Abstract
Objective
To examine theory-based selected factors associated with adherence to mammography screening guidelines in a surveillance database.
Methods
Data from Colorado Mammography Project (CMAP) from 1994–1998 was extracted and analyzed by using SAS statistical software. Based on the Health Belief Model and Behavioral Model of Health Services Utilization a prediction model was developed to examine the mammography utilization patterns and factors influencing the adherence to screening guidelines.
Results
Out of 27,778 women, 41.4% were adherent with mammography screening guidelines. According to the model tested in this study, race/ethnicity (Black vs White, OR=0.76, 95% CI=0.64–0.91); educational attainment (high school vs < high school, OR= 1.10, 95% CI= 1.04–1.18), college graduate vs < high school (OR=1.33, 95% CI=1.25–1.42); insurance status, (any coverage vs no coverage (OR=1.62, 95% CI=1.25–2.12); and community economic status as defined by median income by zip code of residence ($15,000–$24,999 vs <$15,000, OR=0.84, 95% CI=0.76–0.94; >$55,000 vs <$15,000, OR 1.14, 95% CI=1.03–1.26) were statistically significant predictors of adherence to guidelines. Interaction between age and family history of breast cancer was statically significant. Younger females with a family history of breast cancer were less likely to be adherent than their counterparts without a family history (OR=0.93, 95% CI=0.90–0.96). Inclusion or exclusion of women aged 70 years and older did not change the outcome of the analysis.
Conclusion
The prediction model variables such as race/ethnicity, age and family history of breast cancer, educational level and community economic status, are associated with adherence status. Family history of breast cancer needs to be examined very carefully in future studies as it may play negative role in adherence to screening mammography.
Keywords: Adherence, Breast Cancer, Mammography, Screening, Health Belief Model, Andersen’s Model
Introduction
In spite of increasing prevalence of screening mammography, relatively little success has achieved in increasing adherence to recommended guidelines for routine screening beyond initial mammograms. And, although numerous studies have examined factors associated with mammography utilization, [1–5] women ever had mammography, [6–7] and factors associated with women’s adherence to mammography screening guidelines at individual level, [8] less attention, has been directed to mammography surveillance and understanding the factors that influence routine screening mammography utilization in community settings.
Breast cancer is the second leading cause of cancer deaths among women in the United States. The American Cancer Society estimates that 211,240 new invasive cases and 40,410 deaths from breast cancer will occur among women in the United States in 2005. [9] Due to limitations in sensitivity and specificity of clinical breast examination and self- breast examination, mammography remains the most widely recommended screening method for early detection of breast cancer. [9] Mammography allows detection of non-palpable non-invasive and early invasive tumors, Ductal Carcinoma in Situ (DCIS)-an early stage of breast cancer with excellent prognosis [10–11] and mammography is the only examination capable of depicting microcalcifications within breast and, most importantly, those associated with breast cancer.[12] According to the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS), breast calcifications are classified as typically benign, intermediate concern and higher probability of malignancy. [13] Repeated mammograms will provide the full benefit promised from screening, by depicting microcalcifications, by increasing probability of detecting breast masses that are undetectable at one point in time that will grow over time and by differentiating nature of the microcalcifications. Thus, adherence to recommendations for routine mammography has paramount importance to early detection of breast cancer. In this context, understanding factors associated with adherence to mammography screening guidelines is an important element toward improvement of preventive measures for the reduction of mortality and morbidity due to breast cancer.
In this paper, we present the results from a secondary analysis of surveillance data on mammograms conducted in a community setting. A model for predicting adherence to screening mammography guidelines is proposed and evaluated.
Theoretical Underpinnings
For over five decades, Health Belief Model (HBM) has been one of the most widely used conceptual frameworks in health behavior. [14] In predicting preventive behaviors, such as obtaining mammograms, this model has been used at individual level. On the other hand, Behavioral Model of Health Services Utilization (also referred as ‘Andersen’s Model’) [15] has been used in predicting health services utilization behaviors in population-based studies. In the study presented here, we conceptualized a model that incorporates similar constructs or comparable constructs of these two models for predicting screening mammography adherence in a community level surveillance project.
The Health Belief Model [16–17] suggests that the likelihood of performing a behavior is influenced by the combination of perception regarding susceptibility to a particular disease, severity of the disease, barriers and benefits of the behavior and self-efficacy to perform the behavior. This theory has been widely tested in preventive health behaviors including screening.[14] Health Belief Model variables have been previously applied to numerous studies of mammography utilization.[18–25] Figure 1 shows the constructs of Health Belief Model and linkages in between the different constructs. The Andersen’s Model [15] predicts that utilization is affected by three categories of variables. First are predisposing factors that describe the propensity of individuals to use services. In previous studies perceived susceptibility, severity, and benefits of Health Belief Model have been categorized as predisposing factors of Andersen’s model, since they reflect beliefs of the individual about cancer and benefits of mammography.[26] According to Andersen’s Model demographic, social structure, health benefits and attitudinal-belief are all considered as predisposing factors.[15] The barrier component of the Health Belief Model may be conceptualized as enabling factors, since these factors or conditions make it possible for an individual to obtain health services. ‘Cues to action’ of HBM would be compared as need/health status factors of Andersen’s Model, since any physical signs or symptoms, reminders or recommendations from a professional (that considered as cues to action) act as a force to take action for change a behavior or to use a health service. Feelings of vulnerability, concern, or susceptibility were found predictive of use of mammography screening in several studies.[27–31] Risk factors recognized to lay people such as age, family history of breast cancer were used as operational definition of susceptibility or ‘Cues to action’ (Body symptoms used as a proxy) in several studies [32–33] and found them related to the compliance with mammography. [34] In the same way Hormone Replacement Therapy was considered in this study since this is a known risk factor for breast cancer.[35–37] Figure 2 shows the constructs of Andersen’s Model.
Figure 1.
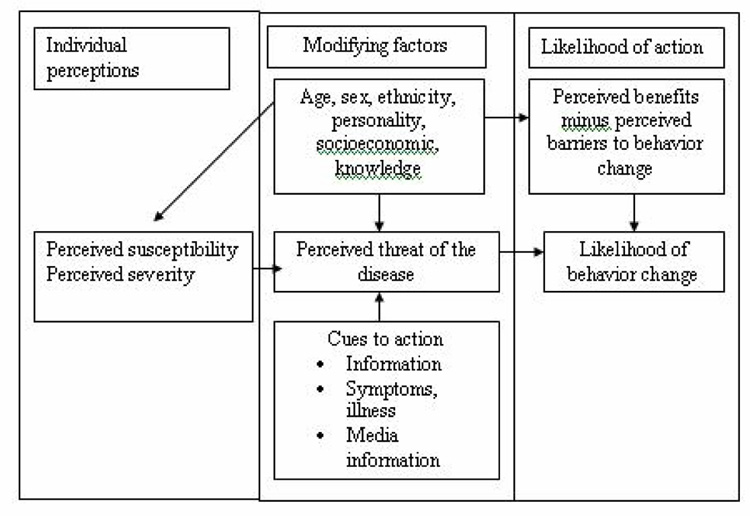
Health Belief Model components and linkages
Figure 2.
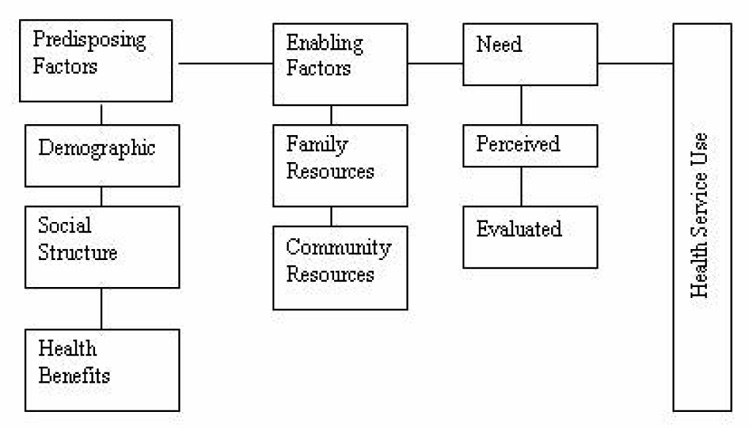
The Behavioral Model of Health Services Use. Adapted from the Behavioral Model of Health Service Use by Andersen, Joana, et al., 1975
Prediction Model
Based on the constructs of Health Belief Model and Behavioral Model of Health Services Utilization, we developed a prediction model with variables that, based on guidance from theory, may have influence on adherence to guidelines of mammography. In Andersen’s model need factors are of two types, perceived needs and evaluated need. Family history of breast cancer and current breast problems is considered as perceived needs since these factors might encourage women to seek mammograms. Hormone replacement therapy and follow-up test recommendation for mammography are considered as evaluated need factors, since these are the needs of women evaluated by health care professionals. Figure 3 shows the different selected factors that have been conceptualized for the Prediction Model and their linkages to obtain an age-appropriate and family history-appropriate screening mammography in this surveillance database.
Figure 3.
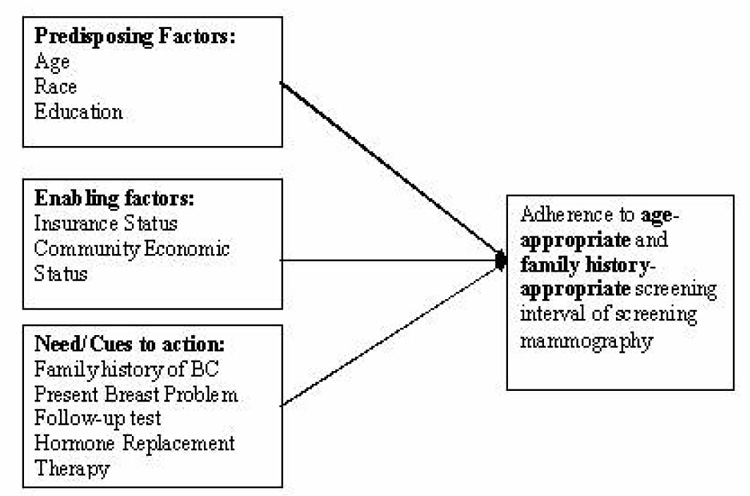
Prediction Model and Variables
Methods
Subjects
The Colorado Mammography Project (CMAP) is a National Cancer Institute (NCI)-funded project that obtains data on mammograms from approximately half of all mammography facilities in the six-county Denver Metropolitan Area of Colorado.[38] This study examined data from 139,445 screening mammograms carried out on women age 40 and older who participated in the Colorado Mammography Project (CMAP) from January 1, 1994 to December 31, 1998.
To incorporate the recommendations by eleven expert organizations including American Cancer Society and National Cancer Institute, the definitions of adherence were considered as follows: For women 50 and over, as well as for women between 40 and 49 with a family history of breast cancer, adherence was considered to be at least two mammograms within a one-year interval. For women between 40 and 49 without a family history of breast cancer, adherence was considered to be at least two mammograms within two years. Although 139,445 women were defined either adherent or nonadherent, only a total of 27,778 women and their multiple mammogram records were included in the final analyses because of complete records and valid values for all the covariates. Data management and all statistical procedures were performed using SAS Software, version 8.1.[39]
Analysis
First, we examined the mammography utilization patterns in the database. A SAS algorithm was developed to create indicators whether a woman had a mammogram in each year of the study (1994–1998). Using this approach, we observed 30 patterns of mammography utilization. These patterns do not include the situation where a woman might have had a mammogram in this project, and another mammogram that was not captured in the database, and re-entered CMAP during the four-year period of this study. To capture this issue and overcome underestimation of actual utilization we created another SAS algorithm where adherence was measured based on women’s age, family history of breast cancer, and date of previous mammogram. For the measurement of adherence, we categorized patterns of mammography utilization as continuous or discontinuous. Using “1” for having a mammogram and “0” for not having a mammogram, women who had utilization patters as, 10, 11, 1110, 1111, 1000 and so on were considered continuous patterns, and 101, 1001, 1010, 1011, 1101, 10001, 11001, 11101 and so on were considered as discontinuous patterns. For the continuous patterns we looked at the previous mammogram date, if the patterns fulfilled the criteria for adherence then the women were coded as adherence=1 (Yes) and if not then adherence=2 (No). For the discontinuous patterns we considered the previous mammography date twice; at the first appearance in the database and last mammography date. Then based on the criteria women were coded as adherent or nonadherent. Women who entered into the surveillance at the age of 40 were considered as adherent.
The dependent variable was dichotomous (where adherence =1 and non-adherence = 2). Independent variables included predisposing (age, race, and education), enabling (Insurance and community economic status) and need factors (Family history of BC, Current breast problem, Follow-up test recommendation, and Hormone Replacement Therapy). A new variable ‘community economic status (CES)’ was created based on the median income of the residences in the zip code of the woman’s residence. Data for CES were obtained from the 1990 US Census, since the study period is 1994–1998. In this model ‘current breast problems’ are those not considered for diagnostic mammography. The Wald Chi-square test was used to assess the main effects and interaction terms. All main effects were kept in the final model and only the significant interaction terms were kept using a two-sided significance level of 5%. Maximum likelihood procedures were used to estimate the model parameters for the logistic model to quantify the odds ratio. A detailed description of the methods is available elsewhere. [40]
Results
A total of 41.3% of the women were adherent to the guidelines of screening mammography as defined in this study. The study population was characterized by participants 40 to 49 (37%), white (91%), had some college or higher education (38%), had some form of health insurance (99%), came from communities having median annual income of $ 25,000 to $34,999 based on zip code of residence (28%), had no family history of breast cancer (80.6%), had no current breast problem (94%), and did not receive a recommended follow-up test (90.6%) (Table 1).
Table 1.
Characteristics of Women (Adherent versus Nonadherent)
| All | Adherent | Nonadherent | ||||
|---|---|---|---|---|---|---|
| (N = 27,778) | (n = 11,486) | (n = 16,292) | ||||
| Factors | N | % | n | % | n | % |
| Predisposing factors | ||||||
| Age | ||||||
| 40–49 | 10,267 | 37.0 | 4,271 | 41.6 | 5,996 | 58.4 |
| 50–59 | 8,031 | 28.9 | 3,309 | 41.2 | 4,722 | 58.8 |
| 60–69 | 4,692 | 16.9 | 2,003 | 42.7 | 2,689 | 57.3 |
| ≥70 | 4,788 | 17.2 | 1,903 | 39.8 | 2,885 | 60.3 |
| Race/Ethnicity | ||||||
| White | 25,274 | 91.0 | 10,543 | 41.7 | 14,731 | 58.3 |
| Black | 587 | 2.1 | 204 | 34.8 | 383 | 65.3 |
| Asian | 342 | 1.2 | 127 | 37.1 | 215 | 62.8 |
| Indian American | 75 | 0.3 | 32 | 42.7 | 43 | 57.3 |
| Hispanic | 1,366 | 4.9 | 520 | 38.1 | 846 | 61.9 |
| Other | 134 | 0.5 | 60 | 44.8 | 74 | 55.2 |
| Education | ||||||
| <High school graduate | 7,670 | 27.6 | 2,899 | 37.8 | 4,771 | 62.2 |
| High school graduate | 9,542 | 34.4 | 3,844 | 40.3 | 5,698 | 59.7 |
| Some college, college, or post graduate | 10,566 | 38.0 | 4,743 | 44.9 | 5,823 | 55.1 |
| Enabling factors | ||||||
| Health insurance | ||||||
| Yes (Medicare, Medicaid or Other) | 27,507 | 99.0 | 11,408 | 41.5 | 16,099 | 58.5 |
| No | 271 | 0.9 | 78 | 28.8 | 193 | 71.2 |
| Community economic status | ||||||
| (Median income per zip code) | ||||||
| < $15,000 | 9,087 | 32.7 | 3,737 | 41.1 | 5,350 | 58.9 |
| $15,000–$24,999 | 1,854 | 6.7 | 676 | 36.5 | 1,178 | 63.5 |
| $25,000–$34,999 | 7,858 | 28.3 | 3,171 | 40.4 | 4,687 | 59.7 |
| $35,000–$44,999 | 3,826 | 13.8 | 1,645 | 43.0 | 2,181 | 57.0 |
| $45,000 –$54,999 | 3,234 | 11.6 | 1,380 | 42.7 | 1,854 | 57.3 |
| ≥$55,000 | 1,919 | 6.9 | 877 | 45.7 | 1,042 | 54.3 |
| Need/Health status factors or cues to action | ||||||
| Family history of breast cancer | ||||||
| Yes | 5,401 | 19.4 | 2004 | 37.1 | 3,397 | 62.9 |
| No | 22,377 | 80.6 | 9,482 | 42.4 | 12,895 | 57.6 |
| Current breast problem | ||||||
| Yes | 1,661 | 6.0 | 693 | 41.7 | 968 | 58.3 |
| No | 26,117 | 94.0 | 10,793 | 41.3 | 15,324 | 58.7 |
| Follow-up test recommended | ||||||
| Yes | 2,621 | 9.4 | 1,082 | 41.3 | 1,539 | 58.7 |
| No | 25,157 | 90.6 | 10,404 | 41.4 | 14,753 | 58.6 |
| Hormone use* | ||||||
| Yes | 10,013 | 49.1 | 4,563 | 45.6 | 5,450 | 54.4 |
| No | 10,376 | 50.9 | 4,257 | 41.0 | 6,119 | 59.0 |
Note. Total number does not add to 27,778 because of missing values.
In the process of examining the prediction model both univariate and multivariate analyses were conducted. Table 2 shows both univariate and adjusted odds ratios for the factors included in the final model. Black women had 24% lower odds of adherence to guidelines than did white women. High school graduates had greater odds of adherence to guidelines than did those who had less than high school education (OR = 1.11, 95% CI = 1.04–1.18). However, more education, i.e., some college, college, and graduate degree attainment also had a positive influence on adherence to mammography guidelines (OR = 1.33, 95% CI = 1.25–1.42). Insurance status also was a significant predictor of adherence (OR = 1.63, 95% CI = 1.25–2.13)
Table 2.
Statistically Aignificant Univarate and Adjusted Odds Ratio for the Factors Influencing Screening Mammography Adherence
| Factors | Univarate OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Age** | ||||
| 40–49 | 1.00 | --- | ||
| 50–59 | 0.98 | 0.93–1.04 | --- | --- |
| 60–69 | 1.05 | 0.98–1.12 | --- | --- |
| 70+ | 0.93 | 0.86–0.99* | --- | --- |
| Race/Ethnicity | ||||
| White | 1.00 | 1.00 | ||
| Black | 0.74 | 0.63–0.88* | 0.76 | 0.64–0.90* |
| Hispanic | 0.86 | 0.77–0.96* | 0.95 | 0.84–1.06 |
| Education | ||||
| <High school graduate | 1.00 | 1.00 | ||
| High school graduate | 1.11 | 1.04–1.18* | 1.11 | 1.04–1.18* |
| Some college, college or postgraduate | 1.34 | 1.26–1.42* | 1.33 | 1.25–1.42* |
| Health Insurance | ||||
| Yes (Medicare, Medicaid or Other) | 1.75 | 1.35–2.28* | 1.62 | 1.25–21.3* |
| No | 1.00 | 1.00 | ||
| Community economic status (Median income per zip code) | ||||
| < $15,000 | 1.00 | 1.00 | ||
| $15,000–$24,999 | 0.82 | 0.74–0.91* | 0.84 | 0.76–0.94* |
| $35,000–$44,999 | 1.08 | 1.00–1.17* | 1.08 | 1.00–1.16* |
| $≥ $55,000 | 1.21 | 1.09–1.33* | 1.14 | 1.03–1.26* |
| Family history of breast cancer** | ||||
| Yes | 0.80 | 0.76–0.85* | --- | --- |
| No | 1.00 | --- | ||
| Age by family history interaction** |
Note OR = Odds ratio, CI = Confidence interval
statistically significant
There was an age/family history interaction in the adjusted model and therefore the results are presented in table 3.
In the final model, community economic status, as estimated by median annual income within zip code [32–33] of residence was also found to be a significant predictor of adherence to mammography guidelines. Those with an annual median income between $15,000 and $24,999 were less likely to be adherent with screening guidelines than women who had an annual median income of less than $15,000 (OR = 0.84, 95% CI = 0.76–0.94). Those with median incomes between $35,000 and $44,999 were more likely to be adherent than did women who had median income of less than $15,000, but this association was at borderline significance (OR = 1.08, 95% CI = 1.00–1.16)
Women with an annual median income greater than $55,000 were more likely to be adherent to guidelines than were women who had median incomes less than $15,000 (OR = 1.14, 95% CI = 1.03–1.26). Follow-up test recommendation and current breast problems were not significantly associated with adherence. There was statistically significant interaction between age and family history. Table 3 summarize the relationship where younger women with a family history of BC were less likely to adhere than their counterparts without a family history (OR=0.93, 95% CI=0.90–0.96) when effects of age were fixed. In general, elderly women with family history of BC were more likely to be adherent with mammography screening guidelines than younger women with family history (OR=1.21, 95% CI=1.11–1.33).
Table 3.
Interaction of Age and Family History on Probability of Adherence to Mammography Screening
| Age | Effect of Family History for a fixed Age Category | Effect of Age for a Fixed Family History | ||||
|---|---|---|---|---|---|---|
| Family History | Family History | |||||
| (Yes\No) | Yes | No | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| 40–49 | 0.93 | (0.90, 0.96) | 1.00 | 1.00 | ||
| 50–59 | 1.02 | (0.92, 1.14) | 1.08 | (0.99, 1.17) | 0.98 | (0.92, 1.03) |
| 60–69 | 0.96 | (0.85, 1.08) | 1.20 | (1.10, 1.32) | 1.16 | (1.09, 1.23) |
| 70+ | 1.06 | (0.94, 1.19) | 1.21 | (1.11, 1.33) | 1.06 | (1.00, 1.13) |
In summary, the results partially support the ‘prediction model’ based on the two behavioral theories that we considered. Predisposing factor Race and Educational level were significant predictor of adherence. Black women had 24% less odds of adherence to guidelines than did white women. High school graduates had greater odds of adherence to guidelines than did those who had less than high school education (OR = 1.11, 95% CI = 1.04–1.18). However, some college, college, and graduate degree attainment also had a positive influence on adherence to mammography guidelines (OR = 1.33, 95% CI = 1.25–1.42)
Discussion
Age, ethnicity, insurance status, educational attainments, community economic status, family history of breast cancer were found to be significantly associated with adherence. The model used age as a predisposing factor, which interacted with one of the need factor- family history. This interaction could be explained by the fact that as age increased, probability of positive family history also increased. The findings of this study are not consistent with previous studies in many areas. For example, in several studies, older women were less likely to be adherent to screening guidelines than were younger women. [41–44] But those studies did not report an interaction between age and family history. In this study age and family history were not independent. But for a fixed family history of breast cancer shows a general trend that elderly women were more likely to be adherent with the screening guidelines than younger women. Previous studies have also shown that African American women used less screening mammography [45–48] and less likely to be adherent to guidelines. [49–50] Minority populations such as Hispanics, Asians, and American Indians are also less likely to have repeat mammograms.[42, 51] In this study black women were the least likely to be adherent with mammography screening guidelines. Both in univariate and adjusted analyses, black women had 24% less odds of adherence to mammography screening guidelines than white women. [52–53]
Educational attainment is consistently associated with healthy behavior. In this study women’s educational attainment was consistently a strong predictor of adherence to screening guidelines.
Like many other previous studies, insurance status was a predictor of mammography utilization. [52–53] Overall presence of any form of insurance had a positive influence on screening mammography behavior. However, in the study database, 99% women were insured and only 0.9 % was uninsured, which indicates presence of selection bias.
Though several factors were not significantly associated with adherence behavior, such as current breast problems, follow-up recommendation and Hormone Replacement therapy as needs factors, the results of this study supported the prediction model that we hypothesized. Previously body symptoms or information such as positive family history were considered as ‘cue to action’ in health behavior. However, several study findings support an alternative explanation that family history of breast cancer might increase fear of getting breast cancer and it is this psychological factor that inhibits women from having mammogram. [53–54] In many studies positive results and anxiety about procedures were negative influencing factors. 53 In this study, younger women with a positive family history of breast cancer were less likely to be adherent to the screening guidelines. This issue needs to be addressed carefully in future behavioral intervention as fear may play significant role.
Community economic status, as defined by median annual income per zip code of residence was consistently associated with adherence to screening guidelines. The pattern of association is noteworthy. Women with median annual incomes of $15,000 to $24,999 were 16% less likely to be adherent with guidelines than were women with median annual incomes less than $15,000. On the other hand, women with median annual income of $35,000 to $44,999 and those with incomes more than $55,000 were 1.08 times and 1.14 times, respectively, as likely to be adherent to screening mammography.
This study had several limitations. One is that our coding of any given women as adherent was based on a relatively short period of time for some but on a longer period in others. Future studies should examine whether the coding of adherence by differential time period leads to differences among explanatory variables in the model. Another limitation is the missing values in covariates used to determine adherence and in fitting the logistic regression model. Because of incomplete data the study sample size of 27,778 women may not be a representative of 139,445 women that we had hoped to include in our analysis. This may lead to a bias that has some inherent limitations, but we can still conclude from this sample size of almost 28,000 women about their adherence to screening mammography guidelines.
Like many other studies, ours did not capture the magnitude of the problem of how many women knew about the recommended guidelines for screening mammography. Future studies will need to assess the status of women’s knowledge about mammography guidelines. Since benefits of screening in older population is controversial [55–56], we analyzed data both including age greater than 70 years and excluding age greater than 70 years but there was no change of results of this study.
Understanding prevention-related behavior requires attention to individual and population factors. The model is tested in a surveillance database and validity of the model should be considered in this particular study context. Future study should focus on direct measure of behavioral perceptions and practices related to screening mammography behavior. Also path analysis would be necessary to understand more precisely how the factors are related with each other in this model.
References
- 1.Glanz K, Resch N, Blake A, Gorchov P, Rimer B. Factors associated with adherence to breast cancer screening among working women. Journal of occupational Medicine. 1992;34(11):1071–1078. doi: 10.1097/00043764-199211000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Bastani R, Kaplan C, Maxwell A, Nisenbaum R, Pearce J, Marcus A. Initial and repeat Mammography Screening in a Low Income Multi-Ethnic Population in Los Angeles. Cancer Epidemiology, Biomarkers and Prevention. 1995;4:161–167. [PubMed] [Google Scholar]
- 3.Laws MB, Mayo SJ. The Latina breast cancer control study, year one: factors predicting screening mammography utilization by urban Latina women in Massachusetts. Journal of Community Health. 1998;23(4):251–267. doi: 10.1023/a:1018776704683. [DOI] [PubMed] [Google Scholar]
- 4.Legler J, Breen N, Meissner H, Malec D, Coyne C. Predicting Patterns of mammography use: a geographic perspective on national needs for intervention research. Health Services Research. 2002;37(4):929–947. doi: 10.1034/j.1600-0560.2002.59.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison RV, Janz NK, Wolf RA, Tedeschi PJ, Huang X, McMahon LF. 5-year mammography rates and associated factors for older women. Cancer. 2003;97(5):1147–1155. doi: 10.1002/cncr.11172. [DOI] [PubMed] [Google Scholar]
- 6.Vernon S. Participation in breast screening programs: a review. Social Science and Medicine. 1990;30(10):1107–1118. doi: 10.1016/0277-9536(90)90297-6. [DOI] [PubMed] [Google Scholar]
- 7.Brown M, Fintor L. Accreditation of mammography facilities by the American College of Radiology: Results of a national survey. American Journal of Preventive Medicine. 1994;10(3):162–167. [PubMed] [Google Scholar]
- 8.Phillips K, Kerlikowske K, Chang S, Baker L, Brown M. Factors associated with women’s adherence to mammography screening guidelines. Health Services Research. 1998;33:29–53. [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. American Cancer Society Facts and Figures. 2005 [Google Scholar]
- 10.Markopoulos C, Kakisis J, Kouskos S, Kontzoglou K, Koufopoulos K, Gogas J. Management of nonpalpable, mammographically detectable breast lesions. World J Surg. 1999 May;23(5):434–438. doi: 10.1007/pl00012323. [DOI] [PubMed] [Google Scholar]
- 11.Parker J, Dance DR, Davies DH, Yeoman LJ, Michell MJ, Humphrey S. Classification of ductal carcinoma in situ by image analysis of calcifications from digital mammograms. Br J Radiol. 1995 Feb;68(806):150–159. doi: 10.1259/0007-1285-68-806-150. [DOI] [PubMed] [Google Scholar]
- 12.Lev-Toaff AS, Feig SA, Saitas VL, Finkel GC, Schwartz GF. Stability of malignant breast microcalcifications. Radiology. 1994 July;192(1):153–156. doi: 10.1148/radiology.192.1.8208928. [DOI] [PubMed] [Google Scholar]
- 13.American College of Radiology, Breast Imaging Reporting and Data System (BI-RADS) Terms and Conditions. 3rd edition 1998. [Google Scholar]
- 14.Janz NK, Becker MH. The Health Belief Model: A decade later. Health Education Quarterly. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 15.Andersen R, Joana K, Anderson O. Equity in health services: empirical analyses in social policy. Cambridge: Ballinger Publishing Co.; 1975. [Google Scholar]
- 16.Hochbaum G. Public Participation in Medical Screening Programs: A sociopsychological study. Washington, DC: Government Printing Office; 1958. [Google Scholar]
- 17.Rosenstock I. Historical origin of Health Belief Model. Health Education Monographs. 1974;2:328–335. [Google Scholar]
- 18.Burack RC, Liang J. The early detection of cancer in the primary care settings: factors associated with the acceptance and completion of recommended procedures. Prev. Med. 1987;16:739–751. doi: 10.1016/0091-7435(87)90014-4. [DOI] [PubMed] [Google Scholar]
- 19.Burack RC, Liang J. The acceptance and completion of mammography by older black women. American Journal of Public health. 1989;79:721–726. doi: 10.2105/ajph.79.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calnan M. The Health Belief Model and participation in programmes for the early detection of breast cancer: A comparative analysis. Soc. Sci. Med. 1984;19:823–830. doi: 10.1016/0277-9536(84)90399-x. [DOI] [PubMed] [Google Scholar]
- 21.Fink R, Shapiro S, Roester R. Impact of Efforts to Increase Participation in Repetitive Screening for Early Breast Cancer Detection. American Journal of Public Health. 1972 March;62:328–336. doi: 10.2105/ajph.62.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane DS, Fine HL. Compliance with mammography referrals. N.Y. State J. M. 1983;83:173–176. [PubMed] [Google Scholar]
- 23.Nattinger AB, Panzer RJ, Janus J. Improving the utilization of screening mammography in primary care practices. Arch. Intern. Med. 1989;149:2087–2092. [PubMed] [Google Scholar]
- 24.Taplin SH, Anderman C, Orothaus L. Breast cancer risk and participation in mammographic screening. American Journal of Public Health. 1989;79:1494–1498. doi: 10.2105/ajph.79.11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayman RB, Baker S, Ephraim R, Moadel A, Phillip J. Health belief model variables as predictors of screening mammography utilization. Journal of Behavioral Medicine. 1994;17:391–406. doi: 10.1007/BF01858010. [DOI] [PubMed] [Google Scholar]
- 26.Aiken LS, Fenaughty AM, West SG, Johnson JJ, Luckett TL. Perceived determinants of risk for breast cancer and relations among objective risk, perceived risk and screening behavior over time. Womens Health Res Gender Behav Policy. 1995;Vol. 1:27–50. [PubMed] [Google Scholar]
- 27.Crane L, A, Kaplan C, P, Bastani R, Scrimshaw S, C, M Determinants of adherence among health department patients referred for a mammogram. Women and Health. 1996;Vol. 24(2):43–64. doi: 10.1300/J013v24n02_03. [DOI] [PubMed] [Google Scholar]
- 28.Andersen R. Revisiting the Behavioral Model Care: Does it Matter? Journal of Health and Social Behavior. 1995;36:1–10. [PubMed] [Google Scholar]
- 29.Hayman RB, Baker S, Ephraim R, Moadel A, Phillip J. Health belief model variables as predictors of screening mammography utilization. Journal of Behavioral Medicine. 1994;17:391–406. doi: 10.1007/BF01858010. [DOI] [PubMed] [Google Scholar]
- 30.Zapka JG, Stoddard AM, Costanza ME, Greene HL. Breast cancer screening by mammography: utilization and associated factors. American Journal of Public Health. 1989;79:1499–1502. doi: 10.2105/ajph.79.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calnan M. The Health Belief Model and participation in programmes for the early detection of breast cancer: A comparative analysis. Society of Science and Medicine. 1984;19:823–883. doi: 10.1016/0277-9536(84)90399-x. [DOI] [PubMed] [Google Scholar]
- 32.Lane D, Fine H. Compliance with mammography referrals. New York State Journal of Medicine. 1983;83:173–176. [PubMed] [Google Scholar]
- 33.Fink R, Shapiro S, Lewison J. The reluctant participant in a breast cancer screening program. Public Health Rep. 1968;83:479–490. [PMC free article] [PubMed] [Google Scholar]
- 34.Taplin S, Anderman C, Orothaus L. Breast cancer risk and participation in mammographic screening. American Journal of Public Health. 1989;79:1494–1498. doi: 10.2105/ajph.79.11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zapka J, Stoddard A, Costanza M, Greene H. Breast cancer screening by mammography: utilization and associated factors. American Journal of Public Health. 1989;79:1499–1502. doi: 10.2105/ajph.79.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schairer C, Lubin J, Troisi R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 37.Colditz G, Hankinson S, Hunter D. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. New England Journal of Medicine. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 38.Jacobellis J, Cutter GR. Mammography screening and differences in Stage of disease by Race/Ethnicity. American Journal of Public Health. Vol. 92(7):1144–1150. doi: 10.2105/ajph.92.7.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SAS software release 8.1. Cary, NC: SAS Institute Inc.; 1999–2000. p. 27513. [Google Scholar]
- 40.Rahman SMM, Dignan MB, Shelton BJ. Factors influencing adherence to guidelines for screening mammography among women aged 40 years and older. Ethnicity and Disease. 2003;Vol. 13(No 4):477–484. [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes C, Lerman C, Lustbader E. Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Research Treatment. 1996;40:25–35. doi: 10.1007/BF01806000. [DOI] [PubMed] [Google Scholar]
- 42.Miller A, Champion V. Attitudes about breast cancer and mammography: racial, income, and educational differences. Women and Health. 1997;26:41–63. doi: 10.1300/J013v26n01_04. [DOI] [PubMed] [Google Scholar]
- 43.Makuc D, Breen N, Fried V. Low income, race and use of mammography. Health Services Research. 1999;34:229–239. [PMC free article] [PubMed] [Google Scholar]
- 44.Breen N, Feuer E, Depuy S, Zapka J. The effect of Medicare reimbursement for screening mammography on utilization and payment. Public Health Report. 1997;112:423–432. [PMC free article] [PubMed] [Google Scholar]
- 45.Kirman-Liff B, Kronenfeld J. Access to cancer screening services for women. American Journal of Public Health. 1992;82:733–735. doi: 10.2105/ajph.82.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zapka J, Hosmer A. Changes in Mammography Use: Economic, Need, and Service Factors. American Journal of Public Health. 1992;82:1345–1345. doi: 10.2105/ajph.82.10.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harper A. Mammography utilization in the poor and medically underserved. Cancer Supplement. 1993;72:1478–1483. doi: 10.1002/1097-0142(19930815)72:4+<1478::aid-cncr2820721411>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 48.Michielutte R, Dignan M, Smith B. Psychosocial factors associated with the use of breast cancer screening by women age 60 years or over. Health Education and Behavior. 1999;26:625–647. doi: 10.1177/109019819902600505. [DOI] [PubMed] [Google Scholar]
- 49.Clemow L, Costanza ME, Haddad W, P, Luckman R, White M, J, Klaus D, Stoddard A, M Underutilizers of mammography screening today: Characteristics of women planning, undecided about, and not planning a mammogram. Annals of Behavioral Medicine. 2000;Vol. 22(1):80–88. doi: 10.1007/BF02895171. [DOI] [PubMed] [Google Scholar]
- 50.Miller AM, Champion VL. Attitudes about breast cancer and mammography: racial, income, and educational differences. Women and Health. 1997;26(1):41–63. doi: 10.1300/J013v26n01_04. [DOI] [PubMed] [Google Scholar]
- 51.Hardy R, Ahmed N, Hargreaves M. Difficulty in reaching low-income women for screening mammography. Journal of Health Care for the Poor and Underserved. 2000;11:45–57. doi: 10.1353/hpu.2010.0614. [DOI] [PubMed] [Google Scholar]
- 52.Harper A. Mammography utilization in the poor and medically underserved. Cancer Supplement. 1993;72:1478–1483. doi: 10.1002/1097-0142(19930815)72:4+<1478::aid-cncr2820721411>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 53.Clemow L, Costanza M, Haddad W, Luckmann R, White M, Klaus D, Stoddard A. Underutilizers of mammography screening today: characteristics of women planning, undecided about, and not planning a mammogram. Annals of Behavioral Medicine. 2000;22:80–88. doi: 10.1007/BF02895171. [DOI] [PubMed] [Google Scholar]
- 54.Rahman SMM, Mohamed I, Dignan MB. Assessment of Perceptions Related to Breast Cancer Prevention and Behavioral Practices in Medically Underserved Women. The Journal of Multicultural Nursing and Health. Vol. 9(No 3):30–39. [Google Scholar]
- 55.Mandelblatt J, Wheat M, Monane M, Moshief R, Hollengerg J, Tang J. Breast cancer screening for elderly women with or without comorbid conditions. Annals of Internal Medicine. 1989;116:722–730. doi: 10.7326/0003-4819-116-9-722. [DOI] [PubMed] [Google Scholar]
- 56.Kerlikowske K, Salzman P, Phillips K, Cauley J, Cummings S. Continuing screening mammography in women age 70 to 79 years: Impact on life expectancy and cost-effectiveness. JAMA. 1999;282:2156–2163. doi: 10.1001/jama.282.22.2156. [DOI] [PubMed] [Google Scholar]


