Abstract
Fluorous-tagged protecting groups are attractive tools for elongating carbohydrate chains in oligosaccharide synthesis. To eliminate the accumulation of failed sequences during automated oligosaccharide synthesis conditions, an additional C8F17 ester derived protecting group was attached to the glycosyl donor to better retain the desired doubly-tagged glycosylation product on fluorous solid phase extraction (FSPE) cartridges. Initial studies show that the double-fluorous-tagging strategy offers a robust enough separation using a commercial FSPE cartridge using simple gravity filtration to separate the desired product from the singly-fluorous-tagged starting materials and their decomposition products. In addition, removal of the fluorous acetate and its byproducts after sodium methoxide treatment and neutralization required only dissolution of the desired sugar in toluene and subsequent removal of the toluene layer from the denser fluorous byproducts.
Keywords: fluorous-assisted separation, carbohydrates, fluorous acetate
1. Introduction
The isolation of pure, structurally well-defined oligosaccharides from nature is generally a tedious and inefficient process, because these biomolecules are often present in low concentrations as microheterogeneous mixtures. A better understanding of the growing numbers of biological processes that carbohydrates mediate [1] demands efficient ways to access these structures. Studies of proteins and nucleic acids have thrived since automated solid-phase chemistry has been discovered [2]. The same principle was applied to automated oligosaccharide synthesis as well to produce various oligosaccharide compounds [3]. However the production of these compounds required a large excess of donor molecules to achieve reasonable coupling yields on a solid support and reaction monitoring is more difficult than in solution. To circumvent some of the inherent limitations of automated solid-phase synthesis, we have pursued a solution-phase approach. A lipid introduced to a monosaccharide building block [4] while maintaining solubility in organic solvents offers a handle to easily purify the desired compound; however, this tag complicates reaction monitoring by proton NMR. Due to its facile purification from non-fluorous compounds and silence in proton NMR spectra, fluorous tags have become an attractive tool for many synthetic processes [5]. Highly fluorinated versions of silyl, ether, and ester protecting groups [6] have been applied to oligosaccharides synthesis using liquid phase extractions. Unfortunately, solubility of compounds with heavy fluorous tags in organic solvents is hard to predict. A light fluorous (C8F17) tag, on the other hand, has been shown to cap failed sequences in solid-phase oligosaccharide synthesis [7] and more recently has been shown [8] to be stable to all the reaction conditions required for the requisite glycosylation and deprotection conditions with no problems with solubility or purification of intermediates using fluorous solid phase extraction [9] (FSPE). The fast and robust separation of the fluorinated compounds also makes the method amenable to automation.
A potential problem with a single-fluorous-tag strategy occurs when a reaction does not go to completion. Addition of a fluorous tag to both rather than just one of the coupling partners offers a chance to eliminate by-product build up during the synthesis without addition of a capping [7] step (Figure 1). The structure/retention trends in fluorous chromatography have been studied previously [10] where the larger the tag size, the stronger its retention on fluorinated silica gel. However, these comparison studies were performed on a FluoroFlash™ HPLC column. Success needs to be demonstrated using a simple gravity elution strategy for ready incorporation into an automation scheme. In addition, the benefit of removing singly-tagged starting materials and their decomposition products from the doubly-tagged desired products after a glycosylation cycle might be lost if the two single-fluorous-tagged products generated during the deprotection cycle are not easily separated by means other than FSPE. Herein we report the introduction of a light fluorous acetate protecting group for oligosaccharide synthesis and the viability of separating mono- from di-tagged saccharides using only simple FSPE cartridges to remove any excess/unreacted reagents in the context of building 1–4-linked glucosamine oligomers. We also report a simple method to remove the fluorous acetate byproduct from the desired fluorous-tagged sugar in the deprotection cycle.
Fig. 1.
Comparison of a single- versus double-fluorous-tag strategy for iterative oligosaccharide synthesis.
2. Results and discussion
In order to investigate the ease of purification of di-tagged saccharide products, the synthesis of a fluorous tagged donor and fluorous-tagged acceptor was necessary. In our previous studies using fluorinated tags for oligosaccharide synthesis [11], fluorous allyl groups are attached at the anomeric position of the reducing end of the oligosaccharide for chain extension at the nonreducing end. This fluorous allyl group would again serve as a good acceptor. The synthesis of an activated glucosamine donor was then undertaken in which a fluorous acetate was installed at the chain extension position. Among the different functionalities of fluorinated-protecting groups that could be applied, an ester derived fluorous protecting group was selected for its simple and readily automated deprotection conditions. The known partially-protected glucosamine building block 1 (Scheme 1) [12] with an easily removable tert-butyl dimethyl silyl group on the anomeric position served as a good starting point. The 4-position hydroxyl 1 was treated with 2H, 2H, 3H, 3H-perfluoro undecanoic acid to provide compound 2. This fluorous-tagged intermediate was then further elaborated to the trichloroacetimidate [13] donor 4 ready for glycosylation.
Scheme 1.
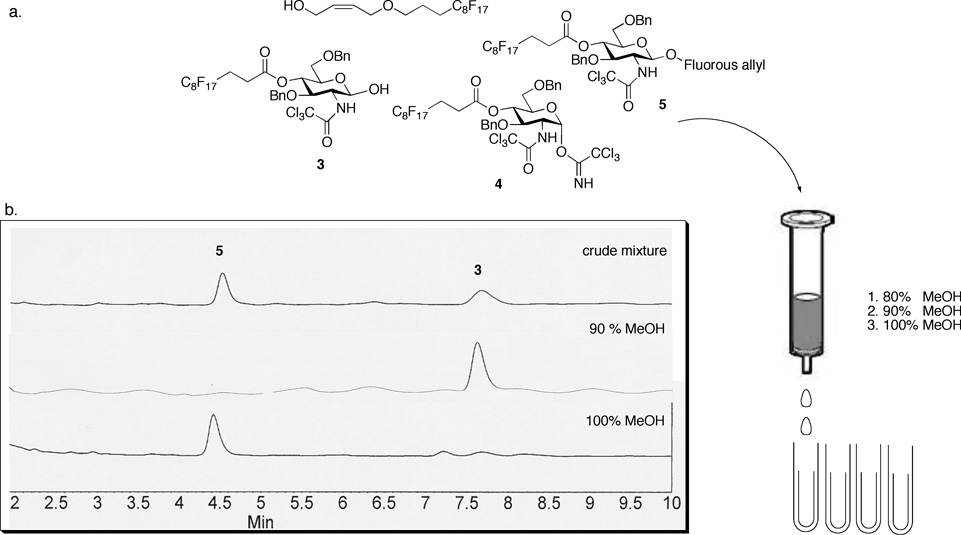
With the fluorous-tagged monosaccharide donor 4 in hand, we next tested the use of the di-tagging strategy by addition of the donor to a fluorous allyl [11d] group (Scheme 2). After the 0.5 h reaction time, the quenched crude mixture was concentrated and then loaded onto a 2-gram fluorous solid phase extraction cartridge (Figure 2). The cartridge was first eluted with 80% methanol/20% water for 5 column volumes. All the non-fluorinated compounds were thereby eluted. The elution solvent was then changed to 90% methanol/10% water for 5 column volumes to investigate any elution of fluorinated compounds. Fortunately, the excess donor that was now decomposed to hydroxyl 3 started to elute out of the cartridge along with a trace amount of the unreacted fluorous allyl tag (Figure 2). The desired di-fluorous tagged compound was readily retained in the FSPE cartridge while all the unreacted/excess reagents were removed. Finally, the desired di-tagged compound 5 was eluted from the cartridge using 100% methanol as eluent. HPLC traces (Figure 2) of the eluents confirmed the complete separation of the desired di-fluorous-tagged compound from the crude glycosylation mixture.
Scheme 2.
Fig. 2.
a) Selective elution of the multiple fluorous-tagged compounds using a FSPE cartridge and various elution conditions. b) HPLC traces of the compounds eluted off the FSPE cartridge with 90% or 100% methanol elution. (HPLC conditions: 15% ethyl acetate/85% hexane, 1ml/min flow rate, injection peak appeared at 1.5 min on a standard silica gel column).
The di-tagged monosaccharide 5 was then deprotected at the 4-position to allow further chain elongation (Scheme 3). Removal of the 2H, 2H, 3H, 3H-perfluoro undecanoyl group was performed under standard sodium methoxide conditions to provide compound 6. Now, of course, the desired product and the by-product both contain just one C8F17 tag and therefore FSPE is no longer a simple strategy for separation. However, alcohol 6, containing a large organic portion in addition to the fluorous tag, readily dissolved in toluene whereas the fluorous acetate byproducts formed a separate denser layer for easy separation. Hence, the physical property differences could be exploited for facile separation. Because growing saccharide chains with the fluorous allyl tag have good solubility in toluene as a rule, this method of fluorous acetate removal from the desired compound after the deprotection reaction should be a general strategy. In addition to fluorous acetate, other fluorous protecting groups have the potential to be used after testing of their solubility and volatility properties.
Scheme 3.
3. Conclusion
In conclusion, we have introduced a fluorous acetate group as a second protecting group to use in conjunction with our previously reported fluorous allyl group for light-fluorous-based carbohydrate synthesis. Use of a fluorous tag on not just the glycosyl acceptor, but also the glycosyl donor proved useful in the separation of the desired glycosylation product from the unreacted or decomposed starting materials with only simple gravity filtration over a commercially available FSPE cartridge. The doubly-tagged carbohydrate compound was retained in the cartridge sufficiently well to allow facile separation with a discrete increase of methanol concentrations in the aqueous eluent. The problem of thereby generating two singly-fluorous tagged components in the deprotection cycle was avoided by exploiting the difference in solubility between the tagged sugar and the fluorous acetate protecting group byproducts. Work is now in progress to test the feasibility of this double-fluorous-tagging approach in the iterative synthesis of oligosaccharides using FSPE separations on a solution-based automation platform.
4. Experimental
4.1 General experimental procedures
Commercial reagents and solvents were used as received without further purification unless otherwise stated. The reactions were monitored and the Rf values determined using analytical thin layer chromatography (tlc) with 0.25 mm EM Science silica gel plates (60F - 254). The developed tlc plates were visualized by immersion in p-anisaldehyde solution followed by heating on a hot plate. Silica gel flash chromatography was performed with Selecto Scientific silica gel, 32–63mm particle size. Fluorous phase chromatography was performed using fluorous solid-phase extraction cartridges containing silica gel bonded with perfluorooctylethylsilyl chains (Fluorous Technologies, Inc.; Pittsburgh, PA). All other fluorous reagents were also obtained from Fluorous Technologies, Inc. All moisture sensitive reactions were performed in flame- or oven-dried glassware under nitrogen atmosphere. Bath temperatures were used to record the reaction temperature. All reactions were stirred magnetically at ambient temperature unless otherwise indicated. 1H NMR and 13C NMR spectra were obtained with a Bruker DRX400 at 400 MHz and 162 MHz. 1H NMR spectra were reported in parts per million (δ) relative to CDCl3 (7.27ppm) as an internal reference. 13C NMR spectra were reported in parts per million (δ) relative to CDCl3 (77.23 ppm). HPLC traces were obtained using a Varian Inc. HPLC with a Waters Nova-pak 4 µl 3.9 ×150 mm silica column. High resolution mass spectrometry was obtained with an Applied Biosystems QSTAR XL Hybrid System.
4.2 Synthesis of tert-butyldimethylsilyl 3,6-di-O-benzyl-2-deoxy-2-trichloroacetimido-4-2H, 2H, 3H, 3H-perfluoroundecanoatyl-β-D-glucopyranoside (2)
To a solution of tert-butyldimethylsilyl 3,6-di-O-benzyl-2-deoxy-2-trichloroacetimido-β-d-glucopyranoside [9] 1 (100 mg, 0.16 mmol) in dichloromethane (10 mL) cooled to 0 °C, was added DCC (66.5 mg, 0.32 mmol), DMAP (19.5 mg, 0.16 mmol) and 2H, 2H, 3H, 3H-perfluoroundecanoic acid (157 mg, 0.32 mmol). The reaction mixture was allowed to warm to room temperature over 2 h. The reaction was diluted with dichloromethane (20 mL), washed with water (20 mL), HCl (2N, 20 mL), and brine (20 mL), and dried over MgSO4. After the solvent was removed under reduced pressure, crude product was purified by flash column chromatography on silica gel using 15% EtOAc/hexane as eluent to obtain the title compound (160 mg, 0.14 mmol, 92%) as colorless oil.
1H NMR (400 MHz, CDCl3): δ 0.23 (6H, d, 2CH3), 0.9 (9H, s, t-Bu-), 2.11–2.29 (4H, m, CH2CH2), 3.45 (1H, m, H-3), 3.56 (2H, m, H-6), 3.74 (1H, m, H-5), 4.35 (1H, dd, J=9.2 Hz, 10.4 Hz, H-2), 4.46–4.74 (4H, m, 2CH2), 5.12 (1H, t, J=9.2 Hz, H-4), 5.23 (1H, d, J=7.8 Hz, H-1), 7.06 (1H, d, J= 7.6 Hz, NH), 7.22–7.40 (10H, m, -CH2C6H5); 13C NMR (162, MHz, CDCl3): δ −5.2, −4.2, 17.9, 25.7, 61.1, 69.8, 72.5, 72.8, 73.6, 74.2, 76.8, 77.1, 77.3, 92.4, 94.0, 127.7, 127.7, 127.8, 128.0, 128.4, 128.5, 137.6, 137.7, 161.8, 170.0; MS (ESI) m/z = calcd. for C39H41Cl3F17NNaO7Si: 1014.1344; found: 1014.1367 [M+Na]+.
4.3 Synthesis of 3,6-di-O-benzyl-2-deoxy-2-trichloroacetimido-4-(2H, 2H, 3H, 3H-perfluoroundecanoyl)-α-d-glucopyranosyl trichloroacetimidate (4)
To a solution of tert-butyldimethylsilyl 3,6-di-O-benzyl-2-deoxy-2-trichloroacetimido-β-d-glucopyranoside 2 (100 mg, 0.09 mmol) in anhydrous THF, 1.0 M TBAF solution in THF (0.18 ml, 0.18 mmol) was added dropwise in a 0 °C ice bath. The reaction mixture was allowed to warm to room temperature over 1 h. After the solvent was removed under reduced pressure, crude reaction mixture was dissolved with DMF (0.4 ml) and loaded onto a 2 g FSPE cartridge. The cartridge was washed with 80% methanol (10 ml) followed by 100% methanol (10 ml) to elute the fluorinated desired product. The solvent was removed under reduced pressure to obtain the product (80 mg, 0.08 mmol, 89%) as colorless oil. The compound was used directly for the next step without further purification.
To a solution of the alcohol (80 mg, 0.08 mmol) in anhydrous DCM (5 ml) was added Cs2CO3 (66.6 mg, 0.2 mmol) and distilled trichloroacetonitrile (0.04 ml, 0.4 mmol). The reaction mixture was stirred at room temperature for 0.5 h and then filtered over a pad of Celite. The filtered solution was concentrated under reduced pressure and the crude product was purified by flash column chromatography on silica gel using 10% EtOAc/hexane as eluent to obtain compound 4 (83 mg, 0.083 mmol, 93%) as a colorless oil.
1H NMR (400 MHz, CDCl3): δ 2.22–2.54 (4H, m, CH2CH2), 3.50–3.67 (2H, m, H-3, H-5), 4.01–4.09 (2H, m, H-6), 4.37 (1H, t, J=10.0 Hz, H-2), 4.43–4.70 (4H, m), 5.40 (1H, t, J=9.9 Hz, H-4), 6.47(1H, d, J=3.6 Hz, H-1), 6.67 (1H, d, J=8.8 Hz, NH), 7.25–7.39 (10H, m, C6H5), 8.80 (1H, s, NH); 13C NMR (162, MHz, CDCl3): δ 61.2, 68.3, 69.8, 69.9, 70.0, 72.1, 73.2, 73.6, 76.0, 90.7, 92.1, 94.3, 127.7, 128.2, 128.3, 128.4, 128.4, 128.7, 137.0, 137.8, 158.9, 161.8, 170.0; MS (ESI) m/z = calcd. for C35H27Cl6F17N2NaO7: 1142.9576; found: 1142.9590 [M+Na]+.
4.4 Synthesis of Cis-4-(1H, 1H, 2H, 2H, 3H, 3H-perfluoroundecyloxy)-2-butenyl-3,6-di-O-benzyl-2-deoxy-2-trichloroacetimido-4-(2H, 2H, 3H, 3H-perfluoroundecanoyl)-β-D-glucopyranoside (5)
To a solution of the imidate (83 mg, 0.07 mmol) in anhydrous toluene (2 ml) at 0 °C was added fluorous allyl alcohol [11d] (25.5 mg, 0.05 mmol) in anhydrous toluene (2 ml) followed by trimethylsilyl trifluoromethanesulfonate (0.9 µl, 5.0 µmol). The reaction mixture was stirred for 0.5 h and then quenched with 3 drops of triethylamine. The reaction mixture was concentrated under reduced pressure and dissolved with DMF (0.4 ml) to load onto a 2 g FSPE cartridge. The loaded cartridge was first washed with 3 ml (1 column volume) 80% methanol/ 20% water mixture 5 times and then was washed with 3 ml 90% methanol/10% water mixture 5 times. After TLC confirmed that no other compounds were eluting from the cartridge, the solvent was changed into 100% methanol (10 ml) to elute out the desired compound. The eluent was concentrated under reduced pressure to provide 5 as a colorless oil (65 mg, 0.044 mmol, 87%).
1H NMR (400 MHz, CDCl3): δ 1.87–1.88 (2H, m, -OCH2CH2CH2C8F17), 2.01–2.43 (6H, m, C(O)CH2CH2C8F17,-OCH2CH2CH2C8F17), 3.46 (3H, m, CH2O-, H-3), 3.56 (2H, d, J=4.8 Hz, H-6), 3.66 (1H, m, H-5), 4.04 (2H, t, J=5.2 Hz, CH2CH=), 4.26–4.38 (3H, m, H-2, CH2CH=), 4.46–4.73 (4H, m, 2 CH2), 5.07 (1H, d, J=8.0 Hz, H-1), 5.12 (1H, t, J=9.6 Hz, H-4), 5.64 – 5.76 (2H, m, -CH=CH-), 7.01 (1H, d, J= 6.8 Hz, NH), 7.27–7.35 (m, 10H); 13C NMR (162, MHz, CDCl3): δ 25.4, 26.3, 64.9, 66.6, 69.0, 69.7, 72.5, 72.7, 73.1, 73.2, 73.8, 74.8, 77.4, 77.5, 97.6, 98.5, 127.7, 128.2, 128.3, 128.4, 128.4, 128.7, 130.8, 130.9,137.0, 137.7, 162.0, 170.2; MS (ESI) m/z = calcd. for C48H38Cl3F34NNaO8:1530.1018; found: 1530.1198 [M+Na]+.
4.5 Synthesis of Cis-4-(1H, 1H, 2H, 2H, 3H, 3H-perfluoroundecyloxy)-2-butenyl-3,6-di-O-benzyl-2-deoxy-2-trichloroacetimido-β-D-glucopyranoside (6)
To a solution of the glycosylated compound 5 (50 mg, 0.03 mmol) in methanol (1 ml) was added 0.5 M NaOMe solution (0.06 ml, 0.03 mmol). The reaction mixture was stirred for 0.5 h and then was neutralized with Amberlyst acidic resin. The reaction mixture was filtered and the solvent was removed under reduced pressure. The crude product was shaken with toluene (1 ml). The clear solution was removed with a pipette from a denser yellow oil and the solvent was removed under reduced pressure to yield 6 as a colorless oil (31 mg, 0.027 mol, 91%).
1H NMR (400 MHz, CDCl3): δ 1.72–1.80 (2H, m, -OCH2CH2CH2C8F17), 2.04–2.17 (2H, m, -OCH2CH2CH2C8F17), 3.01 (1H, d, J=2.2 Hz, -OH), 3.46 (2H, m, CH2O-), 3.60–3.65 (1H, m, H-5), 3.75–3.91 (5H, m, H-4, H-6, CH2CH=), 4.05–4.21 (4H, m, H-2, H-3, CH2CH=), 4.50–4.77 (4H, m, 2CH2), 5.12 (1H, d, J=8.0 Hz, H-1), 5.64 – 5.76 (2H, m, -CH=CH-), 7.00 (1H, d, J= 6.9 Hz, NH), 7.27–7.35 (10H, m); MS (ESI) m/z = calcd. for C37H35Cl3F17NNaO7: 1056.1105; found: 1056.1177 [M+Na]+.
Acknowledgements
This material is based in part upon work supported by the National Institute of General Medical Sciences (1R41M075436-01 and 2R42GM075436-02). N.L.P. is a Cottrell Scholar of Research Corporation and an Alfred P. Sloan Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.a) Nakahara S, Raz A. Anticancer Agents Med. Chem. 2008;8:22–36. doi: 10.2174/187152008783330833. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hart GW, Housley MP, Slawson C. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]; c) Varki A. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]; d) Bishop JR, Schuksz M, Esko JD. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]; e) Scanlon CN, Offer J, Zitzmann N, Dwek RA. Nature. 2007;446:1038–1045. doi: 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]; f) Seeberger PH, Werz DB. Nature. 2007;446:1046–1051. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- 2.a) Merrifield RB. J. Am. Chem. Soc. 1963;85:2149–2154. [Google Scholar]; b) Caruthers MH. Science. 1985;230:281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]; c) Caruthers MH. Acc. Chem. Res. 1991;24:278–284. [Google Scholar]; d) Atherton E, Sheppard RC. Solid-phase peptide synthesis; A practical approach. USA: Oxford University Press; 1989. [Google Scholar]
- 3.a) Plante OJ, Palmacci ER, Seeberger PH. Science. 2001;291:1523–1527. doi: 10.1126/science.1057324. [DOI] [PubMed] [Google Scholar]; b) Seeberger PH. Chem. Soc. Rev. 2008;37:19–28. doi: 10.1039/b511197h. For a recent review see: [DOI] [PubMed] [Google Scholar]
- 4.a) Lohse A, Martins R, Jorgensen MR, Hindsgaul O. Angew. Chem. Int. Ed. 2006;45:4167–4172. doi: 10.1002/anie.200600642. [DOI] [PubMed] [Google Scholar]; b) Bauer J, Rademann J. J. Am. Chem. Soc. 2005;127:7296–7297. doi: 10.1021/ja051737x. [DOI] [PubMed] [Google Scholar]
- 5.a) Luo Z, Zhang Q, Oderaotoshi Y, Curran DP. Science. 2001;291:1766–1769. doi: 10.1126/science.1057567. [DOI] [PubMed] [Google Scholar]; b) Zhang W. Tetrahedron. 2003;59:4475–4489. [Google Scholar]
- 6.a) Curran DP, Ferrito R, Hua Y. Tetrahedron Lett. 1998;39:4937–4940. [Google Scholar]; b) Miura T, Hirose Y, Ohmae M, Inazu T. Org. Lett. 2001;3:3947–3950. doi: 10.1021/ol016838o. [DOI] [PubMed] [Google Scholar]; c) Miura T, Goto K, Hosaka D, Inazu T. Angew. Chem. Int. Ed. 2003;42:2047–2051. doi: 10.1002/anie.200250531. [DOI] [PubMed] [Google Scholar]; d) Miura T, Goto K, Waragai H, Matsumoto H, Hirose Y, Ohmae M, Ishida H-k, Satoh A, Inazu I. J. Org. Chem. 2004;69:5348–5353. doi: 10.1021/jo049425k. [DOI] [PubMed] [Google Scholar]; e) Miuram T, Satoh A, Goto K, Murakami Y, Imai N, Inazu T. Tetrahedron: Asymmetry. 2005;16:3–6. [Google Scholar]
- 7.Palmacci ER, Hewitt MC, Seeberger PH. Angew. Chem. Int. Ed. 2001;40:4433–4437. doi: 10.1002/1521-3773(20011203)40:23<4433::aid-anie4433>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Jaipuri FA, Pohl NL. Org. Biomol. Chem. 2008;6 doi: 10.1039/b803451f. [DOI] [PubMed] [Google Scholar]
- 9.a) Curran DP. Angew. Chem. Int. Ed. 1998;37:1174–1196. doi: 10.1002/(SICI)1521-3773(19980518)37:9<1174::AID-ANIE1174>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; b) Curran DP. Pure Appl. Chem. 2000;72:1649–1653. [Google Scholar]; c) Curran DP, Luo Z. J. Am. Chem. Soc. 1999;121:9069–9072. [Google Scholar]; d) Zhang Q, Luo Z, Curran DP. J. Org. Chem. 2000;65:8866–8873. doi: 10.1021/jo000464f. [DOI] [PubMed] [Google Scholar]; e) Miura T, Inazu T. Tetrahedron Lett. 2003;44:1819–1821. [Google Scholar]; f) Miura T, Goto K, Hosaka D, Inazu T. Angew. Chem. Int. Ed. 2003;42:2047–2051. doi: 10.1002/anie.200250531. [DOI] [PubMed] [Google Scholar]; g) Goto K, Miura T, Mizuno M. Tetrahedron Lett. 2005;46:8293–8297. [Google Scholar]; h) Manzoni L, Castelli R. Org. Lett. 2006;8:955–957. doi: 10.1021/ol060006e. [DOI] [PubMed] [Google Scholar]; i) Mizuno M, Goto K, Miura T, Inazu T. QSAR & Combinatorial Science. 2006;25:742–752. [Google Scholar]; j) Dandapani S. QSAR & Combinatorial Science. 2006;25:681–688. [Google Scholar]; k) Carrel FR, Geyer K, Codee JD, Seeberger PH. Org. Lett. 2007;9:2285–2288. doi: 10.1021/ol0705503. [DOI] [PubMed] [Google Scholar]
- 10.a) Gladysz JA, Curran DP, Horvath IT. Handbook of Fluorous Chemistry. Weinheim: Wiley-VCH; 2004. pp. 111–126. [Google Scholar]; b) Curran DP, Moura-Letts G, Pohlman M. Angew. Chem. Int. Ed. 2006;45:2423–2426. doi: 10.1002/anie.200600041. [DOI] [PubMed] [Google Scholar]; c) Wilcox CS, Gudipati V, Lu H, Turkyilmaz S, Curran DP. Angew. Chem. Int. Ed. 2005;44:6938–6940. doi: 10.1002/anie.200501989. [DOI] [PubMed] [Google Scholar]
- 11.a) Ko K-S, Jaipuri FA, Pohl NL. J. Am. Chem. Soc. 2005;127:13162–13163. doi: 10.1021/ja054811k. [DOI] [PubMed] [Google Scholar]; b) Chen G, Pohl NL. Org. Lett. 2008;10:785–788. doi: 10.1021/ol702915e. [DOI] [PubMed] [Google Scholar]; c) Jaipuri FA, Collet BYM, Pohl NL. Angew. Chem. Int. Ed. 2008;47:1707–1710. doi: 10.1002/anie.200704262. [DOI] [PubMed] [Google Scholar]; d) Mamidyala SK, Ko K-S, Jaipuri FA, Park G, Pohl NL. J. Fluorine Chem. 2006;127:571–579. [Google Scholar]
- 12.Ratner DM, Swanson ER, Seeberger PH. Org. Lett. 2003;5:4717–4720. doi: 10.1021/ol035887t. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt RR, Jung K-H. In: Trichloroacetimidates in Carbohydrates in Chemistry and Biology, Part 1: Chemistry of Saccharides. Ernst B, Hart GW, Sinay P, editors. Vol 1. Weinheim: Wiley-VCH; 2000. pp. 5–59. [Google Scholar]