Abstract
In humans, deficiency of galactose-1-phosphate uridyltransferase (GALT) activity can lead to a potentially lethal disease called Classic Galactosemia. Although a galactose-restricted diet can prevent the acute lethality associated with the disorder, chronic complications persist in many well-treated patients. Approximately 85% of young women with Classic Galactosemia experience hypergonadotropic hypogonadism and premature ovarian failure (POF). Others suffer from mental retardation, growth restriction, speech dyspraxia, and ataxia. Despite decades of intense biochemical characterization, little is known about the molecular etiology, as well as the chronology of the pathological events leading to the poor outcomes. Several hypotheses have been proposed, most of which involved the accumulation of the intermediates and/or the deficit of the products, of the blocked GALT pathway. However, none of these hypotheses satisfactorily explained the absence of patient phenotypes in the GALT-knockout mice. Here we proposed that the gene encoded the human tumor suppressor gene aplysia ras homolog I (ARHI) is a target of toxicity in Classic Galactosemia, and because ARHI gene is lost in rodents in through evolution, it thus accounts for the lack of clinical phenotypes in the GALT-knockout mice.
B. BACKGROUND
B.1 The Galactose metabolic pathway is integrated to other metabolic network
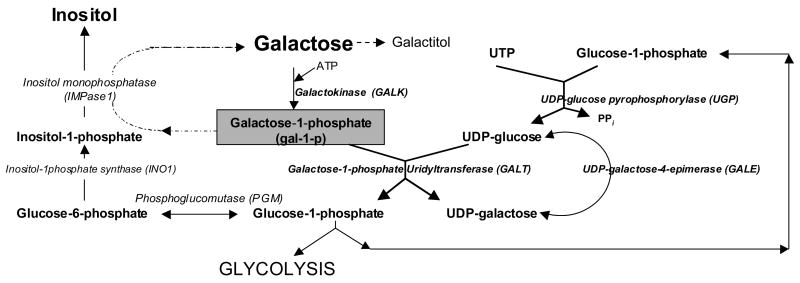
In all living cells, productive utilization of α-D-galactose requires its first conversion to galactose-1-phosphate (gal-1-p) by the enzyme galactokinase (GALK). Then in the presence of a second enzyme, galactose-1-phosphate uridyltransferase (GALT), gal-1-p reacts with UDP-glucose to form UDP-galactose and glucose-1-phosphate [1] (Fig. 1). Glucose-1-phosphate produced can enter glycolysis to yield energy, or react with UTP in the presence of UDP-glucose pyrophosphorylase (UGP) to form a new molecule of UDP-glucose [2]. The other product, UDP-galactose, can act as a galactosyl donor for glycoproteins/glycolipids biosynthesis or be converted to UDP-glucose by UDP-4-galactose epimerase (GALE) [3]. Gal-1-p can also be dephosphorylated by inositol monophosphatase to form galactose [4].
Fig. 1.
The galactose metabolic pathway is linked to uridine nucleotide and inositol metabolism.
B.2 Blockade in the galactose metabolic pathway is detrimental to humans
Deficiency of GALT activity caused by mutations in the human GALT gene can lead to a potentially lethal disorder called Classic Galactosemia [5–7]. For infants deficient in GALT activity (i.e., a G/G biochemical genotype), ingestion of a 60ml-bottle of cow milk will result in a rapid accumulation of 10–20mM (18–36mg/100ml) galactose in their blood and tissues [8]. If galactose is not withdrawn from the diet in time, affected infants will suffer from a range of toxicity syndrome that includes hepatomegaly, bleeding disorder, E. coli sepsis and death [5–7]. Consequently, all 50 states in the U.S. include this disease in their newborn screening programs [7]. Newborns diagnosed with this disease will be put on galactose-restricted diet immediately. Yet despite implementation of newborn screening programs by all U.S. public health departments, infants with G/G galactosemia continue to die [9] and many who are saved from neonatal death have chronic, premature morbidity including cataracts, premature ovarian failure [10–12], growth restriction, dyspraxic speech, ataxia and tremors [13–16]. Thus, it is imperative that we progress in (a) rapid, point-of-care technologies for diagnosis; (b) elucidating the molecular pathophysiology of the disease; and most importantly (c) developing new therapeutic approaches to prevent these poor outcomes.
B.3 Treated GALT-deficient patients are constantly exposed to galactose insult
Before we examine the proposed pathogenic mechanisms for the complications seen in GALT-deficient patients, we must recognize that patients who are on galactose-restricted diet are never truly free from galactose intoxication. Even if patients refrain from all dairy products, significant amounts of bio-available galactose are found in non-dairy foodstuffs such as vegetables and fruits [17, 18]. In addition to these hidden sources of dietary galactose, galactose is produced endogenously from (a) UDP-glucose via the UDP-4-galactose epimerase (GALE) reaction (Fig. 1), and (b) natural turnover of glycoproteins/glycolipids. Using the technique of isotopic labeling, Berry et al. elegantly demonstrated that a 50kg adult male could produce up to 2 grams of galactose per day [19–21].
B.4 Patho-physiological mechanisms for galactose toxicity in GALT deficiency
At least 5 mechanisms producing organ toxicity in GALT deficiency are considered and outlined below:
B.4.1 Accumulation of toxic metabolites in the blocked GALT reaction
Since galactosemic patients are constantly exposed to galactose, they are continually subjected to toxic effect of the intermediates accumulated in the blocked GALT pathway. The metabolites accumulated in the blocked GALT pathway are galactose and gal-1-p (Fig. 1). Untreated GALT-deficient patients accumulated up to 2.5 to 6.5mM of galactose and gal-1-p in their cells. Even on galactose-restricted diets, they continue to amass up to 100–200μM of galactose and gal-1-p [7]
B.4.2 Accumulation of toxic products of alternate galactose catabolism
If galactose is not metabolized efficiently, whether it is due to a block in the GALK or GALT reaction of the galactose metabolic pathway, excess galactose will be catabolized by alternate pathways to form (a) galactitol and (b) galactonate. Galactitol is not further metabolized in the galactosemic patients and the majority formed is excreted in the urine [7]. It remains unclear whether the accumulated galactonate is toxic, and it is thought to be metabolized via the pentose phosphate shunt [7]. On the other hand, researchers have often associated the potential osmotic activity of galactitol with cataract formation seen in both GALK- and GALT-deficient patients [22]. However, studies from Kubo and colleagues suggested that cataract formation through the polyol pathway is predominantly associated with free radical production [23, 24]. These investigators proposed that the concentration of galactitol build-up in the target tissues might not be high enough to evoke significant osmotic stress. Instead, they showed that excess galactitol resulted in activation of aldose reductase, which depleted NADPH and led to lowered glutathione reductase activity. As a result, hydrogen peroxide or other free radicals accumulated and caused serious oxidative damage to the cells.
Nonetheless, as GALK-deficient patients also accumulate high levels of galactose, galactitol and galactonate, but not gal-1-p [7, 25–27], and do not experience the range of complications seen in GALT-deficient patients, one can infer that gal-1-p is a major, if not sole, pathogenic agent for the sufferings seen in patients with galactosemia. In fact, retrospective studies conducted by our group showed that high level of gal-1-p is a risk factor for the development of ovarian failure and dyspraxic speech [13, 16]. Further, while a gal7-deleted (i.e., GALT-deficient) yeast cell model stops growing upon addition of galactose to the growth medium, a gal7 gal1 double mutant strain deficient in both GALT and GALK enzyme activities is no longer sensitive to galactose, and grows well in its presence [28, 29]. Finally, our laboratory recently demonstrated that galactose challenge to isogenic GALT-deficient (but not GALK-deficient) yeast led to overt manifestation of environmental stress response (ESR) [30]. All these studies indicate that gal-1-p is the major, if not sole, culprit for the galactose toxicity observed in GALT-deficient cells.
Yet, despite all these studies, the in vivo target(s) of the presumably toxic gal-1-p in humans has never been confirmed. Various reports suggested that it competitively inhibited phosphoglucomutase [31], inositol monophosphatase [4, 32–34], glycogen phosphorylase [35], UDP-pyrophosphorylase [34, 36, 37], or even glucose-6-phosphatase [38], but none of these hypotheses have been verified in human patients/cells in vivo.
We are aware of a report by Pourci and coworkers, who once proposed that gal-1-p might not be toxic. This was because when they added 2.5mM inosine to the growth medium of GALT-deficient fibroblasts, these cells grew in the presence of 5mM galactose, despite the accumulation of significant amount gal-1-p [39]. However, we believe that their conclusion was premature. In our opinion, the fact that these fibroblasts grew in the presence of high level of intracellular gal-1-p did not necessarily mean that the gal-1-p was not toxic. We suggest that the added inosine might have simply overcome/suppressed the growth sensitivity of the gal-1-p. Moreover, the authors paid little attention to abnormalities other than growth at the time of the study. Therefore, even if the patient fibroblasts could grow does not imply that they were “normal”.
B.4.3 Deficiency of UDP-galactose (and UDP-glucose)
As shown in Fig. 1 above, UDP-galactose is one of the two products of the GALT reaction. It is thus logical to assume that if the GALT reaction is blocked, it will lead to a potential deficit of UDP-galactose [40]. Since UDP-galactose is a galactosyl donor in glycoproteins/glycolipids biosynthesis, UDP-galactose deficiency caused by GALT deficit can theoretically impair the production of these macromolecules. Others suggested that even if the GALT reaction were blocked, UDP-galactose could still be formed via the epimerization of UDP-glucose in the presence of GALE (Fig. 1). However, we found that excess gal-1-p accumulated under GALT deficiency competed with glucose-1-phosphate for the enzyme UDP-glucose pyrophosphorylase (UGP) in vitro [37]. As the UGP reaction is necessary to produce UDP-glucose (Fig. 1), we proposed that the inhibition of this enzyme in vivo by gal-1-p could lead to reduced availability of UDP-glucose [37]. Such decline in UDP-glucose availability will further jeopardize the formation of UDP-galactose from the GALE reaction, and this in turn will lead to aberrant production of glycoproteins/glycolipids macromolecules. Indeed, several groups have reported aberrantly glycosylated glycoproteins such as serum transferrins, lysozomal enzymes, and follicle stimulating hormone receptors, in galactosemic patients [41–44]. In all cases, the oligosaccharide chains of the circulating transferrin or follicle-stimulating hormone (FSH) were found deficient in their penultimate galactose and terminal sialic acids. In case of the aberrant glycosylation of FSH, it has been proposed that the mis-glycosylated FSH out-competed the normal FSH to the hormone receptor, yet it failed to elicit the signaling pathway of the hormone. As a result, this might have led to the under-development of the follicles. Abnormal glycosylation has also been proposed to cause impaired germ cell migration in experimental animals [45].
B.4.4 Prenatal toxicity
As abnormally high level of gal-1-p, galactitol and galactose are found in the cord blood or amniotic fluid in the third trimester of galactosemic pregnancies [46, 47], some suggested that galactose toxicity could be prenatal in origin [47]. Although this hypothesis is logical, recent studies of endogenous production of galactose in normal and galactosemic adults indicated that galactose insult continues after birth [19–21].
B.4.5 Perturbation of inositol metabolism
Several lines of evidence led Bhat to propose that excess gal-1-p accumulated might interfere inositol monophosphatases, thus disrupting the normal phosphatidylinositol bisphosphate [PI(P)2]-dependent signaling pathway in GALT-deficient tissues by limiting inositol phosphates turnover. As free inositol supply in limited in tissues such as brain, any reduction in inositol phosphates turnover can be detrimental [4, 32, 34]. Interestingly, Wells and Wells also showed that developing rats fed with excess galactose had less inositol contents in their brains [48].
B.5 Acute toxicity syndrome/chronic complications of Classic Galactosemia are not seen in animal models
One major obstacle in resolving the above mechanisms for toxicity is the lack of an animal model for Classic Galactosemia. Animal models of Classic Galactosemia so far have failed to mimic either the acute lethality or long-term complications associated with the human disorder. In early studies, investigators fed normal rats with high galactose diets (40% galactose by weight) in an attempt to overwhelm the galactose metabolic pathways [49]. Although these rats gave birth to newborns with cataracts and the female pups had fewer oocytes, these pups remained fertile and had no long-term complications. The authors contended that the endogenous GALT genes in these rats were intact and therefore might have protected them from galactose toxicity. Later, Leslie and coworkers constructed GALT-knockout mice [50–52]. When these mice were fed with a high galactose diet (40% galactose by weight), they showed mildly elevated levels of cellular gal-1-p (~30% of the level seen in untreated human patients), galactitol and galactose. Nevertheless, they remained symptom-free and the female mice were fertile. The fact that they accumulated significant levels of gal-1-p and galactitol suggested that the Leloir pathway of galactose metabolism remained the predominant route of galactose metabolism in these mice. But after noticing that the level of galactitol accumulated in these knock-out mice was only about 10% of that seen in human galactosemic patients, the authors proposed the “two-hit” hypothesis, which stated that the toxic effects of galactose in GALT deficiency manifest themselves only when the level of accumulated gal-1-p and galactitol are both high. It is, however, unclear how the investigators decided that the level of galactitol, albeit at only 10% of what is seen in human patients, was not high enough for these mice. Moreover, the authors have not seriously considered that galactokinase in mice may be less active than in humans and this itself may be “protective” of galactose toxicity caused by high level of gal-1-p. Furthermore, we must emphasize that whether a “high” level of galactitol is required for the manifestation of galactose toxicity, one should not be distracted from the fact that gal-1-p remains a key pathogenic agent under this “two-hit” hypothesis. Lastly, no one has considered the possibility that the target(s) of galactose toxicity in human galactosemic patients are not present in rodents, or the likelihood that these toxicity targets in mice are less susceptible to the toxic galactose metabolites.
C. PROPOSED HYPOTHESIS
In this report, we propose target(s) of galactose toxicity in human galactosemic patients are not present in rodents, thus explaining the absence of clinical phenotypes in mice deficient in galactose-1-phosphate uridyltransferase (GALT) activity.
D. EVIDENCE TO SUPPORT THE PROPOSED HYPOTHESIS
Although the pathogenic mechanisms for Classic Galactosemia outlined above were insightful, they all failed to account for the lack of patient phenotypes in the GALT-knockout mice [50–52]. At the same time, several groups of investigators have downplayed the role of gal-1-p in the pathogenesis of the disorder as they suggested that the modest accumulation of cellular gal-1-p in GALT-knockout mice did not result in any clinical phenotypes [50–52]. Virtually no one has considered the possibilities that the target(s) of galactose toxicity in human galactosemic patients is absent in rodents, or the likelihood that these toxicity targets in mice are less susceptible to the toxic galactose metabolites. Here we report that a human gene called aplysia ras homolog I (ARHI), which is absent in mice, could be a target of galactose toxicity in galactosemic patients. Since this gene is evolutionarily lost in rodents, this will explain why the GALT-knockout mice did not show any patient phenotypes.
D.1 Transcriptome Profiling of human GALT-knockout and normal fibroblasts under galactose challenge
In a recent study, in order to search for unique genes that are either up- or down-regulated in the GALT-knockout cells under galactose challenge, we performed transcriptome profiling of GALT-knockout and control cells at two time points: +2 hours and +24 hours using protocols established in our laboratory [33]. This gave us information on both the acute and chronic response by these cells to galactose challenge. We also tested the sensitivity to galactose by exposing the GALT-knockout cells to two different concentrations of galactose (0.01% and 0.05%). Thus the sensitivity and kinetics of gene expression to galactose challenge could be compared.
We found that when the GALT-knockout cells were transferred from media with 0.1% glucose to medium containing 0.09% glucose plus 0.01% galactose, 1,146 and 1,230 transcripts were up-regulated and down-regulated, respectively by +2 hours. The number of up-regulated and down-regulated transcripts dropped significantly to 302 and 296, respectively, when we sampled the cultures and tested them at +24 hours (Table 1). When the GALT-knockout cells were transferred to medium containing 0.05% glucose and higher, 0.05% galactose, fewer genes were over-expressed at +24 hours than at +2 hours of exposure (Table 1). Interestingly, results from the normal fibroblasts were different from those of the GALT-knockout cells. At +2 hours after transfer of normal cells to medium containing 0.09% glucose plus 0.01% galactose, we observed a modest increase in 312 transcripts, while 420 transcripts were down-regulated. At +24 hours, the number of up-regulated transcripts had increased to 805, but the number of down-regulated transcripts rose slightly to 442 (Table 1).
Table 1. Kinetics of gene expression in GALT-knockout and normal (N/N) fibroblasts upon galactose challenge.
The number of genes under the Common column represent the number of genes that were increased or decreased in common found at +2 hours (+2h) and +24 hours (+24h).
| 0.09% Glucose + 0.01% Galactose | 0.05% Glucose + 0.05% Galactose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Up-Regulated Genes | No. of Down-Regulated Genes | No. of Up-Regulated Genes | No. of Down-Regulated Genes | |||||||||
| +2h | +24h | Common | +2h | +24h | Common | +2h | +24h | Common | +2h | +24h | Common | |
| GALT-knockout | 1146 | 302 | 116 | 1230 | 296 | 142 | 973 | 427 | 195 | 838 | 117 | 56 |
| N/N | 312 | 805 | 33 | 420 | 442 | 32 | Not done | Not done | Not done | Not done | Not done | Not done |
We then cross-compared the transcriptome profiles of normal and GALT-knockout cells after following transfer to medium containing 0.09% glucose and 0.01% galactose. We wanted to identify unique genes that were consistently up-regulated or down-regulated at 2-fold or higher at +2 hours and +24 hours of galactose stress when compared to 0 hour. We found that 116 genes that were in common among the up-regulated genes at +2 hours and +24 hours for the GALT-knockout cells, while 142 genes were in common among the down-regulated genes (Table 1). In contrast, only 33 genes were common among the up-regulated at +2 hours and +24 hours for the normal cells, while 32 genes were common among the down-regulated genes (Table 1).
When we examined the transcriptome profiles of the GALT-knockout cells challenged with higher percentage of galactose (i.e., 0.05% galactose), we found that 195 genes were common among the genes that were up--regulated at +2 hours and +24 hours, and 56 genes were in common at both time points among the down-regulated genes (Table 1).
D.2 Up-regulation of ARHI gene in GALT-knockout, GALT-deficient and normal fibroblasts
When we looked for genes that were up-regulated in the GALT-knockout cells at both time points in the two media containing different galactose concentrations (i.e., to identify the genes that were in common among the 116 genes in and the 195 genes highlighted in Table 1), we found a total of 49 genes. However, the level of up-regulation in 46 of them declined or decreased substantially at +24 hours (Table 2). Only three genes continued an increased level of expression at both +2 hours and +24 hours of galactose stress. None of these three genes were up-regulated in the normal cells challenged with galactose (highlighted in Table 2). These three genes were ZNF141, ZNF223, and the human tumor suppressor gene DIRAS3, which is also known as aplysia ras homolog I (ARHI). Little is known about the specific functions of the two zinc finger proteins, ZNF141 [53] and ZNF223 [54]. Thus, we focused on the ARHI gene. In the microarray data sets, this gene was up-regulated 6.73 fold in the GALT-knockout cells after exposure to 0.01% galactose for two hours. ARHI expression increased to 10.70 fold after 24 hours (Table 2). These microarray data were later confirmed with quantitative real-time PCR (Fig. 2). Here we demonstrated the dose-response to galactose concentrations in the media at 0%, 0.01%, and 0.05% galactose. Gal-1-p concentrations were elevated in the knock-out cells in the absence of added galactose, but not in the control cells. As galactose concentrations increased, the Δ5kb/Δ5kb cells had increased intracellular gal-1-p concentrations to 4.45 and 5.33mM while control cells had less than 0.01mM gal-1-p. There was a direct correlation of this increased intracellular gal-1-p to the increased expression of ARHI that rose two and four fold when quantified by RT-PCR (Fig. 2). No transcripts of ARHI were found in the control cells at these concentrations, or exposure times to galactose in the medium. In separate experiments not shown, we found that ARHI transcripts were also increased in human fibroblasts that were homozygous for the common Q188R mutation in the GALT gene [55–58].
Table 2.
49 genes that were up-regulated in GALT-knockout cells at both time (T) = +2 hours (+2h) and time (T) =+24 hours (+24h) after galactose challenge.
| 0.09% Glucose+0.01% Galactose | 0.05% Glucose+0.05% Galactose | |||||
|---|---|---|---|---|---|---|
| T= +2h | T= +24h | T= +2h | T= +24h | |||
| Gene Symbol/ID | Gene name | Fold Change | Fold Change | Fold Change | Fold Change | |
| 1 | AF118081 | Homo sapiens PRO1900 mRNA, complete cds. [AF118081] | 2.2159 | 4.1903 | 2.2199 | 2.4788 |
| 2 | ATAD2 | Homo sapiens ATPase family, AAA domain containing 2 (ATAD2), mRNA [NM_014109] | 2.5860 | 2.1912 | 2.6624 | 2.9207 |
| 3 | BLM | Homo sapiens Bloom syndrome (BLM), mRNA [NM_000057] | 3.9977 | 3.0104 | 4.1334 | 3.0475 |
| 4 | BRCA1 | Homo sapiens breast cancer 1, early onset (BRCA1), transcript variant BRCA1b, mRNA [NM_007295] | 2.9278 | 2.7657 | 3.1038 | 2.7372 |
| 5 | BTBD11 | Homo sapiens BTB (POZ) domain containing 11 (BTBD11), transcript variant 1, mRNA [NM_152322] | 4.6453 | 3.6777 | 3.8577 | 2.9645 |
| 6 | C14orf145 | Homo sapiens chromosome 14 open reading frame 145 (C14orf145), mRNA [NM_152446] | 2.3419 | 2.3638 | 3.1662 | 2.6317 |
| 7 | C15orf42 | Homo sapiens chromosome 15 open reading frame 42, mRNA (cDNA clone IMAGE:3940845), partial cds. [BC002881] | 2.5754 | 2.6657 | 2.6642 | 2.5498 |
| 8 | C1orf135 | Homo sapiens chromosome 1 open reading frame 135 (C1orf135), mRNA [NM_024037] | 6.0206 | 2.4856 | 4.5836 | 2.1706 |
| 9 | C6orf167 | Homo sapiens chromosome 6 open reading frame 167 (C6orf167), mRNA [NM_198468] | 2.5044 | 2.1085 | 2.9092 | 2.6598 |
| 10 | CALB2 | Homo sapiens calbindin 2, 29kDa (calretinin) (CALB2), transcript variant CALB2, mRNA [NM_001740] | 4.6864 | 3.5229 | 5.6453 | 2.1511 |
| 11 | CENPO | Homo sapiens centromere protein O (CENPO), mRNA [NM_024322] | 3.5715 | 2.6316 | 3.0162 | 2.2971 |
| 12 | CHAF1B | Homo sapiens chromatin assembly factor 1, subunit B (p60) (CHAF1B), mRNA [NM_005441] | 2.7261 | 2.1368 | 2.0194 | 2.2379 |
| 13 | CKAP2L | Homo sapiens cytoskeleton associated protein 2-like (CKAP2L), mRNA [NM_152515] | 2.2616 | 3.9039 | 2.7397 | 3.2746 |
| 14 | COMP | Homo sapiens cartilage oligomeric matrix protein (COMP), mRNA [NM_000095] | 5.2865 | 3.3143 | 3.2940 | 3.0677 |
| 15 | DIRAS3 | Homo sapiens DIRAS family, GTP-binding RAS-like 3 (DIRAS3), mRNA [NM_004675] | 6.7295 | 10.6971 | 7.9285 | 11.6346 |
| 16 | DKFZP564O0523 | Homo sapiens hypothetical protein DKFZp564O0523 (DKFZP564O0523), mRNA [NM_032120] | 2.3763 | 3.9786 | 2.0353 | 3.4618 |
| 17 | DLX2 | Homo sapiens distal-less homeobox 2 (DLX2), mRNA [NM_004405] | 3.4586 | 2.6290 | 2.8188 | 2.2133 |
| 18 | DTL | Homo sapiens denticleless homolog (Drosophila) (DTL), mRNA [NM_016448] | 4.0266 | 2.0709 | 3.3927 | 2.4674 |
| 19 | ENST00000272831 | Homo sapiens cDNA FLJ39660 fis, clone SMINT2006801. [AK096979] | 3.2824 | 3.3055 | 3.3231 | 2.8630 |
| 20 | EXO1 | Homo sapiens exonuclease 1 (EXO1), transcript variant 3, mRNA [NM_003686] | 3.8826 | 2.7400 | 3.3765 | 2.6737 |
| 21 | FANCA | Homo sapiens Fanconi anemia, complementation group A (FANCA), transcript variant 1, mRNA [NM_000135] | 4.4405 | 2.5373 | 3.9381 | 2.0839 |
| 22 | FANCD2 | Homo sapiens Fanconi anemia, complementation group D2 (FANCD2), transcript variant 2, mRNA [NM_001018115] | 2.3172 | 2.7902 | 2.7712 | 2.3316 |
| 23 | FEN1 | Homo sapiens flap structure-specific endonuclease 1 (FEN1), mRNA [NM_004111] | 4.6554 | 2.0057 | 2.9660 | 2.0220 |
| 24 | FLJ25416 | Homo sapiens hypothetical protein FLJ25416 (FLJ25416), mRNA [NM_145018] | 3.3113 | 2.4363 | 4.5802 | 2.9110 |
| 25 | FLJ39660 | Homo sapiens mRNA; cDNA DKFZp434P055 (from clone DKFZp434P055). [AL834537] | 2.7470 | 3.6942 | 2.4951 | 2.8696 |
| 26 | FOXF1 | Homo sapiens forkhead box F1 (FOXF1), mRNA [NM_001451] | 3.8644 | 2.3633 | 3.0667 | 2.0672 |
| 27 | GRB14 | Homo sapiens growth factor receptor-bound protein 14 (GRB14), mRNA [NM_004490] | 11.6293 | 4.3499 | 4.9834 | 3.5137 |
| 28 | GSG2 | Homo sapiens cDNA FLJ32129 fis, clone PEBLM2000213, weakly similar to Mus musculus genes for integrin aM290, hapsin. [AK056691] | 4.0174 | 3.0022 | 3.6721 | 2.4851 |
| 29 | HIST1H4B | Homo sapiens histone 1, H4b (HIST1H4B), mRNA [NM_003544] | 6.0164 | 2.5334 | 4.5282 | 2.5567 |
| 30 | HIST1H4L | Homo sapiens histone 1, H4l (HIST1H4L), mRNA [NM_003546] | 4.9153 | 2.2718 | 4.6618 | 2.4205 |
| 31 | IL8 | Homo sapiens interleukin 8 (IL8), mRNA [NM_000584] | 13.2970 | 3.7871 | 40.6700 | 6.9918 |
| 32 | KIAA1794 | Homo sapiens KIAA1794 (KIAA1794), mRNA [NM_018193] | 2.3180 | 2.2701 | 2.5657 | 2.3368 |
| 33 | KRTAP2-4 | Homo sapiens keratin associated protein 2–4, mRNA (cDNA clone MGC:74790 IMAGE:3907481), complete cds. [BC063625] | 14.5565 | 2.1635 | 8.8681 | 2.2416 |
| 34 | LCP1 | Homo sapiens lymphocyte cytosolic protein 1 (L- plastin) (LCP1), mRNA [NM_002298] | 5.3567 | 3.1974 | 6.5640 | 3.5841 |
| 35 | LIN9 | Homo sapiens lin-9 homolog (C. elegans) (LIN9), mRNA [NM_173083] | 2.0351 | 2.2094 | 2.8722 | 2.5001 |
| 36 | MALL | Homo sapiens mal, T-cell differentiation protein- like (MALL), mRNA [NM_005434] | 3.7111 | 2.1212 | 2.2459 | 2.1592 |
| 37 | MCM8 | Homo sapiens MCM8 minichromosome maintenance deficient 8 (S. cerevisiae) (MCM8), transcript variant 2, mRNA [NM_182802] | 2.4416 | 2.0233 | 2.7290 | 2.1927 |
| 38 | NCF2 | Homo sapiens neutrophil cytosolic factor 2 (65kDa, chronic granulomatous disease, autosomal 2) (NCF2), mRNA [NM_000433] | 4.0104 | 2.3476 | 5.5209 | 2.4254 |
| 39 | PODXL | Homo sapiens podocalyxin-like (PODXL), transcript variant 2, mRNA [NM_005397] | 6.1891 | 3.7635 | 6.9373 | 3.4990 |
| 40 | RBL1 | Homo sapiens retinoblastoma-like 1 (p107) (RBL1), transcript variant 1, mRNA [NM_002895] | 3.1106 | 2.5367 | 3.3424 | 6.0484 |
| 41 | RGS20 | Homo sapiens regulator of G-protein signalling 20 (RGS20), transcript variant 1, mRNA [NM_170587] | 3.2261 | 3.2284 | 3.1573 | 2.8231 |
| 42 | RRM2 | Homo sapiens ribonucleotide reductase M2 polypeptide (RRM2), mRNA [NM_001034] | 4.2204 | 2.3773 | 3.7226 | 3.0227 |
| 43 | SHCBP1 | Homo sapiens SHC SH2-domain binding protein 1 (SHCBP1), mRNA [NM_024745] | 2.0100 | 2.3920 | 2.6868 | 2.7386 |
| 44 | VRK1 | Homo sapiens vaccinia related kinase 1 (VRK1), mRNA [NM_003384] | 2.8160 | 2.1657 | 2.6390 | 2.0119 |
| 45 | WDHD1 | Homo sapiens WD repeat and HMG-box DNA binding protein 1 (WDHD1), transcript variant 1, mRNA [NM_007086] | 2.8091 | 2.2974 | 3.0100 | 2.6967 |
| 46 | XRCC2 | Homo sapiens mRNA; cDNA DKFZp781P0919 (from clone DKFZp781P0919). [CR749256] | 4.9724 | 2.4844 | 2.7891 | 2.8756 |
| 47 | ZNF141 | Homo sapiens zinc finger protein 141 (ZNF141), mRNA [NM_003441] | 7.7618 | 16.5265 | 5.5300 | 12.2949 |
| 48 | ZNF223 | Homo sapiens zinc finger protein 223 (ZNF223), mRNA [NM_013361] | 3.0949 | 8.0183 | 6.0460 | 19.5330 |
| 49 | ZWINT | Homo sapiens ZW10 interactor (ZWINT), transcript variant 4, mRNA [NM_001005414] | 2.2286 | 2.1947 | 2.5364 | 2.4322 |
Fig. 2. Up-regulation of ARHI gene transcription in galactose-challenged GALT-deficient cells.
Fibroblasts derived from normal (N/N) and galactosemic (G/G) patients were cultured in media containing varying galactose concentration for 24 hours. Quantitative RT-PCR was performed on the mRNA harvested from the respective cultures using primers specific for the primers specific for human ARHI gene and the housekeeping gene GAPDH. This figure illustrated the relative abundance of the amplified ARHI transcripts in different samples by electrophoresing the amplified products on 1% agarose gel and stained with ethidium bromide.
ARHI is a human maternally imprinted tumor suppressor gene and it was originally found to be down-regulated in many types of human tumors [59–63]. Not surprisingly, ARHI maps to chromosome 1p31, a hotspot for loss of heterozygosity [63]. This gene encodes a small 26kD protein [64] and when it is over-expressed in cancer cells, it induced apoptosis and reduced growth [63, 64] via a number of well-characterized molecular mechanisms.
Interestingly, this gene was lost as a result of chromosome rearrangement in mice [65]. Over-expression of this transgene in a normal mouse model caused failure of folliculogenesis, loss of neurons in the cerebellar cortex, and stunted growth [66]. These findings are prevalent clinical complications seen in galactosemic patients [7]. Therefore, our data may explain not only the absence of galactose toxicity in the GALT-knockout mice, but also the organ specificity of pathology in ovary and cerebellum [50, 51].
E. CONSEQUNCES OF THE HYPOTHESIS AND DISCUSSION
In the past fours years alone, at least six groups of investigators reported that the health-related quality of life consequences of galactosemic patients and their parents were worse than generally thought [67–72]. Such outcry of concerns for a relatively rare disease suggested that the stressful conditions suffered by the patients and their family members have been overlooked for too long, and swift actions are required to improve the current treatments. In order to achieve this, it will be useful to understand the underlying pathogenic mechanisms of the disease. Yet the investigation of pathogenic mechanisms of Classic Galactosemia has been hampered by the failure of the GALT-knockout mice to replicate the clinical phenotypes manifested in human patients [50–52].
Here we propose the human tumor suppressor gene ARHI as a new target of galactose toxicity in patients with Classic Galactosemia. Although our results on ARHI shown above were preliminary, we believe that ARHI is a target worth pursuing because it was one of the three genes (among 36,000+ transcripts queried) that was consistently up-regulated in galactose-challenged, GALT-knockout cells at both low and high concentrations of galactose challenge and over time (Tables 1 & 2). Furthermore, the facts that rodents have lost the ARHI gene through evolution [65], and the re-expression of this gene in mice causes failure of ovarian follicular maturation, poor growth, and impaired Purkinje cell development [66] lent strong support for the organ specificity of chronic problems in patients with Classic Galactosemia, and explains the lack of a clinical phenotype in the GALT-knockout mice [50, 51].
Clearly, more experiments are required to thoroughly test our hypothesis. For instance, we are currently examining for any abnormal up-regulation of the gene in patient tissues. It will be also useful to introduce the gene back into the GALT-knockout mice under the human ARHI gene promoter to see whether the transgenic mice will manifest any patient phenotypes. Finally, it will be interesting to determine how galactose challenge results in the up-regulation of the ARHI gene expression in human GALT-deficient patients/cells, and the precise roles it plays in the pathophysiology of the disease.
Acknowledgments
Grants support to Kent Lai include NIH grant 1R01 HD054744, American Heart Association South-East Affiliate Scientist Development Grant # 0435267B, and The Woman’s Cancer Association of The University of Miami.
Footnotes
Presented in part at the 2007 Annual Meeting of the American Society for Human Genetics, San Diego, USA. (Platform Presentation Abstract #52)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Leloir LF. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem. 1951;33(2):186–90. doi: 10.1016/0003-9861(51)90096-3. [DOI] [PubMed] [Google Scholar]
- 2.Duggleby RG, et al. Sequence differences between human muscle and liver cDNAs for UDPglucose pyrophosphorylase and kinetic properties of the recombinant enzymes expressed in Escherichia coli. Eur J Biochem. 1996;235(1–2):173–9. doi: 10.1111/j.1432-1033.1996.00173.x. [DOI] [PubMed] [Google Scholar]
- 3.Salo WL, et al. The specificity of UDP-glucose 4-epimerase from the yeast Saccharomyces fragilis. Biochim Biophys Acta. 1968;151(2):484–92. doi: 10.1016/0005-2744(68)90116-2. [DOI] [PubMed] [Google Scholar]
- 4.Parthasarathy R, Parthasarathy L, Vadnal R. Brain inositol monophosphatase identified as a galactose 1-phosphatase. Brain Res. 1997;778(1):99–106. doi: 10.1016/s0006-8993(97)01042-1. [DOI] [PubMed] [Google Scholar]
- 5.Isselbacher KJ, et al. Congenital galactosemia, a single enzymatic block in galactose metabolism. Science. 1956;123(3198):635–6. doi: 10.1126/science.123.3198.635. [DOI] [PubMed] [Google Scholar]
- 6.Kalckar HM, Anderson EP, Isselbacher KJ. Galactosemia, a congenital defect in a nucleotide transferase. Biochim Biophys Acta. 1956;20(1):262–8. doi: 10.1016/0006-3002(56)90285-2. [DOI] [PubMed] [Google Scholar]
- 7.Segal S, Berry GT. Disorders of galactose metabolism. In: Scriver DBA, Sly W, Valle D, editors. The Metabolic Basis of Inherited Diseases. McGraw-Hill; New York: 1995. pp. 967–1000. [Google Scholar]
- 8.Siegel CD, Sparks JW, Battaglia FC. Patterns of serum glucose and galactose concentrations in term newborn infants after milk feeding. Biol Neonate. 1988;54(6):301–6. doi: 10.1159/000242868. [DOI] [PubMed] [Google Scholar]
- 9.Elsas LJ. Personal Experience (4 families) 2006 http://www.savebabies.org.
- 10.Kaufman FR, et al. Hypergonadotropic hypogonadism in female patients with galactosemia. N Engl J Med. 1981;304(17):994–8. doi: 10.1056/NEJM198104233041702. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman FR, et al. Gonadal function and ovarian galactose metabolism in classic galactosemia. Acta Endocrinol (Copenh) 1989;120(2):129–33. doi: 10.1530/acta.0.1200129. [DOI] [PubMed] [Google Scholar]
- 12.Waggoner D, Buist NRM, Donnell GN. Long-term prognosis in Galactosemia: results of a survey of 350 cases. Journal of Inherited Metabolic Disorders. 1990;13:802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- 13.Guerrero NV, et al. Risk factors for premature ovarian failure in females with galactosemia. J Pediatr. 2000;137(6):833–41. doi: 10.1067/mpd.2000.109148. [DOI] [PubMed] [Google Scholar]
- 14.Robertson A, et al. Outcomes analysis of verbal dyspraxia in classic galactosemia. Genet Med. 2000;2(2):142–8. doi: 10.1097/00125817-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Waggoner D, Buist NRM. Long-term complications in treated galactosemia - 175 U.S. cases. International Pediatrics. 1993;8:97–100. [Google Scholar]
- 16.Webb AL, et al. Verbal dyspraxia and galactosemia. Pediatr Res. 2003;53(3):396–402. doi: 10.1203/01.PDR.0000049666.19532.1B. [DOI] [PubMed] [Google Scholar]
- 17.Acosta PB, Gross KC. Hidden sources of galactose in the environment. Eur J Pediatr. 1995;154(7 Suppl 2):S87–92. doi: 10.1007/BF02143811. [DOI] [PubMed] [Google Scholar]
- 18.Berry GT, et al. The effect of dietary fruits and vegetables on urinary galactitol excretion in galactose-1-phosphate uridyltransferase deficiency. J Inherit Metab Dis. 1993;16(1):91–100. doi: 10.1007/BF00711320. [DOI] [PubMed] [Google Scholar]
- 19.Berry GT, et al. The rate of de novo galactose synthesis in patients with galactose-1-phosphate uridyltransferase deficiency. Mol Genet Metab. 2004;81(1):22–30. doi: 10.1016/j.ymgme.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Berry GT, et al. Quantitative assessment of whole body galactose metabolism in galactosemic patients. Eur J Pediatr. 1997;156(Suppl 1):S43–9. doi: 10.1007/pl00014271. [DOI] [PubMed] [Google Scholar]
- 21.Berry GT, et al. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet. 1995;346(8982):1073–4. doi: 10.1016/s0140-6736(95)91745-4. [DOI] [PubMed] [Google Scholar]
- 22.Berry GT. The role of polyols in the pathophysiology of hypergalactosemia. Eur J Pediatr. 1995;154(7 Suppl 2):S53–64. doi: 10.1007/BF02143805. [DOI] [PubMed] [Google Scholar]
- 23.Kubo E, et al. Cataract formation through the polyol pathway is associated with free radical production. Exp Eye Res. 1999;68(4):457–64. doi: 10.1006/exer.1998.0624. [DOI] [PubMed] [Google Scholar]
- 24.Kubo E, et al. Polyol pathway-dependent osmotic and oxidative stresses in aldose reductase-mediated apoptosis in human lens epithelial cells: role of AOP2. Biochem Biophys Res Commun. 2004;314(4):1050–6. doi: 10.1016/j.bbrc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Bosch AM, et al. Clinical features of galactokinase deficiency: a review of the literature. J Inherit Metab Dis. 2002;25(8):629–34. doi: 10.1023/a:1022875629436. [DOI] [PubMed] [Google Scholar]
- 26.Gitzelmann R. Letter: Additional findings in galactokinase deficiency. J Pediatr. 1975;87(6 Pt 1):1007–8. doi: 10.1016/s0022-3476(75)80937-1. [DOI] [PubMed] [Google Scholar]
- 27.Gitzelmann R, Wells HJ, Segal S. Galactose metabolism in a patient with hereditary galactokinase deficiency. Eur J Clin Invest. 1974;4(2):79–84. doi: 10.1111/j.1365-2362.1974.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 28.Douglas HC, Hawthorne DC. Enzymatic Expression And Genetic Linkage Of Genes Controlling Galactose Utilization In Saccharomyces. Genetics. 1964;49:837–44. doi: 10.1093/genetics/49.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas HC, Hawthorne DC. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966;54(3):911–6. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slepak T, et al. Intracellular galactose-1-phosphate accumulation leads to environmental stress response in yeast model. Mol Genet Metab. 2005;86(3):360–71. doi: 10.1016/j.ymgme.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Stempfel RS, Jr, Sidbury JB, Jr, Migeon CJ. beta-Glucuronidase hydrolysis of urinary corticosteroid conjugates: the effect of salicylate glucuronoside as a competing substrate and the effect of enzyme inactivation. J Clin Endocrinol Metab. 1960;20:814–24. doi: 10.1210/jcem-20-6-814. [DOI] [PubMed] [Google Scholar]
- 32.Bhat PJ. Galactose-1-phosphate is a regulator of inositol monophosphatase: a fact or a fiction? Med Hypotheses. 2003;60(1):123–8. doi: 10.1016/s0306-9877(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 33.Slepak TI, et al. Involvement of endoplasmic reticulum stress in a novel Classic Galactosemia model. Mol Genet Metab. 2007;92(1–2):78–87. doi: 10.1016/j.ymgme.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta DV, Kabir A, Bhat PJ. Expression of human inositol monophosphatase suppresses galactose toxicity in Saccharomyces cerevisiae: possible implications in galactosemia. Biochim Biophys Acta. 1999;1454(3):217–26. doi: 10.1016/s0925-4439(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 35.Maddaiah VT, Madsen NB. Kinetics of purified liver phosphorylase. J Biol Chem. 1966;241(17):3873–81. [PubMed] [Google Scholar]
- 36.Lai K, Elsas LJ. Overexpression of human UDP-glucose pyrophosphorylase rescues galactose-1-phosphate uridyltransferase-deficient yeast. Biochem Biophys Res Commun. 2000;271(2):392–400. doi: 10.1006/bbrc.2000.2629. [DOI] [PubMed] [Google Scholar]
- 37.Lai K, et al. GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology. 2003;13(4):285–94. doi: 10.1093/glycob/cwg033. [DOI] [PubMed] [Google Scholar]
- 38.Kalckar HM, Maxwell ES. Biosynthesis and metabolic function of uridine diphosphoglucose in mammalian organisms and its relevance to certain inborn errors. Physiol Rev. 1958;38(1):77–90. doi: 10.1152/physrev.1958.38.1.77. [DOI] [PubMed] [Google Scholar]
- 39.Pourci ML, et al. Culture of galactosaemic fibroblasts in the presence of galactose: effect of inosine. J Inherit Metab Dis. 1990;13(6):819–28. doi: 10.1007/BF01800205. [DOI] [PubMed] [Google Scholar]
- 40.Ng WG, et al. Deficit of uridine diphosphate galactose in galactosaemia. J Inherit Metab Dis. 1989;12(3):257–66. doi: 10.1007/BF01799215. [DOI] [PubMed] [Google Scholar]
- 41.Charlwood J, et al. Defective galactosylation of serum transferrin in galactosemia. Glycobiology. 1998;8(4):351–7. doi: 10.1093/glycob/8.4.351. [DOI] [PubMed] [Google Scholar]
- 42.Dobbie JA, Holton JB, Clamp JR. Defective galactosylation of proteins in cultured skin fibroblasts from galactosaemic patients. Ann Clin Biochem. 1990;27(Pt 3):274–5. doi: 10.1177/000456329002700317. [DOI] [PubMed] [Google Scholar]
- 43.Jaeken J, Kint J, Spaapen L. Serum lysosomal enzyme abnormalities in galactosaemia. Lancet. 1992;340(8833):1472–3. doi: 10.1016/0140-6736(92)92664-2. [DOI] [PubMed] [Google Scholar]
- 44.Prestoz LL, et al. Altered follicle stimulating hormone isoforms in female galactosaemia patients. Eur J Pediatr. 1997;156(2):116–20. doi: 10.1007/s004310050568. [DOI] [PubMed] [Google Scholar]
- 45.Bandyopadhyay S, et al. Prenatal exposure to high galactose adversely affects initial gonadal pool of germ cells in rats. Hum Reprod. 2003;18(2):276–82. doi: 10.1093/humrep/deg058. [DOI] [PubMed] [Google Scholar]
- 46.Segal S. In utero galactose intoxication in animals. Eur J Pediatr. 1995;154(7 Suppl 2):S82–6. doi: 10.1007/BF02143810. [DOI] [PubMed] [Google Scholar]
- 47.Irons M, et al. Accumulation of galactose-1-phosphate in the galactosemic fetus despite maternal milk avoidance. J Pediatr. 1985;107(2):261–3. doi: 10.1016/s0022-3476(85)80143-8. [DOI] [PubMed] [Google Scholar]
- 48.Wells HJ, Wells WW. Galactose toxicity and myoinositol metabolism in the developing rat brain. Biochemistry. 1967;6(4):1168–73. doi: 10.1021/bi00856a029. [DOI] [PubMed] [Google Scholar]
- 49.Chen YT, et al. Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science. 1981;214(4525):1145–7. doi: 10.1126/science.7302587. [DOI] [PubMed] [Google Scholar]
- 50.Leslie ND, et al. A mouse model of galactose-1-phosphate uridyl transferase deficiency. Biochem Mol Med. 1996;59(1):7–12. doi: 10.1006/bmme.1996.0057. [DOI] [PubMed] [Google Scholar]
- 51.Ning C, et al. Galactose metabolism by the mouse with galactose-1-phosphate uridyltransferase deficiency. Pediatr Res. 2000;48(2):211–7. doi: 10.1203/00006450-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Ning C, et al. Galactose metabolism in mice with galactose-1-phosphate uridyltransferase deficiency: sucklings and 7-week-old animals fed a high-galactose diet. Mol Genet Metab. 2001;72(4):306–15. doi: 10.1006/mgme.2001.3152. [DOI] [PubMed] [Google Scholar]
- 53.Tommerup N, et al. A zinc-finger gene ZNF141 mapping at 4p16.3/D4S90 is a candidate gene for the Wolf-Hirschhorn (4p-) syndrome. Hum Mol Genet. 1993;2(10):1571–5. doi: 10.1093/hmg/2.10.1571. [DOI] [PubMed] [Google Scholar]
- 54.Shannon M, et al. Differential expansion of zinc-finger transcription factor loci in homologous human and mouse gene clusters. Genome Res. 2003;13(6A):1097–110. doi: 10.1101/gr.963903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elsas LJ, 2nd, Lai K. The molecular biology of galactosemia. Genet Med. 1998;1(1):40–8. doi: 10.1097/00125817-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Flach JE, Reichardt JK, Elsas LJ., 2nd Sequence of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1990;7(4):365–9. [PubMed] [Google Scholar]
- 57.Reichardt JK, Berg P. Cloning and characterization of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1988;5(2):107–22. [PubMed] [Google Scholar]
- 58.Lai K, Willis AC, Elsas LJ. The biochemical role of glutamine 188 in human galactose-1-phosphate uridyltransferase. J Biol Chem. 1999;274(10):6559–66. doi: 10.1074/jbc.274.10.6559. [DOI] [PubMed] [Google Scholar]
- 59.Dalai I, et al. Low expression of ARHI is associated with shorter progression-free survival in pancreatic endocrine tumors. Neoplasia. 2007;9(3):181–3. doi: 10.1593/neo.06838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hisatomi H, et al. ARHI/NOEY2 inactivation may be important in breast tumor pathogenesis. Oncology. 2002;62(2):136–40. doi: 10.1159/000048259. [DOI] [PubMed] [Google Scholar]
- 61.Luo RZ, et al. ARHI is a Ras-related small G-protein with a novel N-terminal extension that inhibits growth of ovarian and breast cancers. Oncogene. 2003;22(19):2897–909. doi: 10.1038/sj.onc.1206380. [DOI] [PubMed] [Google Scholar]
- 62.Weber F, et al. Silencing of the maternally imprinted tumor suppressor ARHI contributes to follicular thyroid carcinogenesis. J Clin Endocrinol Metab. 2005;90(2):1149–55. doi: 10.1210/jc.2004-1447. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y, et al. NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc Natl Acad Sci U S A. 1999;96(1):214–9. doi: 10.1073/pnas.96.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bao JJ, et al. Reexpression of the tumor suppressor gene ARHI induces apoptosis in ovarian and breast cancer cells through a caspase-independent calpain-dependent pathway. Cancer Res. 2002;62(24):7264–72. [PubMed] [Google Scholar]
- 65.Fitzgerald J, Bateman JF. Why mice have lost genes for COL21A1, STK17A, GPR145 and AHRI: evidence for gene deletion at evolutionary breakpoints in the rodent lineage. Trends Genet. 2004;20(9):408–12. doi: 10.1016/j.tig.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Xu F, et al. The human ARHI tumor suppressor gene inhibits lactation and growth in transgenic mice. Cancer Res. 2000;60(17):4913–20. [PubMed] [Google Scholar]
- 67.Antshel KM, I, Epstein O, Waisbren SE. Cognitive strengths and weaknesses in children and adolescents homozygous for the galactosemia Q188R mutation: a descriptive study. Neuropsychology. 2004;18(4):658–64. doi: 10.1037/0894-4105.18.4.658. [DOI] [PubMed] [Google Scholar]
- 68.Bosch AM, et al. Living with classical galactosemia: health-related quality of life consequences. Pediatrics. 2004;113(5):e423–8. doi: 10.1542/peds.113.5.e423. [DOI] [PubMed] [Google Scholar]
- 69.Lambert C, Boneh A. The impact of galactosaemia on quality of life--a pilot study. J Inherit Metab Dis. 2004;27(5):601–8. doi: 10.1023/b:boli.0000042957.98782.e4. [DOI] [PubMed] [Google Scholar]
- 70.Ridel KR, Leslie ND, Gilbert DL. An updated review of the long-term neurological effects of galactosemia. Pediatr Neurol. 2005;33(3):153–61. doi: 10.1016/j.pediatrneurol.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 71.Waisbren SE, et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. Jama. 2003;290(19):2564–72. doi: 10.1001/jama.290.19.2564. [DOI] [PubMed] [Google Scholar]
- 72.Waisbren SE, et al. Brief report: Predictors of parenting stress among parents of children with biochemical genetic disorders. J Pediatr Psychol. 2004;29(7):565–70. doi: 10.1093/jpepsy/jsh058. [DOI] [PubMed] [Google Scholar]