Summary
There has been a great deal of interest in identifying potential biomarkers of aging (Butler et al. 2004). Biomarkers of aging would be useful to predict potential vulnerabilities in an individual that may arise well before they are chronologically expected, due to idiosyncratic aging rates that occur between individuals. Prior attempts to identify biomarkers of aging have often relied on the comparisons of long-lived animals to a wild-type control (Dhahbi et al. 2004). However, the effect of interventions in model systems that prolong lifespan (such as single gene mutations, or caloric restriction) can sometimes be difficult to interpret due to the manipulation itself having multiple unforeseen consequences on physiology, unrelated to aging itself (Gems et al. 2002; Partridge & Gems 2006). The search for predictive biomarkers of aging therefore is problematic, and the identification of metrics that can be used to predict either physiological or chronological age would be of great value (Butler et al. 2004). One methodology which has been used to identify biomarkers for numerous pathologies is gene expression profiling. Here, we report whole-genome expression profiles of individual wild-type Caenorhabditis elegans covering the entire wild-type nematode life span. Individual nematodes were scored for either age-related behavioral phenotypes, or survival, and then subsequently associated with their respective gene expression profiles. This facilitated the identification of transcriptional profiles that were highly associated with either physiological or chronological age. Overall, our approach serves as a paradigm for identifying potential biomarkers of aging in higher organisms that can be repeatedly sampled throughout their lifespan.
Introduction
Aging is a time dependent increase in fragility that results in acceleration of the mortality rate as a function of time (Finch 1990). Aging is often considered a nonspecific degeneration resulting from accumulated damage due to environmental exposure or normal metabolism (Beckman & Ames 1998; Kenyon & Gerson 2007). However, there is an underlying genetic component to aging, since each species has a characteristic lifespan, and a characteristic rate of aging that can be visualized as a regular doubling of the mortality rate with time(Finch & Pike 1996). Identifying the underlying genetic components and how they interact with environmental forces to cause the changes associated with aging has been the subject of intense research(Antebi 2007).
According to evolutionary theory, selection pressure is reduced once an organism passes reproductive age. Consistent with this, several theories of aging have been proposed, two of the most influential being the theories of mutation accumulation (Medawar 1952), and antagonistic pleiotropy (Williams 1957). Mutation accumulation posits that deleterious mutations whose effects are felt only late in life will accumulate passively in the genome; they are not removed because selective pressure weakens with age. Antagonistic pleiotropy proposes that genes may be actively selected because they have a beneficial effect on reproductive fitness, even if they have negative effects on health in later life, contributing to the negative phenotypes associated with aging. The result, in either case, is the maintenance of genes that might contribute to the pathobiology of aging in the post-reproductive organism. Identifying the expression profiles of such genes with age, has therefore become increasingly important, although there remain a number of technological and methodological impediments to effective experimental design in this context(Melov & Hubbard 2004).
Gene expression profiling provides global insight into complex processes at the transcriptional level. To date, one extensive microarray study of wild-type aging in C. elegans has been conducted on pools of thousands of individual worms, using whole-genome cDNA arrays (Lund et al. 2002). The aggregate gene expression data from this study was a combination of three distinct strains, each infertile due to temperature-sensitive mutations, at four different ages. Lund et al reported that 164 genes changed in expression with age from the pools of nematodes they used. They concluded that the differential expression of these 164 genes is consistent with viewing aging as a generalized degeneration, occurring in a stochastic way in each individual, and so having few common themes that can be detected when studying a large population. In addition to this longitudinal study on aging, a number of other microarray studies have also been published which primarily focused on the insulin-signaling pathway (McElwee et al. 2003; Murphy et al. 2003; McElwee et al. 2004). These studies did not study aging populations of nematodes per se, but contrasted different mutations in the insulin-signaling pathway to gain insight into potential transcriptional targets relating to longevity in C.elegans. At the individual morphological level, C. elegans aging has been described to have a significant stochastic component (Garigan et al. 2002; Herndon et al. 2002). Consistent with these prior morphological observations, we recently described a dramatic increase in variability between individual aging nematodes with regard to nuclear genome copy number, and loss of cellular nuclei with age (Golden et al. 2007). Here, we report a large number of genes whose expression changes dynamically over the lifespan of wild-type C.elegans and are thematically linked via established gene ontologies. We used characteristic gene expression profiles to identify suites of genes that could be used as biomarkers for physiological or chronological age. In order to identify such biomarkers, distinct physiological traits or observable phenotypes that change with increasing age must be highly correlated with an appropriate marker. The main purpose of our study here is to use idiosyncratic differences in gene expression that arise with age in individual nematodes to identify characteristic expression profiles of physiological age, or biomarkers of aging.
Specifically, we associate distinct transcriptional profiles, with a robust observable phenotype that changes with age - behavior. Robust behavioral defects have been previously reported to arise in individual aging C.elegans (Hosono 1978; Herndon et al. 2002). In total, we examined seven distinct ages for gene expression profiling and behavioral analysis, using at least nine and as many as seventeen discrete worms for each age, for a total of 104 individual arrays across the nematode lifespan. Prior to gene expression profiling, each animal was scored for age-related behavior, which has been reported to be intimately associated with physiological age (Herndon et al. 2002). Overall, we find approximately 30% of the C. elegans transcriptome changes significantly with age from young adults through to late life. The profiling of a relatively large number of individuals (104 individual nematodes from 4–24 days of age) allowed us to test the hypothesis that gene expression was associated with behavioral phenotypes previously associated with aging (Hosono et al. 1980; Herndon et al. 2002).
Microarrays have frequently been used to gain insight into biological function, by coupling characteristic expression profiles with functional studies for genes implicated in a specific cellular process. This kind of approach of using a “big net” to identify genes of interest in certain genes or pathways, facilitates subsequent testing of specific hypotheses using other functional approaches, such as GFP reporters, or RNAi screens. However, microarray profiling can also be used to identify suites of genes that are highly correlated with a particular phenotype, and can therefore be used as surrogate biomarkers. Groups of such genes can then be used to predict specific biological outcomes. The nature of this kind of approach generally precludes the use of single genes as biomarkers, but gains power from the combination of many genes being used simultaneously to predict the metric of interest.
Gene expression profiling has been used in such a fashion to evaluate potential biomarkers for pathological conditions, most notably cancer (Foekens et al. 2006; Nagaraja et al. 2006; Spira et al. 2007). The study by Spira et al in this context is particularly noteworthy in that it demonstrated that gene expression profiling from a modestly sized cohort (n=80) in combination with a suitable training set was able to predict to a high degree of certainty the likelihood of smokers developing lung cancer. The number of individuals used in this study demonstrates the necessity of using a sufficient number of microarrays to identify potential biomarkers for predictive purposes. An analogous approach would prove extremely useful to predict physiological or chronological age in human beings. We present here a model system that has been used to develop an approach for identifying potential biomarkers of aging. Using the model system C. elegans, we have a system that can reasonably predict both chronological age, as well as age-specific behavioral status. This implies that our overall methodology could likely be applied to other species that are amenable to repeated sampling over their lifespan, including human beings.
Results
Gene expression changes with age-related behavioral changes
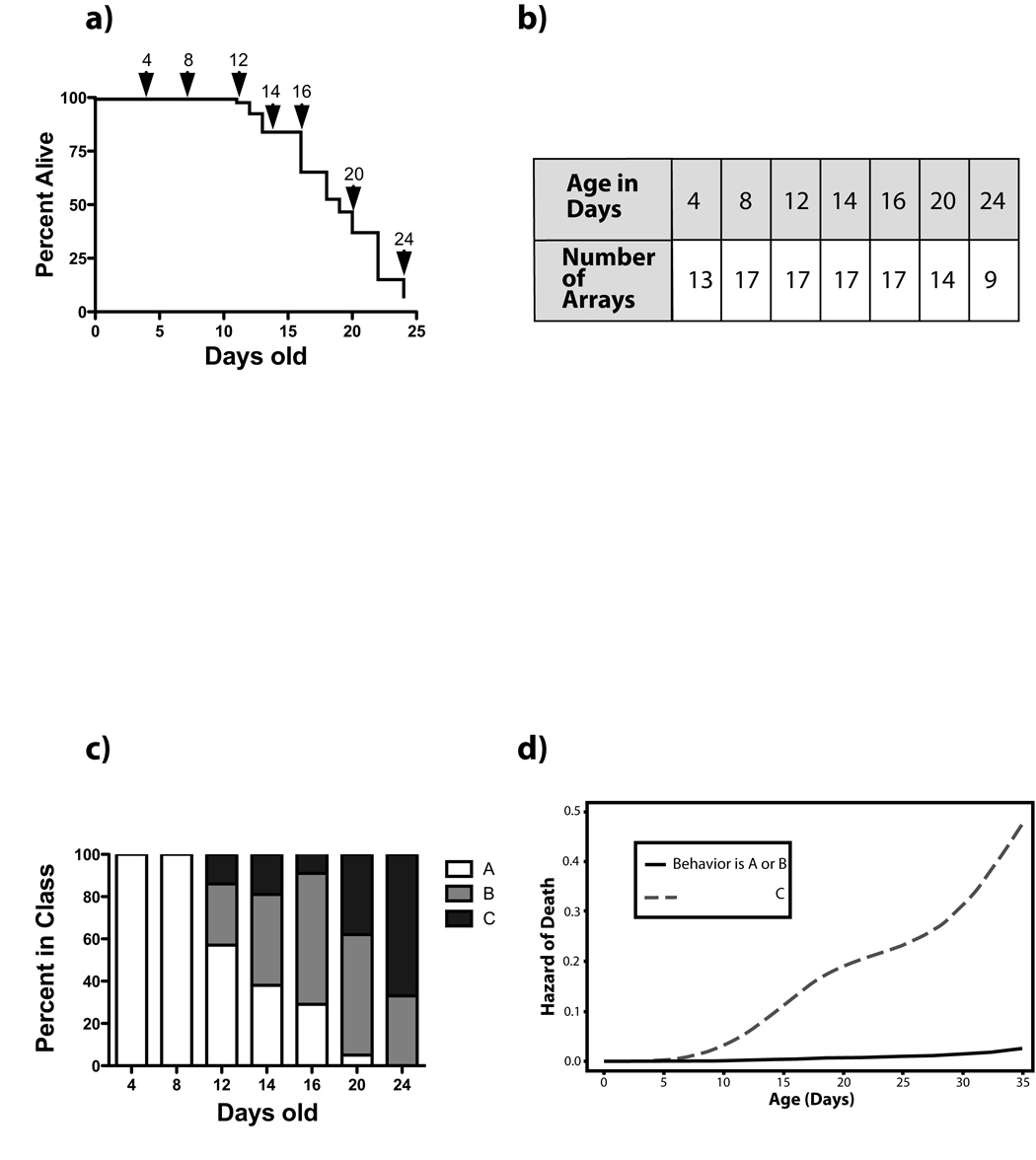
Full-genome gene expression profiles were collected from individual wild type (N2) nematodes at seven ages throughout the C.elegans life span. Individuals were selected from a synchronized population of 700 nematodes. The survival curve for the entire population is shown in Figure 1a We used an individual nematode for each microarray, as this afforded us many advantages over using pools of worms. Firstly, by collecting individual worms, this ensured that each worm was in fact alive prior to harvest, and free from adverse conditions which could confound later analysis such as bagging. Secondly, it allowed us to score individual attributes such as changes in behavior with age, and third, our approach mirrors the vast majority of studies carried out on aging nematodes in that we have a low-density population on the plate, and use standard culture conditions.
Figure 1.
Worms were grown on NGM plates under standard laboratory conditions at 20°C, in the absence of antibiotics or sterility-inducing drugs in order to evaluate gene expression changes under standard conditions. These conditions allow the worm’s access to the food on an ad libitum basis. Worms were counted and moved to fresh plates at least every other day to avoid contamination by progeny. At seven ages (Figure 1a), individual worms were randomly collected from the plates for gene expression profiling, for a total of 104 microarray experiments (Figure 1b). Each worm was filmed on the plate prior to harvesting, and its behavior classified as “A” (youthful, symmetrical, spontaneous movement), “B” (less smooth, uncoordinated movement, frequently must be prodded), or “C” (moves only nose or tail if prodded) as described by Herndon et al. (Herndon et al. 2002). Though not all animals become “C” animals before death, statistically “C” animals have a shorter future life expectancy than “A” animals (Herndon et al. 2002), and the majority of the population progresses from “A” to “B” to “C” with time (Figure 1c). We obtained the raw data for the survivals/behavioral analysis used in Herndon et al., to better understand how robust behavior alone was in terms of predicting death independent of age. We found that although chronological age is still predictive of death, it is not nearly as predictive as behavior (Figure 1d). Animals which are either “A” or “B”, essentially have marginal utility in predicting death, but being a “C” animal results in a greatly increased hazard of death (P<0.0001, Figure 1d). After filming and behavioral categorization, the worm was placed in a tube of nuclease-free water and flash-frozen until RNA was extracted.
Gene expression profiling of each of the 104 individual nematodes was then conducted using full-genome oligo arrays containing 22490 C.elegans predicted genes representing 20333 unique probes, obtained from the Genome Sequencing Center at Washington University (St Louis, MO). RNA from a pool of four-day-old N2 worms served as reference RNA for every individual. At each step (RNA extraction, amplification, labeling, array hybridization), worms were processed in batches containing representatives from every age group, to avoid introducing potential artifacts through day-to-day or batch-to-batch variation. The entire data set is deposited in the GEO database using the accession number GSE12290 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12290).
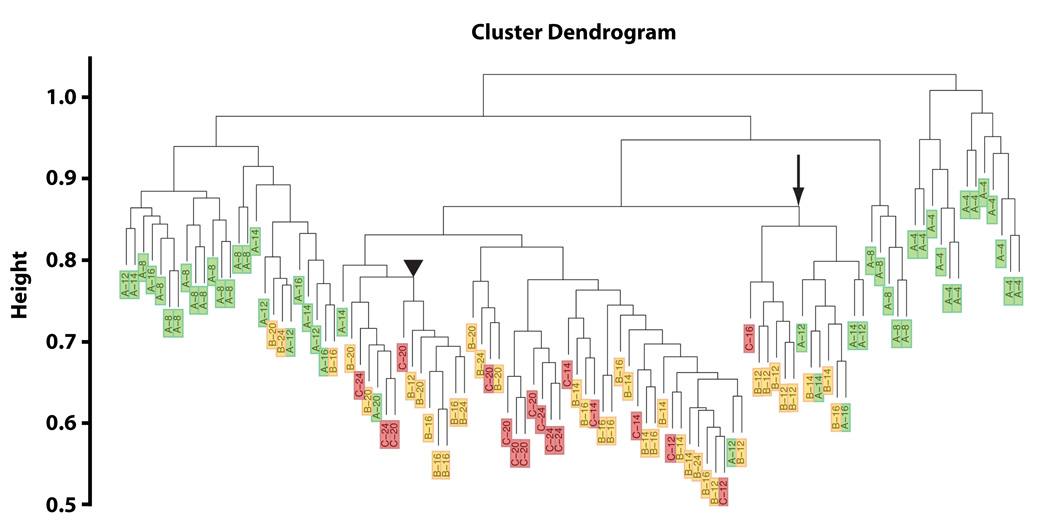
Using the expression data from all genes, we used hierarchical clustering to form groups of these 104 individuals with similar expression profiles across probes to determine whether age and/or behavior had an impact on the global transcriptional profile. It is apparent from this clustering that young worms, 4–8 days old, cluster away from all other individuals (Figure 2). Among those individuals older than 8 days, the clustering is associated in a complex way by behavior and chronological age with some clusters seemingly involving worms of the same age regardless of behavior, and others dominated by behavioral class. For example, seven “B” worms ranging in age from 12 to 24 days, cluster together, rather than with “A” or “C” worms of the same age (Figure 2, arrowhead). Other clusters are seen to be dominated by chronological age, with one cluster containing six worms, all 20 or 24 days old, but including all three behavioral classes (Figure 2, arrow). We conclude from this data that age related phenotypes could be uniquely associated with specific gene expression profiles.
Figure 2.
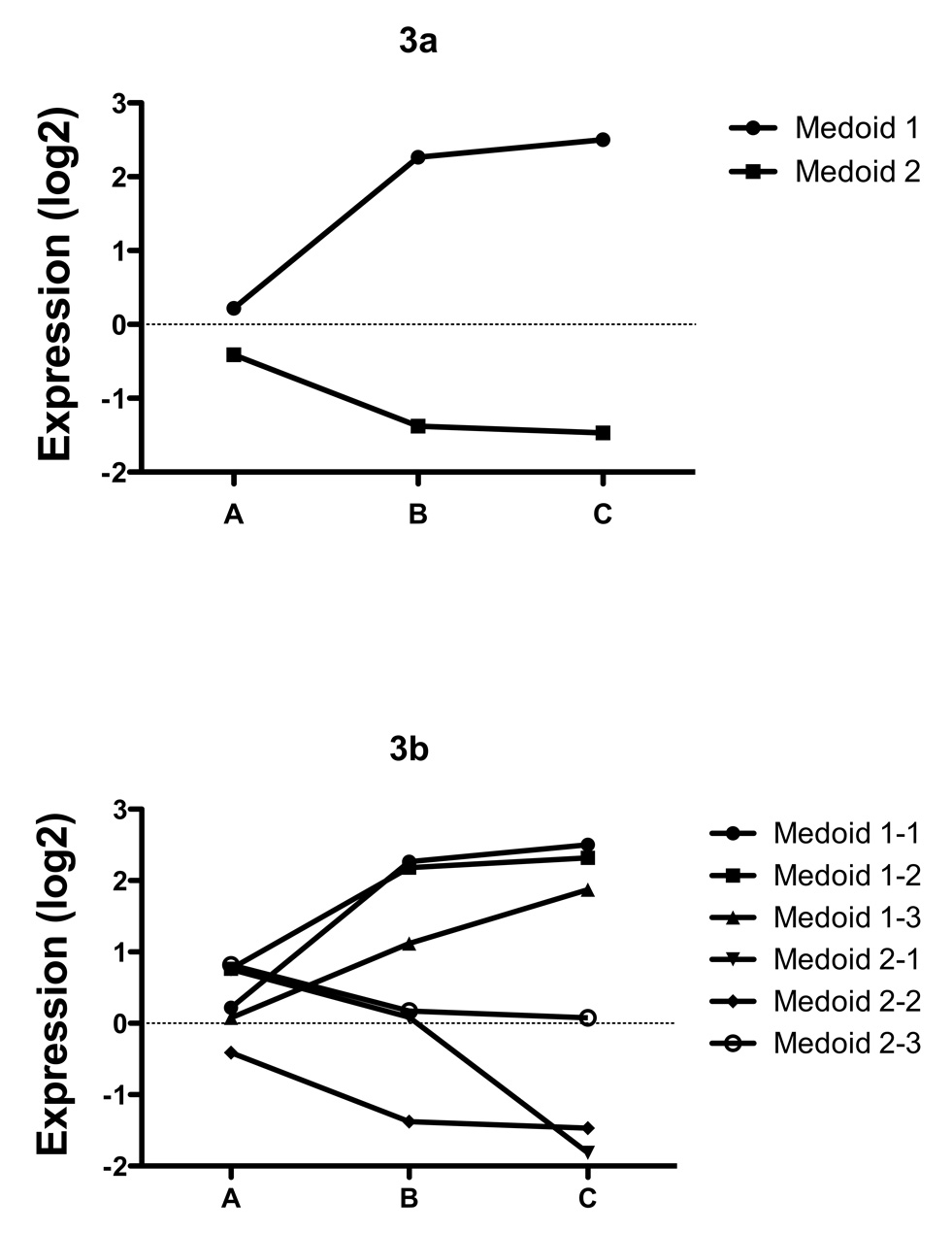
We next identified genes associated with the change in behavioral class, independent of age. We examined the overall association of behavior adjusting (within a linear model) for categorical age to find those genes with an independent association of behavior and expression. Hierarchical clustering (HOPACH (van der Laan & Pollard 2003)); of the resulting genes (corrected for multiple testing using FDR q-value < 0.005; (Benjamini & Hochberg 1995)) resulted in two clusters; one consisting of genes whose expression increases as behavior deteriorates from “A” through to “C” (Medoid 1, Figure 3a), the other consisting of genes whose expression declines from “A” to “C” (Medoid 2, Figure 3a). The Medoid can be thought of as the gene in a cluster that most approximates the behavior of all genes in the cluster, and the basis of the underlying clustering routine. No clusters of genes were identified whose expression was uniquely different in “B” class worms, indicating that overall, the genes identified were associated with a progressive loss of youthful behavior from “A” through “C”. It is interesting to note that “B” worms also do not have an increased risk of death independent of age, similar to “A” worms, but distinct from “C” worms (Figure 1d). The list of genes in each of the two main clusters (Figure 3a), and their respective sub-clusters (Figure 3b) associated with behavioral changes and age was analyzed with Go-Miner (Zeeberg et al. 2003; Zeeberg et al. 2005) to identify gene ontology (GO) categories over-represented in the clusters relative to their representation on the microarray chip as a whole. For a full discussion of the enrichment of biological themes associated with changes in behavior independent of age, see supplemental gene ontology discussion.
Figure 3.
Gene expression changes with age
Next, we identified genes that changed with chronological age over the lifespan of wild-type worms, essentially pooling the individual nematodes of each age “in silico” by averaging the expression values for all members of a given age group (N=9–17 per individuals per timepoint). We carried out several statistical tests to better understand gene expression with age in order to identify potential biomarkers of aging. First, an F test was performed to identify genes whose expression was significantly different at any given age from the control. 7130 probes (31.7% of the probes on the entire chip), representing 6948 unique gene IDs, were found to be significantly associated with age by this test (FDR q-value < 0.05). This represents nearly a third of all genes changing with age over the lifespan of wild-type nematodes.
We then hypothesized that there may be genes which were changing monotonically with age, either induced or repressed over the course of the nematode lifespan. Therefore, we applied a linear model to the data (log2 (relative expression) versus age, data not shown). 5986 genes were identified through this test (FDR q-value < 0.05), 4868 of which (81%) were common with the procedure treating age as categorical (F-test list of genes). Of the 460 most statistically significant genes on this list, only three were not present from the results of our F-test analysis. Since our initial F test results essentially captured the genes identified via the linear analysis, we focused subsequent analysis on the genes we identified via the F test.
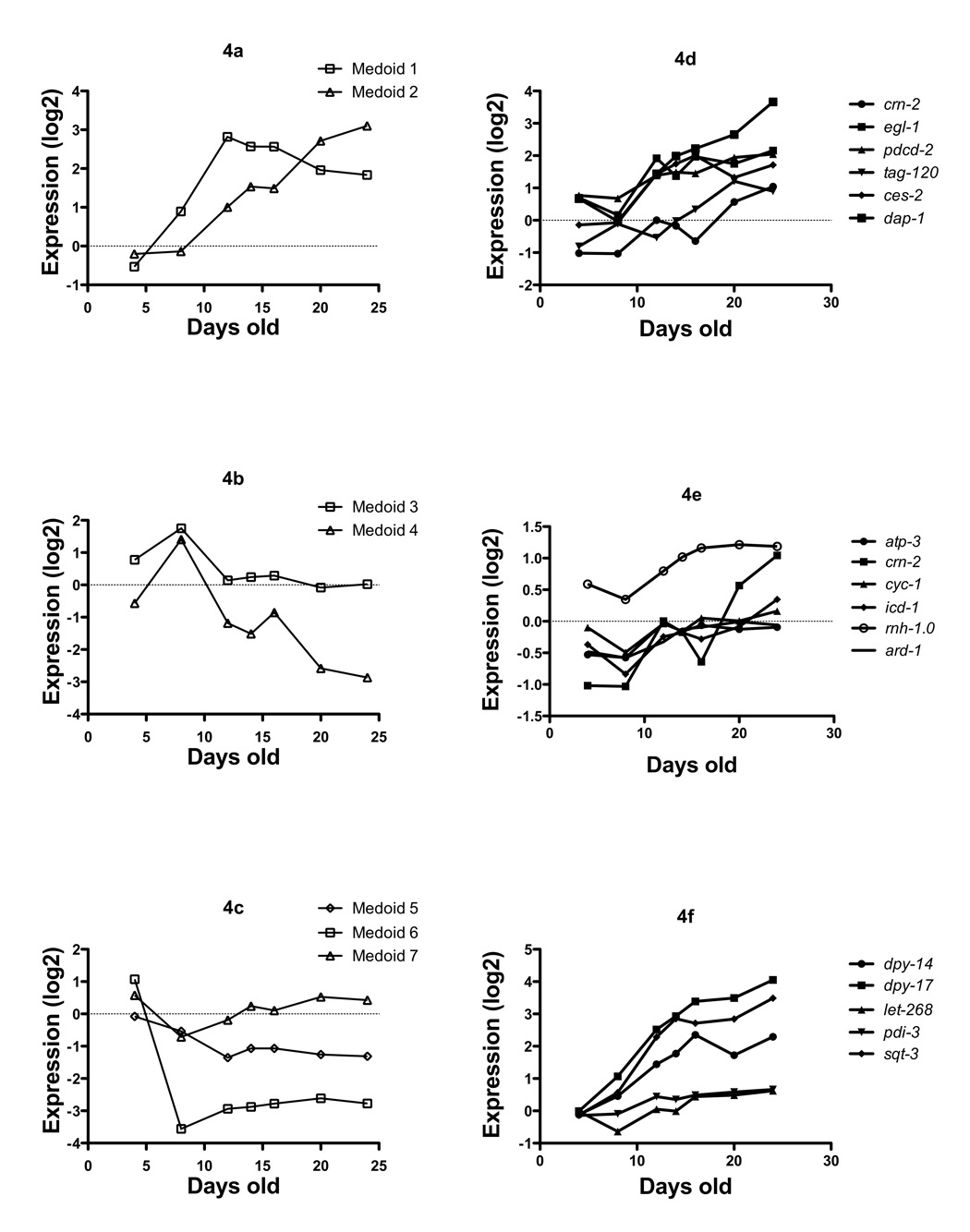
We then clustered these significantly differentially expressed genes with age using the HOPACH algorithm (Hierarchical Ordered Partitioning And Collapsing Hybrid)(van der Laan & Pollard 2003), and this revealed seven distinct clusters at the first split (Figure 4a–c). It can be seen that there are a number of characteristic trends in the data with regards to gene expression and advancing age, clusters of genes which essentially increase with age over the entire lifespan (Figure 4a), and those which increase, then sharply decrease, (Figure 4b), and those which generally decrease with age (Figure 4c).
Figure 4.
Once adult worms are capable of laying eggs (around day 3 after the egg is laid), the next 3–5 days are spent actively laying eggs. Hence wild type worms during this period are filled with eggs in various stages of development. Our study design incorporated 2 time points during this period (days 4 and 8). Hence it is reasonable to assume we would detect gene expression associated with the developing eggs inside the nematodes. Development and reproduction are largely associated with active gene transcription programs, therefore we compared the gene list from Cluster 6 (genes whose expression fell after day 4, (Figure 4, Medoid 6, Supplementary Table 9) with the list of embryonic genes identified by microarray profiling of development (Hill et al. 2000) as expressed more highly in embryos than adult worms (clusters 5.2 through 6.6 from that publication). There was no significant overlap between these groups of genes (data not shown). Interestingly, a large number of collagen genes (20) were identified in Cluster 6, indicating a potential down regulation in overall collagen message with age (Supplementary Table 9). At the morphological level, electron microscopy studies have shown that collagen thickness in the cuticle actually increases with age(Herndon et al. 2002). GO-miner analysis of the entire list of differentially expressed genes with age yielded enrichment for a large number of GO categories using an FDR cut-off of < 5% FDR (Supplementary discussion, and Supplementary Table 5).
Clusters 1 and 2 showed distinct trajectories in the gene expression landscape. Cluster 1 (Medoids 1 & 2 respectively, Figure 4, Supplementary Table 6) increased sharply up to 10 days of age relative to the young control, then leveled off, while as Cluster 2 increased at a constant rate over the entire adult lifespan. GO-miner analysis of Cluster 2 yielded no themes in gene ontologies using a 5% FDR (data not shown). However, examination of the genes associated with Cluster 2, revealed a number of significant functional categories relevant to aging, which prior studies have reported.
Firstly, a number of well characterized genes associated with cell death mediated by either necrosis or apoptosis are increasing with age including - ces-2; a transcription factor required to activate programmed cell death, crn-2; a cell death related nuclease, egl-1; an upstream activator of programmed cell death, pmk-1 a mitogen activated protein kinase which is required for eliciting gonadal programmed cell death in response to infection, F54B8.4; a homolog of death associated protein 1 (dap-1), vha-10 and 8, proteins encoding subunits of the vacuolar proton-translocating ATPase, which is required for necrosis. Ces-2 has been shown to regulate egl-1, which is required for cell death during development (Conradt & Xue). It is particularly noteworthy that these genes are increasing quite dramatically post reproductively, well past the egg-laying window of C.elegans which is less than 10 days of age. The homolog of death-associated protein 1 (dap-1) is the most abundant of these, increasing nearly 30 fold over basal levels when the animals are young, and packed full of developing eggs (Figure 4d, and supplemental Table 6). Taken together, the increase in transcription of these genes with age is suggestive of cell death being mediated by apoptosis and necrosis with advancing age in the post-reproductive worm. The observations we make here about increased abundances of mRNAs associated with cell death mediated by apoptosis and necrosis are consistent with our prior morphological observations of loss of nuclei in aging C.elegans in the post reproductive period (Golden et al. 2007). Secondly, consistent with prior studies using RNAi screens to identify genes involved in modulating longevity (Dillin et al. 2002), we identified a number of mitochondrial genes that are increasing with age (Figure 4e); ard-1 a homolog of a mammalian mitochondrial amyloid-beta binding alcohol dehydrogenase (ABAD, (Lustbader et al. 2004; Takuma et al. 2005)), rnh-1.0, a mitochondrial RNAse H, and cyc-1 and atp-3, two components of the respiratory chain which when inhibited by RNAi result in increased lifespan (Dillin et al. 2002). The third categories of genes in Cluster 2 that are clearly functionally related are those related to collagen metabolism (Supplementary Table 6). Sqt-3, and dpy-17 in particular (Figure 4f), are increasing dramatically in abundance as worms age. This implies these genes could be contributing substantially to the increase in cuticle thickness that occurs with age (Herndon et al. 2002). We used GO-miner to further investigate the biological themes of the genes changing over lifespan, and this is further elaborated on in the supplementary discussion.
Wild-type worms are reproductively active and laying eggs up to about 10 days of age. This active reproductive process (including gamete maturation and egg development) in wild-type worms is frequently inhibited in aging studies in the laboratory by administration of genotoxic chemicals, or the introduction of pleiotropic mutations that impair fertility to facilitate survival analysis of worms. As we carried out our survival analysis and expression studies in the absence of fertility mutations or genotoxic chemicals, we were able to detect many gene expression profiles relating to active reproduction in the wild-type worm prior to 10 days of age (further elaborated on in the supplemental discussion). Therefore, in order to identify genes that are transcriptionally active as a result of aging in the post-reproductive period, we examined gene expression profiles between 12 and 24 days of age.
We repeated the F-test analysis excluding all worms younger than 12 days of age, in order to identify differential gene expression in the age range of 12–24 days of age. This represented 74 individual nematodes and their respective arrays. Using multiple testing procedures based on p-values from a one-way ANOVA (F-test), and using a FDR cut-off of < 5% we identified 447 genes that were differentially expressed relative to the young pooled 4-day-old control between 12 and 24 days of age (Figure 5, Supplementary Table 11). GO-miner analysis of the entire list of Supplementary Table 11 revealed a highly significant over-representation of the GO categories stress response and aging at a FDR cut-off of < 5% FDR (Supplementary Table 12), consistent with prior studies using longevity mutants (McElwee et al. 2003; Murphy et al. 2003). The identity and trajectory of the genes involved in each of these GO’s identified through partitioning the worm lifespan into a post-reproductive phase is of interest, hence Figure 5 shows the genes identified as being associated with aging, ER-nuclear signaling, and chaperones via global GO-miner analysis. This is further elaborated on in the supplementary discussion
Figure 5.
Validation of microarray data
In order to validate our overall approach for identifying genes which were robustly differentially expressed in C. elegans aging, we tested whether or not a subset of genes we had identified via microarrays were also differentially expressed via real-time PCR in a completely biologically independent cohort.
We picked 24 genes to validate (Supplementary Table 14) which were either statistically significantly differentially expressed, or not changed between two time points in our original microarray study; 4 and 20 days of age (young and old respectively). These validation experiments were carried out approximately 12 months after our initial microarray experiments. Our goal was to give us confidence that the genes we determined as being differentially expressed via microarray were in fact reproducible in an independent biological cohort. Nineteen of the 24 genes replicated in the independent cohort in terms of being differentially expressed in the same direction as that we observed previously in our microarray experiments. Possible reasons for the 5 genes that did not replicate may be biological variation between one cohort of nematodes (and the second cohort 1 year later), or platform specific differences between microarrays and real-time PCR in terms of dynamic range and sensitivity.
Identification of characteristic transcriptional profiles, or “biomarkers” of age
A major goal in our studies was to identify whether or not microarray data could be used in conjunction with survival and behavioral classification to identify predicative transcriptional profiles or “biomarkers” that could be used to either assess either chronological or physiological age. Our reasoning was that behavior had already been shown to be a good predictor of physiological age, as worms classified as “C” were likely to die within a few days, regardless of chronological age, where as worms that were “A” were likely to survive for several weeks (Herndon et al. 2002). Our new analysis of the data presented in Herndon et al (Figure 1d) also gave us great confidence that behavioral state “C” was an excellent proxy for imminent death. In addition, we also wished to determine whether the gene expression measures used have any benefit over using age itself to predict behavior. We used a combination of ranking by significance of association of a gene and behavior, a machine learning (black-box) prediction algorithm to determine the “best” predictor of behavior. In order to fairly examine the accuracy of prediction, we performed the analysis nested in a V-fold cross-validation sampling method. Specifically, the sample was broken into V=5 equal parts and each of these served as the so-called validation sample whereas the remainder in each case was the training sample. For each of the training samples, we first used tests of significance (by F-test of behavior versus log2(relative expression)) to rank probes from most to least significant. Then, the top 100 probes were entered as candidate predictors in the chosen prediction/regression algorithm. Since behavior is a categorical outcome with 3 levels (A, B, or C), a machine-learning algorithm that predicts class (POLYCLASS; (Kooperberg et al. 1997)) was used on each of the training samples to predict on the validation sample. We used each of the validation samples (which was not used to either find the candidate probes by multiple testing nor fit the regression model) to determine our prediction accuracy.
Table 1 shows the predicted versus observed behavior class on the validation samples, which yields an overall accuracy of 71%. When chronological age alone was used to predict behavior class (using an equivalent method) we obtained a lower prediction accuracy (65%), which satisfies one criterion of an appropriate biomarker listed in Butler et al.(Butler et al. 2004), namely that the biomarker should predict the domains of physiological aging better than chronological age itself (in our case, we only have one domain, behavior). Essentially this means that from a combination of gene expression measures alone, we are able to predict physiological age (incorporating both behavior and chronological age), to an accuracy of 71%. It is important to note that these gene expression biomarkers are combinatorial, and from this data set no single gene, has any significant contribution to our predictive power. It is the combination of many genes that give us the ability to predict age using this approach.
Table 1.
| Predicted Behavior | ||||
|---|---|---|---|---|
| A | B | C | ||
| True Behavior | A | 41 | 6 | 1 |
| B | 7 | 27 | 4 | |
| C | 0 | 9 | 9 | |
To examine the robustness of the actual list of genes chosen in the biomarker model (note, typical numbers of genes chosen for any one model predicting behavior were 2–5), we performed a type of variable importance analysis using bootstrapping. We simply randomly drew samples of size 104 (the original number of arrays) with replacement, then used the same algorithm (reducing the list by first ranking by p-value and then using POLYCLASS to find the prediction model). The measure of variable importance is simply the proportion of bootstrap samples for which that variable is chosen by the procedure as a predictor. Supplemental Table 15 has the top 50 genes with regards to the proportion of times the gene is included as a predictor, and no probe is chosen a majority of times. This is a typical pattern when correlated predictor variables are used to predict on a finite sample; small changes in the data result in large differences in the model used, even if the models do similarly well at predicting the outcome. It is worth emphasizing that the genes in Supplemental Table 15 do not necessarily have any causal role in the aging process, they are merely strongly associated with the metric of interest, in this case, aging. We expect that if we increased the size of both the training and testing data sets to larger and larger sizes, one would expect that the procedure would converge to a stable predictor (i.e., the same genes would be chosen). Therefore, large sample sizes (i.e. many hundreds of individuals) may be needed to distinguish between candidate predictors with similar performance.
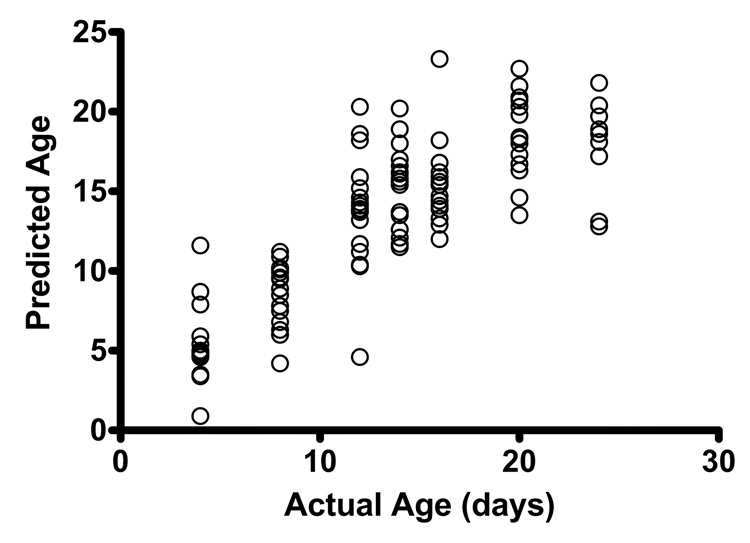
We repeated this procedure predicting continuous age (replacing POLYMARS for POLYCLASS, (Kooperberg et al. 1997)). Figure 6 shows the resulting prediction of age (in the validation sample) versus the true age. This can be viewed as a biomarker for the mean chronological age for a subject with a certain gene expression profile. Note that the relationship between the predicted age and observed age in the validation sample implies that, up to 15 days of age, different ages have different expression profiles as summarized by the predicted age. However, after 15 days, the ages start to look relatively similar, so finding biomarkers to distinguish older ages becomes more problematic.
Figure 6.
Discussion
We examined individual nematodes at seven ages covering wild type C. elegans life span for changes in gene expression and behavior. An advantage to the study of individual nematodes is that transcriptional profiles can be associated with individual phenotypes; in this case, we demonstrated that expression changes could be associated with broadly defined behavioral categories, and in the future this approach could be applied to any individual phenotype that can be assayed. A significant motive for examining individuals was to test the hypothesis that gene expression coupled with some age-specific marker (behavior) could be used to predict either physiological or chronological age.
The 104 individual worms we profiled could be sorted into clusters based on their global transcriptional profiles indicating that there is structure in the expression profiles representative of age and behavior despite the fact that approximately 70% of the genome does not change with age. The majority of the “A” class nematodes form distinct clusters away from the other worms. Among the older nematodes, clusters based on behavioral category or on age are apparent.
It has long been recognized that the majority of individuals in a population of C. elegans will lose mobility and coordination with age (Hosono 1978). Herndon et al. (Herndon et al. 2002) followed individuals throughout their life spans, and determined that statistically behavior is a better predictor of subsequent lifespan than chronological age. Thus, the behavioral category can be considered an indicator of physiological age. This assignment is not perfect; a fraction of worms will die as “B” or even “A”. However, the distribution of behavioral classes at each age shows a progression of the population as a whole from entirely the “A” class to almost entirely “C” with age. This mirrors the result of a similar study some 20 years earlier, when behavioral classification of worms with age was also carried out, except the descriptors were “I”, “II”, and “III”, instead of “A–C” (Hosono 1978). We analyzed the data from Herndon et al., and found that the “C” state was highly associated with death. Therefore, it follows that transcriptional changes associated with “C”, may also be predictive of death.
We assayed behavior using a slight modification to the method of Herndon et al., with “A” worms having symmetric, spontaneous and smooth movement, “C” being those that only move their nose or tail when prodded, and “B” being every behavioral state in-between. We identified genes whose expression changed significantly as a function of behavioral class, controlled for age. No “B” specific genes were identified, indicating that the identified genes are all related to the trend towards decreasing mobility. HOPACH clustering of the behavior-related genes identified two clusters, one of genes that increase in expression and one of genes that decrease in expression with worsening behavior.
Genes that increased in expression with worsening behavior were statistically significantly enriched in actin-related genes, and a number of amino-acid metabolic pathways. The involvement of these gene categories is not surprising, as the behavioral categories can essentially be seen as an assay of muscle function. The fact that these genes are increasing in expression as movement decreases may indicate that physiologically old worms are trying to rebuild their fracturing cytoskeletons, through activation of amino acid metabolism, and actin-related processes. Herndon et al. (Herndon et al. 2002) described a loss of muscle integrity with age, analogous to sarcopenia (a term describing atrophy of muscle arising with age(Melton et al. 2000)), which was delayed in the long-lived strain age-1. The cause of the cytoskeletal problems is unknown.
We found that approximately 30% of the C. elegans genome changes significantly in expression with age in reproductive nematodes. The large number of age-related gene expression changes is consistent with a study of Drosophila aging, which found that 23% of the genome changed expression with age (Pletcher et al. 2002). The data we report here has no overlap with the only previous full-genome microarray study of C. elegans aging, which found only 164 genes to change expression with age (Lund et al. 2002). This earlier study combined data from three different sterile C. elegans strains, used large pools of worms covering 2–3 days per time point, and analyzed gene expression using cDNA arrays. Each of these differences in method is significant and could contribute to the difference in number and identity of genes identified.
We identified a number of substantive gene expression changes in aging nematodes between the ages of 4 and 8 days of age. This period coincides with the cessation of reproductive activity in wild type worms, and indicated that genes which change in intact animals over the reproductive phase of the animals lifespan may play a role in reproduction, or be expressed in developing embryos. To determine whether transcripts in embryos contribute significantly to the transcriptional profiles of 4-day-old worms, we compared the list of genes in Cluster 6 (Figure 4) with the list of genes determined by Hill et al. to be expressed in embryos (Hill et al. 2000) and found no significant overlap. Possibly this could be due to differences in methods between the two studies, with Hill et al using pools of thousands of worms, while as our study uses individual worms raised on standard NGM plates.
We used gene ontologies developed and maintained by the GO consortium (Zeeberg et al. 2003; Zeeberg et al. 2005) to identify a number of biological themes that appear to dominate aging in wild-type C.elegans (discussed in detail in the supplemental discussion). Firstly, we were able to identify reproductive processes that appeared to dominate several groups of genes in the 4–8 day window in the nematode lifespan. Ontologies of development, embryogenesis, and sex determination were clearly prominent in several major clusters we identified in that age range. We detected many genes associated with developmental processes in the reproductive window of 4–8 days of age, and within this age-range, we identified a significant number of genes that continued on the same gene expression trajectory well into the post-reproductive period (after 12 days of age) regardless of the presence of actively developing embryos in 4–8 day old worms. This demonstrates the importance of including the entire lifespan of the animal in our analyses. A large number of transcription factors also increase with age (Cluster 1), as do genes related to development, RNA metabolism, reproduction, and growth (Cluster 7). The transcription factors include many nuclear hormone receptors and homeodomain proteins. The combination of increased expression of transcription factors, and decreased expression of many other genes with age may imply that a major phenomenon associated with aging in C. elegans is substantial DNA damage or chromatin condensation preventing proper expression of many genes, which is consistent with our data.
There have been reports of an increase in heterochromatin with age in the flight muscle of the housefly M. domestica (Panno & Nair 1985), and of changes in nuclear architecture in aging C. elegans (Garigan et al. 2002; Haithcock et al. 2005). In addition to the themes of DNA damage, and chromatin dynamics, we also observed an increase in expression of genes associated with cell death. This is somewhat surprising, as active cell death has not been considered to be a feature of nematode aging (Garigan et al. 2002). However, we recently reported the loss of specific nuclei in wild-type nematodes with age, and the expression of the suites of genes we report here associated with both programmed cell death and necrosis is consistent with our prior morphological observations (Golden et al. 2007). The up-regulation of transcription factors with age may also be a compensatory response to either DNA damage, or increasing chromatin condensation. The status of chromatin condensation with age in somatic nuclei of C. elegans would be of great interest, however, there are a number of technical challenges which prevent detailed exploration of this area at present in discrete cells in the nematode. Overall, our observations are consistent with the massive amount of nucleic acids that accumulate in wild-type nematodes as they age (Golden et al. 2007). The reason for the accumulation of DNA in aging nematodes remains unclear.
We also observed a number of mitochondrial transcripts increasing with age, including several which have previously been reported to be associated with longevity (Dillin et al. 2002; Lee et al. 2003). Given that the increase in expression of mitochondrial respiratory chain subunits appears to be deleterious to lifespan(as RNAi treatment of such subunits results in longer life), our results are consistent with this prior work. One mitochondrial-associated transcript that increased with age in wild-type C. elegans is orthologous to ABAD, a protein that is increasingly related to mitochondrial dysfunction in association with Alzheimers disease (Yan et al. 2006). The function of ABAD in C. elegans aging has yet to be elucidated.
By segmenting aging nematodes into 2 discrete populations corresponding to developmentally active worms, versus post-reproductive (pre 12 days of age, and post 12 days of age respectively), we were able to uncover a number of other classes of interesting gene expression changes. Induction of stress response was clearly apparent in the post-reproductive phase of the nematodes life, as was a downregulation of genes involved in glycosylation (supplementary discussion). The induction of stress response genes is consistent with much prior work in this area(Walker et al. 2001; Walker & Lithgow 2003), but a downregulated transcriptional response to glycosylation is puzzling, and the reason for this remains unknown.
Many aspects of metabolism including carbohydrate, DNA, tRNA metabolism, amino acid and peptidoglycan metabolism, and lipid metabolism and proteolysis decrease with age at the message level (Supplementary Tables 7–9, Medoids 4–6, Figure 4). We had previously described a decrease in expression of proteases with age in wild-type nematodes (Golden & Melov 2004). This decrease did not occur in a long-lived strain daf-2 at the same ages, indicating that this decrease in expression correlates with the shorter life span of N2 compared to daf-2. Supporting an important role for proteases in maintaining longer life spans, RNAi of an aspartyl protease reduced lifespan to a greater extent in daf-2 than N2 worms (McElwee et al. 2003).
The C. elegans genome has approximately 154 collagen encoding genes (Johnstone 2000). In our study, we observed a number of collagen genes falling dramatically in expression between days 4 and 8 (Supplementary Table 9, Medoid 6, Figure 4). Eight of the genes in this group have been found to alter adult nematode body shape when mutated. C. elegans synthesizes a new cuticle at five times during development; before hatching, and prior to each larval molt. The expression of only six C. elegans collagen genes during development has been published(Johnstone & Barry 1996), one of which, dpy-13, is in Cluster 6 (Figure 4). Interestingly, Johnstone et al found dpy-13 to peak in expression just before the L4 to adult molt, and then to fall to very low levels immediately upon molting, and to remain low for at least the first 8 hours of adulthood (at 25°C). Our data is consistent with this prior observation. We propose that the other collagens in this group are also important for synthesis of the adult cuticle. Despite this down regulation at the transcriptional level, the amount of collagen is known to increase substantially in worms with age, such that the cuticle of old adults can be ten times thicker than that of young adults (Herndon et al. 2002). We also observed a massive increase in the expression of several genes relating to collagen metabolism (Figure 4). We propose that these specific collagens, combined with a decrease in protease expression, may be responsible for the increase in cuticle thickness with age.
The main emphasis of this work was to determine whether or not gene expression could be used to identify biomarkers that could predict age, with a view to applying this approach in organisms that were amenable to repeated sampling. Here, we used a machine-learning algorithm in association with both behavioral descriptors as well as gene expression profiling to identify a suite of genes that were predictive of both chronological, as well as physiological (behavior) age. Using both metrics resulted in an accuracy of 71%, for predicting both physiological and chronological age, a mild improvement over either method alone. Although it is intellectually appealing to infer mechanistic relationships between the top genes in our predictive list (Supplemental Table 15), and the process of aging itself, it is worth emphasizing that the predictive genes in Supplemental Table 15 change each time the analysis is rerun with a slightly altered data set (a bootstrapped sample). By increasing the number of samples in the training set (for example to hundreds of individuals which is clearly feasible), one can asymptotically focus the top genes in the list to a smaller and smaller number, such that with a large training set, the same genes would likely always arise as being the best predictors in repeated experiments. In a typical case where there are many highly correlated gene expressions that serve as candidate predictors, the problem of finding a stable model (a reproducible set of genes consistently chosen as the best predictors of physiological age) is akin to accurately estimating the joint distribution of many variables. Such a problem requires a very large sample size (a product of the so-called curse of dimensionality); note that no straightforward sample size calculations are available as they would be based on properties of this joint distribution, such as the variance-covariance matrix, and on how these properties interact with the specific algorithm we used to identify the predictors (in this case the machine learning tool). If the sample size was large enough that algorithm always converged to the same set of genes, such genes could then be used as markers for predicting physiological age, in drug screens, and other related high throughput studies to identify compounds that may retard aging, without necessarily carrying out survival analysis. This is clearly a desirable goal, but at this stage we cannot yet predict how many individuals would need to be profiled in order to identify a small list of genes with the best predictive value as biomarkers of aging. It is worth re-emphasizing that genes associated with specific behavioral states may merely be proxies for that physiological state, and not causally associated with the metric of interest, in this case, aging.
A number of prior efforts to develop approaches for identifying biomarkers of physiological or chronological age have been made (Harrison & Archer 1988; Randerath et al. 1993; Lipman et al. 1999; Ingram et al. 2001; Dhahbi et al. 2004), and the problem remains difficult. It is clear that robust methods that can predict remaining lifespan, and/or age-related physiological decline are desirable (Butler et al. 2004). What has been lacking is an approach that can give reasonable expectation that such a paradigm is practical. The results we present here we believe give such hope. Although the system is simple, and the animals themselves are used to derive the molecular metrics which eventually give rise to a predictive biomarker of age, we propose that our approach is an appropriate starting point for carrying out similar studies in human beings in tissues which are relevant such as skin and skeletal muscle. Repeated sampling of specific tissues over the course of life would clearly be an interesting study, using approaches analogous to that we describe here. Skeletal muscle in particular can be associated with a specific metric (strength), is assayable via biopsy for gene expression profiling, responds to intervention (exercise), and the characteristic age-specific gene expression profile can be somewhat reversed back to youthful levels coupled with improvements in strength (Melov et al. 2007). Therefore it would be of great interest to carry out similar studies to those we describe here in human skeletal muscle, to determine the likelihood of identifying suites of genes that were predictive of both age, and strength, potentially being combined to yield a predictive biomarker of frailty, and physiological age. At present, we do not yet know how generalizeable such a suite of biomarkers would be to other tissues in human beings.
In summary, we identified a number of novel themes of gene expression including cell death and collagen deposition by carrying out whole-genome expression profiling across the lifespan of wild-type C. elegans. We also coupled age-related behavioral phenotypes with quantitative gene expression metrics to predict both behavior, as well as chronological age in this model system. When we combined the two approaches, we were able to predict both chronological age, as well as physiological age with an accuracy of up to 71%. This is a significant step in the search for approaches that can predict remaining lifespan, as well as age-related physiologies, and we feel our approach has some potential for predicting similar outcomes in organisms that can have tissues repeatedly sampled over the course of their lifespan, including human beings.
Experimental Procedures
Culture of nematodes
Wild-type (N2) C. elegans were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). The worm population was initiated with a two-hour timed lay. Worms were cultured at 20°C on nematode growth media (NGM) plates seeded with OP50 bacteria. Seven plates containing 100 nematodes each were maintained; plates were checked at least every other day, live and dead worms were counted, and live worms moved to fresh plates to avoid contamination by progeny. All seven plates had virtually identical survival curves. At the specified ages, 20 individuals were picked at random from the seven plates. Each individual was placed on an NGM plate without an OP50 lawn and filmed. The worm was then picked into a tube containing 5µl of nuclease-free water and flash-frozen and stored at −80°C until RNA was prepared.
RNA preparation
Total RNA was extracted from individual worms using the AbsolutelyRNA™ Nanoprep kit from Stratagene. Reference RNA was a pool of RNA from15 four-day-old nematodes. Each sample, including the reference pool, was subjected to two rounds of linear amplification using the MessageAmp™ kit from Ambion for the first round and the Amino Allyl Message Amplification II kit from Ambion for the second round. After amplification, each sample was assessed for yield and overall quality via Bioanlayzer™(Amersham). Samples that did not pass QC were discarded, and not used in the final analysis. Post Labeling Reactive Dyes (Cy3 and Cy5) from Amersham were used in conjunction with the reagents and protocol from the Ambion Amino Allyl Message Amplification II Kit to label the aRNA. 5µg of aRNA plus aRNA spike were labeled (aRNA Spike: equal amounts of the 10 Stratagene Spot Repot Spikes were combined and then amplified 1 round, to make an aRNA Spot Report Spike mix). All controls were labeled with the Cy3 (red) dye and all experimental samples were labeled with the Cy5 (blue) dye. The Cy3 (red) and Cy5 (blue) sample pairs were combined and purified. The Ambion RNA Fragmentation kit was used to “fragment” the aRNA, to improve hybridization.
Arrays
Oligonucleotide arrays were obtained from the Washington University Microarray Core Facility, Washington University, St. Louis. The arrays contained 22490 C. elegans features, and 124 controls, both positive and negative. Additional information can be found at http://genome.wustl.edu/genome/celegans/microarray/ma_gen_info.cgi.
Hybridization was carried out using a Lucidea Slide Pro machine. All wash solutions were made using Ambion Pre-Made Solutions (Nuclease-free Water, 20% SDS and 20XSSC) and filtered sterilized with a 500ml, 0.22uM filter unit. All C. elegans arrays from the Washington University in St. Louis Genomic Sequencing Center arrive pretreated; no blocking step was necessary. To prepare the aRNA sample for hybridization, the labeled aRNA sample was brought up to a volume with Nuclease-free water and Dextran Sulfate/ Hybe Buffer mix was added to the aRNA sample. The mixture was warmed to 50°C. Using a Hamilton syringe the labeled sample was loaded into the Lucidea chamber. The hybridization program was run for 16hrs at 42°C with a cycling speed of 10µl/sec every 30 minutes. The array was then washed at a speed of 50µl/sec with the following wash buffers in succession: 2X SSC +0.1% SDS (warmed to 42°C for 1st wash solution), 2X SSC, 0.2XSSC, and a 95% ethanol drying step. The slide was removed for scanning. The array slide was loaded into a Perkin Elmer Scan Array Express 2-Laser Scanner. A "QuickScan" was performed, which scans the first 3–4 rows of the array. A surface calibration was then performed using the Spot Report control spots on the array. After surface calibration, the PMT and POW settings were adjusted to have the control spots match in intensity. The array was then scanned. Images were quantitated using GenePix 5.0 software package which overlays the GAL file (provided by the Washington University in St. Louis Genomic Sequencing Center) on the two array TIFF images (Cy3 and Cy5). Pixels per spot, per channel were counted by the software and a gpr file was generated.
104 arrays were conducted (13 4-day-old, 17 each of 8-, 12-, 14-, and 16-day old, 14 20-day-old, and 9 24-day-old). RNA labeling and array processing were organized such that samples from each age group were processed simultaneously. This was to avoid introducing any day- or batch-dependant bias by preparing all members of one group together.
All arrays have been deposited in the GEO database and have been assigned the series identifier GSE12290 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12290) (both normalized and raw data) for ease of access.
Validation of microarray results via Microfluidic PCR
We used a BioMark™ real-time PCR system (Fluidigm Inc.), which has high throughput due to its Integrated Fluidic Circuit feature(Spurgeon et al. 2008). Validation of our microarray results was carried out on an independent biological cohort by performing more than 2000 PCR’s in a single run using the Biomark™ system in conjunction with a BioMark 48.48 Dynamic array. A cohort of N2 nematodes was grown in a similar fashion to that described above, and individual nematodes were flash frozen in water at either 4 or 20 days of age. Worms were then lysed as described above.
We then picked a range of genes that we had identified as being differentially expressed in our microarray experiment (Supplementary Table 14) for validation via Dynamic Array using pre-designed Taqman primers from Applied Biosystems. First strand cDNA from 15–18 individual C. elegans samples per timepoint was prepared using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR according to manufacturer’s recommendations (Invitrogen, Carlsbad, CA 92008). Prior to analysis, the cDNA from each individual C. elegans was preamplified using a 0.2x mixture of 25 gene expression assays and TaqMan® PreAmp Master Mix, both from Applied Biosystems (Foster City, CA 94404). A 1.25 µL volume of cDNA was amplified in a 5 µL reaction for 14 cycles according to manufacturer’s recommendations. At the end of thermal cycling, the reactions were diluted 1:5 in low EDTA TE (10mM Tris-HCl, pH 8.0, 0.1mM EDTA, pH 8.0).
Samples were prepared for loading into the Dynamic Array by mixing 2.5 µL of TaqMan® Universal Master Mix (Applied Biosystems), 0.25 µL of DA Sample Loading Reagent (Fluidigm PN 85000735) and 2.25 µL of preamplified cDNA. The 48.48 Dynamic Arrays (Fluidigm PN BMK-M-48.48) were primed according to the manufacturer’s recommendations. A 5 µL volume of each C. elegans sample was loaded into separate sample inlets on the dynamic array. The 20x gene expression assays (Applied Biosystems) were diluted to 10x using the DA Assay Loading Reagent (Fluidigm PN 85000735). A 5 µL volume of 10x assay reagent, for each of the 25 gene expression assays, was loaded into separate reagent inlets on the dynamic array. The dynamic array was placed on the Nanoflex™ 4-IFC Controller for loading and mixing. After loading, the dynamic array was placed on the BioMark™ Real-Time PCR System for thermal cycling and real-time imaging of the reactions. Thermal cycling conditions were: 50°C for 2 minutes AmpErase UNG sterilization step, 95 °C for 10 minutes hot start, and 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. Data was analyzed using the BioMark Real-Time PCR Analysis software. Nineteen of the twenty four genes we assessed via the Biomark™ system were validated between the 4 day old group versus the 20 day old group (Supplementary Table 14), comparing the two ages via a permutation distribution with two-sample t statistic using unequal variances. Five of the genes did not replicate in this separate experiment, perhaps due to biological variability between the two distinct microarray and real-time PCR experiments carried out 12 months apart, or alternatively differing thresholds of sensitivity between the platforms for detecting differential expression.
Statistical Analysis
Arrays were normalized using LIMMA (Smyth & Speed 2003).
Clustering Worms (using all genes)
We applied hierarchical clustering to find groups of worms with similar expression profiles across the genes.
Behavior (adjusting for Age)
For these analyses, the parameter of interest is the association (slope) of behavior vs. expression, within age strata. So, we want to define the independent association of behavior and expression that is not simply accounted for by age. We thus fit a model, for each gene, g, the following model:
where the parameter of interest is β1 is and Beh = 1(A), 2(B) or 3(C).
To define differentially expressed genes, we used first used a classic F-test, treating the 7 ages as a categorical variable (4, 8, 12, 14, 16, 20 and 24 days) as using an FDR (Benjamini & Hochberg 1995) cut-off of 5%. For these genes, we then applied a clustering technique, HOPACH, to find groups of similarly expressed genes.
We repeat this using two more testing/ranking procedures: 1) using the test that the slope =0 of relative expression over time (days) and 2) using the test that the variance in expression is constant over time using a linear model on the residuals
Supervised Clustering
There is a proposed general method of supervised clustering that is based on a transformation of the raw data. The goal is to create a transformation such that simple clustering on this transformed data will create groups that are similar with regard to an association with some other variable (phenotype), rather than similar in the profile across arrays. Specifically, we want a transform of the data, so that each element of the new data matrix (genes by arrays) represents the contribution of an individual arrays contribution to the association of the variable (in our case age or behavior) and gene expression. If the Xij are the gene expressions for the ith gene, jth array one can use the influence curve for estimation of the slope in a linear of expression on age:
representing the conditional expectation of expression for the ith gene, given the age of the jth array, Zj=z, can be described by a simple linear regression equation with two parameters for each gene, the intercept, αi and the slope, βi.
The goal is to create a transformation, βij such that, in this case:
| (1) |
(where the ^ denotes estimate), which provides an estimate of the association at the individual/gene level of some fixed characteristic of the array with gene expression. In this case, the transformation one uses is
which provides the property (1). When this new data matrix is clustered, then the resulting clusters represent genes that have a similar association with Z. We also wanted to cluster genes that were related to behavior (using a continuous scale) after adjusting for age. In this case, we fit models, for each gene, of the type:
where now Uj is the behavior of the worm in the jth array. In this case, we can get a similar transformation (γij)for the contribution of the jth array to the adjusted (for age) association of expression on behavior as:
Re-analysis of Herndon data
To examine the patterns of survival within both age and behavior groups simultaneously, we re-analyzed the Herndon data using a generalized additive models (GAM) approach (Hastie & Tibshirani 1990). Specifically, we re-formatted the data to have an observation for every day at risk for a worm and an indicator of whether or not the worm died on that particular day. By performing smooth, logistic regression (using GAM) stratified by behavior (A vs. B/C) on this data we obtain the daily average hazard of death within behavior groups. Simply plotting these results (which assume no particular pattern) provides a semi-parametric model of the hazard of death.
Acknowledgements
We would like to thank Elaine Mardis and the Washington University Microarray Core Facility for providing the full-genome C. elegans oligo arrays, and Krysta Felkey for conducting the microarray hybridizations and quantifications. We are grateful to Lawreen Asuncion and Nancy Dudek for excellent technical assistance, and to Ken Beckman for helpful discussion. We would also like to thank Monica Driscoll, and Laura Herndon for providing us with the raw data from their 2002 publication.
Funding
This work was supported by NIH grants AG24385 (SM), RL1 AG032117 (SM) and P30AG025708 (SM).
References
- Antebi A. Genetics of Aging in Caenorhabditis elegans. PLoS Genetics. 2007;3 doi: 10.1371/journal.pgen.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Butler RN, Sprott R, Warner H, Bland J, Feuers R, Forster M, Fillit H, Harman SM, Hewitt M, Hyman M. Biomarkers of aging: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci. 2004;59:B560–B567. doi: 10.1093/gerona/59.6.b560. [DOI] [PubMed] [Google Scholar]
- Conradt B, Xue D. Programmed cell death. In: TCeR Community, editor. WormBook. WormBook; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004;101:5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. Chicago: The University of Chicago Press; 1990. [Google Scholar]
- Finch CE, Pike MC. Maximum life span predictions from the Gompertz mortality model. Journals of Gerontology Series A: Biological and Medical Sciences. 1996;51:183–194. doi: 10.1093/gerona/51a.3.b183. [DOI] [PubMed] [Google Scholar]
- Foekens JA, Atkins D, Zhang Y, Sweep F, Harbeck N, Paradiso A, Cufer T, Sieuwerts AM, Talantov D, Span PN. Multicenter Validation of a Gene Expression-Based Prognostic Signature in Lymph Node-Negative Primary Breast Cancer. Journal of Clinical Oncology. 2006;24:1665. doi: 10.1200/JCO.2005.03.9115. [DOI] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic Analysis of Tissue Aging in Caenorhabditis elegans. A role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Pletcher S, Partridge L. Interpreting interactions between treatments that slow aging. Aging Cell. 2002;1:1–9. doi: 10.1046/j.1474-9728.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- Golden TR, Beckman KB, Lee AH, Dudek N, Hubbard A, Samper E, Melov S. Dramatic age-related changes in nuclear and genome copy number in the nematode Caenorhabditis elegans. Aging Cell. 2007;6:179–188. doi: 10.1111/j.1474-9726.2007.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden TR, Melov S. Microarray analysis of gene expression with age in individual nematodes. Aging Cell. 2004;3:111–124. doi: 10.1111/j.1474-9728.2004.00095.x. [DOI] [PubMed] [Google Scholar]
- Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, Mattout A, Gruenbaum Y, Liu J. From the Cover: Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:16690–16695. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Biomarkers of aging: tissue markers. Future research needs, strategies, directions and priorities. Exp Gerontol. 1988;23:309–325. doi: 10.1016/0531-5565(88)90034-4. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Monographs on statistics and applied probability. London, New York: Chapman and Hall; 1990. Generalized additive models. [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hill AA, Hunter CP, Tsung BT, Tucker-Kellogg G, Brown EL. Genomic analysis of gene expression in C. elegans. Science. 2000;290:809–812. doi: 10.1126/science.290.5492.809. [DOI] [PubMed] [Google Scholar]
- Hosono R. Age dependent changes in the behavior of Caenorhabditis elegans on attraction to Escherichia coli. Exp Gerontol. 1978;13:31–36. doi: 10.1016/0531-5565(78)90027-x. [DOI] [PubMed] [Google Scholar]
- Hosono R, Sato Y, Aizawa SI, Mitsui Y. Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol. 1980;15:285–289. doi: 10.1016/0531-5565(80)90032-7. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol. 2001;36:1025–1034. doi: 10.1016/s0531-5565(01)00110-3. [DOI] [PubMed] [Google Scholar]
- Johnstone IL. Cuticle collagen genes. Expression in Caenorhabditis elegans. Trends Genet. 2000;16:21–27. doi: 10.1016/s0168-9525(99)01857-0. [DOI] [PubMed] [Google Scholar]
- Johnstone IL, Barry JD. Temporal reiteration of a precise gene expression pattern during nematode development. Embo J. 1996;15:3633–3639. [PMC free article] [PubMed] [Google Scholar]
- Kenyon J, Gerson SL. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Research. 2007;35:7557. doi: 10.1093/nar/gkm1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooperberg C, Bose S, Stone C. Polychotomouse Regression. Journal of the American Statistical Association. 1997;92:117–127. [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Dallal GE, Bronson RT. Lesion biomarkers of aging in B6C3F1 hybrid mice. J Gerontol A Biol Sci Med Sci. 1999;54:B466–B477. doi: 10.1093/gerona/54.11.b466. [DOI] [PubMed] [Google Scholar]
- Lund J, Tedesco P, Duke K, Wang J, Kim S, Johnson T. Transcriptional Profile of Aging in C. elegans. Curr Biol. 2002;12:1566. doi: 10.1016/s0960-9822(02)01146-6. [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Medawar PB. An Unsolved Problem ofBiology. London, UK: HK Lewis; 1952. [Google Scholar]
- Melov S, Hubbard A. Microarrays as a tool to investigate the biology of aging: a retrospective and a look to the future. Sci Aging Knowledge Environ. 2004;2004:re7. doi: 10.1126/sageke.2004.42.re7. [DOI] [PubMed] [Google Scholar]
- Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE. 2007;2:e465. doi: 10.1371/journal.pone.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nagaraja GM, Othman M, Fox BP, Alsaber R, Pellegrino CM, Zeng Y, Khanna R, Tamburini P, Swaroop A, Kandpal RP. Gene expression signatures and biomarkers of noninvasive and invasive breast cancer cells: comprehensive profiles by representational difference analysis, microarrays and proteomics. Oncogene. 2006;25:2328–2338. doi: 10.1038/sj.onc.1209265. [DOI] [PubMed] [Google Scholar]
- Panno JP, Nair KK. Age-related chromatin condensation in flight muscle nuclei of the adult male housefly, Musca domestica. Exp Gerontol. 1985;20:341–345. doi: 10.1016/0531-5565(85)90014-2. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Trends in ecology & evolution (Personal edition) Vol. 21. 2006. Beyond the evolutionary theory of ageing, from functional genomics to evo-gero; pp. 334–340. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-Wide Transcript Profiles in Aging and Calorically Restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Randerath K, Zhou GD, Hart RW, Turturro A, Randerath E. Biomarkers of aging: correlation of DNA I-compound levels with median lifespan of calorically restricted and ad libitum fed rats and mice. Mutat Res. 1993;295:247–263. doi: 10.1016/0921-8734(93)90024-w. [DOI] [PubMed] [Google Scholar]
- Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nature Medicine. 2007;13:361–366. doi: 10.1038/nm1556. [DOI] [PubMed] [Google Scholar]
- Spurgeon SL, Jones RC, Ramakrishnan R. High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PLoS ONE. 2008;3:e1662. doi: 10.1371/journal.pone.0001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. Faseb J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- van der Laan MJ, Pollard KS. A new algorithm for hybrid hierarchical clustering with visualization and the bootstrap. Journal of Statistical Planning and Inference. 2003;117:275–303. [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Walker GA, White TM, McColl G, Jenkins NL, Babich S, Candido EP, Johnson TE, Lithgow GJ. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2001;56:B281–B287. doi: 10.1093/gerona/56.7.b281. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Yan SD, Xiong WC, Stern DM. Mitochondrial amyloid-beta peptide: Pathogenesis or late-phase development? Journal of Alzheimer's Disease. 2006;9:127–137. doi: 10.3233/jad-2006-9205. [DOI] [PubMed] [Google Scholar]
- Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Narasimhan S, Kane DW, Reinhold WC, Lababidi S. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeberg BR, Qin H, Narasimhan S, Sunshine M, Cao H, Kane DW, Reimers M, Stephens RM, Bryant D, Burt SK. High-Throughput GoMiner, an ‘industrial-strength’integrative gene ontology tool for interpretation of multiple-microarray experiments, with application to studies of Common Variable Immune Deficiency (CVID) BMC Bioinformatics. 2005;6:168. doi: 10.1186/1471-2105-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]