Abstract
Signaling molecules, such as ROP/RAC GTPases and their regulators, reactive oxygen species (ROS) and phospholipids, play pivotal roles in the control of tip growth in pollen tubes and root hairs. They are often localized to the apical growing region of these cells, where their functions are tightly interconnected with cytoskeletal rearrangement and polar vesicle trafficking, which participate in tip growth as well as affect the generation and maintenance of the apical growing region. Recent advances in our understanding of the interface between these cellular activities and signaling in tip growth will be discussed.
Introduction
As an extreme form of polarized cell growth, tip growth is a common mode of cell expansion and morphogenesis in eukaryotic kingdoms such as in cell shape formation and hyphal growth in fungi and formation of root hairs and directional extension of pollen tubes in plants, and extension of neuronal axons in animals [1–3]. Tip growth is strictly dependent on polarized exocytosis of vesicles to the apical growth domain, which provide new plasma membrane (PM) and cell wall precursors. The central questions about the mechanism of tip growth are how cells generate and maintain this growth domain, how this domain is spatiotemporally regulated by extracellular/intracellular signals that control tip growth, and how this growth domain signals to underlying cellular machinery required for polarized exocytosis.
Root hairs and pollen tubes provide important models for addressing these questions. Studies in these systems have revealed an increasing number of signaling molecules and emerging pathways underlying the formation and the maintenance of the apical growth domain [4–6]. Signaling in this domain has been connected to the cytoskeleton and vesicular trafficking required for polarized exocytosis. Evidence suggests that vesicular trafficking pathways not only provide structural requirements for tip growth, but plays an important role in the feedback regulation of the tip-localized signaling for the generation and the maintenance of the apical growth domain [7,8]. These advances have lead to the formulation of the concept of LENS (localization enhancing network, self-sustaining), which provides an important framework for our understanding of tip growth [4]. Here, we highlight recent advances in the studies of cellular activities, key nodes, and emerging signaling pathways in the growing tip. The current review will emphasize how these key nodes and signaling pathways regulate central components of the tip-growth machinery, such as cytoskeleton, vesicular trafficking, and exocytosis.
FINE TUNING OF CELLULAR ACTIVITIES AT THE TIP
The prominent roles of cytoskeletal reorganization and dynamics, exocytosis, and endocytosis in tip growth have been recognized. However, their spatiotemoral regulation and precise roles in tip growth remained vague until several recent studies.
F-actin dynamics
The investigation of signaling mechanisms that control tip growth, such as ROP/Rac GTPase-dependent signaling, have revealed the behavior and the function of apical F-actin as well as the mechanism underlying the regulation of apical F-actin [7,9•]. These studies have identified highly dynamic populations of F-actin localized to the apical region of the pollen tubes [7,9•], some of which have been confirmed by using enhanced fixation techniques [10]. The dynamic apical F-actin is regulated by ROP GTPase signaling at the tip of pollen tubes and root hairs [7,11]. However, specific actin-binding proteins involved and their connection to the signaling mechanisms remain largely uncharacterized. An important latest advance in this area is a study on a new actin binding protein, ABP29, from lily pollen [9•]. ABP29 is the villin/gelsolin/framin superfamily protein. ABP29 binds and severs F-actin filaments in the presence of Ca2+, whereas phosphatidylinositol diphosphate (PIP2) inhibits the severing function of ABP29, suggesting Ca2+ and PIP2 control F-actin dynamics by promoting depolymerization and stabilization, respectively. Moreover, Ca2+ also modulates ABP29 capping activity to prevent elongation or depolymerization from the barbered ends. Transient expression of ABP29 disrupts the actin cytoskeleton and inhibits pollen tube growth, indicating that ABP29-mediated actin reorganization is important for tip growth. ROP signaling controls two downstream pathways leading to actin dynamics: RIC4-mediated F-actin polymerization and RIC3-mediated calcium promoting actin depolymerization [7]. It would be interesting to see whether APB29 acts downstream of the RIC3-calcium pathway.
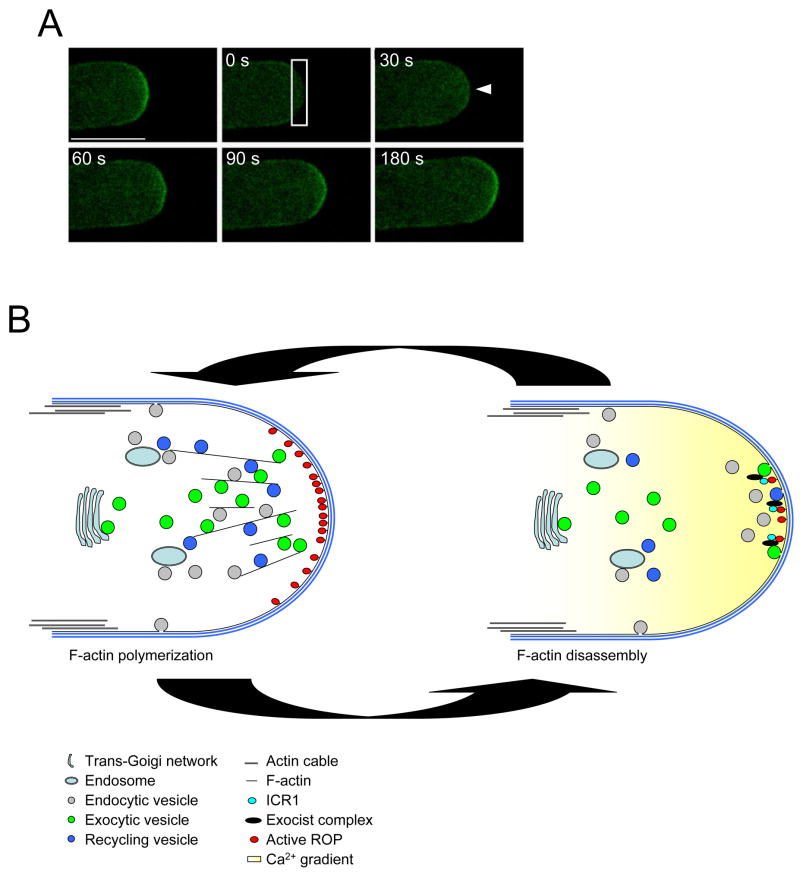
Although earlier pharmacological studies have implied the importance of the apical F-actin in pollen tube growth [12], its precise function was unknown until analysis of tip growth signaling mechanisms. ROP/RIC4-dependent apical F-actin has been implicated in the positive feedback regulation of ROP/Rac signaling [8]. Recent studies suggest that the RIC4-dependent apical F-actin promotes polar accumulation of exocytic vesicles at the tip. First, tip accumulation of post-Golgi compartments is dependent on the apical F-actin [13••]. Second, oscillation of this F-actin and vesicle accumulation at the tip is associated with oscillatory tip growth [13••]. Moreover, F-actin accumulation at the tip is ahead of the growth burst during oscillation [8]. Finally, overexpression of RIC4 or a constitutively active mutant of ROP1 (CA-rop1) enhanced the accumulation of exocytic vesicles to the apical cortex of pollen tubes [13••]. All these findings support the idea that the accumulation of exocytotic vesicles to the apex requires RIC4-dependent tip F-actin. In addition, apical F-actin appears to be important for vesicle recycling during tip growth. Highly motile endosomes accumulate in the apex of root hair, and disruption of F-actin results in inhibition of the motility of these endosomes [14]. Although the basis for F-actin action in endocytosis remains to be investigated, the cortical F-actin network may contribute to endocytosis by pushing coated endocytic vesicles away from the PM.
Microtubules
Pharmacological studies suggest an interaction between microtubules and actin filaments in the regulation of tip growth polarity in root hairs [15]. This is further supported by recent genetic work from the Zheng group. They isolated a mutation that enhanced depolarization of root hair tip growth induced by the expression of a constitutively active ROP2 mutant [16•]. ROP2 was implicated in the control of root hair tip growth at least in part through the regulation of tip actin filaments [11]. This mutation (cae1, also designated as mrh2-3) turned out to be within the MRH2 gene, which encodes an armadillo (ARM) domain-containing kinesin-like protein [17]. The mrh2-3 mutation induced a wavy root hair phenotype and caused fragmentation and random orientation of microtubule [16•]. These results suggest a role for MRH2 in stabilizing microtubule or maintaining microtubule orientation, which is important for determination of orientation of root hair tip growth [16•]. Interestingly, the ARM domain-containing MRH2 fragment binds to actin filaments, and mrh2-3 root hairs shows increased sensitivity to LatB, suggesting that MRH2 may coordinate actin microfilaments and microtubules during tip growth of root hairs. However the mechanism by which MRH2 coordinates these cytoskeletal elements and interact with ROP2 signaling remains to be determined.
Exocytosis
Localized exocytosis provides materials for the expansion of the PM and the assembly of new cell wall during tip growth. It has been proposed that ROP1 activity may play an important role in the control of vesicle exocytosis because active ROP1 localizes to the apical region of the pollen tube PM, where growth takes places [7]. However, the spatiotemporal activity of exocytosis has been elusive, because of lack of methods for measuring exocytic activity in the tip of pollen tubes or root hairs. A fluorescence recovery after photobleaching (FRAP)-based method has recently been developed for the analysis of vesicle exocytosis in pollen tube tips [13••]. PM localization of receptor-like kinase (RLK)-GFP is dependent on exocytosis. Thus, exocytic activity at the photo-bleached region of the RLK-GFP containing PM can be measured by the rate of its FRAP. This study reveals that exocytosis of the RLK-GFP tracer protein is indeed restricted to the PM apex with a tip-high gradient, which corresponds to the gradient of active ROP1 (Figure 1A). Moreover, CA-rop1 expression induced depolarization of exocytosis associated with growth depolarization, whereas DN-rop1 expression inhibited exocytosis. These results suggest ROPs are required for exocytosis and that the spatial distribution of ROP1 activity determines the site of exocytosis. Lee et al’s study also provides insights into the mechanism by which ROP1 regulates polarized exocytosis [13••]. It is ROP1-dependent dynamics of the apical F-actin that is required for polarized exocytosis. ROP1/RIC4-dependent assembly of the apical F-actin allows exocytic vesicles to accumulate at the tip, whereas ROP1/RIC3-dependent actin depolymerization is required for the docking and/or fusion of these vesicles. Cortical F-actin seems to act as a barrier for vesicle docking/fusion to the target PM, as found in animal cells [18]. Indeed, RIC4 dependent cortical F-actin polymerization inhibited vesicle docking/fusion to the PM and this inhibition was suppressed by Latrunculin B (LatB) or RIC3 expression [13••]. Both LatB and RIC3-mediated Ca2+ influx have been shown to disrupt tip F-actin [7]. Thus, RIC4-mediated F-actin assembly and RIC3-mediated F-actin delpolymerization both appear to be required for RLK-GFP delivery, suggesting that ROP1 governs exocytosis to the apical PM through regulating F-actin dynamics.
Figure 1. Cellular activities in the tip.
(A) Exocytosis at the tip of pollen tube. Exocytic activity was monitored by fluorescence recovery of RLK-GFP after photo-bleaching [13••]. Bleached area is marked by solid lined box. Arrow head indicates a starting site of fluorescence recovery. Numbers on each image indicate elapsed time (seconds) after bleaching.
(B) A simplified schematic representation of the cellular processes involved in pollen tube tip growth. Pollen tip growth is controlled by F-actin dynamics. RIC4 dependent F-actin polymerization is required for both accumulation of exocytotic vesicles (both secretory and recycling vesicles) to the tip and trafficking of endocytic vesicles to the endosome (left). Exocytic vesicle tethering and fusion to apical PM occurs when RIC3-dependent high Ca2+ promotes F-actin disassembly (right). ICR1 involvement in this process is hypothetical.
The exocyst, an octameric protein complex, is also involved in polarized exocytosis [19]. In animal cells, the exocyst facilitates the targeting and tethering of secretory vesicles to the PM [20]. The latest report reveals existence of such a functional protein complex in Arabidopsis [21••]. Chromatographic fractionation identified a high molecular mass complex containing exocyst subunits. Immunolocalization of SEC6, SEC8, and EXO70 demonstrated that exocyst subunits have overlapping localization patterns at the tip of pollen tubes, supporting its role in exocytosis. Indeed, their loss of function mutants exhibited defect in pollen germination and tube growth [21••]. Together with root hair growth defects in an Arabidopsis exo70A1 mutant [22], these findings implicate a common function for these proteins in plant tip growth. Interestingly, an Arabidopsis SEC3 homolog was reported to interact with a novel ROP effector, ICR1, which raises an interesting possibility that an additional downstream pathway may also participate in the ROP GTPase regulation of polar exocytosis by recruiting the exocyst to the site of tip growth [23,24].
Endocytosis
Mathematical modeling predicts a crucial role for endocytosis in cell polarity establishment in yeast [25••], and in plants endocytosis has been shown to be critical for establishment of the polarity of PIN localization [26]. Emerging evidence suggests that endocytosis may also be important for the maintenance of cell polarity in pollen tubes. Using the pollen tube system, Raikhel and colleagues identified a small molecule, endosidin1 (ES1) that altered the apical PM localization of a ROP interacting partner 1 (RIP1) and induced growth depolarization [27,28]. ES1 induced aggregation of PIN2, AUX1 and BRI1, but not PIN1 and PIN7, into distinct endosomal compartments. These results not only reveal the complex endosomal sorting pathways in plant cells, but importantly show that a specific endosomal trafficking pathway regulates cell polarity in tip-growing cells.
Recent studies have identified two different types of endocytosis in pollen tubes [29•-31]. Endocytosis probed by negatively charged nanogold takes place along the extreme apical PM [29•]. These particles were also observed in the lumen of vacuole-like structures but not in the ER or Golgi bodies, suggesting this type of endocytosis may be involved in the degradation pathway. The second endocytic pathway occurred just behind the extreme apex, which was stained with positively charged gold particles [29•,30]. This pathway seems to be clathrin-mediated because clathrin-coated vesicles and high density of clathrin-coated pits were found in the region just behind the extreme apex. Cytochalasin D completely abolished this type of PM retrieval, suggesting endocytosis in this region requires actin filaments [31]. Tracking of fluorescent phospholipid revealed that retrieved phospholipid was redistributed to the base and apex of pollen tube [31]. Electron microscopic analysis demonstrated that positively charged gold particles also stain the early endosome and vesicles associated with the Golgi body [29•]. Taken together these results suggest that this pathway may be involved in recycling of PM materials to the apical PM. An important remaining question is which of these two types of endocytosis are important for cell polarity in pollen tubes.
REGULATION OF DYNAMIC TIP-LOCALIZED ROP SIGNALING
As a critical regulator of tip growth, ROP1 is active in the PM apex of pollen tubes as a dynamic apical cap [8]. During oscillatory growth, the ROP1 apical cap is highly dynamic with maximum accumulation forming ahead of growth burst [8]. The maximum cap determines the region of tip growth (i.e., exocytosis) [Hwang et al., unpublished]. These observations suggest a self-organizing mechanism for the control of the activation and down-regulation of ROP1 to generate and limit the apical cap so that its dynamics can be maintained for continuous tip growth. Recent studies on several regulators, including guanine nucleotide exchange factor (GEF), which activates ROP signaling by promoting GDP to GTP exchange, and GTPase-activating protein (GAP), which promotes GTP hydrolysis, have generated some important insights into these mechanisms [5].
A plant-specific family of ROP activators, RopGEFs, appears to be responsible for the ROP activation during tip growth in plants [32,33]. RopGEFs are also localized to the pollen tube tip as an apical cap [33,34••]. Understanding how RopGEFs are regulated can provide insights into mechanisms behind the initiation of ROP activation to generate the apical cap of active ROP1. PM-localized receptor-like kinases (RLK) may transmit an extracellular signal to ROP GTPase through RopGEFs. Recent work from McCormick’s group suggests that a pollen RLK, PRK2a, appears to regulate RopGEFs in the spatial control of ROP signaling [34••]. When transiently overexpressed in tobacco pollen tubes, PRK2a and RopGEF12 apparently had a synergistic effect, producing isotropic pollen tube growth similar to that induced by CA-rop1. PRK2a may regulate the pollen-specific RopGEF12 by phosphorylating the C-terminal regulatory region of RopGEF12. The kinase domain of PRK2a interacted with this region of RopGEF12. Based on sequence alignment of pollen-specific RopGEFs, several C-terminal conserved serine residues were identified. The phospho-mimic S510D mutation of RopGEF12 increased its membrane localization and its induction of growth depolarization in pollen tubes. The authors propose that the phosphorylated C-terminal region recruits RopGEF12 to the apical PM of pollen tubes. It remains to be determined whether the C-terminal region is indeed phosphorylated by PRK2a and whether this phosphorylation regulates RopGEF12 activity. It will also be interesting to investigate the potential extracellular signal that activates PRK2a and RopGEF12. Several candidate ligands for RLK have been isolated from tomato mature pollen and stigma. One of these, a stigma cysteine-rich protein (LeSTIG1), interacts with the extracellular domain of tomato PRKs and promotes pollen tube growth [35]. Thus STIG1 might be a ligand for PRKs in the regulation of ROP1-dependent tip growth.
How ROP GTPases are recruited specifically to the apical PM remains to be an interesting unanswered question. A recent study suggests that RIP1/ICR1 may play a role in recruiting ROP1 to the pollen tube PM in addition to its potential role as a ROP1 effector [23,28]. RIP1/ICR1 is localized to the cortex of pollen germination sites and the apical PM of pollen tubes. Its overexpression induced pollen tube growth depolarization and enhanced GFP-ROP1 recruitment to the PM. A C-terminal polybasic motif of RIP1/ICR1, which may potentially bind phospholipids, is required for RIP1/ICR1 localization to the PM and its function. The precise function of this interesting ROP1-interacting protein needs further investigation.
Visualization of active ROP1 by the localization of RIC4ΔC shows that ROP1 overexpression induces lateral expansion of the ROP1 apical cap, causing growth depolarization in pollen tubes [8]. Thus, restricting ROP activity to a localized PM domain is required for polarized tip growth. Two ROP negative regulators, guanine nucleotide dissociation inhibitor (GDI) and GAP appear to be important for this process. GDI sequesters ROP in the cytosol and is important for limiting ROP signaling to the tip in both root hairs and pollen tubes [36,37, Hwang et al., unpublished]. GFP-tagged NtRhoGAP1 was reported to preferentially localize to the flanks of the tip, and this localization was proposed to restrict ROP activation to the apex of pollen tubes [38]. However, lack of growth depolarization in Arabidopsis pollen, in which homologs of this RopGAP are knocked out, suggests that these RopGAPs do not play a primary role in restricting ROP activity to the pollen tube tip [Hwang et al., unpublished]. A new family of RhoGAPs in Arabidopsis pollen has been shown to play a prominent role in restricting active ROP1 to the tube apex [39, Hwang et al., unpublished].
ROS SIGNALING IN TIP GROWTH
An important advance in the tip growth field in recent years has been the revelation of a tight connection between reactive oxygen species (ROS) and tip-focused Ca2+ gradients in the regulation of tip growth. Earlier studies demonstrated that ROS produced by RHD2 [40], a PM localized NADPH oxidase, activates Ca2+ permeable channels required to generate the tip-focused Ca2+ gradient in root hairs [41]. A recent study by Potocký also support a role for ROS in pollen tube tip growth [42•]. ROS was found to localize to the tip of growing pollen tubes, and NADPH oxidase (NOX) inhibitor, diphenylene iodonium chloride, inhibited ROS formation and pollen tube growth, as did suppression of NtNOX expression. The effect of NtNOX suppression was rescued by addition of exogenous H2O2, implying NtNOX is the source of the tip-focused ROS formation. Exogenous CaCl2 treatment to pollen tubes resulted in increased ROS at the tip of pollen tubes. Given the positive effect of Ca2+ on RDH2 activity at the root hair tip [43••], increased Ca2+ probably activates pollen NOX activity in the same manner. Taken together, these data suggest that tip accumulation of ROS is another hallmark of tip growing cells and that NADPH oxidase-dependent ROS provides a common regulatory mechanism for tip growth in plant cells.
Tip-localized ROS formation in root hairs is spatially regulated by a RhoGDI, hinting at a functional link between ROP signaling and ROS production [44]. Indeed ROP may play a pivotal role in the spatial regulation of ROS production [43••]. Cytochalasin D induced cytoplasmic accumulation of RHD2, a NADPH oxidase. Given that ROP regulates F-actin dynamics in tip-growing cells [7], apical F-actin might be the key regulator in RHD2 mediated tip accumulation of ROS. This notion is further supported by the finding that RHD2 accumulated in the cytoplasm in the deformed root hairs 1 (der1) mutant, which lacks the ACTIN2 protein [45].
In addition to spatial regulation of ROS production, ROP seems to regulate the enzyme activity of NADPH oxidase. CA-rop2 increases ROS production in root hairs, but DN-rop2 decreases ROS formation [46•]. The rhd2-1 loss function mutation suppressed ROS formation induced by CA-rop2 expression in root hairs. These observations suggest that NADPH oxidase may be a downstream target of ROP signaling. This is further supported by direct interaction between ROP and NADPH oxidase ([47], see below). CA-rop2 stimulated swelling formation and promoted root hair tip growth, whereas rhd2-1 root hairs were tip growth defective [46•]. Multiple swelling formation and defect in root hair tip growth in CA-rop2 rhd2-1 plants indicate RDH2 activity is involved in tip growth but not in swelling formation. These findings further support the idea that ROP2 acts earlier than RHD2 in root hair formation and RDH2 activity is critical for ROP2 mediated root hair tip elongation.
How might ROS production by RHD2 be mediated by ROP activity? Evidence suggests that ROP activity regulates pollen tube growth by promoting Ca2+ influx [7]. Thus, it is possible that ROP-mediated Ca2+ influx may promote activation of RDH2 activity, which creates a positive feedback loop between RDH2 and the Ca2+ channel (see below).
Alternatively or additionally, ROP may directly regulate RDH2 activity. Work from Wong et al demonstrated that OsRac1 binds to the N-terminal extension of rice NADPH oxidase in vivo [47]. Transient coexpression of CA-OsRac1 and OsrbohB (a rice NADPH oxidase) enhances ROS production, suggesting that Rac and Rboh interaction may activate its Rboh activity in plants. Interestingly high Ca2+ concentration suppresses OsRac1 binding to OsrbohB. Thus, in addition to its direct role in activity regulation of NAPDH oxidase, Ca2+ also seems to modulate NAPDH oxidase activity by regulating interaction between ROP and NAPDH oxidase.
An interesting aspect of ROS signaling is the Ca2+ dependent regulation of RHD2 NADPH oxidase activity. Dolan’s group recently reported that expression of a RHD2 mutant, substitutions in conserved amino acid residues within the EF hand motif, did not complement tip growth defects of a rhd2 knockout mutant [43••]. This implies that Ca2+ binding to the RHD2 is required for ROS production and root hair tip growth. Moreover, Ca2+ dependent phosphorylation of RHD2 is also involved in the regulation of its activity. Treatment with calyculin A, a phosphatase inhibitor, stimulated ROS production by RHD2. The two Ca2+-dependent mechanisms, Ca2+ sensing through the EF hands and phosphorylation by a Ca2+-dependent protein kinase, synergistically activate ROS production [43••]. On the basis of these observations, the Dolan group proposed that RHD2-mediated ROS production activates PM-localized Ca2+ influx, which in turn promotes RHD2 activation, forming a ROS-Ca2+-ROS positive feedback loop that rapidly establishes a tip growth site and activates root hair tip growth. This finding is significant, because it is the first ROS-mediated positive feedback loop shown to control cell polarity. Given a common role for Rho GTPases in the positive feedback regulation of cell polarity in many systems [48], an important future direction is to elucidate how ROP signaling is integrated into the ROS-Ca2+-ROS positive feedback loop in the regulation of tip growth.
In addition to its signaling role in mediating tip-focused Ca2+ gradients, ROS has also been implicated in the regulation of cell wall extensibility during tip growth. Monshausen et al. reported higher apoplastic ROS present along the root hair flanks, whereas low ROS levels were found in the rapidly expanding region of the root hair tip [49]. Interestingly, levels of apoplastic ROS oscillate in the same periodicity as the tip growth oscillation. Peaks of apoplastic ROS most likely lag peaks in growth rate, suggesting increased ROS production restrict cell expansion after a peak of elongation. Based on these findings, they proposed that extracellular ROS promote the rigidification of the cell wall. Given the role of the tip-localized ROS in the regulation of tip-focused calcium required for growth, it appears that there exist at least two different pools of ROS, which are differentially regulated spatially or spatiotemporally. Future studies should determine the mechanism behind the production of the ROS pool involved in wall rigidity regulation and the relationship of the extracellular pool of ROS to the intracellular pool regulating Ca2+ influx.
PHOSPHOINOSITIDE SIGNALING
By association with various signaling proteins on the cell membranes and participating in membrane microdomain formation, phosphoinositides have been shown to be important factors for the regulation of actin dynamics, vesicle trafficking, ion transport, and cell polarity formation. Thus, phosphoinositides are excellent candidates for an important role in the regulation of tip growth in plants. The first hint to a potential role for PIPs in the control of tip growth in pollen tubes was Kost et al’s demonstration that a PIPK activity is associated with ROPs [50]. Recent work has demonstrated their diverse roles in both root hairs and pollen tubes (Table 1).
Table 1.
Lipid signaling: enzymes, messengers, and their mutant phenotype in root hair and pollen tube.
| Enzyme | substrate | Product | Messenger | Mutant phenotype | References |
|---|---|---|---|---|---|
| PI3K | PI | PI(3)P | ROS | short root haira | 51 |
| PI4K | PI | PI(4)P | NO | short, bulged, branched root hair | 63 |
| PI(4) P phosphatase | PI(4)P | PI | - | short, bulged, branched root hair | 57 |
| PI4PK | PI(4)P | PK(4,5)P2 | ABPs | reduced tip growth in pollen tube and root hair | 59, 60 |
| PLC | PI(4,5)P2 | DAG | - | - | |
| IP3 | Ca2+ flux | reduced tip growth in pollen tube b | 64 |
phenotype produced by overexpression of FYVE domain
phenotype induced by PLC inhibitor, U-73343
Phosphatidylinositol 3-phosphate (PI3P) was implicated in tip growth by a study using transgenic plants expressing the PI3P-binding FYVE domain [51]. Probably by blocking PI3P signaling, expression of the FYVE domain resulted in inhibition of root hair growth. In animals, PI3P is concentrated in endosomal membranes and recruits PI3P binding proteins that regulate endocytosis and endosomal trafficking [52]. Thus, it is speculated that PI3P is important for vesicle recycling in tip growth process. In support of this idea, treatment with LY294002, a phosphatidylinositol-3 kinase (PI3K) specific inhibitor, decreases ROS levels in root hairs [51], which is consistant with a recent report that both ROS production and endocytosis are suppressed in PI3K mutants [53]. It has also been suggested PI3P regulates actin dynamics in guard cells [54]. Thus, cellular function for PI3P in tip growth might also be interconnected with ROS-mediated tip focused Ca2+ gradients and F-actin dynamics.
PI4P is a precursor of the versatile signaling molecule phosphatidylinositol 4,5 diphosphate and is known to have an important role in the Golgi compartment [55]. The activity of phosphatidylinositol-4 kinase (PI4K), has been shown to be required for vesicle trafficking to the PM in yeast [56]. In plants, a cellular role for PI4P in root hair tip growth was recently demonstrated. PI4P is localized to the apical PM in growing root hairs [57•] and a knockout mutation in PI4P phosphatase (rhd4) exhibits short, bulged, and branched root hairs. These results suggest PI4P is involved in the regulation of tip growth polarity. However, it is unclear whether the rhd4-1 mutant phenotype is the result of altered PI4P levels in the apical PM or the indirect result of malfunctioning of the Golgi apparatus. Identification of PIP4 binding proteins involved in tip growth will be important for addressing this question.
An increasing number of reports supports a link between PI4,5P2-mediated signaling pathways and tip growth [58–61]. PI4,5P2 accumulates at the apical PM of pollen tubes and root hairs, and local release of PI4,5P2 modulates the growth direction of pollen tubes, suggesting a role for PI4,5P2 in tip growth [58,59]. Recently, two independent reports examined the cellular function of PI4,5P2 in root hair tip growth [59,60]. In growing root hairs, PI(4)P-5 kinase is localized to the apical PM, whereas tip localization disappears in mature root hairs, suggesting that a tight spatial and temporal regulation of PI4,5P2 may be critical for tip growth [59]. T-DNA insertion-mediated knockdown a PI(4)P-5 kinase gene inhibits root hair tip growth, whereas expression of a gain-of-function PI(4)P-5 kinase mutant causes thicker, curling, and depolarized root hairs, a phenotype similar to that observed in PI4,5P2 overproducing pollen tubes [59–61]. These results imply the importance of a proper level of PI4,5P2 in tip growth in both root hairs and pollen tubes. In animal cells, PI4,5P2 interacts with various proteins that affect multiple cellular processes [62]. One of the possible targets in tip growth might be actin binding proteins [9•], but the in vivo function of these proteins in tip growth is not clear. Consequently the precise role of PI4,5P2 in the regulation of tip growth is unknown.
One complication in elucidating the specific function of a given PIP is that manipulation of a particular PIP by using genetic mutant or transgenic expression of a PIP-metabolizing enzyme not only alters the level of the immediate products (and usually subsequent derivatives) but also of the substrates. As such, it is difficult to pinpoint the substrate or the product that directly causes the defect. For example, PIPK overexpression may increase not only PIP2 but also subsequent products such as diacyl glycerol (DAG), and inositol 1,4,5-trisphosphate (IP3), which have also been implicated in tip growth. In yeast and animal cells, transient activation of a PIP-metabolizing enzyme (such as PI4,5P2 phosphatase) has been used to acutely alter the level of PIPs, helping to obviate this problem [65]. However, identification and functional analysis of specific PIP-binding proteins will ultimately be necessary to fully understand the roles of specific PIPs in the regulation of tip growth.
CONCLUDING REMARKS
Studies in the past few years have generated significant progress in our understanding of signaling mechanisms underlying tip growth. Many signaling components have been identified and connected within pathways that connect intracellular signals to F-actin dynamics and actin-mediated vesicular trafficking (Figure 2). Despite these substantial advances, many questions, particularly those related to the initiation of tip growth, are yet to be answered. For example, what are molecular mechanisms that control F-actin dynamics in tip growing cells? What are the extrinsic or intrinsic cues that initiate tip growth? Innovative genetic screens to identify mutants defective in tip growth will expand our understating of signaling underlying tip growth process. Nonetheless, it is clear that more comprehensive and quantitative approaches, such as real time quantification of signaling molecules combined with mathematical modeling of the complex tip growth signaling network, will help to raise our understanding of tip growth to a new level.
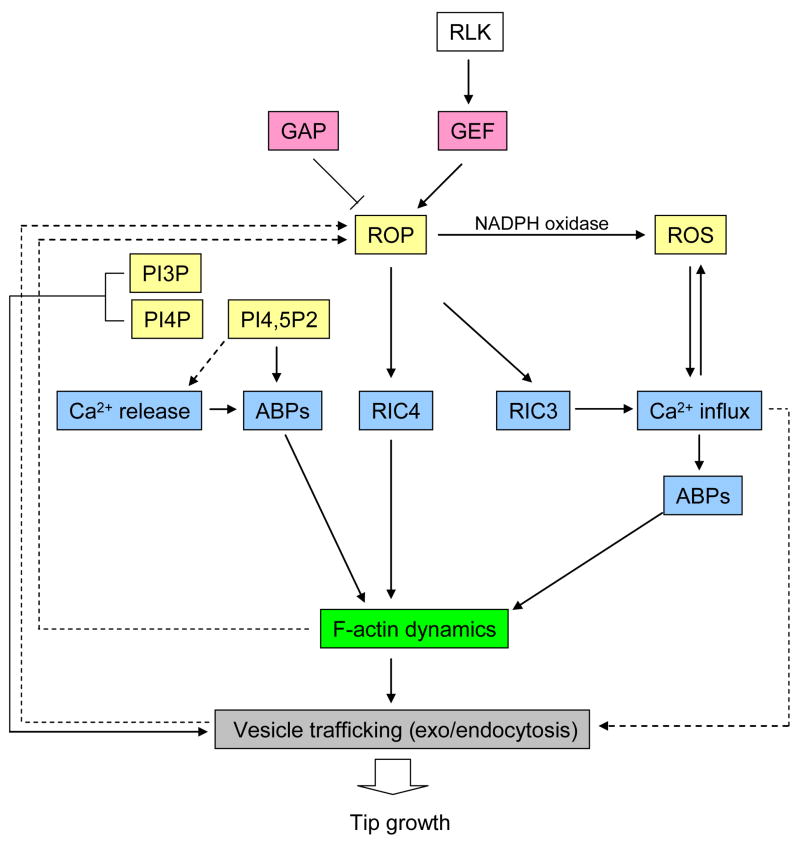
Figure 2. Signaling pathways regulating tip growth.
The available data indicate that multiple pathways are converged in F-actin dynamics, which is critical for tip growth. ROP activity has been shown to oscillate at the tip, suggesting a self-organizing mechanism involving feedback loops underlies the regulation of the apical ROP activity. ROP downstream events, such as vesicle trafficking, calcium signaling, and F-actin dynamics, are most likely to contribute to the feedback regulation of ROP activity. Phsopholipids signaling was proposed to act downstream of ROP signaling, but no direct evidence is available. Actin binding proteins (ABPs) represented here is including ADF, profilin, and villin/gelsolin/fragmin superfamily proteins, whose activities are regulated by Ca2+ and/or PIP2. Ins(1,4,5)P3-induced-Ca2+ release was observed in pollen tubes, but IP3-sensitive calcium channel has not been identified. Solid arrows represent pathways that supported by experimental data, whereas dotted arrows represent more hypothetical pathways.
Abbreviations
- PI3K
phosphatidylinositol-3-kinase
- PI4K
phosphatidylinositol-3-kinase, PI4PK, phosphatidylinositol-4-phosphate-kinase
- PLC
phospholipase C
- PI
phosphatidylinositol
- PI(3)P
phosphatidylinositol-3-phosphate
- PI(4)P
phosphatidylinositol-4-phosphate
- PI(4
5)P2, phosphatidylinositol-4,5-diphosphate
- DAG
diacylglycerol
- IP3
inositol 1,4,5-triphosphate
- ROS
reactive oxygen species
- ABPs
actin binding proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. doi: 10.1016/s0959-4388(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 2.Momany M. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr Opin Microbiol. 2002;5:580–585. doi: 10.1016/s1369-5274(02)00368-5. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z. Signaling tip growth in plants. Curr Opin Plant Biol. 1998;1:525–530. doi: 10.1016/s1369-5266(98)80046-0. [DOI] [PubMed] [Google Scholar]
- 4.Cole RA, Fowler JE. Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol. 2006;9:579–588. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Kost B. Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends Cell Biol. 2008;18:119–127. doi: 10.1016/j.tcb.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Krichevsky A, Kozlovsky SV, Tian GW, Chen MH, Zaltsman A, Citovsky V. How pollen tubes grow. Dev Biol. 2007;303:405–420. doi: 10.1016/j.ydbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169:127–138. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang JU, Gu Y, Lee YJ, Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Xiang Y, Huang X, Wang T, Zhang Y, Liu Q, Hussey PJ, Ren H. ACTIN BINDING PROTEIN 29 from Lilium pollen plays an important role in dynamic actin remodeling. Plant Cell. 2007;19:1930–1946. doi: 10.1105/tpc.106.048413. This paper presents data suggesting that calcium and PI4,5P2 play an important role in the regulation of ABP29 activity, which controls actin remodeling in pollen tube growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK. Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta. 2005;221:95–104. doi: 10.1007/s00425-004-1423-2. [DOI] [PubMed] [Google Scholar]
- 11.Jones MA, Shen JJ, Fu Y, Li H, Yang Z, Grierson CS. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 2002;14:763–776. doi: 10.1105/tpc.010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Mol Biol Cell. 2001;12:2534–2545. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Lee YJ, Szumlanski A, Nielsen E, Yang Z. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol. 2008;181:1155–1168. doi: 10.1083/jcb.200801086. This report provides evidence that ROP1-mediated F-actin dynamics control pollen tube growth through its effect on trafficking and exocytosis of vesicles at the tip. The authors demonstrate that RIC4-dependent F-actin polymerization promotes polar accumulation of exocytic vesicles at the tip and that RIC3-dependent Ca2+ influx, which promotes the depolymerization of apical actin filaments, is required for exocytosis of vesicles to the apical PM. A new technique developed to quantify exocytosis in this paper, which was based on FRAP analysis of a GFP-tagged PM targeted protein, should add an important tool for the study of tip growth in plants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voigt B, Timmers AC, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, et al. Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol. 2005;84:609–621. doi: 10.1016/j.ejcb.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Ketelaar T, de Ruijter NC, Emons AM. Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell. 2003;15:285–292. doi: 10.1105/tpc.007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Yang G, Gao P, Zhang H, Huang S, Zheng ZL. A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PLoS ONE. 2007;2:e1074. doi: 10.1371/journal.pone.0001074. This report demonstrates armadillo domain-containing protein, MRH2, controls microtubule organization in root hair and provides evidence suggesting MRH2 is involved in the coordination of microtubule and actin filaments during tip growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai T, Honing H, Nishioka M, Uehara Y, Takahashi M, Fujisawa N, Saji K, Seki M, Shinozaki K, Jones MA, Smirnoff N, Okada K, Wasteneys GO. Armadillo repeat-containing kinesins and a NIMA-related kinase are required for epidermal-cell morphogenesis in Arabidopsis. Plant J. 2008;53:157–171. doi: 10.1111/j.1365-313X.2007.03327.x. [DOI] [PubMed] [Google Scholar]
- 18.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- 20.Zajac A, Sun X, Zhang J, Guo W. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol Biol Cell. 2005;16:1500–1512. doi: 10.1091/mbc.E04-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Hala M, Cole R, Synek L, Drdova E, Pecenkova T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, et al. An Exocyst Complex Functions in Plant Cell Growth in Arabidopsis and Tobacco. Plant Cell. 2008;20:1330–1345. doi: 10.1105/tpc.108.059105. An important and extensive study suggesting that plant homologs of exocyst subunits function together in vivo. This work demonstrates functional exocyst plays important roles in pollen tube growth and hypocotyl elongation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Synek L, Schlager N, Elias M, Quentin M, Hauser MT, Zarsky V. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J. 2006;48:54–72. doi: 10.1111/j.1365-313X.2006.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Fu Y. ROP/RAC GTPase signaling. Curr Opin Plant Biol. 2007;10:490–494. doi: 10.1016/j.pbi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. By measuring parameters from experiments, authors develop a mathematical model for the dynamic distribution of polarized proteins. The modeling suggests that endocytosis plays a pivotal role in determining a polarized cortical region whereas positive feedback loops are important for polarity maintenance in yeast cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 27.Robert S, Chary SN, Drakakaki G, Li S, Yang Z, Raikhel NV, Hicks GR. Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci U S A. 2008;105:8464–8469. doi: 10.1073/pnas.0711650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Gu Y, Yan A, Lord EM, Yang Z. RIP1 (ROP interactive partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen tube growth. Mol Plant. 2008 doi: 10.1093/mp/ssn051. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Moscatelli A, Ciampolini F, Rodighiero S, Onelli E, Cresti M, Santo N, Idilli A. Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J Cell Sci. 2007;120:3804–3819. doi: 10.1242/jcs.012138. This elegant study using charged nanogold particle and in vivo time-lapse experiments provides evidence that two distinct endocytic pathways exist at the specific regions of the pollen tube. [DOI] [PubMed] [Google Scholar]
- 30.Zonia L, Munnik T. Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot. 2008;59:861–873. doi: 10.1093/jxb/ern007. [DOI] [PubMed] [Google Scholar]
- 31.Lisboa S, Scherer GE, Quader H. Localized endocytosis in tobacco pollen tubes: visualisation and dynamics of membrane retrieval by a fluorescent phospholipid. Plant Cell Rep. 2008;27:21–28. doi: 10.1007/s00299-007-0437-1. [DOI] [PubMed] [Google Scholar]
- 32.Berken A, Thomas C, Wittinghofer A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 2005;436:1176–1180. doi: 10.1038/nature03883. [DOI] [PubMed] [Google Scholar]
- 33.Gu Y, Li S, Lord EM, Yang Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18:366–381. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2007;104:18830–18835. doi: 10.1073/pnas.0705874104. This exciting paper provides evidence that phosphorylation of a C-terminal conserved serine residue in RopGEF12 has an important influence on its function and localization. Based on the co-overexpression phenotype of RopGEF12 and pollen RLK (PRK2a), the authors propose that PM-localized receptor-like kinases may be involved in the apical PM localization of RopGEFs and thus the initiation and maintenance of ROP signaling at the tip of pollen tubes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S. LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J. 2004;39:343–353. doi: 10.1111/j.1365-313X.2004.02139.x. [DOI] [PubMed] [Google Scholar]
- 36.Klahre U, Becker C, Schmitt AC, Kost B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant J. 2006;46:1018–1031. doi: 10.1111/j.1365-313X.2006.02757.x. [DOI] [PubMed] [Google Scholar]
- 37.Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 38.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell. 2006;18:3033–3046. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z. Cell polarity signaling in Arabidopsis. Ann Rev Cell Dev Biol. 2008 doi: 10.1146/annurev.cellbio.23.090506.123233. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 41.Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- 42•.Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zarsky V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. This study shows that tip-focused reactive oxygen species generated by pollen specific NADPH oxidase is required for pollen tube growth, suggesting that it could be a general mechanism that controls tip growth in plant cells. [DOI] [PubMed] [Google Scholar]
- 43••.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. This is a highly significant study, which elegantly shows that determination and maintenance of root hair growth sites is regulated by ROS produced by the RHD2 NADPH oxidase and Ca2+-mediated positive feedback loop. The authors proposed local production of ROS activates calcium channels and stimulates Ca2+ influx that in turn activates RHD2 activity to produce more ROS to establish tip growing site in root hair cells. [DOI] [PubMed] [Google Scholar]
- 44.Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 45.Ringli C, Baumberger N, Diet A, Frey B, Keller B. ACTIN2 is essential for bulge site selection and tip growth during root hair development of Arabidopsis. Plant Physiol. 2002;129:1464–1472. doi: 10.1104/pp.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Jones MA, Raymond MJ, Yang Z, Smirnoff N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot. 2007;58:1261–1270. doi: 10.1093/jxb/erl279. This interesting work provides evidence that activity of ROP is involved in root hair growth by regulating superoxide production at the tip. [DOI] [PubMed] [Google Scholar]
- 47.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frantz C, Karydis A, Nalbant P, Hahn KM, Barber DL. Positive feedback between Cdc42 activity and H+ efflux by the Na-H exchanger NHE1 for polarity of migrating cells. J Cell Biol. 2007;179:403–410. doi: 10.1083/jcb.200704169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci U S A. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee Y, Bak G, Choi Y, Chuang WI, Cho HT, Lee Y. Roles of phosphatidylinositol 3-kinase in root hair growth. Plant Physiol. 2008;147:624–635. doi: 10.1104/pp.108.117341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 53.Leshem Y, Seri L, Levine A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- 54.Choi Y, Lee Y, Jeon BW, Staiger CJ, Lee Y. Phosphatidylinositol 3- and 4-phosphate modulate actin filament reorganization in guard cells of day flower. Plant Cell Environ. 2008;31:366–377. doi: 10.1111/j.1365-3040.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Sun HQ, Macia E, Kirchhausen T, Watson H, Bonifacino JS, Yin HL. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol Biol Cell. 2007;18:2646–2655. doi: 10.1091/mbc.E06-10-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sciorra VA, Audhya A, Parsons AB, Segev N, Boone C, Emr SD. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol Biol Cell. 2005;16:776–793. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Thole JM, Vermeer JE, Zhang Y, Gadella TW, Jr, Nielsen E. Root hair defective4 encodes a phosphatidylinositol-4-phosphate phosphatase required for proper root hair development in Arabidopsis thaliana. Plant Cell. 2008;20:381–395. doi: 10.1105/tpc.107.054304. This paper presents data suggesting that both the level of PI4P and its distribution in the cell are important for polarized secretion of vesicles, which is important for root hair morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteiro D, Castanho Coelho P, Rodrigues C, Camacho L, Quader H, Malho R. Modulation of endocytosis in pollen tube growth by phosphoinositides and phospholipids. Protoplasma. 2005;226:31–38. doi: 10.1007/s00709-005-0102-x. [DOI] [PubMed] [Google Scholar]
- 59.Kusano H, Testerink C, Vermeer JE, Tsuge T, Shimada H, Oka A, Munnik T, Aoyama T. The Arabidopsis Phosphatidylinositol Phosphate 5-Kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell. 2008;20:367–380. doi: 10.1105/tpc.107.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stenzel I, Ischebeck T, Konig S, Holubowska A, Sporysz M, Hause B, Heilmann I. The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 2008;20:124–141. doi: 10.1105/tpc.107.052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dowd PE, Coursol S, Skirpan AL, Kao TH, Gilroy S. Petunia phospholipase c1 is involved in pollen tube growth. Plant Cell. 2006;18:1438–1453. doi: 10.1105/tpc.106.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- 63.Preuss ML, Schmitz AJ, Thole JM, Bonner HK, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helling D, Possart A, Cottier S, Klahre U, Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell. 2006;18:3519–3534. doi: 10.1105/tpc.106.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golub T, Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol. 2005;169:151–165. doi: 10.1083/jcb.200407058. [DOI] [PMC free article] [PubMed] [Google Scholar]