Abstract
Salmonella enterica serovar Choleraesuis is an enteric pathogen of swine, producing septicemia, enterocolitis, pneumonia, and hepatitis. The initial molecular events at the site of Salmonella infection are hypothesized to be critical in the initiation of innate and adaptive immune responses; however the acute immune response elicited by porcine intestinal tissues is not well understood. To address this need, we employed explants of jejunal Peyer’s patch (JPP) mucosa from pigs to examine Salmonella-induced immune responses under controlled conditions as well as to overcome limitations of whole animal approaches. JPP explants mounted in Ussing chambers maintained normal histological structure for 2 h and stable short-circuit current and electrical conductance for 2.5 h. After ex vivo luminal exposure to Salmonella serovar Choleraesuis, JPP responded with an increase in mRNA expression of IL-1β and IL-8, but not TNFα. Increased IL-1β and IL-8 expression were dependent on efficient Salmonella adhesion and internalization, whereas mutant Salmonella did not induce inflammatory cytokine expression. Commensal enteric bacteria, present in some experiments, also did not induce inflammatory cytokine expression. These findings indicate that Salmonella uptake by Peyer’s patch is important in the induction of an innate response involving expression of IL-1β and IL-8, and that ex vivo intestinal immune tissue explants provide an intact tissue model that will facilitate investigation of mucosal immunity in swine.
Keywords: animal models, swine, cytokines, interleukins, mucosal immunology
1.1.1.2 Introduction
Salmonella enterica represents a serologically diverse group of enteric pathogens capable of infecting both animals and humans. Salmonella serovar Choleraesuis is a major enteric pathogen in swine which produces septicemia, enterocolitis, pneumonia, and hepatitis, and is a common cause of systemic disease (Schwartz, 1999). Ingested Salmonella invade epithelial cells of the small intestine, including those of the gut-associated lymphoid tissue (GALT). Peyer’s patches and mesenteric lymph nodes constitute a part of the organized GALT which provides specific, acquired immune defense against bacterial pathogens (Mowat, 2003).
To date, the innate mucosal immune response to Salmonella has not been described in pigs. In this species, studies have focused primarily on vaccine development (Kennedy et al., 1999; Maes et al., 2001) and the characteristics of Salmonella infection in different intestinal regions (Bolton et al., 1999; Meyerholz and Stabel, 2003). Within 10 minutes of Salmonella serovar Choleraesuis or Typhimurium exposure in porcine ileal and jejeunal loop models, bacteria adhere to the brush border of enterocytes and invade epithelial cells. After 1 to 2 h, Salmonella are detected within epithelial cells and the lamina propria (Meyerholz and Stabel, 2003; Schauser et al., 2004). Studies of porcine Peyer's patches indicate that the follicle-associated epithelium within jejunal Peyer's patches preferentially takes up inert particles in comparison to the neighboring absorptive epithelium (Beier and Gebert, 1998; Liebler et al., 1995). Peyer’s patches constitute the main portal of entry early in infection by Salmonella servar Typhimurium (Jones et al., 1994; Schauser et al., 2004).
Virulent Salmonella inject protein effectors via a type III secretion system to induce actin rearrangements and macropinocytosis by epithelial cells (Cossart, 2004). Salmonella flagellin is translocated to the epithelial basolateral membrane and binds to type 5 Toll-like receptors (TLR5)(Gewirtz et al., 2001a; Gewirtz et al., 2001b). Binding of flagellin toTLR5 activates NF-κB and MAPK signaling pathways and induces expression of inflammation-associated genes, including IL-8 and other chemokines (CXCL8) (Elewaut et al., 1999; Gewirtz et al., 2001a; Medzhitov, 2001). Injection of SipB protein by Salmonella serovar Typhimurium or Dublin induces caspase-1-mediated cell death of macrophages and dendritic cells (Hersh et al., 1999; van der Velden et al., 2003; Watson et al., 2000). Caspase-1 enzymatically cleaves IL-1β and IL-18 to their bioactive forms, and IL-1β additionally activates NF-κB-mediated transcription of chemokines (Lee et al., 2004; Monack et al., 2000). The combined secretion of IL-8 (CXCL8), MIP-2 (CXCL2), and MIP-3α (CCL20) induces chemotaxis of neutrophils and dendritic cells to the site of Salmonella invasion (Coates and McColl, 2001; McCormick et al., 1993; Sierro et al., 2001; Zhang et al., 2003).
The early, innate host response to infection is critical for the development of an effective adaptive immune response (Medzhitov and Janeway, 2000). Clarification of the acute molecular and cellular events occurring in response to enteric infection is important in understanding the mechanisms underlying innate immune responses to Salmonella. Although simplified cell culture systems are useful in identifying Salmonella virulence factors required for invasion of host cells (McCormick, 2003), the complexity of the Peyer’s patch cannot be readily recreated in vitro. In addition, many studies utilize cell lines originating from outside of the intestine, and thus may exhibit markedly different phenotypes compared to cells residing in the GALT. For example, intestinal macrophages do not function like monocytes in their responses to lipopolysaccharide (Spottl et al., 2001). In addition, epithelial cells exhibit polarized expression of surface proteins (Gewirtz et al., 2001a), and Salmonella infection of non-polarized epithelial cell layers may induce responses which would not occur in vivo.
An investigation of intact mucosal tissues may reveal integrated signaling between cells residing in the Peyer’s patches which would not be evident when studying isolated cell types. Therefore, we have examined acute cytokine responses of intact porcine JPP mucosa to Salmonella exposure ex vivo in an Ussing chamber. Intestinal tissues were obtained from pigs conventionally colonized with commensal microflora, as a competent intestinal immune system was necessary in these studies. Enteric commensal bacteria are critical to the development of Peyer’s patches (Pabst and Rothkotter, 1999), production of antibodies (Butler et al., 2002; Cukrowska et al., 2001), and migration of B and T lymphocytes to the Peyer’s patches (Yamanaka et al., 2003). Our findings indicate that intact JPP respond rapidly to Salmonella infection by increased expression of IL-1β and IL-8 mRNAs, and efficient Salmonella adhesion and internalization are required to induce these changes in mRNA expression.
1.1.1.3 Materials and Methods
Animals
Weaned Yorkshire-Landrace outbred pigs of each sex (5 – 8 weeks of age) were clinically healthy, had continuous access to water and nonmedicated pig feed, were not fasted prior to sacrifice, and were under veterinary care. Randomly selected pigs were further screened for Salmonella by an invA-based PCR assay and by bacterial isolation from intestinal tissues. All procedures were conducted on animals anesthetized by an intramuscular injection of tiletamine hydrochloride-zolazepam (Telazol®; 8 mg/kg; Fort Dodge Laboratories, Inc, Fort Dodge, IA) in combination with xylazine, and subsequently euthanized with intravenous Beuthanasia®-D Special (0.5 ml/kg; Schering-Plough Animal Health, Union, NJ) in accordance with approved University of Minnesota IACUC protocols. Segments of jejunum containing Peyer's patches were removed within 15 min after death and placed in ice-cold, oxygenated Kreb's bicarbonate buffer (composition: 118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 0.5 mM MgCl2, 25 mM NaHCO3, 1.0 mM NaH2PO4, and 11.0 mM D-glucose). Intestinal segments were incised longitudinally along the mesenteric border and stripped of the circular and longitudinal muscle layers by blunt dissection.
Bacterial phenotypes, growth and ex vivo inoculation
Salmonella serovar Choleraesuis strain χ3246 is a virulent porcine field isolate (Kennedy et al., 1999). Salmonella serovar Choleraesuis strain χ4522 is Δcya Δ (crp-cdt) pmi-3834 vpl+. Both strains of Salmonella were grown statically overnight in Luria-Bertani broth (LB) at 37 °C (≥2 ×108 CFU ml−1).Overnight cultures of strain χ3246 were diluted 1:100 in fresh LB media and allowed to grow in static culture at 37°C for two h until they reached the early logarithmic phase of growth (≤1 ×108 CFU ml−1).
Bacterial quantitation
To quantitate intracellular bacteria, the follicle-containing region of the tissue was incubated at 37°C under 5% CO2 in PBS containing 100 µg/ml gentamicin for 80 min to kill extracellular bacteria (Elsinghorst, ; Green et al., 2003). For assessment of total bacteria (adherent and intracellular), the follicle-containing JPP tissue was washed in three separate aliquots of PBS. Tissues were homogenized, serially diluted, and spread-plated in triplicate on differential media, XLD and Brilliant Green (BG) agar (Becton Dickinson, Sparks, MD), to differentiate Salmonella from enteric rapid lactose fermenting bacteria. Comparison of growth on XLD and BG additionally differentiated wild type and mutant Salmonella strains. Strain χ3246 grew equally well on XLD and BG and formed black, H2S positive colonies on XLD, while strain χ4522 grew better on BG and formed red, H2S negative colonies on XLD. The amount of adherent Salmonella was determined by calculating the total of adherent and intracellular Salmonella minus the number of intracellular Salmonella, in matched samples.
Ussing chamber and measurement of transepithelial ion transport
Explants of proximal jejunum containing Peyer's patches with smooth muscle layers removed, as described above, were mounted in Ussing chambers with a flux area of 2 cm2 and short circuited (O'Grady, 1996). Between four and nine tissue pieces from one animal were analyzed in each experiment. Tissues were bathed on both sides in a physiological salt solution (composition: 130 mM NaCl, 6 mM KCl, 3 mM CaCl2, 0.7 mM MgCl2, 0.29 NaH2PO4, and 1.3 mM Na2HPO4; pH 7.4) that was circulated in water-jacketed reservoirs maintained at 39 °C (porcine core temperature) and gassed with 95% O2/ 5% CO2 by gas lift. Ten mM mannitol or D-glucose was added to the luminal or contraluminal sides of the tissue, respectively. The antibiotics polymyxin B (50 µg/ml) and trimethoprim (50 µg/ml) (Fisher, Pittsburgh, PA) were added to the contraluminal bathing medium. Preliminary experiments demonstrated that addition of contraluminal antibiotics decreased the numbers of internalized commensal bacteria, but had no effect on Salmonella internalization (data not shown).
Tissues were incubated for 30–45 min for stabilization of baseline electrical parameters before bacteria were added to the luminal reservoir and permitted to interact with the mucosa for periods from 1 to 3.5 h. Short-circuit current (Isc, in µA/cm2), a measure of active ion transport, was measured continuously in each JPP tissue by an automatic voltage clamp apparatus (Model TR100, JWT Engineering, Overland Park, KS). Tissues were subjected to a 5 mV pulse every 100 sec to permit determination of tissue electrical conductance (Gt, in mS/cm2), a measure of ionic permeability, as previously described (O'Grady, 1996). Mock infected JPP controls were incubated in Ussing chambers for identical time intervals in the absence of luminal Salmonella. To assess tissue viability, active glucose-stimulated Na+ absorption was measured at the end of each experiment by the rapid elevation in Isc occurring in response to the luminal addition of 10 mM glucose. Electrophysiological data were collected and analyzed by PowerLab Chart software (Version 4.0, ADInstruments, Colorado Springs, CO).
Histology
Tissues containing JPP follicles were excised from the flux area, fixed in 10% buffered formalin, embedded in paraffin, sectioned (5–7 µm) and stained with hematoxylin and eosin. Two to three sections from each of two or three JPP samples from 2 pigs were examined at each time point.
RNA isolation
Tissues were stored in RNAlater (Ambion, Austin, TX) at −20 °C until total RNA isolation with RNeasy midi columns (Qiagen, Valencia, CA). RNA quality was determined by Agilent 2100 Bioanalyzer analysis with a RNA Nano chip (Agilent Technologies, Palo Alto, CA) and RNA 6000 ladder (Ambion, Austin, TX).
Real time quantitative RT-PCR
The relative expression of messenger RNA transcripts from specific genes was measured in triplicate by real-time quantitative RT-PCR. Reverse transcriptase reactions were performed following a modified Superscript II RNaseH- protocol (Invitrogen, Carlsbad, CA) with 2 µg of total RNA and random hexamer primers (Applied Biosystems, Foster City, CA). Approximately 30 ng of converted total RNA, diluted in 10 mM Tris-HCl, 1 mM EDTA, was mixed with the SYBR Green I Master Mix kit (Applied Biosystems, Foster City, CA), and porcine specific primers designed by Primer3 (Rozen and Skaletsky, 2000) (Table 1). The reactions were denatured at 95°C for 10 minutes followed by 50 cycles of 95°C for 15 seconds and 60 °C for 1 minute in the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). PCR efficiencies were calculated by linear regression of the log transformed, background subtracted relative fluorescence values (LinRegPCR program, ver. 7.4) (Ramakers et al., 2003), then outlier efficiency values were excluded (Bar et al., 2003), and the mean efficiency (E) was calculated (Table 1). Relative starting template fluorescence (R0) was determined using the method of Peirson et al. (Peirson et al., 2003). R0 values for specific genes were normalized by dividing with the R0 values of cyclophilin A or β2-microglobulin, and these normalized values were expressed relative to those of untreated control samples, pooled from the JPP of three pigs. To confirm amplicon specificity, the PCR products were subjected to melting curve analysis (Schmittgen et al., 2000) at 95°C for 15 seconds, 60°C for 20 seconds, then ramping from 60°C to 90°C over 20 minutes or were analyzed by agarose gel electrophoresis.
Table 1.
Porcine primer sequences for real-time quantitative RT-PCR analysis.
| Gene | Sequence 5′ to 3′ | GenBank Accession No. |
Final conc. (nM) |
|---|---|---|---|
| β2 microglobulin | F1 TCTACCTTCTGGTCCACACTGAG | L13854 | 30 |
| R1 TCATCCAACCCAGATGCA | 65 | ||
| cyclophilin A | F GCGTCTCCTTCGAGCTGTT | AY008846 | 100 |
| R CCATTATGGCGTGTGAAGTC | 100 | ||
| IL-1β | F CCTCCTCCCAGGCCTTCTGT | M86725 | 30 |
| R GGGCCAGCCAGCACTAGAGA | 65 | ||
| IL-6 | F ATTAGTACCAAAGCACTGATCC | M86722 | 100 |
| R TGAGAATGATCTTTGTGTTCTTC | 100 | ||
| IL-8 | F AACTGAGAGTGATTGAGAGTGGA | M86923 | 100 |
| R GCTGTTGTTGTTGCTTCTCAGTT | 100 | ||
| TNF α | F TTCCAGCTGGCCCCTTGAGC | X57321 | 70 |
| R GAGGGCATTGGCATACCCAC | 30 | ||
F= forward, R= reverse
Statistics
Statistical analyses of data were performed using the PRISM computer software program (Version 3.03; GraphPad Software, Inc., San Diego, CA), and analyzed by an unpaired t-test, ANOVA, or correlation where appropriate. P values less than 0.05 were considered significant.
1.1.1.4 Results
Electrophysiological and morphological characteristics of the ex vivo model
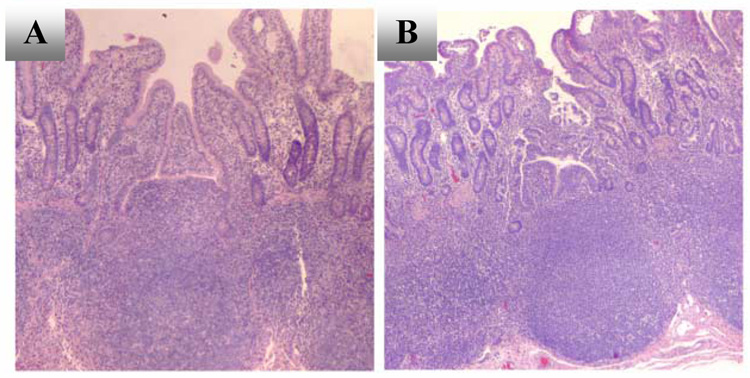
JPP tissues maintained in Ussing chambers displayed no statistically significant changes in Isc or Gt over time (Table 3). Although the difference between Gt of mock-infected and Salmonella-exposed JPP was statistically significant (Table 3), there was no significant difference in Gt before and after the addition of the Salmonella culture to the luminal reservoir (15.3 ± 0.9 mS/cm2, 15 min in the Ussing chamber, compared to 16.8 ± 0.8 mSiemens/cm2 at 1.0 h, unpaired t-test). Luminal infection of JPP with Salmonella serovar Choleraesuis did not alter Isc for periods up to 2.5 h (Table 3, two-way ANOVA). These data indicate that spontaneous active ion transport and ionic permeability were intact during this time period and Salmonella infection had no overt effect on tissue function. Histological examination of mucosal tissue indicated that after 2 h in the chamber, the epithelium was intact (Figure 1). Peak elevations in Isc after luminal addition of 10 mM glucose were not significantly different in tissues exposed to Salmonella (20.5 ± 4.0 µA/cm2, 19 JPP/ 5 pigs) or mock-infected (19.7 ± 4.7 µA/cm2, 9 JPP/ 6 pigs) at time points from 1.5 to 2.5 h in the chamber.
Table 3.
Electrophysiological parameters of JPP after Salmonella exposure
| Time in the Ussing chamber |
|||||
|---|---|---|---|---|---|
| Condition | t=30 min | t=1 h | t=2 h | t=2.5 h | n/N2 |
| Salmonella1, Gt (mS/cm2) | 16.6 ±0.7* | 16.8 ±0.8* | 16.7 ±0.9* | 16.5 ±0.9* | 34/ 8 |
| Control, Gt (mS/cm2) | 14.2 ±0.7* | 14.7 ±1.0* | 14.7 ±0.8* | 14.3 ±1.0* | 23/ 10 |
| Salmonella1, Isc (µA/cm2) | 14.8 ±2.4 | 15.7 ±2.6 | 14.0 ±2.0 | 12.6 ±2.2 | 30/ 9 |
| Control, Isc (µA/cm2) | 16.9 ±4.1 | 16.7 ±4.3 | 14.1 ±4.3 | 13.5 ±4.4 | 20/ 11 |
After a 30- minute equilibration period, Salmonella serovar Choleraesuis strain SC-54 or strain χ3246 were added to the luminal aspect of the tissue
Number of tissues (n) examined from N pigs for each condition.
p < 0.0001, control vs. Salmonella-exposed at each time point indicated, two-way ANOVA
Figure 1.
Histology of Peyer’s patch mucosa mounted in Ussing chambers and bathed in oxygenated physiological salt solution. Representative photomicrographs (40X magnification) of H & E-stained jejunal Peyer's patches in cross-section immediately after isolation (A), and after incubation in Ussing chamber for 2 h (B). Photomicrographs are representative of observations in 2–3 tissues from two pigs.
Bacterial internalization and the innate response of isolated JPP
Salmonella grown to early log phase and exposed to JPP at a density of 104–106 CFU/ml were internalized, but mRNA levels of inflammatory cytokines were not greatly altered relative to mock-infected JPP (Figure 2, low inoculum of 106 CFU/ml). Salmonella serovar Choleraesuis strain χ3246, both after early log and overnight growth, invaded JPP and induced cytokine responses which were dependent on the time of exposure. Incubation of tissue with more Salmonella (107 to108 CFU/ml) in either log or stationary growth phase for 3.5 h maximally increased mRNA levels of IL-1β, IL-6 and IL-8 in JPP (Figure 2).
Figure 2.
Virulent Salmonella serovar Choleraesuis strain χ3246 induced time dependent changes in cytokine gene expression of JPP. Salmonella grown to early log phase were added at a low inoculum (“low”, 106 CFU/ml) and Salmonella grown to early log or overnight growth were added at a 10-fold higher inoculum (“high”, 107 CFU/ml) for 1 or 3.5 h. Levels of IL-1β, IL-6, IL-8, and TNFα mRNA transcripts were measured by real-time quantitative PCR, and fold changes are expressed relative to cyclophilin A mRNA levels and an untreated control JPP. The results are representative of experiments in two pigs. Commensal bacteria were detected at 3.6 ± 0.3 log10 CFU/g (mean ± SE) in 13 of 19 JPP from two pigs.
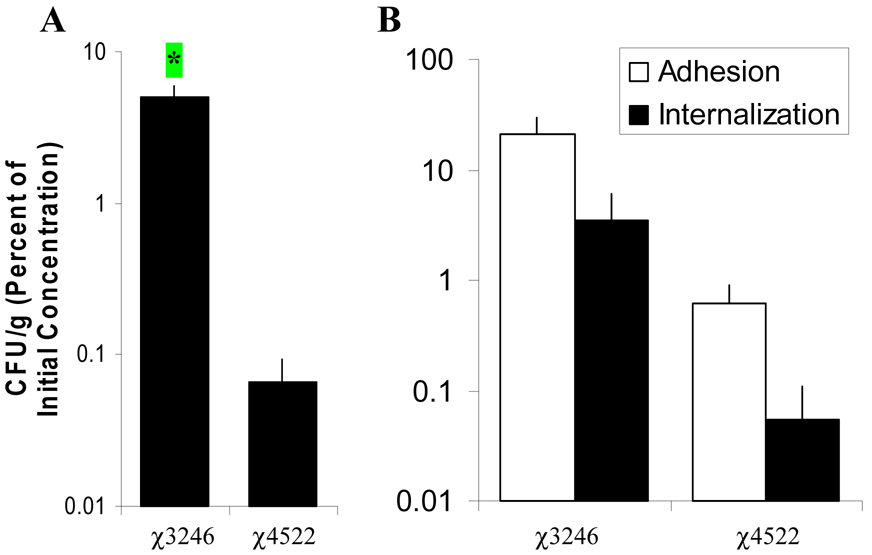
Conditions of overnight growth and a high amount (107CFU/ml) of Salmonella were selected to further characterize the interaction of Salmonella and JPP. Salmonella serovar Choleraesuis strain χ3246 was significantly internalized by JPP after contact with the luminal aspect for 2 h compared to strain χ4522, p <0.05 (Figure 3A). Strain χ3246 also adhered to JPP at a greater level than strain χ4522, although these differences were not statistically significant (3 JPP preparations from 3 pigs) (Figure 3B). No internalized Salmonella were detected in mock-infected or uninfected JPP (15 and 12 JPP/ 8 pigs, respectively). After 2 h incubation in the luminal bathing media, numbers of Salmonella serovar Choleraesuis strain χ3246 and χ4522 were increased two-fold compared to the initial inoculum (7.3 ± 0.1 log10 CFU/ml at time 0 and 7.6 ± 0.1 log10 CFU/ml at 2 h, 26 JPP/ 7 pigs). These data indicate that the bathing media supported minimal, if any, Salmonella replication.
Figure 3.
Virulent Salmonella serovar Choleraesuis strain χ3246 is internalized by JPP more efficiently than attenuated strain χ4522 after exposure 2 h ex vivo. A. Tissues were exposed on their luminal aspect to 7.3 ± 0.1 log10 CFU/ml (mean ± SE) of strain χ3246 (21 JPP/10 pigs) or strain χ4522 (11 JPP/ 7 pigs) for 2 h. * p < 0.05. Commensal bacteria were detected in 9 of 31 Salmonella -infected JPP from 10 pigs, with a mean of 3.1 ± 0.5 log10 CFU/g (mean ± SE). B. Salmonella serovar Choleraesuis strain χ4522 adheres to and is internalized poorly by JPP tissue. Tissues were exposed on their luminal aspect to 6.7 ± 0.1 log10 CFU/ml (mean ± SE) of strain χ3246 or strain χ4522, both grown overnight in LB (3 JPP/3 pigs) for 2 h. Values are CFU/g tissue after 2 h, expressed as a percentage of the Salmonella inoculum in the luminal bathing medium at time zero, and the error bars show standard error. The open bar (□) represents adherent bacteria and the solid bar (■) represents internalized Salmonella.
Indigenous enteric bacteria were detected from tissues incubated in Ussing chambers (48% of JPP) and in untreated JPP (50% of JPP). However, there was no relationship between the cytokine mRNA levels in mock-infected JPP and the numbers of commensal bacteria after 2 h incubation in the Ussing chamber, indicating that the presence of commensal bacteria did not induce changes in cytokine mRNA (Figure 4). Moreover, the numbers of internalized commensal bacteria measured in JPP were not significantly altered by the presence (3.3 ± 1.4 log10 CFU/g commensal bacteria) or absence (3.5 ± 1.1 log10 CFU/g commensal bacteria) of Salmonella.
Figure 4.
Virulent Salmonella serovar Choleraesuis strain χ3246 infection for 2 h induces increased expression of IL-1β and IL-8 mRNA compared to mock infect JPP and attenuated strain χ4522. Tissues were not mounted in the Ussing chamber (8 JPP/6 pigs untreated JPP), or mock-infected by mounting in the Ussing chamber for 2 h (13 JPP/6 pigs JPP), or luminally exposed to 7.5 ± 0.1 log10 CFU/ml (mean ± SE) of strain χ3246 (16 JPP/6 pigs) or strain χ4522 (7 JPP/4 pigs) for 2 h. Levels of IL-1β, IL-6, IL-8, and TNFα mRNA transcripts were measured by real-time quantitative PCR, and fold changes were expressed relative to β2-microglobin mRNA levels and to pooled untreated control JPP. The error bars indicate standard error. Commensal bacteria were detected in 22 of 44 JPP from 6 pigs, with a mean of 3.2 ± 0.3 log10 CFU/g (mean ± SE). * p<0.01 compared to mock-infected by unpaired t-test.
Infection with Salmonella serovar Choleraesuis strain χ3246 (14 JPP/ 7 pigs) induced a significant increase in IL-1β and IL-8 mRNA levels compared to cytokine mRNA levels in mock-infected JPP (13 JPP/6 pigs) (t-test, p<0.01) (Figure 5). Luminal exposure to the same amount of mutant strain χ4522 (7 JPP/4 pigs) induced an increase in cytokine mRNA expression, which was comparable to that of mock-infected controls. Changes in TNFα gene expression were insignificant in all treatments (Figure 5).
Figure 5.
JPP mounted in the Ussing chamber for two h express the similar amounts of IL-1β, IL-6 and IL-8 mRNA when commensal bacteria were present or not. JPP were mounted in the Ussing chamber for two h and the numbers of intracellular commensal bacteria were determined. Levels of IL-1β, IL-6, and IL-8 mRNA transcripts were measured by real-time quantitative PCR, and fold changes are expressed relative to β2-microglobin mRNA levels and to pooled untreated control JPP. Error bars represent standard error of 5 JPP without any detectable commensal bacteria (□) and 10 JPP with greater than 50 CFU/g commensal bacteria (■).
Discussion
In the present study, we have shown that IL-1β and IL-8 are important components of the innate response of porcine jejunal Peyer’s patches to Salmonella infection. Induction of IL-1β and IL-8 mRNA in ileal PP correlated with migration of neutrophils to the mucosa, 6 h after intragastric inoculation of pigs with Salmonella serovar Choleraesuis χ3246 (Hyland et al., unpublished data). Salmonella-mediated induction of IL-1β and IL-8 has been demonstrated in epithelial cell lines and in the jejunum of chickens, which do not have Peyer’s patches (McCormick et al., 1993; Withanage et al., 2004; Zeng et al., 2003). Salmonella serovar Typhimurium infection of ligated intestinal loops in mice increases mRNA levels of IL-1, TNFα, and IL-6 along with secretion of TNFα protein (Arnold et al., 1993; Klimpel et al., 1995). However, in this study, TNFα mRNA levels were not significantly increased after ex vivo infection, indicating that TNFα may not be as critical to the early response to Salmonella in pigs as it is in mice. In the ex vivo model, we observed an increase in IL-6 mRNA levels in both mock- and Salmonella-infected JPP. Thus, it may represent a tissue response to the nonspecific stress of incubation in the Ussing chamber similar to its induction in keratinocytes after skin damage for enhanced wound healing (Wang et al., 2004).
After luminal exposure to JPP ex vivo, virulent Salmonella serovar Choleraesuis strain χ3246 was internalized intracellularly within one hour of exposure. Attenuated strain χ4522 was selected since it did not protect pigs against challenge with virulent Salmonella serovar Choleraesuis (Kennedy et al., 1999). Salmonella serovar Choleraesuis strains such as χ4522, which lack cyclic AMP (cAMP) and the cAMP receptor protein (CRP) global regulatory system, are defective in transport and catabolism of carbohydrates, amino acids and peptides as well as diminished in their ability to attach to and invade cells (Botsford and Harman, 1992; Kelly et al., 1992). Although strain χ4522 is attenuated in both growth characteristics and invasion of cells, it is likely that cell adhesion and invasion are more important early in the pathogenic process. The numbers of both Salmonella strains in the luminal bathing medium did not change significantly during 2 h, increasing on average only 2-fold. In addition, growth of Salmonella serovar Typhimurium inside cells is inhibited, with one division occuring every 1.5 to 2.5 h in peritoneal macropages (Lowrie et al., 1979).
These data indicate that a high level of Salmonella adhesion and internalization was necessary for the induction of IL-1β and IL-8. These results suggest that Salmonella strains with mutations in invasion-associated genes would not be expected to induce an effective innate or adaptive response. This knowledge should aid in efforts directed toward rational vaccine design since inflammatory cytokine expression is important for the induction of adaptive immune responses (Murtaugh and Foss, 2002).
For several decades, active and paracellular solute transport has been investigated across sheets of intestinal mucosa mounted in Ussing chambers (Lindemann, 2001). In previous studies of human intestinal mucosa maintained in Ussing chambers, Isc and Gt were stable for at least 2 h and the epithelial surface remained histologically intact for at least 2.5 h (Larsen et al., 2001; Soderholm et al., 1998). Because epithelial degradation and edema occur after several hours of incubation (Figure 2 and (Larsen et al., 2001; Soderholm et al., 1998), experiments in the Ussing chamber were limited to 2 to 3 h. Glucose-stimulated Na+ absorption at the end of the incubation period demonstrated that the JPP was functionally intact (Larsen et al., 2001).
Salmonella serovar Choleraesuis exposure did not alter electrophysiological parameters, Isc or Gt. By contrast, Salmonella serovars Typhimurium and Dublin decrease transepithelial resistance (TER) across T84 or MDCK monolayers (Otte and Podolsky, 2004; Tafazoli et al., 2003). However, Salmonella serovar Typhimurium exposure did not significantly alter short circuit current or conductance of porcine ileal absorptive epithelial mucosa during a 90 min period (K.L. Scheiber and D.R. Brown, unpublished observations). Exposure of porcine JPP to Salmonella serovar Choleraesuis strain SC-54 also did not significantly alter tissue conductance (Green et al., 2003). Comparisons of Salmonella serovars Choleraesuis, Typhimurium and Dublin exposure to porcine ligated intestinal loops indicated that serovar Dublin induced maximum tissue damage, while serovar Typhimurium induced greater amounts of epithelial swelling than serovar Choleraesuis (Bolton et al., 1999; Meyerholz and Stabel, 2003). It has been suggested that the minimal cell damage induced by Salmonella serovar Choleraesuis may allow it to more easily enter the systemic circulation due to a reduced inflammatory response (Meyerholz and Stabel, 2003). These observations are consistent with and help explain the lack of effect of Salmonella serovar Choleraesuis on the electrophysiological responsiveness of intact mucosal tissue.
An ex vivo model such as the one thus described provides a controlled setting to investigate early mucosal immune responses to Salmonella and other enteric pathogens. The Ussing chamber model reduces physiological and animal-to-animal variation within each experiment, as JPP from an individual animal can be exposed to different treatment regimens. The presence of intracellular rapid lactose fermentors, which likely represent nonpathogenic intestinal commensal bacteria, in the JPP did not interfere with the studies. Mock-infected JPP demonstrated a slight increase in cytokine mRNA levels relative to untreated JPP as shown in Figure 5, but there was no relationship between the presence or absence of internalized commensal bacteria and changes in cytokine mRNA.
Inflammatory cytokine responses, which are essential to the induction of effective adaptive immunity, were rapidly increased after infection of jejunal Peyer’s patches ex vivo with virulent Salmonella serovar Choleraesuis. IL-1β and IL-8 mRNA levels were both consistently increased after Salmonella exposure, but TNFα was not changed. These results indicate that the ex vivo JPP tissue preparation retains the ability to respond innately with specific cytokine and chemokine signaling. In addition, the expression of IL-6 under all conditions of tissue incubation is consistent with a stress response that must be considered in the model.
Table 2.
PCR reaction efficiency and basal gene expression levels in untreated porcine jejunal Peyer’s patches
| Gene | Size (bp)1 |
Product Tm (°C)2 |
PCR Reaction Efficiency3 |
Relative Template Abundance4 |
Number of Pigs |
|---|---|---|---|---|---|
| β2 microglobulin | 161 | 81.77 ± 0.02 | 1.92 ± 0.01 | 2,607.0 ± 558.5 | 5 |
| cyclophilin A | 160 | 80.46 ± 0.04 | 1.61 ± 0.01 | 2,894.0 ± 405.4 | 2 |
| IL-1β | 178 | 81.20 ± 0.03 | 1.87 ± 0.01 | 19.3 ± 5.1 | 7 |
| IL-6 | 148 | 82.89 ± 0.03 | 1.57 ± 0.01 | 8.5 ± 1.3 | 7 |
| IL-8 | 157 | 79.08 ± 0.03 | 1.87 ± 0.01 | 218.7 ± 45.8 | 7 |
| TNF α | 146 | 83.78 ± 0.07 | 1.79 ± 0.01 | 46.9 ± 12.6 | 6 |
PCR product size
Tm (mean ±SE, n= 26–55 samples), determined experimentally by melting curve analysis (Schmittgen et al., 2000).
PCR reaction efficiency (mean ±SE, n= 24–53 samples)(Ramakers et al., 2003)
Relative gene abundance in 109 fluorescence units (mean ±SE, n= 2–7 pigs) (Peirson et al., 2003)
Acknowledgements
This work was partially supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant numbers 2002-35204-11705 and 2003-35205-2840 and by National Institutes of Health grant R01 DA-10200. KAH was supported by the NIH/NIDA training grant T32-DA-07239. We thank Dr. Roy Curtiss III (Department of Biology, Washington University, St. Louis, MO), who generously provided the Salmonella enterica serovar Choleraesuis strains χ3246 and χ4522, and Dr. Benedict T. Green (U.S.D.A.-Meat Animal Research Center, Clay Center, NE) for technical assistance and methods development. We would also like to thank LaRae Peterson and Colleen Finnegan for technical assistance.
Abbreviations
- AE
absorptive epithelium
- Isc
short circuit current
- GALT
gut-associated lymphoid tissue
- Gt
conductance
- JPP
jejunal Peyer’s patches
- IL
interleukin
- LB
Luria-Bertani broth
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold JW, Niesel DW, Annable CR, Hess CB, Asuncion M, Cho YJ, Peterson JW, Klimpel GR. Tumor necrosis factor-α mediates the early pathology in Salmonella infection of the gastrointestinal tract. Microb.Pathog. 1993;14:217–227. doi: 10.1006/mpat.1993.1021. [DOI] [PubMed] [Google Scholar]
- Bar T, Stahlberg A, Muszta A, Kubista M. Kinetic Outlier Detection (KOD) in real-time PCR. Nucleic Acids Res. 2003;31:e105. doi: 10.1093/nar/gng106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer's patches. Am. J. Physiol. Gastrointest. Liver Physiol. 1998;38:G130–G137. doi: 10.1152/ajpgi.1998.275.1.G130. [DOI] [PubMed] [Google Scholar]
- Bolton AJ, Osborne MP, Wallis TS, Stephen J. Interaction of Salmonella choleraesuis, Salmonella dublin and Salmonella typhimurium with porcine and bovine terminal ileum in vivo. Microbiology. 1999;145:2431–2441. doi: 10.1099/00221287-145-9-2431. [DOI] [PubMed] [Google Scholar]
- Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol. Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Weber P, Sinkora M, Baker D, Schoenherr A, Mayer B, Francis D. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J. Immunol. 2002;169:6822–6830. doi: 10.4049/jimmunol.169.12.6822. [DOI] [PubMed] [Google Scholar]
- Coates NJ, McColl SR. Production of chemokines in vivo in response to microbial stimulation. J. Immunol. 2001;166:5176–5182. doi: 10.4049/jimmunol.166.8.5176. [DOI] [PubMed] [Google Scholar]
- Cossart P. Bacterial invasion: a new strategy to dominate cytoskeleton plasticity. Dev Cell. 2004;6:314–315. doi: 10.1016/s1534-5807(04)00072-3. [DOI] [PubMed] [Google Scholar]
- Cukrowska B, Kozakova H, Rehakova Z, Sinkora J, Tlaskalova-Hogenova H. Specific antibody and immunoglobulin responses after intestinal colonization of germ-free piglets with non-pathogenic Escherichia coli O86. Immunobiology. 2001;204:425–433. doi: 10.1078/0171-2985-00052. [DOI] [PubMed] [Google Scholar]
- Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. NF-κ B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 1999;163:1457–1466. [PubMed] [Google Scholar]
- Elsinghorst EA. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 2001a;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Invest. 2001b;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BT, Lyte M, Kulkarni-Narla A, Brown DR. Neuromodulation of enteropathogen internalization in Peyer's patches from porcine jejunum. J. Neuroimmunol. 2003;141:74–82. doi: 10.1016/s0165-5728(03)00225-x. [DOI] [PubMed] [Google Scholar]
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasion SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. U.S A. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SM, Bosecker BA, Curtiss R. Characterization and protective properties of attenuated mutants of Salmonella choleraesuis. Infect. Immun. 1992;60:4881–4890. doi: 10.1128/iai.60.11.4881-4890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Yancey RJ, Sanchez MS, Rzepkowski RA, Kelly SM, Curtiss R. Attenuation and immunogenicity of ΔcyaΔcrp derivatives of Salmonella choleraesuis in pigs. Infect. Immun. 1999;67:4628–4636. doi: 10.1128/iai.67.9.4628-4636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel GR, Asuncion M, Haithcoat J, Niesel DW. Cholera toxin and Salmonella typhimurium induce different cytokine profiles in the gastrointestinal tract. Infect. Immun. 1995;63:1134–1137. doi: 10.1128/iai.63.3.1134-1137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R, Mertz-Nielsen A, Hansen MB, Poulsen SS, Bindslev N. Novel modified Ussing chamber for the study of absorption and secretion in human endoscopic biopsies. Acta Physiol. Scand. 2001;173:213–222. doi: 10.1046/j.1365-201X.2001.00865.x. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim SH, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1β and IL-18. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8815–8820. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler EM, Lemke C, Pohlenz JF. Ultrastructural study of the uptake of ferritin by M cells in the follicle-associated epithelium in the small and large intestines of pigs. Am. J. Vet. Res. 1995;56:725–730. [PubMed] [Google Scholar]
- Lindemann B. Hans Ussing, experiments and models. J. Membr. Biol. 2001;184:203–210. doi: 10.1007/s00232-001-0103-4. [DOI] [PubMed] [Google Scholar]
- Lowrie DB, Aber VR, Carrol ME. Division and death rates of Salmonella typhimurium inside macrophages: use of penicillin as a probe. J Gen Microbiol. 1979;110:409–419. doi: 10.1099/00221287-110-2-409. [DOI] [PubMed] [Google Scholar]
- Maes D, Gibson K, Trigo E, Saszak A, Grass J, Carlson A, Blaha T. Evaluation of cross-protection afforded by a Salmonella Choleraesuis vaccine against Salmonella infections in pigs under field conditions. Berl Munch Tierarztl Wochenschr. 2001;114:339–341. [PubMed] [Google Scholar]
- McCormick BA. The use of transepithelial models to examine host-pathogen interactions. Curr Opin Microbiol. 2003;6:77–81. doi: 10.1016/s1369-5274(02)00003-6. [DOI] [PubMed] [Google Scholar]
- McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J.Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immunity. N. Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Stabel TJ. Comparison of early ileal invasion by Salmonella enterica serovars Choleraesuis and Typhimurium. Vet. Pathol. 2003;40:371–375. doi: 10.1354/vp.40-4-371. [DOI] [PubMed] [Google Scholar]
- Monack DM, Hersh D, Ghori N, Bouley D, Zychlinsky A, Falkow S. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 2000;192:249–258. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Murtaugh MP, Foss DL. Inflammatory cytokines and antigen presenting cell activation. Vet. Immunol. Immunopathol. 2002;87:109–121. doi: 10.1016/s0165-2427(02)00042-9. [DOI] [PubMed] [Google Scholar]
- O'Grady SM. Assessment of Intestinal Electrolyte Transport In Vitro. In: Gaginella TS, editor. Handbook of Methods in Gastrointestinal Pharmacology. Boca Raton: CRC Press; 1996. pp. 101–121. [Google Scholar]
- Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–G626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- Pabst R, Rothkotter HJ. Postnatal development of lymphocyte subsets in different compartments of the small intestine of piglets. Vet. Immunol. Immunopathol. 1999;72:167–173. doi: 10.1016/s0165-2427(99)00129-4. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Schauser K, Olsen JE, Larsson LI. Immunocytochemical studies of Salmonella Typhimurium invasion of porcine jejuna1 epithelial cells. J Med Microbiol. 2004;53:691–695. doi: 10.1099/jmm.0.45582-0. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: Comparison of endpoint and real-time methods. Anal. Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- Schwartz KJ. Salmonellosis. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Ames, IA: Iowa State University Press; 1999. pp. 535–551. [Google Scholar]
- Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13722–13727. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm JD, Hedman L, Artursson P, Franzen L, Larsson J, Pantzar N, Permert J, Olaison G. Integrity and metabolism of human ileal mucosa in vitro in the Ussing chamber. Acta Physiol. Scand. 1998;162:47–56. doi: 10.1046/j.1365-201X.1998.0248f.x. [DOI] [PubMed] [Google Scholar]
- Spottl T, Hausmann M, Kreutz M, Peuker A, Vogl D, Scholmerich J, Falk W, Andreesen R, Andus T, Herfarth H, Rogler G. Monocyte differentiation in intestine-like macrophage phenotype induced by epithelial cells. J. Leukoc. Biol. 2001;70:241–251. [PubMed] [Google Scholar]
- Tafazoli F, Magnusson KE, Zheng L. Disruption of epithelial barrier integrity by Salmonella enterica serovar typhimurium requires geranylgeranylated proteins. Infect Immun. 2003;71:872–881. doi: 10.1128/IAI.71.2.872-881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden AW, Velasquez M, Starnbach MN. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J. Immunol. 2003;171:6742–6749. doi: 10.4049/jimmunol.171.12.6742. [DOI] [PubMed] [Google Scholar]
- Wang XP, Schunck M, Kallen KJ, Neumann C, Trautwein C, Rose-John S, Proksch E. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J Invest Dermatol. 2004;123:124–131. doi: 10.1111/j.0022-202X.2004.22736.x. [DOI] [PubMed] [Google Scholar]
- Watson PR, Gautier AV, Paulin SM, Bland AP, Jones PW, Wallis TS. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect. Immun. 2000;68:3744–3747. doi: 10.1128/iai.68.6.3744-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withanage GS, Kaiser P, Wigley P, Powers C, Mastroeni P, Brooks H, Barrow P, Smith A, Maskell D, McConnell I. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar typhimurium. Infect. Immun. 2004;72:2152–2159. doi: 10.1128/IAI.72.4.2152-2159.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Helgeland L, Farstad IN, Fukushima H, Midtvedt T, Brandtzaeg P. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J. Immunol. 2003;170:816–822. doi: 10.4049/jimmunol.170.2.816. [DOI] [PubMed] [Google Scholar]
- Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, Gewirtz AT, Neish AS. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- Zhang S, Adams LG, Nunes J, Khare S, Tsolis RM, Baumler AJ. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 2003;71:4795–4803. doi: 10.1128/IAI.71.8.4795-4803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]