Abstract
Because subjective tinnitus is typically localized to the ear with hearing loss, tinnitus was traditionally thought to originate from neural hyperactivity in the damaged ear. However, most studies have found that hearing loss reduces the neural outputs from the damaged cochlea. These negative findings led to the hypothesis that rinnitus arises from aberrant neural activity in the central auditory system. Positron emission tomography imaging studies performed on tinnitus patients that could modulate their tinnitus provide evidence showing that the aberrant neural activity that gives rise to tinnitus resides in the central auditory pathway. To investigate the biological basis of tinnitus in more detail, an animal model was developed that allowed behavioral measures of tinnitus to be obtained from individual rats after inducing tinnitus with high doses of salicylate or high-intensity noise. This behavioral model was used to test the efficacy of memantine, an N-methyl-D-aspartate antagonist, and scopolamine, an anticholinergic, in suppressing salicylate-induced tinnitus. Neither drug completely suppressed salicylate-induced tinnitus. To detect the physiological changes associated with tinnitus, chronic microwire electrodes were implanted in the auditory cortex and measurements were obtained from the auditory cortex before and after salicylate and noise exposures known to induce tinnitus. High doses of salicylate or high-level noise exposure generally resulted in sound-evoked hyperactivity in the electrophysiological responses recorded from the auditory cortex of awake-animals. However, anesthetic tended to suppress or abolish the hyperactivity.
Keywords: Tinnitus, salicylate, noise exposure, auditory cortex, spontaneous activity, positron emission tomography
The neural generator for tinnitus was traditionally believed to reside in the cochlea. This hypothesis arose from several scientific and clinical observations. Because noise-induced temporary threshold shift was believed to originate in the hair cells and noise-induced tinnitus was nearly always associated with temporary threshold shift, it was assumed that tinnitus also originated in the cochlear hair cells. 1 Moreover, most patients claim that their tinnitus sounds as if it is originating inside the ear, typically in the ear with hearing loss. Some support for a cochlear generator for tinnitus emerged from early studies that found an increase in spontaneous activity in auditory nerve fibers of cats that had been treated with high doses of sodium salicylate (aspirin), a well-known inducer of tinnitus.2 However, cats lack a key enzyme needed to metabolize salicylate, raising doubts about the validity of these measurements. In contrast, more recent studies have shown that high doses of salicylate reduce the rate of spontaneous activity in the auditory nerve of gerbils.3 In addition, other studies have found no change or a reduction in spontaneous activity in the auditory nerve after the cochlear hair cells were damaged by noise exposure or ototoxic drugs such as carboplatin and aminoglycosides that often induce tinnitus.4-7 These results suggest that tinnitus may originate in the central nervous system.
If the neural generator for tinnitus was in the cochlea, then severing the auditory nerve should eliminate the neural activity that gives rise to the phantom sound of tinnitus. In one large clinical report involving hundreds of patients with tinnitus, surgical sectioning of the auditory nerve failed to suppress the tinnitus or made it worse in > 50% of patients.8 Moreover, surgical removal of the auditory nerve during acoustic neuroma surgery often leads to the emergence of tinnitus in the ear that is surgically disconnected from the brain. Obviously, in such cases, the tinnitus generator must reside in the central auditory pathway because of the absence of any neural activity emanating from the “dead ear.”
Gaze-Evoked Tinnitus
Interestingly, many acoustic neuroma patients develop gaze-evoked tinnitus, an unusual condition in which the loudness or pitch of the tinnitus changes with lateral eye gaze.9 Using positron emission tomography (PET) and radiolabeled water as a tracer to measure neural activity, we identified the regions of the brain activated by lateral eye gaze that induced changes in tinnitus loudness and pitch. In some patients, the neural sites activated by gaze-evoked tinnitus included the auditory lateral pontine tegmentum encompassing the dorsal cochlear nucleus and auditory cortex (AC).10 To identify the effects of unilateral deafness caused by acoustic neuroma surgery on brain activity, tone bursts (500 Hz) were presented to the intact ear of patients with gaze-evoked tinnitus, and the pattern of activation was compared with patients with normal hearing. Unilateral sound stimulation (500-Hz tone bursts) delivered to the right ear of normal subjects produced bilateral activation in both ACs.10 The same stimulus delivered to the intact ear of the gaze-evoked tinnitus patients also produced bilateral activation of the AC; however, the degree of activation was significantly larger than normal in both left and right AC and the left medial geniculate body (MGB). These results suggest that the auditory cortex had become hyperactive due to the loss of neural input from the ear destroyed by acoustic neuroma surgery. The PET imaging data together with other physiological studies in animals with sensorineural hearing loss suggest that the central auditory system can become hyperactive to sound stimulation following partial cochlear deafferentation and that the hyperactivity may be a neural marker of hyperacusis.11-13
Somatic Tinnitus and Cortical Activation
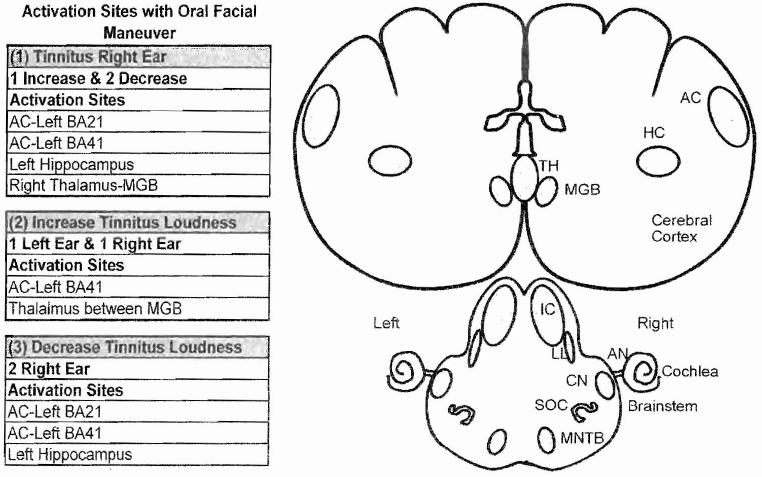
A surprisingly large percentage (60 to 80%) of patients are able to modulate the loudness or pitch of their tinnitus by movements of the head, neck, jaw, or mouth, a condition referred to as somatic tinnitus.14,15 To capitalize on this condition, we used PET imaging to identify the regions of the brain that showed significant changes in activity in four patients who could modulate the loudness of their tinnitus with an oral facial maneuver (jaw clench). As expected, the oral facial maneuver produced strong bilateral activation in the somatosensory cortex and supplementary motor areas of normal control subjects and tinnitus patients.16 Three of the patients localized their tinnitus to the right ear; two showed a reduction in tinnitus loudness during the oral facial maneuver, and one showed an increase in loudness. The statistical analysis showed that the change in loudness regardless of whether it was an increase or decrease resulted in a significant change in activity in the left AC (Brodmann area 21 and 41), the left hippocampus (HC), and the right thalamus (TH) between the MGBs (Fig. 1, list 1). However, in the two patients who could increase the loudness of their tinnitus with the oral facial maneuver, the maneuver caused activation in the posterior thalamus between the two MGBs and in the left primary AC (Brodmann area 41) (Fig. 1, list 2). In the other two patients with somatic tinnitus in the right ear, the oral facial maneuver decreased the loudness of their tinnitus; this was associated with decreased activity in the left middle temporal gyrus (Brodmann area 21), left transverse temporal gyrus (Brodmann area 41), and left hippocampus (Fig. 1, list 3). All four patients in this particular imaging study were severely troubled by their tinnitus and found that the phantom sound disrupted their lives. It has been suggested that a patient’s perception of the severity of tinnitus is not due to its loudness or psychoacoustic properties, but rather its emotional or affective component that link the phantom sound to memories of previous stressful situations or events.17,18 When viewed in this context, the activation seen in the hippocampus, a part of the limbic system involved in emotion and memory, may reflect the affective features rather than auditory features of their tinnitus.
Figure 1.
Regions of the brain that showed significant changes in activity with positron emission tomography during oral facial maneuver Table: (1) Shows activation sites in subjects with right ear tinnitus; one subject shows loudness increase; two subjects show loudness decrease. (2) Shows activation sites in subjects that experience an increase in tinnitus loudness; one subject localized tinnitus to left ear and one to right ear. (3) Shows activation sites in subjects that experience a decrease in tinnitus loudness in the right ear. AN, auditory nerve; BA. Brodmann’s area; CN, cochlear nucleus; HC, hippocampus; IC, inferior colliculus; LL, lateral lemniscus; MGB, medial geniculate body; MNTB, medial nucleus of trapezoid body; SOC, superior olivary complex; TH, thalamus.
The regions of the brain activated by 2000-Hz tone bursts were evaluated in patients and controls to determine if there was evidence of hyperactivity in tinnitus patients. When the brain activity of patients was compared with normal controls during 2000-Hz tone burst stimulation, patients showed significantly greater activity in the left HC and left lenticular nuclei. When the effects of resting brain activity in patients and controls were removed from the analysis to identify only the changes in activity due to sound stimulation, the analysis showed significantly greater activity in the left primary AC (Brodmann area 41) and anterior part of the temporal lobe and insula (Brodmann area 38).
These results suggest that the neural generator responsible for tinnitus may reside in the central portion of the auditory brain. Because all of the tinnitus patients in this study had high-frequency sensorineural hearing loss, the event that triggers the perception of tinnitus may arise from cochlear damage that abolishes or reduces the neural output flowing from the auditory nerve into the brain. In some individuals with hearing loss, the partial or complete deafferentation may stimulate or unmask existing neural circuits or give rise to aberrant neural reorganization and plastic changes that result in the phantom sound of tinnitus that can be modulated by eye gaze or maneuvers of the head, neck, mouth, or face.19-22 In addition, cochlear damage may alter the normal balance between inhibition and excitation so that sound stimulation produces more activity in the central auditory pathway despite a reduced neural input from a damaged cochlea as reported in animal studies.11,23
Lidocaine Modulates Tinnitus
In the otology literature, intravenous lidocaine is sometime used to treat and temporarily suppress tinnitus.24-25 If the tinnitus generator were in the cochlea, then application of lidocaine to the inner ear should be an extremely effective method to suppress tinnitus. However, round window application of lidocaine had little or no effect on tinnitus.26 Moreover, intravenous lidocaine suppresses tinnitus in patients whose auditory nerve had been sectioned to treat a vestibular schwannoma.27 These results suggest that the tinnitus generator is not in the cochlea and that lidocaine acts centrally rather than on the cochlea to suppress tinnitus.
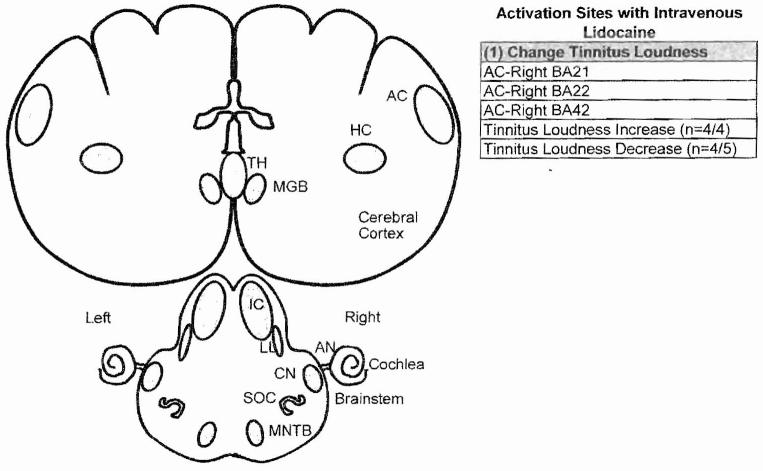
To identify the neural generators of tinnitus, we used PET imaging and radiolabelcd water to identify the changes in neural activity induced by lidocaine in normal subjects and conventional tinnitus patients with intravenous lidocaine. When we administered intravenous lidocaine to tinnitus patients or normal subjects, we unexpectedly found that lidocaine exerted bidirectional effects on the phantom sound of tinnitus.28 In roughly half the cases, lidocaine induced tinnitus in normal subjects or made the preexisting tinnitus louder, consistent with earlier results.29 In contrast, intravenous lidocaine reduced tinnitus loudness in the other half consistent with earlier results.27,30 Importantly, PET imaging showed that the increase in tinnitus loudness was associated with increased neural activity in the right auditory association cortex, whereas decreases in tinnitus loudness, which were larger than the increases, were associated with decreased activity in the right AC (Fig. 2, list 1).28 The change in tinnitus loudness, regardless of whether it increased or decreased, was confined largely to the right AC. Collectively, these results suggest that intravenous lidocaine may suppress or enhance the phantom sound of tinnitus through its effects on the central auditory system, in particular the AC.
Figure 2.
Regions of auditory pathway that showed a significant change in activity (regional cerebral blood flow) measured with positron emission tomography during intravenous lidocaine treatment. Intravenous lidocaine increased tinnitus loudness in four patients; this was associated with increased activity in the right auditory cortex (AC). In four of five subjects, intravenous lidocaine decreased tinnitus; this was associated with decreased neural activity in the right AC. AN, auditory nerve; BA, Brodmann’s area; CN, cochlear nucleus; HC, hippocampus; IC, inferior colliculus; LL, lateral lemniscus; MGB, medial geniculate body; MNTB, medial nucleus of trapezoid body; SOC, superior olivary complex; TH, thalamus
Animal Models of Tinnitus
Human brain imaging studies have proved extremely useful in identifying regions of the auditory pathway involved in tinnitus; however, the precise neurophysiological, anatomical, biochemical, and neuropharmacological mechanisms that give rise to tinnitus are very difficult or almost impossible to study in humans. Consequently, much effort has gone into developing animals that can “tell us” if they are experiencing tinnitus. To address this problem, we have developed two behavioral models, one referred to as schedule-induced polydipsia avoidance conditioning (SIPAC) and the other called gap prepulse inhibition of acoustic startle reflex.
Schedule-Induced Polydipsia Avoidance Conditioning
The SIPAC procedure for training rats to report on the presence of tinnitus has been described previously.31 SIPAC is a combination of two behavioral paradigms, schedule-induced polydipsia and shock avoidance conditioning. Polydipsia, which refers to excessive drinking, is induced by delivering food pellets on a schedule, once per minute for 120 minutes, to a food-deprived rat. Following 2 to 3 days on this schedule, rats begin spontaneously to lick for water between pellet deliveries; licking occurs even though they are not water deprived. A 30-second noise stimulus occurs before the delivery of each food pellet and is followed by either 30 seconds of noise or quiet after each food pellet. After a week or two of training, rats produce a high rate of licking following food pellet delivery. In the second training stage, the licking behavior is placed under stimulus control by pairing brief aversive foot shocks with licks that only occur during sound stimulation (six different narrow band sound stimuli or 16 kHz tone, 40 to 60 dB sound pressure level [SPL]). Shock is not presented during quiet intervals that are considered safe periods. Under these conditions, the rats learn to stop licking for water when one of the six sounds is present and to only lick when it is quiet.
Salicylate Treatment
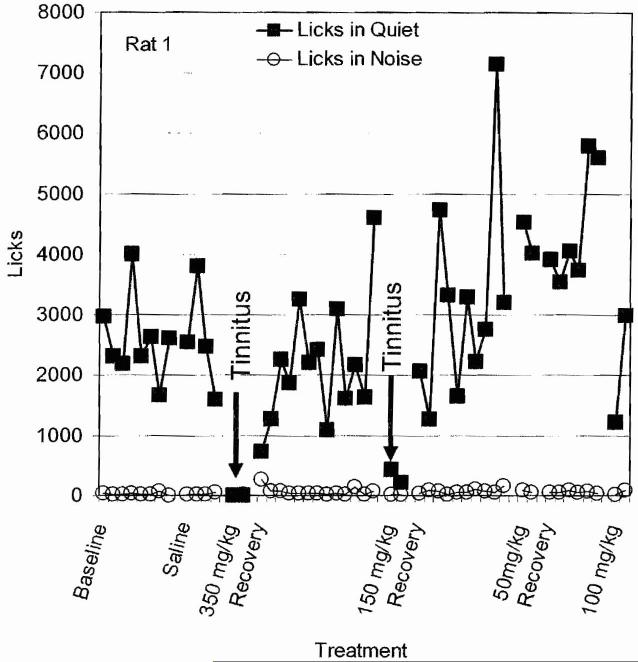
High doses of sodium salicylate, the active ingredient in aspirin, reliably induce tinnitus in humans and animals.32,33 To determine the dose of sodium salicylate that reliably induced tinnitus, we trained rats with the SIPAC paradigm and administered (intraperitoneal [i.p.]) saline, 50, 100, 150, or 350 mg/kg sodium salicylate for 2 consecutive days. Behavioral testing began ∼40 minutes following treatments. Treatments were separated by a washout period of ∼5 days. During baseline training, licks-in-quiet ranged between 1500 and 4000, whereas licks-in-noise were nearly zero (Fig. 3). Subsequent treatment with saline had no effect on licks-in-quiet or licks-in-noise; this rules out the possibility of behavioral artifact due to the injection. However, when the rat was given 350 mg/kg of salicylate on two consecutive days, licks-in-quiet dropped to zero, behavior consistent with the presence of tinnitus (Fig. 3). Importantly, no change was observed for licks-in-noise. During the washout period, licks-in-quiet recovered to baseline levels. When the salicylate dose was reduced to 150 mg/kg for two consecutive days, licks-in-quiet again dropped to nearly zero but then recovered to baseline levels during the washout period. Administration of 50 mg/kg salicylate had no effect on licks-in-quiet, behavior consistent with the absence of tinnitus. Treatment with 100 mg/kg caused a reduction oflicks-in-quiet on the first day, but on the second treatment day, licks-in-quiet was essentially normal indicating an absence of tinnitus. These results indicate that high doses of salicylate can induce tinnitus-like behavior in this rat and that repeated estimates of tinnitus can be obtained from the same animal using SIPAC.
Figure 3.
Plot of licks-in-quiet and licks-in-noise during baseline, saline treatment, 350 mg/kg salicylate, recovery, 150 mg/kg salicylate, recovery, 50 mg/kg salicylate, recovery, and 100 mg/kg salicylate. Arrows show salicylate treatments that induced a significant decrease in licks-in-quiet, behavior consistent with the presence of tinnitus.
Six rats were treated with different doses of salicylate to determine the dose-response curve and to identify the doses that caused a statistically significant decrease in licks-in-quiet and thus behavioral evidence of tinnitus. As shown in Fig. 4, saline and 50 mg/kg of salicylate did not cause a decrease in licks-in-quiet; 100 mg/kg of salicylate caused only a slight decrease. The 150 and 350 mg/kg doses of salicylate both caused a significant decrease in licks-in-quiet but did not have any clear effect on licks-in-noise. These results indicate that the 150 mg/kg is the minimum dose that can reliably induce tinnitus-like behavior in most rats.
Figure 4.
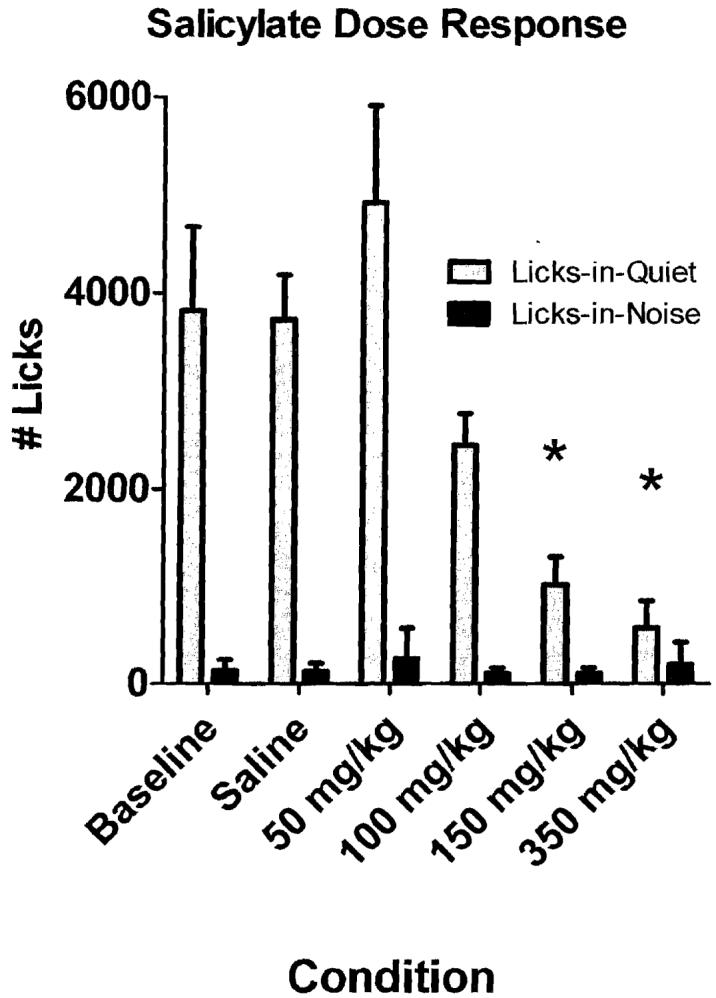
Mean licks-in-noise (n = 6; standard deviation [SD]) and mean licks-in-quiet (n = 6, SD) during baseline and saline conditions and treatment with 50, 100, 150, and 350 mg/kg sodium salicylate. Asterisks indicate conditions that were significantly different (p < 0.051 from baseline.
Memantine and Salicylate-Induced Tinnitus
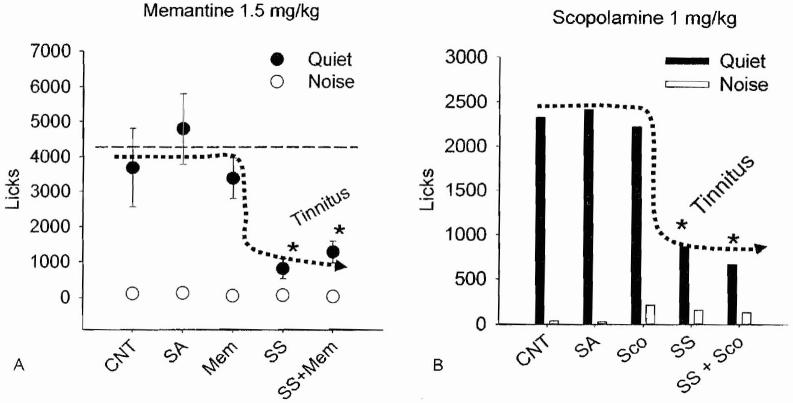
Tinnitus may arise from an increase in excitatory neurotransmission. Because glutamate is a major neurotransmitter in the inner ear and central auditory system, it has been proposed that antiglutamatergic drugs such as memantine might be effective in treating tinnitus.34 Some evidence suggests that salicylate-induced tinnitus might develop from increased glutamate neurotransmission through N-methyl-D-aspartate (NMDA) receptors35 and that salicylate-induced tinnitus can be suppressed by blocking cochlear NMDA receptors with antiglutamatergic drugs such as memantine:36 To test this hypothesis, rats were treated with a high dose of salicylate, saline as a control condition, memantine alone to control potential side effects, or the combination of the salicylate plus memantine to determine if memantine would suppress tinnitus.37 As shown in Fig. 5A, licks-in-quiet during saline or memantine alone (3 mg/kg, i.p.) remained similar to values obtained during the baseline control. This indicates that the 3 mg/kg dose of memantine had no adverse effects on behavior (note: higher dose of memantine alone disrupted behavior). When salicylate was administered alone, licks-in-quiet decreased significantly, behavior consistent with the presence of tinnitus. Finally, when memantine was administered together with salicylate, licks-in-quiet remained low, similar to salicylate alone. These results suggested that 3 mg/kg dose of memantine employed is unable to block salicylate-induced tinnitus. It is conceivable that memantine can suppress other forms of tinnitus or that higher doses of memantine may suppress tinnitus.
Figure 5.
(A) Mean licks-in-noise (n = 6; standard deviation [SD]) and mean licks-in-quiet (n = 6. SD) during control (CNT), saline (SA), memantine (Mem; 1.5 mg/kg intraperitoneal [i.p.]), sodium salicylate (SS; 150 mg/kg, i.p.), and coadministration of salicylate and memantine. Asterisks indicate conditions significantly different (p < 0.05) from baseline. (B) Same as in (A) except that rats were treated with scopolamine (Sco; 1 mg/kg, i.p.) instead of memantine
Scopolamine and Salicylate
High doses of salicylate that induce tinnitus upregulate the expression of the plasticity and excitation-related proteins arg3.1 and c-fos proteins in the AC. Interestingly, coscopolamine, which blocks muscarinic acetylcholine receptors, blocks expression of arg3.1 and c-fos protein in the AC. On the basis of these results, it was suggested that scopolamine might be used to treat salicylate-induced tinnitus.38 To test this hypothesis, rats were tested with the SIPAC before and then afterward with salicylate alone, scopolamine alone, or salicylate plus scopolamine. Rats were tested with several doses of scopolamine and it was found that ≤ 1 mg/kg (i.p.) of scopolamine did not disrupt SIPAC performance. As shown in Fig. 5B, licks-in-quiet were high during the baseline control, during saline treatment, and during 1 mg/kg scopolamine (i.p.). However, when the rats were treated with sodium salicylate (150 mg/kg), large decrease of licks-in-quiet were observed, behaviors consistent with the present of tinnitus. Licks-in-quiet remained low during coadministration of 1 mg/kg scopolamine with 150 mg/kg salicylate; these results indicate that this dose of scopolamine did not suppress salicylate-induced tinnitus.
Noise-Induced Tinnitus
Although salicylate-induced tinnitus is an interesting scientific phenomenon, a more clinically relevant problem is noise-induced tinnitus that affects many individuals in high-noise environments. To address the issue of noise-induced tinnitus, rats were trained on the SIPAC behavioral paradigm to distinguish between periods of quiet and noise. Afterward, the rats were anesthetized and monaurally exposed for 2 hours to a 120 dB SPL narrow band noise centered at 11 kHz. The purpose of using a monaural noise exposure was to induce tinnitus on the side of the exposure while preserving essentially normally hearing in the opposite ear; the preservation of hearing in the unexposed ear insured that the rat was able to perceive the sounds used in SIPAC testing. Figure 6A shows the typical behavior of a rat that developed persistent noise-induced tinnitus. During baseline testing before the noise exposure, licks-in-quiet ranged from 3000 to 4000 licks per session, whereas licks-in-noise values were nearly zero. As illustrated in Fig. 6A, licks-in-quiet decreased dramatically immediately after the noise exposure, behavior consistent with the perception of tinnitus_ In contrast, licks-in-noise remained unchanged, indicating the rats can perceive real sounds. Figure 6B shows the behavior from a rat that developed transient noise-induced tinnitus. Licks-in-quiet were high before the exposure but decreased dramatically 1 and 2 days following the exposure, behavior indicative of tinnitus. However, on day 3 following the exposure, licks-in-quiet returned to normal levels, indicating that the tinnitus had disappeared. However, licks-in-noise remained low during baseline testing and all the days following the exposure, indicating that the rats were able to perceive the real sounds. Figure 6C shows the data from a rat that failed to develop tinnitus following the exposure. Licks-in-quiet were high both before and after the exposure, indicating the rat failed to develop tinnitus-like behavior, whereas licks-in-noise remained low before and after the exposure, indicating the rat was able to detect real sounds. Several aspects of these results are important. First, when rats developed noise-induced tinnitus, the tinnitus appeared immediately after the exposure consistent with other results from human and animal studies of noise-induced tinnitus.39,40 Second, some noise-exposed rats failed to develop tinnitus and others only developed transient tinnitus. These results are clinical observations showing that only a subset of patients with noise-induced hearing loss have persistent tinnitus. One of the unsolved mysteries is why some humans and rats develop tinnitus and others do not.
Figure 6.
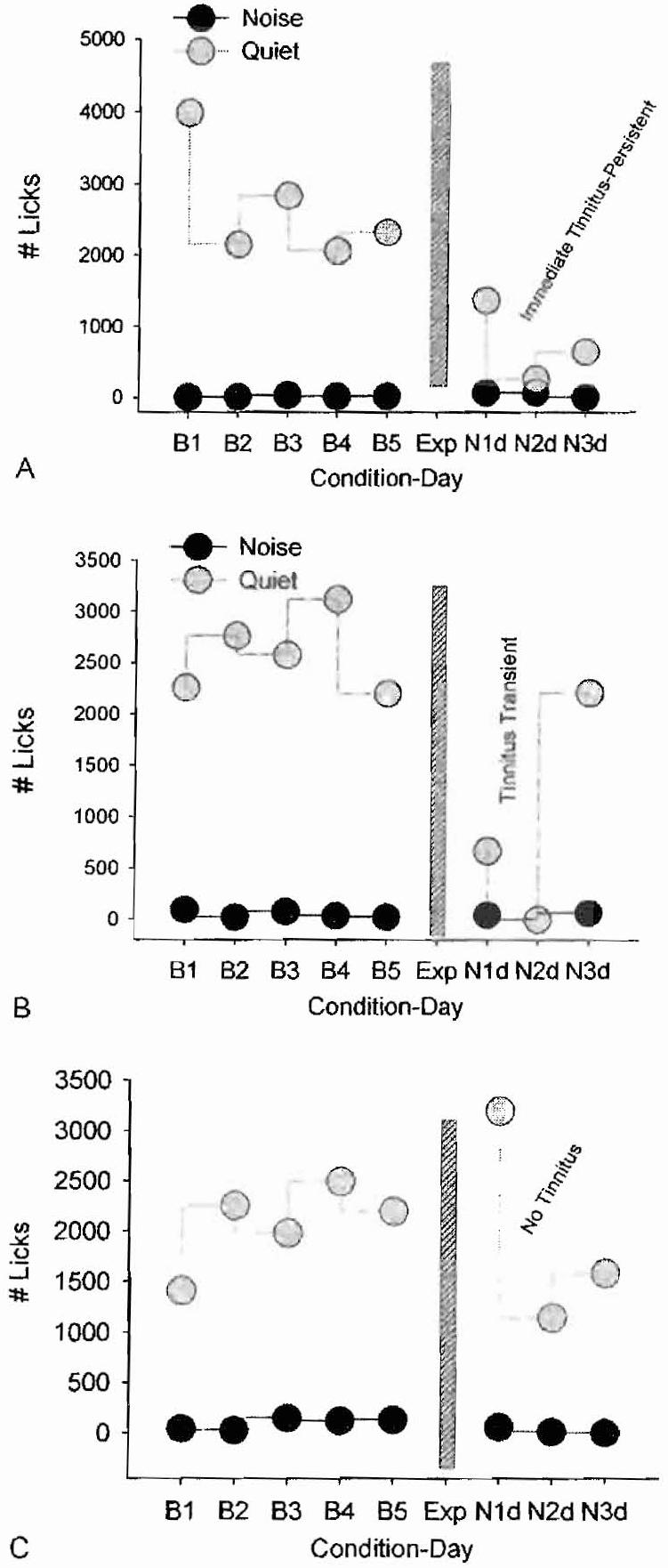
Licks-in-quiet (gray symbols) and licks-in-noise (black symbols) before and after rats were exposed (Exp; vertical bar; 2 hours, 120 dB SPL, narrow band noise centered at 11 kHz). (A) Rat develops tinnitus-like behavior immediately after the exposure that persists for ≥3 days. (B) Rat develops transient tinnitus 1 to 2 days immediately after the exposure that disappears 3 days after the exposure. (C) Rat fails to develop tinnitus after the exposure.
Tinnitus and Neural Activity
Animal models can be used to investigate the neural correlates of tinnitus. Previous neurophysiological studies of tinnitus have typically involved recording from anesthetized animals; because anesthetics have a profound effect on neural activity in the auditory pathway41-43 and are likely to abolish the perception of tinnitus, we have focused our efforts on making electrophysiological recordings from unanesthetized animals before and after inducing tinnitus with salicylate or acoustic overstimulation. To accomplish this goal, a silver ball electrode is implanted on the AC to record the local field potential. In other cases, a 16-channel microwire electrode (Fig. 7A) is implanted into the AC. With suitable filtering these electrodes can be used to record the local field potential (Fig. 7B) or action potential or spike discharges from single neurons or small clusters of neurons (Fig. 7C). After a suitable postsurgical recovery period, rats are placed into an acoustically transparent wire mesh cage that limits their movements in the calibrated sound field.44 In some case, rats are allowed to move about freely in a small plastic cage while recordings are made.
Figure 7.
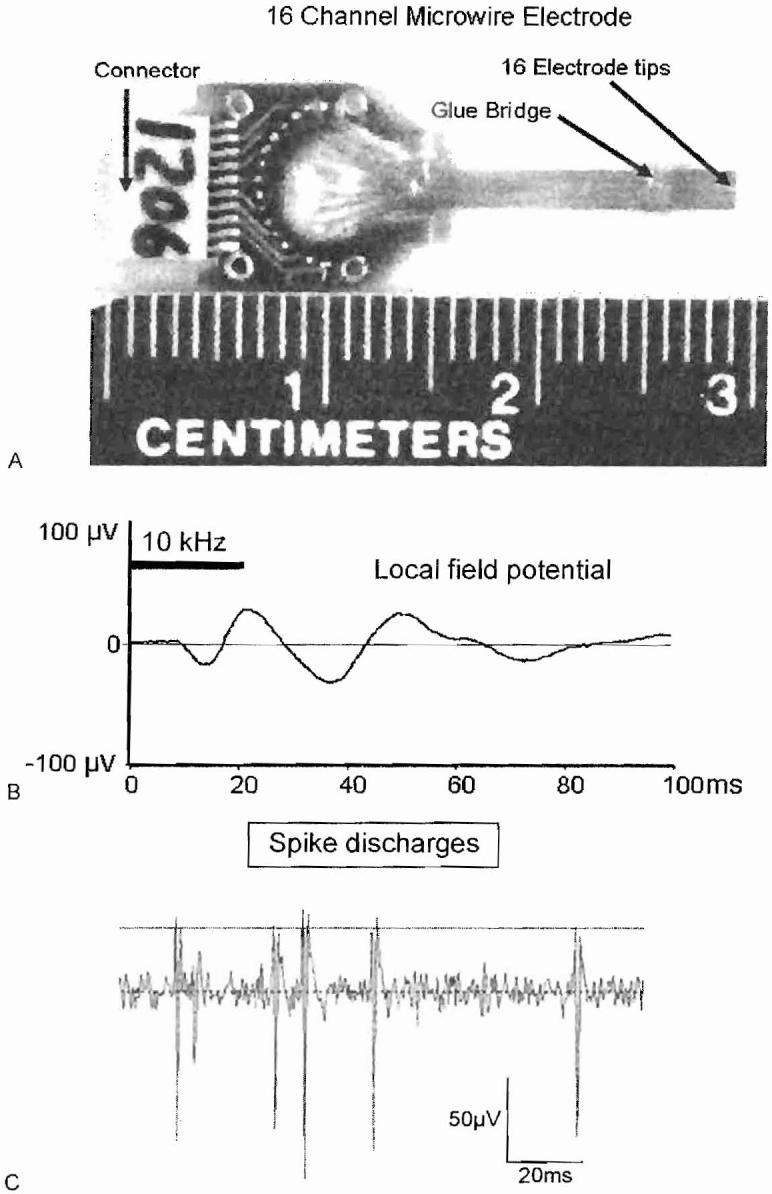
(A) Sixteen-channel microwire electrode. (B) Tone burst evoked local field potential recorded from microwire electrodes implanted in auditory cortex (filtering. 30 to 300 Hz). (C) Spike discharges recorded from small cluster of neurons in auditory cortex using microwire electrodes.
Salicylate-Induced Tinnitus
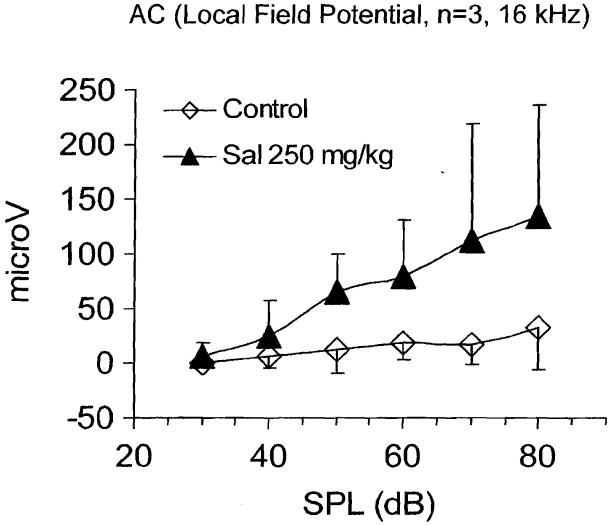
High doses of salicylate are known to reduce distortion product otoacoustic emissions and elevate threshold at the level of the cochlea; however, the central effects of salicylate are less well known. To gain insight into its central effects, we measured the local field potentials from the AC of unanesthetized rats (n = 3) before and after treatment with 250 mg/kg sodium salicylate (i.p.); a dose that reliably induces tinnitus. Figure 8 shows the mean amplitude of the local field potentials obtained with a 16-kHz tone burst at sound intensities ranging from 30 to 80 dB SPL. The maximum amplitude before salicylate treatment was ∼40 μV at 80 dB SPL. Three hours after the salicylate treatment, the slope of the input/output function at 16 kHz had increased significantly. More importantly, the maximum amplitude had increased to approximately 125 μV, roughly a threefold increase. The salicylate-induced amplitude enhancement of the evoked response from the AC was greatest at 16 and 20 kHz and somewhat less at the low frequencies (5 to 12 kHz).37,44 This amplitude enhancement could be related to tinnitus or hyperacusis; however, further work is needed to elucidate the mechanisms underlying this phenomenon. Parenthetically, when similar experiments were performed on rats anesthetized with isofluorane, salicylate treatment failed to induce an enhancement of the cortical evoked response amplitude. These results indicate that anesthetics have a significant effect on the action of salicylate in the central auditory pathway.
Figure 8.
Local field potential recorded from the auditory cortex (AC) of awake rats (n = 3) before and 3 hours following salicylate (Sal) injection. Evoked response input-output functions measured from 30 to 80 dB sound pressure level [SPL] using 16-kHz tone burst (20 ms duration, 5 ms rise/fall time). Response measured from largest positive peak to following negative trough.
Salicylate and Neural Activity in the Auditory Cortex
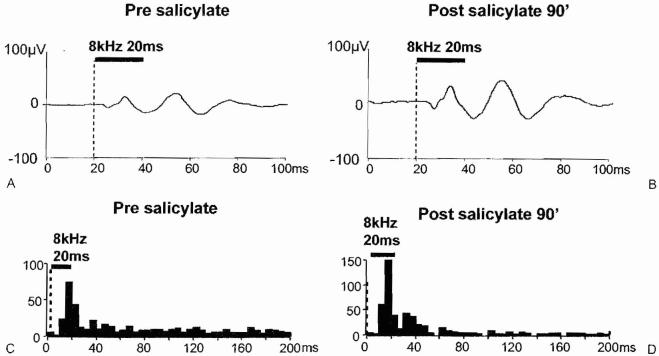
In preliminary studies, the local field potential and multiunit spike activity were recorded from the AC before and after treatment with a high dose of sodium salicylate. Figure 9 shows the local field potential and spike discharge patterns recorded from the same electrode in the AC; recordings were obtained from the same awake animal before and 90 minutes after treatment with 250 mg/kg sodium salicylate. The amplitude of the local field potential evoked by an 8-kHz tone burst (20 milliseconds [ms] duration, 5 ms rise/fall time) increased significantly after treatment with 250 mg/kg sodium salicylate (Fig. 9A,B). These results are consistent with the local field potentials recorded from the surface of the AC (Fig. 8). Figure 9C,D compares the poststimulus time histograms recorded from a multiunit cluster before and 90 minutes after the salicylate treatment. The poststimulus time histogram to the 8-kHz tone burst shows an onset peak near 20 ms before and after salicylate treatment; however, the peak is slightly higher after salicylate treatment. The most noticeable change in spike activity, however, occurs after the offset of the stimulus, Prior to salicylate treatment, numerous spontaneous spikes occurred between 40 and 200 ms; however, 90 minutes after salicylate treatment, spontaneous spikes were greatly reduced. The slight increase in the onset peak after salicylate treatment combined with the dramatic decline in spontaneous activity after salicylate treatment results in a significant increase in the signal to noise ratio; that is the size of the onset peak in the histogram is now much larger than the spontaneous rate. Preliminary results similar to this were seen in three of four units in AC following salicylate treatment.
Figure 9.
Local field potential recorded from the auditory cortex using a 16-channel microwire electrode (filter, 30 to 300 Hz) (A) before and (B) 90 minutes after treatment with 250 mg/kg of sodium salicylate. Response evoked by 8-kHz tone burst (20 ms duration) Poststimulus time histograms recorded from same channel of the electrode (filter, 300 to 3000 Hz); response elicited with 8 kHz, 20 ms tone burst.
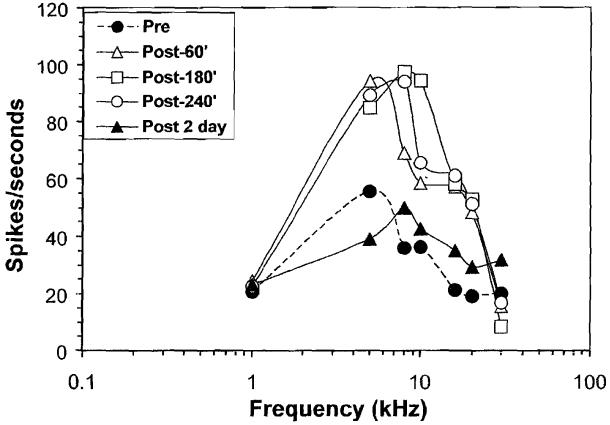
Figure 10 shows the sound-evoked discharge rate from a multiunit cluster in the AC before and after treatment with 250 mg/kg of sodium salicylate. Prior to salicylate treatment, the maximum spike rate, 55 spikes/second, was evoked by a 5-kHz tone burst. Spike rate decreased significantly at higher and lower frequencies, resulting in a frequency tuned response area with a best frequency near 5 kHz. Spike rate to stimuli in the 5-to 10-kHz range nearly doubled 60 to 240 minutes after salicylate treatment; however, there was little change in driven spike rate at 1 kHz and 30 kHz. Salicylate was discontinued and the rat was allowed to recover for 2 days. The response area (spike rate versus frequency) map measured 2 days post-treatment was nearly the same as the one measured before salicylate treatment. These results illustrate how the microwire technique can be used to monitor long-term changes in neural activity and neural plasticity in the central auditory pathway over days, weeks, and, in some cases, months.
Figure 10.
Spike rate recorded from a multiunit cluster in the auditory cortex of an awake rate before and 60, 180, and 240 minutes and 2 days after treatment with 250 mg/kg of sodium salicylate.
Effect of Isofluorane Anesthesia
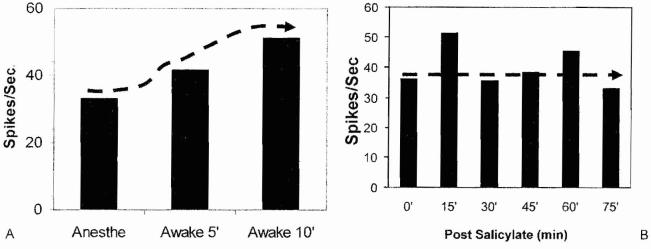
Most electrophysiological studies of noise and salicylate-induced tinnitus have been performed on anesthetized animals; however, anesthetics can exert a profound effect on neural activity in the central auditory pathway41,43,45 and Will abolish the perception of tinnitus. To evaluate the effects of anesthetics on spontaneous spike rate in the AC, we first recorded the multiunit spike activity in the AC of rats anesthetized with 1% isofluorane and then turned off the anesthetic to determine how the spike rate would change when the rat was awake. Figure 11A shows the average spontaneous rate from a typical multiunit cluster in the rat AC during 1% isofluorane anesthesia and the spontaneous rate measured 5 and 10 minutes after the anesthetic was turned off and after the rat was awake. The spontaneous spike rate increased from 5 to 10 minutes following the termination of the anesthetic. These results show that 1% isofluorane suppresses spontaneous activity in auditory cortex, possibly by increasing GABA-mediated inhibitory neurotransmission.46
Figure 11.
(A) Spontaneous spike rate obtained from multiunit cluster in auditory cortex (AC) during 1% isofluorane anesthesia and 5 and 10 minutes after isofluorane anesthesia was terminated. Spike rate increases by nearly 60% after anesthesia was terminated. (B) Spontaneous spike rate recorded from multiunit cluster in AC of rat anesthetized with 1 % isofluorane. Measurements obtained before and from 15 to 75 minutes after administration of 250 mg/kg of salicylate
Effect of Salicylate on Spontaneous Activity under Anesthesia
To determine if isofluorane anesthesia would modify the effects of salicylate, we recorded the spontaneous spike rate from a multiunit cluster in the AC during 1% isofluorane anesthesia before and from 0 to 70 minutes following treatment with 250 mg/kg of salicylate. As shown in Fig. 11B, the spontaneous firing rate increased slightly 15 minutes after salicylate injection; however, the spontaneous rate returned to the pretreatment baseline rate between 30 and 75 minutes post-treatment. These results suggest that 1% isofluorane anesthesia suppresses the effect of salicylate on spontaneous activity in the AC. The weak effect of salicylate on spontaneous activity in AC may occur because isofluorane enhances GABA-mediated inhibition, thereby suppressing the effects of salicylate.46
Effects of Acoustic over Stimulation on Neural Activity
High-level noise exposure is one of the most frequent causes of hearing loss.47 Well-controlled laboratory studies performed on humans have provided insights on the relationships between the characteristics of the traumatizing stimulus, the temporary threshold shift, and the pitch of the transient tinnitus. When pure tone exposures were used to induce temporary hearing loss, the pitch of the tinnitus was located above the frequency of maximum hearing loss. However, when narrow band noise exposures were used to induce temporary hearing loss, the tinnitus pitch was located below the frequency of maximum loss.1,40 The transient tinnitus always emerged immediately after the traumatizing exposure and had a tonal or ringing sensation.
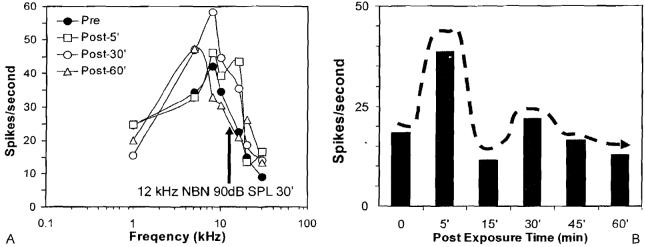
To determine what effects short-term acoustic overstimulation would have on neural activity in the AC, we recorded the spike discharge rates from neurons in the AC before and after moderate intensity sound exposure using a narrow band noise with two different bandwidths. Figure 12A shows the tone burst evoked spike rate versus frequency plot for a multiunit cluster in the AC. Before the exposure, the maximum spike rate was ∼45 spikes/second at 8 kHz. The spike rate dropped off rapidly at higher and lower frequencies so that the response was tuned to 8 kHz, the best frequency. The ear was then exposed at 90 dB SPL for 30 minutes to a narrow band noise centered at 12 kHz (above the best frequency). Interestingly, 5 minutes after the exposure, there was an increase in spike rate evoked by tone bursts between 10 and 16 kHz but little change at other frequencies. At 30 minutes postexposure, large increases in spike rate were evident over a much larger frequency range from 6 to 16 kHz. At 60 minutes postexposure, the spike rate versus frequency plot was nearly normal with the exception of 6 kHz where the spike rate was still elevated. Figure 12B shows the spontaneous spike rate before and after the exposure. The spontaneous rate showed a transient increase 5 minutes postexposures, but the rate fell back toward the baseline rate from 15 to 60 minutes postexposure. Contrary to expectation, these results from the multiunit cluster in Fig. 12A show that high-level noise exposure, which traumatizes the cochlear, can cause some neurons in the AC to become hyperactive to sound stimulation. The hyper-activity seen in this neuron cluster is reminiscent of the hyperactivity to sound stimulation seen in the human brain imaging studies of tinnitus described earlier.16
Figure 12.
(A) Response area map showing spike rate versus stimulus frequency of multiunit cluster in the auditory cortex. Recordings obtained before (filled symbols) and 5, 30, and 60 minutes (open symbol;, see inset) after 30 minutes of exposure to a 90-dB sound pressure level narrow band noise (center frequency, 12 kHz). (B) Spontaneous spike rate from same multiunit cluster before and after the noise exposure, NBN, narrow band noise; SPL, sound pressure level.
DISCUSSION
Because of its subjective nature, tinnitus has long been viewed as an extremely difficult phenomenon to study because of the lack of objective tools to quantify and measure this phantom percept. However, during the past two decades neuroscientists have developed or employed an array of new tools to assess tinnitus objectively. One of the most significant advances has been the use of functional imaging techniques to identify areas of aberrant neural activity in the human brain associated with the perception of tinnitus or hyperacusis. These functional imaging tools not only involve PET, but also functional magnetic resonance imaging, neuromagnetic imaging, and quantitative electroencephalography.48-50
Although human imaging studies have the greatest clinical relevance, more detailed analysis at the cellular or biological level are difficult because of the inability to acquire tissue samples for more detailed analysis. Consequently, investigators have turned to animal models of tinnitus.
Developing an animal model that can report on the presence of tinnitus provides a unique challenge due to the subjective nature of the phenomenon. In the past 20 years, many different animal models have been developed, and improvements have advanced to the point that it is now possible to obtain behavioral measures of tinnitus in individual animal subjects before and after the induction of tinnitus.33,39,44
The SIPAC described earlier is a powerful technique for assessing tinnitus. Behavioral measures of tinnitus can be obtained from individual animals without the need for a control group. Moreover, repeated measurements can be obtained from the same animal because the behavior does not extinguish over time. These behavioral assessment techniques represent a powerful new tool for screening therapeutic compounds to treat tinnitus. Identification of therapeutic compounds that work in an animal model would provide a strong rationale for performing expensive large-scale clinical trials in humans. The development of behavioral models of tinnitus is an obligatory step needed to understand the biological mechanisms that give rise to tinnitus. Specifically, what physiological changes are associated with tinnitus; what region(s) of the brain is responsible for tinnitus, and what biochemical events produce these changes?
Tinnitus is perceived only during states of consciousness, not during sleep or in an unconscious state induced by anesthesia. Anesthetics exert profound and varied effects on neural activity in many regions of the brain including the auditory pathway.42,43,45,51 Unfortunately, most studies that have attempted to identify the physiological correlates of tinnitus have been performed in heavily anesthetized animals.52-55 We do not yet know the extent to which anesthetics perturb the physiological generators that give rise to tinnitus, but clearly it would be prudent to eliminate this potential confounding effect. Our results presented earlier indicate that even moderate levels of isofluorane anesthesia can greatly suppress spontaneous activity in the AC consistent with previous results.56 Equally important, anesthetics may alter the effects that tinnitus-inducing agents, such as salicylate, have on sound-evoked and spontaneous activity. The development of animal models that can tell us if they are experiencing tinnitus combined with advanced electrophysiological recording techniques that allow measurements to be obtained from awake animals with tinnitus should provide important new insights regarding the neural basis of tinnitus.
ACKNOWLEDGMENTS
Research supported in part by past or present grants from the American Tinnitus Association, Tinnitus Research Consortium Tinnitus Research Initiative, and NIH (R01DC00909101, R01DC009219).
Footnotes
Tinnitus: Part II; Guest Editors, Richard Salvi, Ph.D., Wei Sun, Ph.D., and Edward Lobarinas, Ph.D.
Learning Outcomes: As a result of this activity, the participant will be able to (1) discuss how positron emission tomography brain imaging is used to assess neural structures involved in tinnitus in patients, (2) discuss how behavioral techniques are used to assess tinnitus in animals, and (3) discuss how the electrophysiological properties of the auditory cortex are changed by two tinnitus-inducing agents, sodium salicylate or high-level noise exposure.
REFERENCES
- 1.Atherley GR, Hempstock TI, Noble WG. Study of tinnitus induced temporarily by noise. J Acoust Soc Am. 1968;44(6):1503–1506. doi: 10.1121/1.1911288. [DOI] [PubMed] [Google Scholar]
- 2.Myers EN, Bernstein JM. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol. 1965;82(5):483–493. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- 3.Muller M, Klinke R, Arnold W, Oestreicher E. Auditory nerve fibre responses to salicylate revisited. Hear Res. 2003;183(12):37–43. doi: 10.1016/s0378-5955(03)00217-x. [DOI] [PubMed] [Google Scholar]
- 4.Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41(2):365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- 5.Kiang NYS, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Wolstenholme GEW, Knight J, editors. Sensorineural Hearing Loss. J & A Churchill; London, UK: 1970. pp. 241–273. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Powers NL, Hofstetter P, et al. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res. 1997;107(12):67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 7.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hear Res. 1984;16(1):43–53. doi: 10.1016/0378-5955(84)90024-8. [DOI] [PubMed] [Google Scholar]
- 8.House JW, Brackmann DE. Tinnitus: surgical treatment. Ciba Found Symp. 1981;85:204–216. doi: 10.1002/9780470720677.ch12. [DOI] [PubMed] [Google Scholar]
- 9.Coad ML, Lockwood A, Salvi R, Burkard R. Characteristics of patients with gaze-evoked tinnitus. Otol Neurotol. 2001;22(5):650–654. doi: 10.1097/00129492-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Lockwood AH, Wack DS, Burkard RF, et al. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56(4):472–480. doi: 10.1212/wnl.56.4.472. [DOI] [PubMed] [Google Scholar]
- 11.Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–258. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- 12.Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res. 2000;139(12):153–171. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 13.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147(12):261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 14.Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153(4):643–648. doi: 10.1007/s00221-003-1747-3. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez TG, da Silva Lima A, Brandao AL, Lorenzi MC, Bento RF. Somatic modulation of tinnitus: test reliability and results after repetitive muscle contraction training. Ann Otol Rhinol Laryngol. 2007;116(1):30–35. doi: 10.1177/000348940711600106. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood AH, Salvi RJ, Coad ML, et al. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50(1):114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 17.Hallam R, Rachman S, Hinchcliffe R. Psychological aspects of tinnitus. In: Rachman S, editor. Contributions to Medical Psychology. Pergamon Press; Oxford, UK: 1984. pp. 31–53. [Google Scholar]
- 18.Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236–240. [PubMed] [Google Scholar]
- 19.Illing RB, Horvath M. Re-emergence of GAP-43 in cochlear nucleus and superior olive following cochlear ablation in the rat. Neurosci Lett. 1995;194(12):9–12. doi: 10.1016/0304-3940(95)11706-3. [DOI] [PubMed] [Google Scholar]
- 20.Irvine DR, Rajan R, Smith S. Effects of restricted cochlear lesions in adult cats on the frequency organization of the inferior colliculus. J Comp Neurol. 2003;467(3):354–374. doi: 10.1002/cne.10921. [DOI] [PubMed] [Google Scholar]
- 21.Vasama JP, Marttila T, Lahin T, Makela JP. Auditory pathway function after vestibular schwannoma surgery. Acta Otolaryngol. 2001;121(3):378–383. doi: 10.1080/000164801300102833. [DOI] [PubMed] [Google Scholar]
- 22.Harrison RV, Ibrahim D, Mount RJ. Plasticity of tonotopic maps in auditory midbrain following partial cochlear damage in the developing chinchilla. Exp Brain Res. 1998;123(4):449–460. doi: 10.1007/s002210050589. [DOI] [PubMed] [Google Scholar]
- 23.Salvi RJ, Ding D, Wang J, McFadden SL, Sun W. Functional changes in peripheral and central auditory pathways following selective inner hair cell loss. Semin Hear. 2003;24:135–146. [Google Scholar]
- 24.Otsuka K, Pulec JL, Suzuki M. Assessment of intravenous lidocaine for the treatment of subjective tinnitus. Ear Nose Throat J. 2003;82(10):781–784. [PubMed] [Google Scholar]
- 25.Rudack C, Hillebrandt M, Wagenmann M, Hauser U. Treatment of tinnitus with lidocaine? A report of clinical experiences. HNO. 1997;45(2):69–73. doi: 10.1007/s001060050091. [DOI] [PubMed] [Google Scholar]
- 26.Schwab B, Lenarz T, Heermann R. Use of the round window micro cath for inner ear therapy—results of a placebo-controlled, prospective study on chronic tinnitus. Laryngorhinootologie. 2004;83(3):164–172. doi: 10.1055/s-2004-814278. [DOI] [PubMed] [Google Scholar]
- 27.Baguley DM, Jones S, Wilkins I, Axon PR, Moffat DA. The inhibitory effect of intravenous lidocaine infusion on tinnitus after translabyrinthine removal of vestibular schwannoma: a double-blind, placebo-controlled, crossover study. Otol Neurotol. 2005;26(2):169–176. doi: 10.1097/00129492-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Reyes SA, Salvi RJ, Burkard RF, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171(12):43–50. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- 29.Hargarten K, Chapman PD, Stueven HA, et al. Prehospital prophylactic lidocaine does not favorably affect outcome in patients with chest pain. Ann Emerg Med. 1990;19(11):1274–1279. doi: 10.1016/s0196-0644(05)82287-5. [DOI] [PubMed] [Google Scholar]
- 30.Murai K, Tyler RS, Harker LA, Stouffer JL. Review of pharmacologic treatment of tinnitus. Am J Otol. 1992;13(5):454–464. [PubMed] [Google Scholar]
- 31.Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190(12):109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- 32.Mongan E, Kelly P, Nies K, Porter WW, Paulus HE. Tinnitus as an indication of therapeutic serum salicylate levels. JAMA. 1973;226(2):142–145. [PubMed] [Google Scholar]
- 33.Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98(3):280–286. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Oestreicher E, Arnold W, Ehrenberger K, Felix D. Memantine suppresses the glutamatergic neurotransmission of mammalian inner hair cells. ORL J Otorhinolaryngol Relat Spec. 1998;60(1):18–21. doi: 10.1159/000027556. [DOI] [PubMed] [Google Scholar]
- 35.Lin X. Action potentials and underlying voltage-dependent currents studied in cultured spiral ganglion neurons of the postnatal gerbil. Hear Res. 1997;108(12):157–179. doi: 10.1016/s0378-5955(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 36.Guitton MJ, Caston J, Ruel J, et al. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23(9):3944–3952. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobarinas E, Yang G, Sun W, et al. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl. 2006;(556):13–19. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- 38.Wallhausser-Franke E, Cuautle-Heck B, Wenz G, Langner G, Mahlke C. Scopolamine attenuates tinnitus-related plasticity in the auditory cortex. Neuroreport. 2006;17(14):1487–1491. doi: 10.1097/01.wnr.0000230504.25102.26. [DOI] [PubMed] [Google Scholar]
- 39.Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002;170(12):83–95. doi: 10.1016/s0378-5955(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 40.Loeb M, Smith R. Relation of induced tinnitus to physical characteristics of the inducing stimuli. J Acoust Soc Am. 1967;42:453–455. doi: 10.1121/1.1910600. [DOI] [PubMed] [Google Scholar]
- 41.Zurita P, Villa AE, de Ribaupierre Y, de Ribaupierre F, Rouiller EM. Changes of single unit activity in the cat’s auditory thalamus and cortex associated to different anesthetic conditions. Neurosci Res. 1994;19(3):303–316. doi: 10.1016/0168-0102(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 42.Astl J, Popelar J, Kvasnak E, Syka J. Comparison of response properties of neurons in the inferior colliculus of guinea pigs under different anesthetics. Audiology. 1996;35(6):335–345. doi: 10.3109/00206099609071954. [DOI] [PubMed] [Google Scholar]
- 43.Anderson MJ, Young ED. Isoflurane/N20 anesthesia suppresses narrowband but not wideband inhibition in dorsal cochlear nucleus. Hear Res. 2004;188(12):29–41. doi: 10.1016/S0378-5955(03)00348-4. [DOI] [PubMed] [Google Scholar]
- 44.Yang G, Lobarinas E, Zhang L, et al. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226(12):244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Gaese BH, Ostwald J. Anesthesia changes frequency tuning of neurons in the rat primary auditory cortex. J Neurophysiol. 2001;86(2):1062–1066. doi: 10.1152/jn.2001.86.2.1062. [DOI] [PubMed] [Google Scholar]
- 46.Verbny YI, Merriam EB, Banks MI. Modulation of gamma-aminobutyric acid type A receptor-mediated spontaneous inhibitory postsynaptic currents in auditory cortex by midazolam and isoflurane. Anesthesiology. 2005;102(5):962–969. doi: 10.1097/00000542-200505000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Axelsson A, Prasher D. Tinnitus induced by occupational and leisure noise. Noise Health. 2000;2(8):47–54. [PubMed] [Google Scholar]
- 48.Weisz N, Wienbruch C, Dohrmann K, Elbert T. Neuromagnetic indicators of auditory cortical reorganization of tinnitus. Brain. 2005;128(Pt 11):2722–2731. doi: 10.1093/brain/awh588. [DOI] [PubMed] [Google Scholar]
- 49.Cacace AT, Lovely TJ, Winter DF, Parnes SM, McFarland DJ. Auditory perceptual and visual-spatial characteristics of gaze-evoked tinnitus. Audiology. 1994;33(5):291–303. doi: 10.3109/00206099409071889. [DOI] [PubMed] [Google Scholar]
- 50.Ashton H, Reid K, Marsh R, et al. High frequency localised “hot spots” in temporal lobes of patients with intractable tinnitus: a quantitative electroencephalographic (QEEG) study. Neurosci Lett. 2007;426(1):23–28. doi: 10.1016/j.neulet.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 51.Pockett S. Anesthesia and the electrophysiology of auditory consciousness. Conscious Cogn. 1999;8(1):45–61. doi: 10.1006/ccog.1998.0373. [DOI] [PubMed] [Google Scholar]
- 52.Jastreboff PJ, Sasaki CT. Salicylate-induced changes in spontaneous activity of single units in the inferior colliculus of the guinea pig. J Acoust Soc Am. 1986;80(5):1384–1391. doi: 10.1121/1.394391. [DOI] [PubMed] [Google Scholar]
- 53.Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995;82(2):158–178. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- 54.Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear Res. 1998;117(12):149–160. doi: 10.1016/s0378-5955(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett. 1998;250(3):197–200. doi: 10.1016/s0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- 56.Cheung SW, Nagarajan SS, Bedenbaugh PH, et al. Auditory cortical neuron response differences under isoflurane versus pentobarbital anesthesia. Hear Res. 2001;156(12):115–127. doi: 10.1016/s0378-5955(01)00272-6. [DOI] [PubMed] [Google Scholar]