamber and a known amount of positive and negative pressure before tests.
Fig. 1.
Spine testing apparatus
Biomechanical Testing
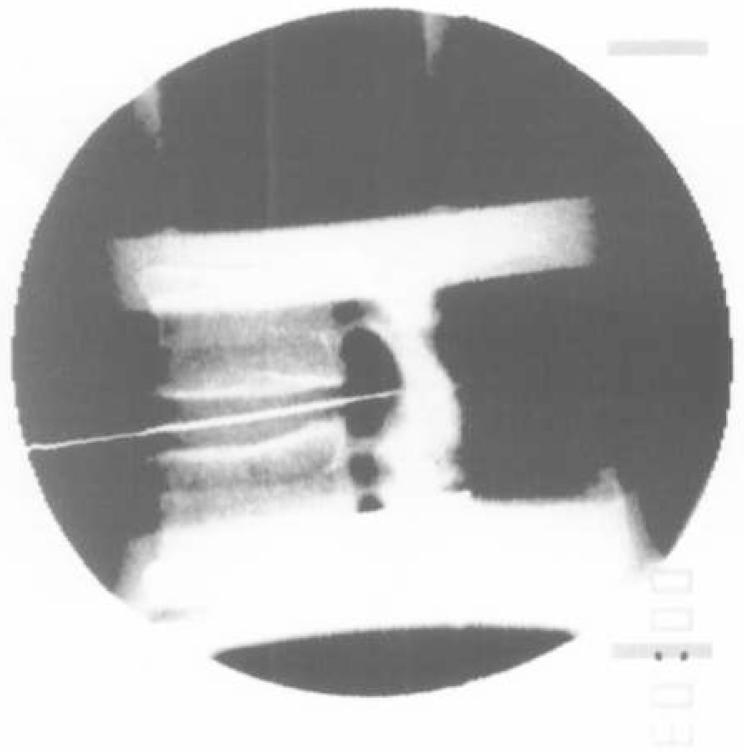
The 21 remaining motion segments were tested in the same manner. A preload of 300 N compression was applied for 30 minutes to expel excess fluid [20]. A 1.3 mm spinal needle with stylet (ground to a point) was then introduced into the anterior disc and advanced in the mid-sagittal plane through the posterior anulus under fluoroscopic guidance (Figure 2). This created a track for the transducer midway between the vertebral endplates. The guide needle was removed and the blunt transducer needle with transducer inserted and oriented to measure the vertical component of stress. The first condition was then applied to the motion segment. A cable from the needle hub to a stepper motor/pulley was properly aligned and the transducer withdrawn at 2mm/second. The needle was then reinserted to measure the horizontal component of stress and again extracted. Each of 5 test conditions were applied in a constant order: 1) axial compression 300 N (simulation of non-weight bearing or lying) [25], 2) axial compression 500 N (simulation of relaxed standing) [25], 3) Axial distraction 90 N (simulation of axial distraction in neutral or traction), 4) Axial distraction 90 N and extension 5 Nm (simulation of extension-distraction), and 5) Axial distraction 90 N and flexion 5 Nm (simulation of flexion-distraction). There was at least 1 minute between conditions to allow for viscoelastic recovery.
Fig. 2.
Path of guide needle and transducer in the intervertebral disc
Grading of disc degeneration
After testing, each disc was sectioned in sagittal and coronal planes and graded by 2 observers (an orthopedic spine surgeon and a rehabilitation physician) as normal (grade 1), mild, moderate, or severe (grades 2, 3, or 4 respectively) according to the scale of Adams et al [5]. In the case of a disagreement between observers a third observer (an orthopedic spine surgeon) determined the final grade. All graders were blinded to results of individual motion segment tests.
Data Reduction and Analysis
The relative stress values in the posterior, middle and anterior disc regions were examined by partitioning the data into thirds. Since these regions could best be identified on profiles collected during compressive loading, each 500 N stress profile was reviewed to ensure that the middle third of the data was consistent with the hydrostatic region, which represented the functional nucleus pulposus [20]. The anterior and posterior thirds of the data (excluding the outermost data points with a precipitous drop in stress) was taken to represent the anterior and posterior disc regions (anulus fibrosus). Vertical and horizontal data were analyzed separately for each test condition in each motion segment. Peak vertical stress values were calculated for the anterior and posterior regions by averaging the single highest point value with the point values before and after it (an average of 3 point values).
The effect of the 5 conditions on regional vertical and horizontal stress values was examined using repeated measures ANOVA. When global F-tests were significant (p<0.05) pair-wise comparisons (contrasts) of nucleus stress (pressure) were made between the axial compression (500 N and 300 N) and each of the 3 distracted conditions. Because of the limited number of motion segments the degenerative grades were collapsed into low degeneration (grades 1 and 2) and high degeneration (grades 3 and 4) groups. The effect of test condition and degeneration were evaluated using two-way ANOVA with repeated measures; generalized estimating equations were employed to account for the correlation of the data within motion segments. Following this, analysis using one-way ANOVA for repeated measures was performed by degenerative group. Finally, the distribution of vertical stress among the anterior, nucleus and posterior disc regions was qualitatively examined in each of the 5 conditions. Analyses were carried out with SAS (SAS Institute Inc., Cary, NC). An α level of 0.05 (two-tailed) was used for all tests.
Results
Three motion segments from a single spine (L1-2, L3-4, and L5-S1) were excluded due to unexpected pathology found upon grading. Two more L5-S1 motion segments could not be tested due to difficulty obtaining stable potting, so the one remaining L5-S1 segment was excluded. Data from the remaining 15 motion segments (9 lumbar spines) were analyzed. Distribution of disc levels was L1-2 (2), L2-3 (5), L3-4 (3), and L4-5 (5). The effect of disc level was not formally examined due to the small sample size and the risk of type II error; nonetheless, ANOVA indicated no large differences between disc levels suggesting that pooling of the levels was appropriate.
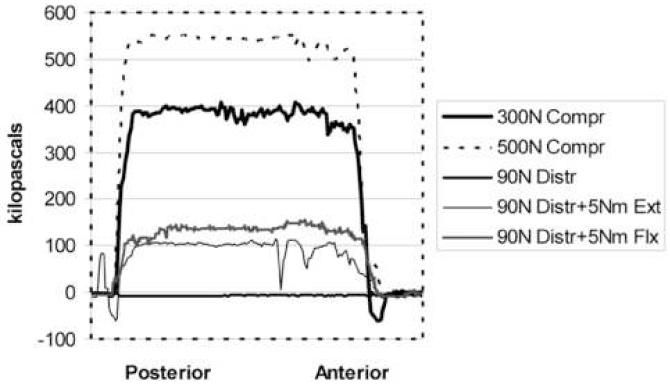
Distribution of degenerative grades was grade 1 (3), grade 2 (5), grade 3 (4), and grade 4 (3). This resulted in 8 in the low degeneration group and 7 in the high degeneration group. Only one cadaver was female. Figure 3 shows a representative set of vertical stress profiles for 5 conditions recorded from a single disc with mild (grade 2) degeneration. These profiles are representative of the raw data collected.
Fig. 3.
Vertical stress profiles in a grade 2 (mildly degenerated) L3-4 motion segment (5 conditions)
Regional vertical and horizontal stress values
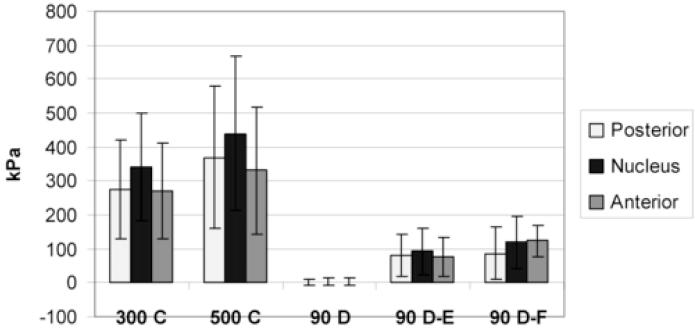
Table 1 shows the regional vertical and horizontal stress values for the 5 conditions for all specimens combined, low degeneration discs (n = 8) and high degeneration discs (n = 7). The regional (mean) vertical stress values for all specimens combined during each of the 5 conditions are shown graphically in Figure 4. The vertical and horizontal stress values in each disc region are compared in Table 2. Vertical and horizontal values were statistically different (paired t-tests) only in the anterior disc region and only for some conditions. Vertical and horizontal peak values in the posterior and anterior disc regions are also included in Table 2 but no statistical comparison was made as the peak values within a region did not always coincide with the same point value position.
Table 1.
Mean (SD) vertical and horizontal stress values (kPa) in 3 disc regions for 5 test conditions
| 300 N Compression | 500 N Compression | 90 N Distraction | 90 N Distraction, 5 Nm extension | 90 N Distraction, 5 Nm flexion | |
|---|---|---|---|---|---|
| Anterior-horizontal | |||||
| All | 231.4 (139.9) | 305.0 (188.8) | -0.7 (9.1) | 61.9 (59.0) | 104.6 (44.9) |
| Low | 302.4 (134.3) | 383.2 (202.3) | 2.1 (10.7) | 94.2 (53.0) | 111.8 (49.2) |
| High | 150.3 (101.0) | 215.6 (134.1) | -3.8 (6.1) | 25.0 (43.1) | 96.3 (41.6) |
| Anterior-vertical | |||||
| All | 269.9 (141.0) | 331.3 (185.6) | 3.1 (11.8) | 76.4 (56.5) | 124.6 (46.2) |
| Low | 345.7 (121.5) | 411.5 (190.0) | 5.5 (8.9) | 107.8 (48.5) | 135.6 (56.5) |
| High | 183.2 (112.8) | 239.7 (141.0) | 0.4 (14.8) | 40.6 (43.6) | 112.0 (30.3) |
| Nucleus-horizontal | |||||
| All | 337.9 (160.4) | 447.6 (228.8) | 0.9 (17.2) | 89.7 (74.1) | 120.7 (73.5) |
| Low | 434.1 (115.4) | 563.5 (222.8) | 7.3 (15.9) | 132.9 (66.1) | 146.6 (72.8) |
| High | 227.9 (134.2) | 315.2 (160.8) | -6.4 (16.7) | 40.3 (48.8) | 91.1 (67.2) |
| Nucleus-vertical | |||||
| All | 341.7 (158.1) | 439.9 (228.6) | 2.9 (10.3) | 92.5 (68.1) | 119.3 (76.6) |
| Low | 430.4 (123.1) | 552.9 (223.5) | 6.5 (9.1) | 130.2 (64.2) | 149.2 (76.3) |
| High | 240.3 (134.7) | 310.8 (164.9) | -1.3 (10.7) | 49.4 (44.1) | 85.1 (65.9) |
| Posterior-horizontal | |||||
| All | 287.6 (125.0) | 391.9 (207.5) | -0.7 (14.7) | 84.7 (59.9) | 91.1 (78.2) |
| Low | 355.3 (97.3) | 478.1 (211.9) | 2.0 (18.0) | 120.0 (51.6) | 123.3 (70.2) |
| High | 210.1 (110.9) | 293.5 (163.9) | -3.9 (10.3) | 44.4 (41.2) | 54.3 (74.5) |
| Posterior-vertical | |||||
| All | 275.5 (144.9) | 369.0 (210.3) | 1.3 (8.9) | 82.1 (62.5) | 87.3 (75.7) |
| Low | 345.4 (120.2) | 449.8 (217.6) | 2.3 (11.7) | 112.6 (66.2) | 113.8 (77.6) |
| High | 195.6 (134.7) | 276.8 (171.2) | 0.2 (4.8) | 47.3 (36.9) | 57.1 (66.1) |
All = all specimens combined (n = 15), Low = low degeneration (grades 1 & 2, n = 8), High = high degeneration (grades 3 & 4, n = 7), Posterior = posterior annulus, Nucleus = nucleus pulposus, Anterior = anterior annulus
Fig. 4.
Mean (SD) regional vertical (compressive) stress during 5 load conditions (all specimens, n = 15)
(C=compression, D=distraction, E=extension, F=flexion)
Differences between compression (either 300 N or 500 N) and each distraction condition were statistically significant in all disc regions (Repeated measures ANOVA with post hoc contrast tests, p < 0.001 for all comparisons)
Table 2.
Comparison of mean (SD) and peak vertical and horizontal stress (kPa) values in 5 conditions (n = 15)
| 300 N Compression | 500 N Compression | 90 N Distraction | 90 N Distraction, 5 Nm extension | 90 N Distraction, 5 Nm flexion | |
|---|---|---|---|---|---|
| Anterior v | 269.9 (141.0) | 331.3 (185.6) | 3.1 (11.8) | 76.4 (56.5) | 124.6 (46.3) |
| Anterior h | 231.4 (139.8) | 305.0 (188.8) | -0.7 (9.1) | 61.9 (58.0) | 104.6 (44.9) |
| P value* | 0.008 | 0.139 | 0.226 | 0.004 | 0.009 |
| Nucleus v | 341.7 (158.1) | 439.9 (228.6) | 2.9 (10.3) | 92.5 (68.1) | 119.3 (76.6) |
| Nucleus h | 337.9 (160.4) | 447.6 (228.8) | 0.9 (17.23) | 89.7 (74.1) | 120.7 (73.6) |
| P value* | 0.574 | 0.244 | 0.435 | 0.334 | 0.737 |
| Posterior v | 275.5 (144.9) | 369.0 (210.3) | 1.3 (8.9) | 82.1 (62.5) | 87.3 (75.7) |
| Posterior h | 287.6 (125.0) | 391.9 (207.5) | -0.7 (14.7) | 84.7 (59.9) | 91.1 (78.2) |
| P value* | 0.435 | 0.177 | 0.597 | 0.625 | 0.589 |
| Peak | |||||
| Posterior v | 380.7 (161.6) | 505.0 (220.3) | 42.8 (67.9) | 130.9 (59.4) | 123.9 (77.5) |
| Peak | |||||
| Posterior h | 388.4 (158.2) | 505.1 (236.7) | 21.7 (24.1) | 121.1 (72.5) | 146.2 (101.9) |
| Peak | |||||
| Anterior v | 367.5 (138.5) | 476.7 (207.7) | 31.1 (22.9) | 117.2 (66.5) | 198.8 (92.3) |
| Peak | |||||
| Anterior h | 336.9 (153.9) | 441.2 (223.1) | 11.9 (11.1) | 95.3 (70.9) | 159.6 (54.1) |
h = horizontal, v = vertical,
Paired t-test between vertical and horizontal values
Effect of distraction on disc stress measures
Vertical and horizontal stress values in the nucleus pulposus were nearly the same (Table 1) suggesting that the measures from the nucleus were consistent with nucleus pressure. Nucleus pressure, posterior vertical stress and anterior vertical stress were all significantly decreased in the 3 distracted conditions as compared to either 300 N or 500 N compression (pair-wise post hoc contrasts, p < 0.001 for each comparison). This was also true when both low and high degeneration groups were analyzed separately with 1 exception. Comparison of anterior vertical stress in high degeneration discs between 300 N compression and flexion-distraction was not significant (p = 0.76). Axial-distraction (without flexion or extension) yielded the lowest mean nucleus pressure. Compared to 300 N compression (simulated lying) nucleus pressure decreased 99% with axial-distraction, 73% with extension-distraction, and 65% with flexion-distraction. Statistical analysis of the differences between disc regions was not carried out. Axial-distraction (without flexion or extension) yielded the lowest mean nucleus pressure. Compared to 300 N compression (simulated lying) nucleus pressure decreased 99% with axial-distraction, 73% with extension-distraction, and 65% with flexion-distraction. Statistical analysis of the differences between disc regions was not carried out.
Effect of flexion-distraction and extension-distraction on stress distribution
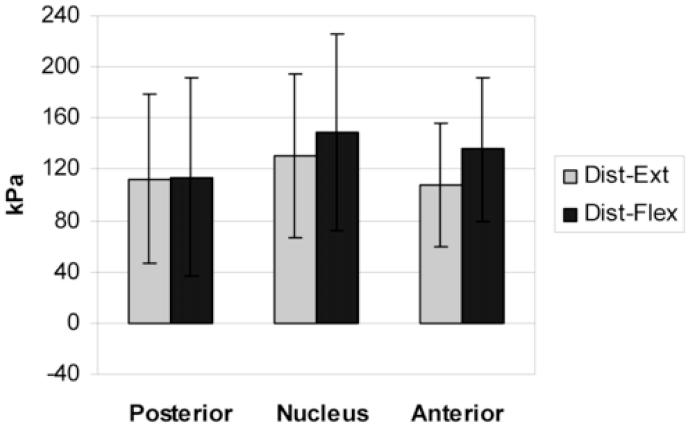
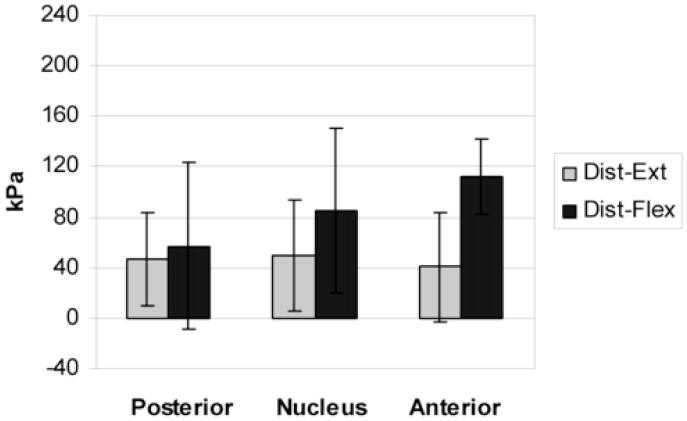
The mean vertical stress values in each disc region of low degeneration discs (grades 1 and 2) during flexion-distraction and extension-distraction are shown in Figure 5. The highest mean value for both conditions was in the nucleus. There was little difference between the 2 conditions in any disc region. The same data for high degeneration discs (grades 3 and 4) are shown in Figure 6. A formal statistical comparison was not made due to the small numbers in each group. Inspection of Figure 6 suggests no statistical difference in vertical (compressive) stress between distraction with flexion and distraction with extension. Yet, vertical stress appeared to be distributed differently in these conditions when the trend from anterior to posterior was considered. During extension-distraction of high degeneration discs vertical stress was greater in the posterior and nucleus regions and least in the anterior region. A very different pattern was seen during flexion-distraction. The vertical stress appears to decrease from anterior to posterior suggesting a gradient.
Fig. 5.
Mean (SD) regional vertical stress in low degeneration discs (grade 1 and 2) during extension-distraction and flexion-distraction (n = 8)
Fig. 6.
Mean (SD) regional vertical stress in high degeneration discs (grade 3 and 4) during extension-distraction and flexion-distraction (n = 7)
Discussion
In this experiment all 3 distraction conditions temporarily significantly reduced nucleus pressure compared to simulated standing and lying. The largest effect was observed during axial-distraction without flexion or extension which reduced pressure to near zero. Degenerated discs responded differently than relatively normal discs; they had greater temporary net reductions in nucleus pressure. Although not examined quantitatively, the distribution of stress among disc regions in normal or minimally degenerated discs (grade 1 or 2) was similar in flexion-distraction and extension-distraction. This could be due to the nucleus being pressurized and efficiently distributing the stress. In discs with higher amounts of degeneration (grades 3 and 4) the nucleus had much less pressure when extension or flexion was introduced indicating that stress distribution may have been dependent on the moment applied to the segment. Flexion-distraction resulted in compressive stress being temporarily qualitatively lower in the posterior region compared to the nucleus and anterior regions. Conversely extension-distraction of degenerated discs yielded similar vertical stress in all 3 regions.
Nucleus pulposus pressure has been used to calculate axial loads on the spine [25, 27]. This is appropriate because the normal nucleus acts as a fluid with the stress being hydrostatic or isotropic (equal in all directions). As such it is a scalar quantity that can be measured with strain gauge technology. Quantifying stress in the anulus is more problematic. Annular stress is not isotropic but anisotropic with different vertical and horizontal components [5]. Pressure and stress have the same SI unit of measure (Pascal). Although strain gauge transducers have been used to estimate stress in the anulus it is debatable exactly what the measurements represent. Rao et al interpreted the output from strain gauges placed in the anulus (to detect vertical stress) to be “intradiscal pressure in the axial direction” [28] despite the fact that pressure is non-directional. McMillan et al attempted to determine the validity of strain gauge transducer measures in the anulus and found the output of their transducer to be linearly proportional to the vertical force applied to the disc. They reasoned that the output was also proportional to the compressive stress perpendicular to the transducer membrane [21]. Interestingly, they found that the same calibration coefficient was applicable to liquids, nucleus pulposus, and all but the outer 2 to 4 mm of the anulus fibrosus. Although we recorded both horizontal and vertical stress components in this experiment, we were primarily interested in the ability of distraction to ‘unload’ the disc, i.e., reduce the vertical or compressive stress. Therefore we have referred to the vertical measures as vertical stress. Vertical stress measures in the nucleus were essentially the same as the horizontal measures, and therefore were interpreted as nucleus pressure.
The normal lumbar nucleus is displaced anteriorly by extension and posteriorly by flexion when lying [29, 30] but changes in nucleus pressure and position in degenerated discs are not as predictable [29, 31, 32] and degenerated discs have been noted to bulge posteriorly with extension [30, 33]. Our findings are consistent with reports that degenerated discs may respond differently than healthy discs to flexion and extension [30, 31, 33] and extends that observation to include flexion and extension combined with distraction. The qualitative differences we observed in stress distribution between relatively healthy and degenerated discs might be due to the degenerated discs being unable to generate or maintain nucleus pressure. They may also be explained in part by anatomy. When the motion segment is extended the facet joints contact each other and the center of rotation moves posteriorly toward the facets, causing the anterior disc space to widen. This effectively shields the posterior disc from further compression [32]. Conversely, flexion-distraction of degenerated discs may result in anterior compression and an anterior shift of the center of rotation. This appears to produce a stress distribution with the least compressive force in the posterior anulus. These observations suggest that the normal response of lumbar discs to flexion and extension is dependent to some extent on the health of the disc.
The primary mechanical theory underlying the use of distraction therapies for disc herniation is that they reduce nucleus pressure and pull peripheral nucleus tissue toward the center of the disc [34-36]. Distraction has been shown to produce temporary negative pressure in the nucleus of living patients [18]. Nucleus pressure in the present experiment became negative during axial-distraction in 4 of 8 low degeneration discs but in only 1 of 7 high degeneration discs. Gudavalli et al [19 177], recorded negative pressures during flexion-distraction in a whole cadaver model but we did not observe that in this study. This may have been due to violation of the annular ‘seal’ with the transducer, but that is unlikely considering the instruments used by Gudavalli et al were similar to the ones we used. Other possible explanations include dissimilar forces used during flexion-distraction or the difference between whole cadaver and single motion segment models. Gudavalli used intermittently applied, short-duration forces and continuous measurement. We measured pressures 1 to 2 minutes after the force was applied which might also explain this difference.
This study has several weaknesses that should be considered. First, a cadaver model may not accurately represent the response of the disc to loading in vivo. At this time there is no safe and acceptable method of obtaining similar in vivo measurements in humans. The age of tissue donors was generally older than persons presenting with discogenic back pain. The effects of freezing and thawing lumbar spine tissues is not thought to significantly affect the physical properties of human spine specimens [37]. Yet, dehydration and prolonged exposure to room temperatures are known to affect their material properties. The specimens in this experiment were kept moist [38] and the exposure to room temperature minimized. Our results were not likely affected by soft-tissue changes due to exposure. Second, the method we used to simulate treatments is most consistent with intermittent traction and lasting 1 to 2 minutes. It may not reflect the exact time course of stress change during shorter treatments such as distraction-manipulation. Third, although the output of the transducer we used has been shown to be proportional to the applied compressive stress (perpendicular to the sensing element), it may not provide a highly accurate measure of compressive stress. Nonetheless, it provides a reasonable measure of stress change within specimens [21]. Fourth, we excluded all L5-S1 motion segments from our data. The L5-S1 segment has different ligamentous anatomy and slightly different kinematics than the other lumbar segments. Further, it can be difficult to secure and test. We did encounter difficulties with potting and as a result elected to exclude the single L5-S1 motion segment with usable data from analysis. Fifth, the results must be considered carefully in light of the small sample size and risk of error. Yet, the study was designed as a repeated measures study to maximize the power.
Our findings provide insight into the mechanical effects of distraction therapies but they do not establish a mechanism by which distraction might benefit those with back pain or sciatica due to disc injury. It is possible that the motion or change in stress results in mechanobiological events that lead to pain relief or promote disc health [39, 40]. Studies using both animal and in vitro models have demonstrated that mechanical stress may play a role in the regulation of both degradative and anabolic processes in discs [41-43]. Kroeber et al [42] using a rabbit model found that degenerated discs (created by compression) treated with distraction had restoration of disc height and histological evidence of regeneration. Although the method of producing degeneration in that model can be questioned, the results provide preliminary evidence that distraction might potentially have a beneficial affect on disc physiology. Distraction might reduce local stress peaks in the anulus fibrosus which are thought to produce lower back pain [44]. Further studies are needed to establish a clear clinical benefit of distraction therapies. Additionally studies are needed to examine the relationship between stress distribution and clinical markers of disc biology such as the degree of nucleus hydration [45].
Acknowledgements
The authors would like to thank Jonathan Bridges for technical assistance. This work was supported by the National Center for Complementary and Alternative Medicine (NCCAM) grant # K07-AT00972.
All work was performed at Mayo Clinic, Rochester, MN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frymoyer JW, Durett CL. The economics of spinal disorders. In: Frymoyer JW, editor. The adult spine: Principles and practice. 2 ed. Vol. 1. Lippincott-Raven; Philadelphia: 1997. pp. 144–149. [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine. 1999;24:2468–2474. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20:2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara A, Tamai K, An HS, Kurihashi T, Lim TH, Yoshida H, et al. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J Spinal Disord. 2000;13:444–450. doi: 10.1097/00002517-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Okawa A, Shinomiya K, Komori H, Muneta T, Arai Y, Nakai O. Dynamic motion study of the whole lumbar spine by videofluoroscopy. Spine. 1998;23:1743–1749. doi: 10.1097/00007632-199808150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Takayanagi K, Takahashi K, Yamagata M, Moriya H, Kitahara H, Tamaki T. Using cineradiography for continuous dynamic-motion analysis of the lumbar spine. Spine. 2001;26:1858–1865. doi: 10.1097/00007632-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 9.Clarke JA, van Tulder MW, Blomberg SE, de Vet HC, van der Heijden GJ, Bronfort G. Traction for low-back pain with or without sciatica. Cochrane Database of Systematic Reviews. 2005:CD003010. doi: 10.1002/14651858.CD003010.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Gudavalli MR, Cambron JA, McGregor M, Jedlicka J, Keenum M, Ghanayem AJ, et al. A randomized clinical trial and subgroup analysis to compare flexion-distraction with active exercise for chronic low back pain. Eur Spine J. 2006;15:1070–1082. doi: 10.1007/s00586-005-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljunggren AE, Weber H, Larsen S. Autotraction versus manual traction in patients with prolapsed lumbar intervertebral discs. Scand J Rehabil Med. 1984;16:117–124. [PubMed] [Google Scholar]
- 12.Cambron JA, Gudavalli MR, Hedeker D, McGregor M, Jedlicka J, Keenum M, et al. One-year follow-up of a randomized clinical trial comparing flexion distraction with an exercise program for chronic low-back pain. J Altern Complement Med. 2006;12:659–668. doi: 10.1089/acm.2006.12.659. [DOI] [PubMed] [Google Scholar]
- 13.Sherry E, Kitchener P, Smart R. A prospective randomized controlled study of vax-d and tens for the treatment of chronic low back pain. Neurol Res. 2001;23:780–784. doi: 10.1179/016164101101199180. [DOI] [PubMed] [Google Scholar]
- 14.Christensen MG, Kerkhoff D, Kollasch MW. Job analysis of chiropractic. National Board of Chiropractic Examiners; Greeley, Colorado: 2000. [Google Scholar]
- 15.Cox JM, Feller J, Cox-Cid J. Distraction chiropractic adjusting: Clinical application and outcomes of 1,000 cases. Top Clin Chiropr. 1996;3:45–59. [Google Scholar]
- 16.Gudavalli MR. Biomechanics research on flexion-distraction procedure. In: Cox JM, editor. Low back pain: Mechanisms, diagnosis and treatment. 6 ed. Lippincott Williams & Wilkins; Philadelphia: 1998. pp. 263–268. [Google Scholar]
- 17.Knutsson E, Skoglund CR, Natchev E. Changes in voluntary muscle strength, somatosensory transmission and skin temperature concomitant with pain relief during autotraction in patients with lumbar and sacral root lesions. Pain. 1988;33:173–179. doi: 10.1016/0304-3959(88)90088-7. [DOI] [PubMed] [Google Scholar]
- 18.Ramos G, Martin W. Effects of vertebral axial decompression on intradiscal pressure. J Neurosurg. 1994;81:350–353. doi: 10.3171/jns.1994.81.3.0350. [DOI] [PubMed] [Google Scholar]
- 19.Gudavalli MR, Cox JM, Baker JA, Cramer GD, Patwardhan AG. Intervertebral disc pressure changes during a chiropractic procedure. Adv Bioeng. 1997;36:215–216. [Google Scholar]
- 20.McNally DS, Adams MA. Internal intervertebral disc mechanics as revealed by stress profilometry. Spine. 1992;17:66–73. doi: 10.1097/00007632-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 21.McMillan DW, McNally DS, Garbutt G, Adams MA. Stress distributions inside intervertebral discs: The validity of experimental “stress profilometry’. Proc Inst Mech Eng [H] 1996;210:81–87. doi: 10.1243/PIME_PROC_1996_210_396_02. [DOI] [PubMed] [Google Scholar]
- 22.Twomey LT. Sustained lumbar traction. An experimental study of long spine segments. Spine. 1985;10:146–149. doi: 10.1097/00007632-198503000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Awad EA. Effects of pelvic traction on the intervertebral disc, a radiological study. Arch Phys Med Rehabil. 1988;69:785. [Google Scholar]
- 24.Mathews JA. The effects of spinal traction. Physiotherapy. 1972;58:64–66. [PubMed] [Google Scholar]
- 25.Nachemson AL. Disc pressure measurements. Spine. 1981;6:93–97. doi: 10.1097/00007632-198101000-00020. [DOI] [PubMed] [Google Scholar]
- 26.McNally DS, Adams MA, Goodship AE. Development and validation of a new transducer for intradiscal pressure measurement. J Biomed Eng. 1992;14:495–498. doi: 10.1016/0141-5425(92)90102-q. [DOI] [PubMed] [Google Scholar]
- 27.Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Rao RD, Wang M, Singhal P, McGrady LM, Rao S. Intradiscal pressure and kinematic behavior of lumbar spine after bilateral laminotomy and laminectomy. Spine J. 2002;2:320–326. doi: 10.1016/s1529-9430(02)00402-3. [DOI] [PubMed] [Google Scholar]
- 29.Fennell AJ, Jones AP, Hukins DW. Migration of the nucleus pulposus within the intervertebral disc during flexion and extension of the spine. Spine. 1996;21:2753–2757. doi: 10.1097/00007632-199612010-00009. [DOI] [PubMed] [Google Scholar]
- 30.Beattie PF, Brooks WM, Rothstein JM, Sibbitt WL, Jr., Robergs RA, MacLean T, et al. Effect of lordosis on the position of the nucleus pulposus in supine subjects: A study using magnetic resonance imaging. Spine. 1994;19:2096–2102. doi: 10.1097/00007632-199409150-00017. [DOI] [PubMed] [Google Scholar]
- 31.Merriam WF, Quinnell RC, Stockdale HR, Willis DS. The effect of postural changes on the inferred pressures within the nucleus pulposus during lumbar discography. Spine. 1984;9:405–408. doi: 10.1097/00007632-198405000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Adams MA, May S, Freeman BJ, Morrison HP, Dolan P. Effects of backward bending on lumbar intervertebral discs. Relevance to physical therapy treatments for low back pain. Spine. 2000;25:431–437. doi: 10.1097/00007632-200002150-00007. [DOI] [PubMed] [Google Scholar]
- 33.Schnebel BE, Simmons JW, Chowning J, Davidson R. A digitizing technique for the study of movement of intradiscal dye in response to flexion and extension of the lumbar spine. Spine. 1988;13:309–312. doi: 10.1097/00007632-198803000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Cox JM, Hazen LJ, Mungovan M. Distraction manipulation reduction of an l5-s1 disk herniation. J Manipulative Physiol Ther. 1993;16:342–346. [PubMed] [Google Scholar]
- 35.Quellette JP. Low back pain: An orthopedic medicine approach. Can Fam Physician. 1987;33:693–694. [PMC free article] [PubMed] [Google Scholar]
- 36.Cyriax J. Textbook of orthopaedic medicine. 8th ed. Vol. 1. Bailliere Tindall; London: 1982. [Google Scholar]
- 37.Panjabi MM, Krag M, Summers D, Videman T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J Orthop Res. 1985;3:292–300. doi: 10.1002/jor.1100030305. [DOI] [PubMed] [Google Scholar]
- 38.Pflaster DS, Krag MH, Johnson CC, Haugh LD, Pope MH. Effect of test environment on intervertebral disc hydration. Spine. 1997;22:133–139. doi: 10.1097/00007632-199701150-00003. [DOI] [PubMed] [Google Scholar]
- 39.Iatridis JC, MacLean JJ, Roughley PJ, Alini M. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88(Suppl 2):41–46. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–981. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 41.MacLean JJ, Lee CR, Alini M, Iatridis JC. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–1127. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Kroeber M, Unglaub F, Guehring T, Nerlich A, Hadi T, Lotz J, et al. Effects of controlled dynamic disc distraction on degenerated intervertebral discs: An in vivo study on the rabbit lumbar spine model. Spine. 2005;30:181–187. doi: 10.1097/01.brs.0000150487.17562.b1. [DOI] [PubMed] [Google Scholar]
- 43.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–337. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 44.McNally DS, Shackleford IM, Goodship AE, Mulholland RC. In vivo stress measurement can predict pain on discography. Spine. 1996;21:2580–2587. doi: 10.1097/00007632-199611150-00007. [DOI] [PubMed] [Google Scholar]
- 45.Han SM, Lee SY, Cho MH, Lee JK. Disc hydration measured by magnetic resonance imaging in relation to its compressive stiffness in rat models. Proc Inst Mech Eng [H] 2001;215:497–501. doi: 10.1243/0954411011536091. [DOI] [PubMed] [Google Scholar]