Abstract
Background
Radiographic joint changes are used to diagnose osteoarthritis; however, they alone do not adequately predict who experiences symptoms.
Purpose
To examine psychological risk and resilience factors in combination with an objective indicator of disease severity (knee X-rays) to determine what factors best account for pain and physical functioning in an early knee osteoarthritis (KOA) population.
Methods
Structural equation modeling was used to analyze data from 275 men and women with early KOA.
Results
Structural equation modeling yielded a fair to good fit of the data, suggesting that both risk and resilience were important in predicting pain and physical functioning over and above disease severity in the expected directions. Resilience’s effect on pain was mediated through self-efficacy, suggesting that higher self-efficacy was linked to lower pain and better physical functioning.
Conclusions
Results provide an integrative model of adjustment to early KOA and may be important to the prevention of disability in this population.
Keywords: Osteoarthritis, Pain, Physical functioning, Self-efficacy, Resilience
Introduction
Although radiographic joint changes are used to diagnose knee osteoarthritis (KOA) and can contribute to pain and functional ability [1, 2], they do not appear to predict who experiences symptoms (e.g., pain, distress, physical disability) [3, 4]. In fact, population studies have shown that only about half of adults who demonstrate radiographic joint damage experience osteoarthritis (OA) symptoms [5, 6]. Clinical signals, such as joint damage or clinician ratings, usually only account for 25% of the variance in disability associated with these conditions [7].
Some studies have even found that psychological factors predict disability over and above disease activity, objective disease markers, and articular and kinesiological factors [4, 8]. Nevertheless, psychological factors have rarely been studied in the prediction of progression of pain and disability for KOA patients, directly or in comparison to the impact of disease severity. This study is designed to examine how psychological factors impact pain and disability in a KOA sample.
Risk and resilience factors have been examined as independent and separable constructs with differential relationships to adaptation to chronic pain [9-11]. Risk and resilience can be viewed as two sets of predisposing individual characteristics that can exist within a person prior to the experience of pain, yet they may impact cognitive–behavioral responses to pain and ultimately physical and psychological outcomes. Turk [12] describes a diathesis-stress model for the progression of pain and disability following a traumatic injury that provides a framework for how psychological risk may impact chronic pain adaptation. In this model, he proposes that individual differences in traits, such as anxiety, act as diatheses that predispose one to the progression of pain and disability. Individual resilience factors may operate in parallel to risk factors as a potential protection against pain chronicity and disability.
Previous literature has found that negative affective states, depression, and neuroticism are highly associated with pain and disability among OA patients [13, 14]. These variables likely go together to form a broader composite of psychological risk that is important in the prediction of pain and related disability [15, 16]. In a longitudinal community study, those who reported knee pain without radiographic joint changes had higher levels of anxiety and depression, indicating that, even without physiological evidence of tissue damage, pain can exist and is often correlated with psychological distress [17]. In prospective studies, depression has been shown to predict pain and disability over and above disease severity for chronic pain populations [4, 18]. Comorbid depression has also been shown to worsen the effects of medical illnesses and depressive effects were found to be comparable to those of arthritis, diabetes, and hypertension for health-related quality of life [19]. Neuroticism was related to patients’ ratings of pain unpleasantness, suffering from pain, and illness behavior and predicted higher levels of joint pain 23 years later in a prospective study [20].
On the other hand, positive affect and extraversion have been found to relate to improvements in pain and disability over time in arthritis populations [21, 22]. These variables together with vitality, which has been described as a positive and restorative state [23, 24], can be considered a composite of resilience in adjustment to chronic pain. Positive affect (PA) has been found to be related to reduced pain, increased functional ability, and improved perceptions of health [22]. Further, the benefits of PA have been observed over time [21].
More work is needed to evaluate the relative contributions of risk and resilience factors; however, in some studies with OA patients, PA has influenced pain above and beyond negative affect (NA) [11, 25]. Not only do risk and resilience indicators relate to the outcomes of pain and physical functioning in OA patients, but they have also been linked to cognitive-behavioral factors that mediate risk and resilience on pain and physical functioning. Risk factors, such as NA, depression, and neuroticism, have been shown to lead to reduced self-efficacy and physical activity [26-28]. Resilience factors, such as PA, vitality, and extraversion, have been shown to predict increased self-efficacy, physical activity, and more active coping [2, 29-31].
Two theories can help us understand the potential impact of psychological risk and resilience on pain and physical functioning through self-efficacy and physical activity. According to Lazarus and Folkman’s model of stress and coping [32], both the primary appraisal of the threat of a stressor, and the secondary appraisal of one’s own resources to cope with a stressor, impact how well one adapts to a chronic illness. Risk factors, such as the ones described herein, impact both primary and secondary appraisal [26-28, 33]. Another perspective that can provide structure to the mechanisms by which risk and resilience relate to pain chronicity and disability is the fear-avoidance model [34]. The basic premise of this model is that negative emotions, such as pain-related fear or anxiety, are usually associated with hypervigilance to pain and fear of movement or reinjury. For example, arthritis patients may be fearful of moving in a way that has caused them pain in the past or catastrophize about the potential consequences of such movements. These responses result in behavioral avoidance of movement and activity, which leads to muscle atrophy from disuse, a lower tolerance for pain, and a likely increase in both physical and social disability.
This study examines psychological risk and resilience factors that have been shown to be predictive of pain and disability in chronic pain patients to help determine the relative contribution of risk and resilience to adjustment to early KOA. In addition, this study probes possible mechanisms commonly targeted in interventions through which factors of risk, resilience, and disease severity may have their effect. The following hypotheses are tested: (1) The latent factors that are composed of variables indicating risk and resilience will be separable constructs; (2) risk will be related to lower self-efficacy and physical activity, while resilience will be related to higher self-efficacy and physical activity; (3) the mediating variables, self-efficacy and physical activity, will be related to higher physical functioning and inversely related to pain; and (4) the relations between risk (controlling for resilience and disease severity) and resilience (controlling for risk and disease severity) on pain and physical functioning will be mediated by self-efficacy and physical activity.
Method
Participants
This study utilized initial data from 275 participants from the KNEE study, a large randomized, controlled, longitudinal (24-month) intervention study aimed at reducing levels of pain and disability in an early KOA population. Eligibility criteria included: (a) age between 35 and 64 years old; (b) pain on most days (i.e., four or more days in a week) in one or both knees for at least 4 months during the previous year; (c) duration of symptoms of less than 5 years; (d) radiographic evidence of knee OA of grade II in at least one knee [35]; and (e) a rating of disability of 3 or higher on a 0-to-10 scale for at least three of the following on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC; [36]) items: descending or ascending stairs, walking, bending, and performing daily activities. Participants were excluded (1) if they had an uncontrolled medical condition that would preclude participation in an exercise program (i.e., uncontrolled heart disease, blood pressure or respiratory conditions, or a neurological condition that affected coordination); (2) if they had inflammatory arthritis, such as rheumatoid or psoriatric arthritis; (3) if they exercise regularly (i.e., aerobic activity or resistance training of more than 120 min per week); (4) if they had previous knee surgery; (5) if they had radiographic grade III–IV based on the Kellgren and Lawrence classification in either knee; (6) if they had BMI≥37.5 kg/m2; (7) if they had a knee corticosteroid injection in the last 3 months; or (8) if they had plans to move from the local area or planned to become pregnant during the study period.
Participants were recruited from the Tucson metropolitan area and surrounding communities through mass mailings, media advertisements, and physician referral. Of the 294 eligible participants who were randomized, 6% dropped out due to problems with noncompliance, the time commitment of the intervention, losing interest in the project, and leaving the local area. Participants were included in the analyses if they completed at least one measure on the prebaseline or baseline assessments. The final sample was comprised of 26% men (n=71) and 74% women (n=204).
Procedure
Participants were screened by telephone to determine eligibility and, if eligible, were invited to an orientation session where the scope and commitment of the program was described to them. Those wishing to enroll provided informed consent and study personnel reviewed their medical records and verified inclusion and exclusion criteria. Participants then underwent a prebaseline assessment and were scheduled for a baseline assessment within 4 weeks.
Prebaseline Assessment
As part of this assessment, semiflex X-rays were taken on both knees and evaluated using the Kellgren and Lawrence Atlas of Standard Radiographs of Arthritis [35]. Diagnoses were made based on criteria from the American College of Rheumatology and were determined by review of the participants’ medical records and semiflex X-rays of both knees by two of the study’s physicians. As necessary, the two physicians conferred with each other to assure diagnosis. Participants were assessed by a physical therapist for posture, function, gait biomechamics, range of motion, and quadriceps strength. Pain was also assessed after each of these physical function tests utilizing a visual analog scale (VAS), which is a 100-point scale that ranged from “No pain” to “Extreme pain” on the left and right extremes, respectively. Participants completed self-report questionnaires to assess demographics, depressive symptoms (Center for Epidemiological Studies Depression Scale, CES-D; [37]), and personality traits, such as neuroticism and extraversion [38].
Baseline Assessment
At the baseline assessment, participants completed a self-report questionnaire that assessed pain and disability (WOMAC [36]), mental and physical functioning (The Short-Form-36 Health Survey, SF-36 [39]), positive and NA (Positive and Negative Affect Schedule, PANAS [40]), arthritis self-efficacy (Arthritis Self-Efficacy Scale, ASES [41]), and physical activity (Aerobics Center Longitudinal Study, ACLS [42]). Participants were also given pedometers to measure their level of physical activity over seven consecutive days.
Measures
Depressive Symptoms
Depressive symptoms were measured by the CES-D [37]. The CES-D is a 20-item measure of depressive symptoms. Prior research has demonstrated that this scale loads onto four different factors, including NA, PA, somatic/vegetative, and interpersonal factors [43, 44]. For the purposes of this study, the NA and PA subscales were explored.1 The seven items in the NA subscale assessed depressive symptoms, including feelings of sadness, loneliness, fear, and failure. The four items in the PA subscale included feelings of being as good as others, happiness, enjoyment in life, and hopefulness about the future. Participants rated how often they felt each symptom during the past week on a five-point scale, with 0 representing “Rarely or none of the time (less than 1 day)” to 4 representing “Most or all of the time (5–7 days).” Scores on the CES-D were computed by summing each participant’s score on each item, taking into account the number of symptoms experienced, as well as the persistence throughout the week. Cronbach’s alpha for this sample was 0.82 for the NA subscale and 0.79 for the PA subscale.
Extraversion and Neuroticism
Neuroticism and extraversion were assessed in the baseline questionnaire using the 12 neuroticism and the 12 extraversion items from the McCrae and Costa [38] measure of the Big 5 traits. Participants rated their agreement with each statement beginning with “I see myself as someone who…” on a five-point scale, with 1 representing “Disagree strongly,” and 5 representing “Agree strongly.” Examples of neuroticism items include “can be moody,” “can be tense,” and “gets nervous easily.” Extraversion, on the other hand, can be described as high levels of sociability, excitement-seeking, and activity levels. Examples of these items include “is talkative,” “is outgoing,” and “has an assertive personality.” Cronbach’s alpha is 0.83 for neuroticism and 0.77 for extraversion in the present study.
Positive and Negative Affect
PA and NA were measured by the PANAS [40]. The PANAS is a 20-item self-report measure that assesses two primary dimensions of mood, PA and NA. Each item is rated using a five-point scale with 1 indicating “None of the time” and 5 indicating “All of the time” to the extent that they have experienced each mood state during the previous week. The PA scale reflects mood states such as “enthusiastic,” “active,” and “alert,” while the NA scale reflects mood states such as “distressed,” “irritable,” and “afraid.” Cronbach’s alpha was 0.87 for NA and 0.91 for PA for the present study.
Vitality
Vitality was measured by a subscale of the SF-36 [39]. The four-item vitality subscale includes items such as “feels full of pep” and “has a lot of energy.” Each item is rated using a six-point scale with 1 indicating “All of the time” and 6 indicating “None of the time” to the extent that they felt each mood or experience during the last 4 weeks. Cronbach’s alpha was 0.85 for the present study.
Disease Severity
Disease severity of KOA was estimated based on semiflex X-rays of both knees that were evaluated on the Kellgren and Lawrence classification [35]. In this study, participants were limited to radiographic status of grade II in one knee and a grade II or lower in the other knee. Since some of the participants have both knees affected, which potentially constitutes greater disease severity, the radiographic status of both knees was taken into account for the approximation of disease severity for all of the participants. Hence, the radiographic status of both of the knees was aggregated as an estimate of disease severity. Thus, the range of potential radiographic status scores was grades II–IV.
Self-efficacy
Self-efficacy was measured by the ASES [41]. The ASES is a 20-item measure of self-efficacy developed specifically for people with arthritis and assesses self-efficacy for physical function, pain management, and controlling other arthritis symptoms. The ASES reflects items such as “How certain are you that you can get out of an armless chair quickly, without using your hands for support?,” “How certain are you that can make a large reduction in your arthritis pain by using methods other than taking extra medication?,” and “How certain are you that you can control your fatigue?” Participants evaluated these items based on a visual analog 100-mm scale (VAS) that ranged from “Very uncertain” to “Very certain.” Cronbach’s alpha of self-efficacy was 0.89 in this study.
Physical Activity
Both self-report and objective measures of physical activity were used. A modified version of the ACLS [42] was used to ask participants about the frequency, duration, and intensity of their current physical activity and exercise habits over the last 3 months. Some examples of exercise habits and physical activities included walking, swimming, jogging, weight training, and household activities. Energy expenditure was calculated and used as an estimate of physical activity. To calculate energy expenditure, total metabolic equivalent (MET) values were first assigned to each reported activity with the following formula: [sessions/week] × [min/session] × [h/min] × [METS’s] × [body weight (kg)]. Then, a weekly total of physical activity was calculated by summing the energy expenditure values across all of the activities. The total weekly physical activity was used in this study’s analyses.2 Cronbach’s alpha for physical activity was 0.51 in the present study. Although reliability for this type of measure is predictably low, studies have shown that the ACLS has adequate validity compared to other physical activity measures [45, 46]. The second measure of physical activity was objectively measured via a pedometer that each participant wore over their hip for seven consecutive days from the time of waking until bedtime, except for showering or swimming. The pedometer ratings were averaged across the 7 days for each participant for use in these analyses.3 Cronbach’s alpha for the pedometer measure was 0.92 in the present study.
Self-report Physical Functioning
Physical functioning was measured using a subscale of SF-36 [39]. This subscale is a 12-item measure that asks participants to rate how much their health limits them in a variety of daily activities on a typical day, such as “climbing one flight of stairs;” “bending, kneeling, or stooping;” and “bathing or dressing yourself.” All the responses were rated based on a three-point scale, where 1 indicates “Yes, limited a lot;” 2 indicates “Yes, limited a little;” and 3 indicates “No, not at all limited.” Cronbach’s alpha for physical functioning was 0.85 in this study.
Observed Physical Functioning
Objective measures of physical functioning (knee and quadriceps strength and flexibility) were taken using the ERGOS™ Work Simulator, a standardized and reliable functional capacity evaluation instrument [47, 48]. For this study, the crouching subtest was utilized because it most directly accesses the functioning of the knee. The exercise test required participants to grasp a series of 5-in. steel discs and move them along a metal bar while in a crouching position. Results are measured in elapsed time to complete the exercise and converted to a methods–time–measurement (MTM) standard, an internationally accepted industrial measurement of physical performance. The MTM values were rescaled and aggregated to yield a score of objective functioning.
Pain
Pain was assessed in two ways. A five-item WOMAC pain index [36] asked participants to rate how much pain they have during specific activities, such as walking on a flat surface, ascending or descending stairs, and sitting or lying down. Participant responses are rated by using a 100-mm VAS, ranging from “No pain” to “Extreme pain.” Cronbach’s alpha was 0.82 for the scale in the present study. Also, a pain composite was formed by taking an average of four pain assessments taken after each physical function test in the prebaseline assessment. Participants responded to the question, “How bad was your pain during this test?” and used a VAS, which is a 100-mm scale that ranges from “No pain” to “Extreme pain.” Cronbach’s alpha for pain post-function was 0.69 in this study.
Results
Correlations between all relevant variables along with means and standard deviations are provided in Table 1. In this study, participants were included in the analyses if they had completed data from at least the prebaseline or the baseline assessment. Given this criterion, the final sample sizes ranged from n=179 to n=275, with missing data ranging from 2.5% to 34.9%. The larger categories of missing data were from the objective measures, the ERGOS function test (15.6%) and the pedometer measure (34.9%). Missing data from the self-report measures ranged from 2.5% to 7.6%. Estimation of missing data was handled using the full information maximum likelihood method.
Table 1.
Means, standard deviations, and correlations between study variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age (M=54.40, SD=7.17) | – | |||||||||||||||
| 2. Gender | 0.05 | – | ||||||||||||||
| 3. Dx Severity (M=3.33, SD=0.71) | 0.04 | −0.05 | – | |||||||||||||
| 4. Depressive Sx (M=1.80, SD=2.79) | −0.13* | −0.12 | −0.06 | – | ||||||||||||
| 5. Neuroticism (M=2.26, SD=0.59) | −0.10 | −0.03 | 0.05 | 0.53** | – | |||||||||||
| 6. Negative Affect (M=1.67, SD=0.51) | −0.13* | −0.00 | 0.06 | 0.56** | 0.63** | – | ||||||||||
| 7. Vitality (M=52.50, SD=19.44) | 0.07 | 0.09 | −0.05 | −0.38*** | −0.43** | −0.44** | – | |||||||||
| 8. Extraversion (M=3.43, SD=0.48) | −0.05 | −0.08 | −0.04 | −0.33** | −0.45** | −0.32** | 0.46** | – | ||||||||
| 9. Positive Affect (M=3.46, SD=0.48) | 0.15* | −0.00 | −0.01 | −0.38** | −0.44** | −0.40** | 0.65** | 0.51** | – | |||||||
| 10. Arthritis SE (M=75.14, SD=13.58) | 0.02 | 0.06 | −0.06 | −0.35** | −0.32** | −0.26** | 0.44** | 0.17** | 0.38** | – | ||||||
| 11. ACLS PA (M=26.83, SD=21.33) | −0.11 | 0.19** | −0.02 | 0.00 | −0.14* | −0.09 | 0.12 | 0.11 | 0.03 | 0.10 | – | |||||
| 12. Pedometer (M=46.69, SD=22.60) | −0.15* | 0.15* | 0.05 | −0.03 | −0.09 | −0.10 | 0.16* | 0.05 | 0.06 | 0.20** | 0.17* | – | ||||
| 13. WOMAC Pain (M=17.76, SD=14.47) | −0.00 | −0.03 | 0.05 | 0.21** | 0.09 | 0.15* | −0.16* | 0.07 | 0.03 | −0.31** | 0.06 | 0.02 | – | |||
| 14. Pain Post-function (M=21.82, SD=15.40) | 0.03 | −0.08 | 0.04 | 0.18** | 0.07 | 0.06 | −0.18** | 0.02 | −0.03 | −0.25** | −0.03 | −0.06 | 0.43** | – | ||
| 15. SF36 Function (M=65.79, SD=19.32) | −0.13* | 0.03 | −0.06 | −0.22** | −0.17** | −0.22** | 0.34** | 0.07 | 0.11 | 0.47** | 0.07 | 0.21** | −0.53** | −0.47** | – | |
| 16. ERGOS (M=117.82, SD=27.80) | −0.13* | 0.22** | −0.08 | −0.11 | −0.00 | −0.05 | 0.17** | 0.11 | 0.02 | 0.21** | 0.03 | 0.09 | −0.21** | 0.30** | 0.29** | – |
Gender was coded 0 for females and 1 for males.
Dx disease, Sx symptoms, SE self-efficacy, PA physical activity
p<0.05;
p<0.01
Measurement Model
Preliminary analyses were conducted to select risk and resilience variables for use in model building. In order to test the first hypothesis, confirmatory factor analysis techniques were utilized to determine whether latent factors for risk and resilience were separable constructs. Both the single-factor and the two-factor model were evaluated for goodness of fit. In the single-factor model, all indicators were permitted to freely load onto a common factor. For the two-factor model, the variables were assigned to a single latent factor: either psychological risk or resilience. It was hypothesized that neuroticism, depressive symptoms (NA subscale), and NA would load on the latent risk factor and extraversion, vitality, and PA would load on a separate latent resilience factor. NA and PA were chosen as reference variables based on theory, as they are the most central indicators of the competing one- vs two-factor models [9, 10].
Several fit indices were utilized to examine the omnibus fit of the models. These include the chi-square test, root mean square error approximation (RMSEA), the standardized mean square residual (SRMSR), and the comparative fit index (CFI). The chi-square test provides a measure of exact fit of the model to the data. The RMSEA provides an estimate of the average size of the residual adjusting for the degrees of freedom; values of less than or equal to 0.05 indicate that the model fits the data well. The SRMSR is a standardized version of the average size of the residuals, where values less than 0.05 are considered a good fit and those between 0.05 and 0.08 are considered a fair fit [49]. The CFI is a measure of how well the model is improved in comparison to the null model; values greater than or equal to 0.95 are considered a good fit of the model.
The hypothesized two-factor model had a better fit than the competing single-factor model. For the congeneric two-factor model (see Fig. 1), the fit indices were: χ2(8)=13.08 (p=0.11); CFI=0.99; RMSEA=0.05 (90% CI: 0.00–0.09); SRMSR=0.03. The correlation between the two factors was high (r=−0.70), suggesting that, although they share common variance, there is unique variance of each factor not accounted for by the single-factor model.
Fig. 1.
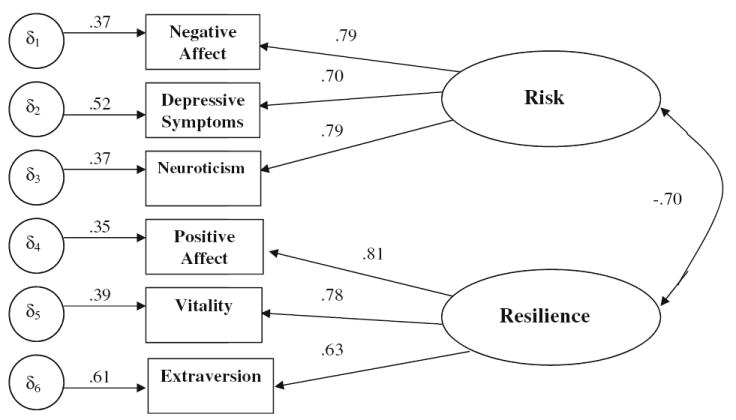
Measurement model of risk and resilience. Note: All estimates in the model are based on the values from the completely standardized solution
Structural Equation Model
Structural equation modeling (SEM) was used to develop a model of adjustment to early KOA. Unlike standard regression analyses, SEM with multiple measures for latent constructs reduces measurement error and improves reliability, while assessing direct and indirect effects simultaneously. Models were tested using Mplus 3.01 [50] on the variance–covariance matrix for the variables of study. Disturbance terms were allowed to correlate between the mediators and between the outcomes. In addition, disease severity was modeled with the same pathways as risk and resilience in order to control for this covariate. Age and gender were also included as covariates with paths to the outcomes of pain and physical functioning based on prior research findings [51, 52]. Changes to the models were made incrementally and each iteration was evaluated and reported.
Hypothesized Model with Arthritis Self-efficacy Scale
The first hypothesized model had a poor to fair fit on the indices: χ2(48)=127.09, p<0.001; CFI=0.92; RMSEA= 0.08 (90% CI: 0.06–0.09); SRMSR=0.05. This model accounted for 25% of the variance in subjective physical functioning, 12% of the variance in objective physical functioning, 15% of the variance in the WOMAC pain measure, and 7% of the variance in the pain postfunction test. The model was replicated substituting pedometer data, a more objective physical activity measure for self-report physical activity. This replication was conducted to determine if the fit or the prediction of the model changed with this different measure of physical activity. The model fit did not improve with this revision and continued to provide a poor to fair fit of the data: χ2(48)=118.34, p<.001; CFI= 0.93; RMSEA=0.07 (90% CI: 0.06–0.09); SRMSR=0.05. In addition, this model did not provide any additional significant paths compared to the hypothesized model.
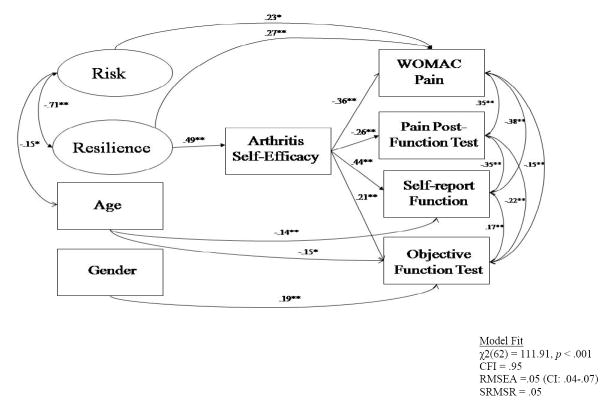
To revise the model, paths were omitted if they were not significant and their omission retained a model consistent with theory. Modification indices also supported these omissions. As a result of model respecification, disease severity and physical activity were removed from the model. Removing the nonsignificant paths allowed the model to be more parsimonious and improved overall model fit as follows: χ2(62)=111.64, p<.001; CFI=0.95; RMSEA= 0.05 (90% CI: 0.04–0.07); SRMSR=0.05. This model, as depicted in Fig. 2, provided a fair to good fit to the data and accounted for 25% of the variance in subjective physical functioning, 10% of the variance in objective physical functioning, 13% of the variance in the WOMAC pain measure, and 7% of the variance in the pain postfunction test.
Fig. 2.
Final model of risk and resilience on pain and physical functioning. Note: All estimates in the model are based on the values from the completely standardized solution. Single asterisks, p<0.05; double asterisks, p<0.01
As hypothesized, resilience was positively related to self-efficacy, suggesting that those higher in psychological resilience will, in turn, have higher self-efficacy. Although risk was not directly related to self-efficacy, it was directly related to increased WOMAC pain, as anticipated. As expected, self-efficacy was related to higher subjective and objective physical functioning, suggesting that those with higher levels of self-efficacy also show improved physical functioning. In addition, self-efficacy was related to decreased pain for both pain measures. Age was negatively related to risk and subjective, as well as objective, physical functioning. All relationships for risk, resilience, age, gender, pain, and physical functioning were consistent with hypotheses. There was one finding that was inconsistent with the hypothesized model. Contrary to hypotheses, resilience was directly related to high levels of pain on the WOMAC scale.
Four tests of mediation were conducted and all of these tests found significant effects. Table 2 depicts the unstandardized direct, indirect, and total effects of the variables in the respecified model. Results indicated that resilience had significant indirect effects on both pain measures and both physical function measures through self-efficacy. These results suggest that resilience predicts higher levels of self-efficacy, which in turn relate to reduced pain and enhanced physical functioning over and above the effects of risk and disease severity. The direct effect of resilience on WOMAC pain remained significant even after this indirect effect was taken into account. Risk also had a direct effect on WOMAC pain.
Table 2.
Indirect and direct effects on physical functioning and pain
| Effect | Effects on the Model with Arthritis Self-efficacy
|
||
|---|---|---|---|
| Indirect | Direct | Total | |
| Risk → SF-36 Physical Function | – | −1.76 | −1.76 |
| Risk → ERGOS Function | −2.35** | 1.21 | 0.75 |
| Resilience→ SF-36 Physical Function | 5.27** | – | 5.27** |
| Resilience → ERGOS Function | 3.00** | – | 3.00** |
| Risk → WOMAC Pain | – | 2.34* | 2.34* |
| Resilience → WOMAC Pain | −4.20** | 2.65** | 1.10 |
| Resilience → Pain Post-Fxn Test | −3.71** | – | −3.71** |
Pathways in the table are unstandardized estimates.
p<0.05;
p<0.01
Supplementary Analyses
To further probe the contradictory finding of resilience being positively related to WOMAC pain, the above model was tested with and, alternately, without risk or resilience factors to determine the singular effects of each. Without resilience, the model had a good fit on the indices: χ2(31)= 32.93, p=0.37; CFI=0.996; RMSEA=0.02 (90% CI: 0.001–0.05); SRMSR=0.03. In this analysis, risk was directly related to self-efficacy but was not directly related to pain or physical functioning. As hypothesized, self-efficacy was negatively related to both pain measures and positively related to subjective and objective physical functioning. Age and gender maintained their previous relationships. The outcome variables were all related to each other. The hypothesis that the relation between risk on WOMAC pain and pain postfunction was mediated by self-efficacy was supported. Further, the hypothesis that the relation between risk and subjective and objective functioning was mediated by self-efficacy was found.
The model without risk demonstrated a fair to good fit on the indices: χ2(31)=61.70, p<.001; CFI=0.95; RMSEA= 0.06 (90% CI: 0.04–0.08); SRMSR=0.05. Resilience was again positively related to self-efficacy. In this model, resilience was now unrelated to WOMAC pain, as well as the other outcomes of pain and physical functioning. Consistent with the hypotheses, self-efficacy was again negatively related to both pain measures and positively related to subjective and objective physical functioning. The hypotheses that the relation between resilience on WOMAC pain, pain postfunction, and subjective and objective physical functioning was mediated by self-efficacy was supported.
Discussion
Confirmatory factor analyses supported the first hypothesis that risk and resilience provided a better fit as separable factors, rather than a single bipolar factor similar to previous findings for the positive and negative affective systems [9-11]. This study hypothesized that risk and resilience would have parallel and opposing relationships, where risk would be related to decreased self-efficacy and physical activity and resilience would be related to increases in these measures. The findings were consistent with the fundamental principle that risk and resilience impacted self-efficacy, pain, and physical functioning over and above disease severity. As hypothesized, resilience was related to increased self-efficacy. In terms of mediation, resilience demonstrated indirect effects on all of the pain and physical functioning outcomes through self-efficacy, suggesting that those higher in resilience had higher levels of arthritis self-efficacy and in turn had decreased pain and improved functioning. When risk was tested without resilience in the model, it also was indirectly related to pain and physical functioning through self-efficacy. However, risk was unrelated to self-efficacy when resilience was included in the model, indicating that when both risk and resilience were taken into account, the resilience factor had a greater impact on arthritis self-efficacy. This finding makes sense theoretically as physical functioning is measured as the participants’ ability to function in spite of pain or distress.
Risk demonstrated direct effects on pain in the expected direction, and resilience scores showed a similar effect, contrary to prediction. Since the relation between resilience and WOMAC pain was no longer significant when risk was taken out of the model, this relationship may best be thought of as an artifact of the estimation procedures that included variables with a higher inverse correlation than expected. However, there may be some unique variance in resilience that is not shared with risk that contributes to its positive relationship with pain. For example, there is an element of optimism, positive unrealistic thinking, that may actually elevate pain if these patients do not make appropriate self-care or health decisions [53]. In any event, this unexpected finding underscores a need for replication.
For the most part, self-report and objective physical activity were unrelated to the variables of interest. The hypotheses that risk and resilience would be related to physical activity and, in turn, physical activity would be related to pain and physical functioning were not supported. However, the complementary hypothesis that self-efficacy would mirror these hypothesized relationships was supported. Self-efficacy was related to decreased pain and increased physical functioning over and above physical activity. These results were found for both subjective and objective physical functioning. Although not modeled in this study, self-efficacy may also relate to physical activity as shown by Keefe and colleagues [54], who reported that improvements in self-efficacy had a direct impact on physical fitness, as well as decreases in disability and improvements in pain coping.
Self-efficacy may be particularly important at early stages of chronic illness, and the findings from the present study may have important implications for programs designed to minimize impairment and pain long-term. A major contribution of this study is the finding that risk and resilience predict self-efficacy. This result informs future intervention research, suggesting that participants with different profiles of risk and resilience may respond differently to interventions aimed to improve self-efficacy and, in turn, impact pain or physical functioning. It may be that interventions also need to enhance resilience as a means of improving pain and functional outcomes, as opposed to simply reducing risk or focusing on biomedical factors. Future research exploring risk, resilience, and self-efficacy will need to address these questions.
Two key demographic covariates, age and gender, were tested in relation to risk, resilience, and disease severity. Older participants were found to have lower levels of psychological risk. This finding is consistent with previous research that has shown that there is a significant decrease in negative emotion in older adults [55]. Older participants reported greater difficulty in their physical functioning. Men performed better on objective physical functioning test of quadriceps strength and flexibility than women did. In general, men have greater physical strength than women do and it would be expected that this effect would also be captured by the ERGOS measure. These results confirm that age and gender should continue to be included in studies as they have important effects on the outcomes of pain and physical functioning.
There are limitations that should be considered when interpreting the results of this study. First, a cross-sectional design was used to test relationships that likely change over time. It is likely that some of the relationships modeled are bidirectional in nature, such as the relationship between risk factors and pain [56]. Second, participants with moderate to high levels of physical activity at baseline were excluded from the study. This exclusion limits the generalizability of the results and may have contributed to the absence of effects for self-report or observed physical activity. Future research should extend these findings to the range of arthritis patients that engage in light, infrequent to vigorous, regular physical activity, as well as for those who have had symptoms for a longer period of time. Comparison of these differences would allow us to determine if these relationships hold or if other factors are more important to the maintenance of pain tolerance and physical functioning.
Third, the amount of variance accounted for in pain was relatively small compared to the variance accounted for in physical functioning. Similar discrepancies between pain and disability have been found in previous studies when looking at psychological and radiologic determinants [4]. Other variables not included in this analysis may have contributed more to pain reports and should be examined in future studies. Physiological processes in the knee joint, such as secondary inflammation, or additional psychological factors, such as pain catastrophizing, fear of pain, and fear avoidance, may contribute more to the prediction of pain [34, 57]. In addition, the relationships between self-efficacy and the objective outcome measures, ERGOS function test, and pain postfunction were relatively small compared to the relationships between self-efficacy and self-reports of pain and functioning. Again, it may be that other variables would have had a stronger association with the objective outcomes, such as a more specific measure of self-efficacy for function or pain.
Finally, although not measured in the present study, there are other variables that have been shown to be relevant in pain and disability research that may allow for a more complete picture of risk and resilience in this population. The role of the social environment has been found to be important, especially for disability and recovery [13, 58-60]. Cognitive factors such as optimism and locus of control have also been shown to improve self-care and functioning [53, 61].
Despite these limitations, this study has provided valuable information for an understudied population that has implications for future research and clinical practice. To our knowledge, this was the first study to examine how both risk and resilience factors influence pain and physical functioning in an early KOA population. In addition, we have used both self-report and objective measures of physical activity and physical functioning to study KOA. Utilizing SEM to analyze the data was also a strength of this study, as it more closely mirrors real life by demonstrating how all variables interact and relate to one another as a whole and identifies the relative importance of each path within the model. Results from this study provide information about adaptation to pain and arthritis symptoms experienced by an early KOA population that engages in little physical activity. Risk and resilience proved to be directly and indirectly related to pain and physical functioning over and above disease severity, suggesting that these factors should be considered in determining the effectiveness of interventions.
An interesting finding from this study is that, even at this early stage of disease, a process of response to KOA has become enacted that may predict future levels of pain and physical functioning. If psychological risk and resilience are already showing important impacts on pain and physical functioning at this early stage, they may have even greater effects when the disease has progressed and the cognitive/affective responses to KOA have become more established. Some researchers have found that, with persistence of pain, psychological factors play an even greater role in disability [4, 62]. Thus, it may be even more important to intervene before the condition becomes chronic. This is especially true if bidirectional relationships of pain and psychological risk exist. For example, if psychological risk is already contributing to increasing levels of pain at an early stage of disease and pain worsens over time, it is likely that psychological risk will also increase, contributing to this downward spiral of increasing pain and distress. However, since resilience and self-efficacy were found to have significant influences on pain independent of risk, the coexistence of resilience and self-efficacy could also be protective in slowing or curtailing this downward spiral. These results provide important information for clinicians on the front lines to encourage patients to believe in their abilities to care for themselves, as it may mark a vast difference in their illness trajectory. Enhancing resilience and self-efficacy at this early stage may be critical to preventing or reducing levels of future pain and disability.
Footnotes
It was determined that the fit of the CFA model was worsened with the CES-D positive affect subscale in the model and was ultimately omitted. The CES-D negative affect subscale, on the other hand, improved the fit of the CFA model over and above the model fit with the full CES-D measure included. Therefore, this subscale was utilized in this study within the risk factor. No other variations of the measurement model were tested.
This value was rescaled by dividing each value by 100 so that the range of this measure would be more similar to the other variables in the model.
This value was rescaled by dividing each averaged value by 553 so that the range of this measure would be more similar to the other variables in the model.
Contributor Information
Lisa Johnson Wright, University of California, San Diego, San Diego, CA 92093-0738, USA, e-mail: lmjwright@ucsd.edu
Alex J. Zautra, Arizona State University, Tempe, AZ 85287-1104, USA
Scott Going, Department of Nutritional Sciences, University of Arizona, Tucson, AZ 85721-0038, USA
References
- 1.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;418:1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Williams DA, Farrell MJ, Cunningham J, et al. Knee pain and radiographic osteoarthritis interact in the prediction of levels of self-reported disability. Arthritis Rheum. 2004;514:558–561. doi: 10.1002/art.20537. [DOI] [PubMed] [Google Scholar]
- 3.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford) 2000;395:490–496. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 4.van Baar ME, Dekker J, Lemmens JA, Oostendorp RA, Bijlsma JW. Pain and disability in patients with osteoarthritis of hip or knee: The relationship with articular, kinesiological, and psychological characteristics. J Rheumatol. 1998;251:125–133. [PubMed] [Google Scholar]
- 5.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann Intern Med. 2000;1339:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 6.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;276:1513–1517. [PubMed] [Google Scholar]
- 7.Lorish CD, Abraham N, Austin J, Bradley LA, Alarcon GS. Disease and psychosocial factors related to physical functioning in rheumatoid arthritis. J Rheumatol. 1991;188:1150–1157. [PubMed] [Google Scholar]
- 8.McFarlane AC, Brooks PM. Determinants of disability in rheumatoid arthritis. Br J Rheumatol. 1988;271:7–14. doi: 10.1093/rheumatology/27.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Cacioppo JT, Gardner WL. Emotion. Annu Rev Psychol. 1999;50:191–214. doi: 10.1146/annurev.psych.50.1.191. [DOI] [PubMed] [Google Scholar]
- 10.Reich JW, Zautra AJ, Davis MC. Dimensions of affect relationships: Models and their integrative implications. Rev Gen Psychol. 2003;71:66–83. [Google Scholar]
- 11.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;732:212–220. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turk DC. A diathesis-stress model of chronic pain and disability following traumatic injury. Pain Res Man. 2002;71:9–19. doi: 10.1155/2002/252904. [DOI] [PubMed] [Google Scholar]
- 13.Keefe FJ, Smith SJ, Buffington AL, Gibson J, Studts JL, Caldwell DS. Recent advances and future directions in the biopsychosocial assessment and treatment of arthritis. J Consult Clin Psychol. 2002;703:640–655. doi: 10.1037//0022-006x.70.3.640. [DOI] [PubMed] [Google Scholar]
- 14.Zautra AJ, Smith BW. Depression and reactivity to stress in older women with rheumatoid arthritis and osteoarthritis. Psychosom Med. 2001;634:687–696. doi: 10.1097/00006842-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci. 2001;1151:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Watson D, Clark LA. On traits and temperament: General and specific factors of emotional experience and their relation to the five-factor model. J Pers. 1992;602:441–476. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 17.Creamer P, Lethbridge-Cejku M, Costa P, Tobin JD, Herbst JH, Hochberg MC. The relationship of anxiety and depression with self-reported knee pain in the community: Data from the Baltimore Longitudinal Study of Aging. Arthritis Care Res. 1999;121:3–7. doi: 10.1002/1529-0131(199902)12:1<3::aid-art2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: A daily diary study of people with reflex sympathetic dystrophy syndrome. J Consult Clin Psychol. 1999;675:776–785. doi: 10.1037//0022-006x.67.5.776. [DOI] [PubMed] [Google Scholar]
- 19.Gaynes BN, Burns BJ, Tweed DL, Erickson P. Depression and health-related quality of life. J Nerv Ment Dis. 2002;19012:799–806. doi: 10.1097/00005053-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Charles ST, Gatz M, Pedersen NL, Dahlberg L. Genetic and behavioral risk factors for self-reported joint pain among a population-based sample of Swedish twins. Health Psychol. 1999;186:644–654. doi: 10.1037//0278-6133.18.6.644. [DOI] [PubMed] [Google Scholar]
- 21.Fisher MN, Snih SA, Ostir GV, Goodwin JS. Positive affect and disability among older Mexican Americans with arthritis. Arthritis Rheum. 2004;511:34–39. doi: 10.1002/art.20079. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva I, Cornett M, Yocum D, Castro WL. Living healthy with arthritis: Individual’s positive affect predicts outcomes of a community based multidisciplinary interventional program focusing on wellness and preventive care in arthritis. Arthritis Rheum. 1999;42:S1244. [Google Scholar]
- 23.Rozanski A, Kubzansky LD. Psychologic functioning and physical health: A paradigm of flexibility. Psychosom Med. 2005;67(Suppl 1):S47–S53. doi: 10.1097/01.psy.0000164253.69550.49. [DOI] [PubMed] [Google Scholar]
- 24.Ryan RM, Frederick C. On energy, personality, and health: Subjective vitality as a dynamic reflection of well-being. J Pers. 1997;653:529–565. doi: 10.1111/j.1467-6494.1997.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 25.Porter LS, Gil KM, Carson JW, Anthony KK, Ready J. The role of stress and mood in sickle cell disease pain: An analysis of daily diary data. J Health Psychol. 2000;51:53–63. doi: 10.1177/135910530000500109. [DOI] [PubMed] [Google Scholar]
- 26.Asghari A, Nicholas MK. Personality and pain-related beliefs/coping strategies: A prospective study. Clin J Pain. 2006;221:10–18. doi: 10.1097/01.ajp.0000146218.31780.0b. [DOI] [PubMed] [Google Scholar]
- 27.Maly MR, Costigan PA, Olney SJ. Determinants of self efficacy for physical tasks in people with knee osteoarthritis. Arthritis Rheum. 2006;551:94–101. doi: 10.1002/art.21701. [DOI] [PubMed] [Google Scholar]
- 28.Turner JA, Ersek M, Kemp C. Self-efficacy for managing pain is associated with disability, depression, and pain coping among retirement community residents with chronic pain. J Pain. 2005;67:471–479. doi: 10.1016/j.jpain.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Engel C, Hamilton NA, Potter P, Zautra AJ. Impact of two types of expectancy on recovery from total knee replacement surgery (TRK) in adults with osteoarthritis. Behav Med. 2004;30:113–123. doi: 10.3200/BMED.30.3.113-123. [DOI] [PubMed] [Google Scholar]
- 30.Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol. 2001;563:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingledew DK, Markland D, Sheppard KE. Personality and self-determination of exercise behavior. Pers Ind Diff. 2004;368:1921–1932. [Google Scholar]
- 32.Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 33.Goubert L, Crombez G, Van Damme S. The role of neuroticism, pain catastrophizing and pain-related fear in vigilance to pain: A structural equations approach. Pain. 2004;1073:234–241. doi: 10.1016/j.pain.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;853:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 35.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;164:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;1512:1833–1840. [PubMed] [Google Scholar]
- 37.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;13:385–401. [Google Scholar]
- 38.McCrae RR, Costa PT. Handbook of Personality: Theory and Research. New York, NY: Guilford; 1999. [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;306:473–483. [PubMed] [Google Scholar]
- 40.Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affect. Psychol Rev. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- 41.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;321:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 42.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;251:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Moskowitz JT, Epel ES, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychol. 2008;271(Suppl):S73–S82. doi: 10.1037/0278-6133.27.1.S73. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 19995;643:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 45.Bowles HR, Fitzgerald SJ, Morrow JR, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;1603:279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 46.Jurca R, Jackson AS, LaMonte MJ, et al. Assessing cardiovascular fitness without performing exercise testing. Am J Prev Med. 2005;293:185–193. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Innes E, Straker L. Reliability of work-related assessments. Work. 1999;132:107–124. [PubMed] [Google Scholar]
- 48.Reneman MF, Jaegers SM, Westmaas M, Goeken LN. Test–retest reliability of lifting and carrying in a 2-day Functional Capacity Evaluation. J Occup Rehab. 2002;124:269–275. doi: 10.1023/a:1020274624791. [DOI] [PubMed] [Google Scholar]
- 49.Browne MW, Cudeck R. Alternative ways of assessing model fit. Soc Meth Res. 1992;212:230–258. [Google Scholar]
- 50.Muthén LK, Muthén BO. Mplus User’s Guide. 4. Vol. 2005 Los Angeles: Muthén and Muthén; [Google Scholar]
- 51.Affleck G, Tennen H, Keefe FJ, et al. Everyday life with osteoarthritis or rheumatoid artphritis: Independent effects of disease and gender on daily pain, mood and coping. Pain. 1999;833:601–609. doi: 10.1016/S0304-3959(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 52.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: Cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;1101–2:361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 53.Fournier M, de Ridder D, Bensing J. Optimism and adaptation to chronic disease: The role of optimism in relation to self-care options of type-1 diabetes mellitus, rheumatoid arthritis, and multiple sclerosis. Br J Health Psychol. 2002;74:409–432. doi: 10.1348/135910702320645390. [DOI] [PubMed] [Google Scholar]
- 54.Keefe FJ, Blumenthal J, Baucom D, et al. Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: A randomized controlled study. Pain. 2004;110:539–549. doi: 10.1016/j.pain.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. J Pers Soc Psychol. 2000;794:644–655. [PubMed] [Google Scholar]
- 56.Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;921–2:195–200. doi: 10.1016/s0304-3959(00)00483-8. [DOI] [PubMed] [Google Scholar]
- 57.France CR, Keefe FJ, Emery CF, et al. Laboratory pain perception and clinical pain in post-menopausal women and age-matched men with osteoarthritis: Relationship to pain coping and hormonal status. Pain. 2004;1123:274–281. doi: 10.1016/j.pain.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Jette AM, Keysor JJ. Disability models: Implications for arthritis exercise and physical activity interventions. Arthritis Rheum. 2003;491:114–120. doi: 10.1002/art.10909. [DOI] [PubMed] [Google Scholar]
- 59.Keefe FJ, Caldwell DS, Baucom D, et al. Spouse-assisted coping skills training in the management of knee pain in osteoarthritis: Long-term followup results. Arth Care Res. 1999;122:101–111. doi: 10.1002/1529-0131(199904)12:2<101::aid-art5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 60.Stephens MAP, Druley JA, Zautra AJ. Older adult’s recovery from surgery for osteoarthritis of the knee: Psychosocial resources and constraints as predictors of outcomes. Health Psychol. 2002;21:377–383. doi: 10.1037//0278-6133.21.4.377. [DOI] [PubMed] [Google Scholar]
- 61.Cross MJ, March LM, Lapsley HM, Byrne E, Brooks PM. Patient self-efficacy and health locus of control: Relationships with health status and arthritis-related expenditures. Rheumatology. 2006;451:92–96. doi: 10.1093/rheumatology/kei114. [DOI] [PubMed] [Google Scholar]
- 62.Kroner-Herwig B, Jakle C, Frettloh PK, Seeman H, Franz C, Basler HD. Predicting subjective disability in chronic pain patients. Int J Behav Med. 1996;31:30–41. doi: 10.1207/s15327558ijbm0301_3. [DOI] [PubMed] [Google Scholar]