Abstract
The present study evaluates the central circuits that are synaptically engaged by very small subsets of the total population of geniculate ganglion cells to test the hypothesis that taste ganglion cells are heterogeneous in terms of their central connections. We used trans-synaptic anterograde pseudorabies virus labeling of fungiform taste papillae to infect single or small numbers of geniculate ganglion cells, together with the central neurons with which they connect, to define differential patterns of synaptically linked neurons in the taste pathway. Labeled brain cells were localized within known gustatory regions, including the rostral central subdivision (RC) of the nucleus of the solitary tract (NST), the principal site where geniculate axons synapse, and the site containing most of the cells that project to the parabrachial nucleus (PBN) of the pons. Cells were also located in the rostral lateral NST subdivision (RL), a site of trigeminal and sparse geniculate input, and the ventral NST (V) and medullary reticular formation (RF), a caudal brainstem pathway leading to reflexive oromotor functions. Comparisons among cases, each with a random, very small subset of labeled geniculate neurons, revealed “types” of central neural circuits consistent with a differential engagement of either the ascending or the local, intramedullary pathway by different classes of ganglion cells. We conclude that taste ganglion cells are heterogeneous in terms of their central connectivity, some engaging, predominantly, the ascending “lemniscal,” taste pathway, a circuit associated with higher order discriminative and homeostatic functions, others engaging the “local,” intramedullary “reflex” circuit that mediates ingestion and rejection oromotor behaviors.
Indexing terms: taste, nucleus of the solitary tract, gustatory system, taste bud, virus, synaptic organization, pathways, geniculate ganglion, connections, tract tracing, cell types
The taste system begins with specialized cells of the taste buds. These receptor cells are innervated by sensory ganglion cells that transmit taste information to the brain; specifically, in mammals, to the nucleus of the solitary tract (NST) of the medulla. This nucleus, which extends for the length of the medulla, consists of a complex of cytoarchitectonic subdivisions. Previous anatomical studies have shown that the subdivisions have different connections. For example, one subdivision receives most of the input from the gustatory ganglion cells. That subdivision and others engage in various output connections, including ascending projections to higher centers (e.g., the pontine parabrachial nucleus), local intramedullary projections (e.g., connecting with the preoromotor reticular formation), and intra-NST projections (e.g., linking rostral gustatory subdivisions with caudal, general viscerosensory subdivisions; e.g., for review see Whitehead and Finger, 2008). However, these various connections have been described in only a general sense, i.e., for large populations of neurons, because of limitations associated with traditional neuroanatomical studies. The methods used to date typically labeled large populations of neurons at the various levels of the taste system. Thus, the rostral NST subdivision that receives massive input from taste ganglion cells has been identified after bulk labeling of entire gustatory nerves (see, e.g., Whitehead and Frank, 1983, in hamster; Hamilton and Norgren, 1984; May and Hill, 2006, in rat; Ganchrow et al., 2007, in mouse). Projections from the NST to other nuclei, similarly, have been mapped with large tracer injections that are often larger than any single nucleus or subnuclear domain and that spuriously label neurons that border the intended target (see, e.g., Beckstead et al., 1980, in monkey; Herbert et al., 1990, in rat; Beckman and Whitehead, 1991, in hamster). Moreover, the markers used in traditional neuroanatomical mapping studies can inadvertently label neurons unrelated to taste function by involving their fibers of passage with injected tracers. Altogether, mapping of taste pathways from the taste nerves to the NST and beyond has, until now, relied entirely on tracing connections of large populations of neurons labeled solely on the basis of their proximity to boluses of injected markers.
In contrast to previous anatomical information on connections of neuron groups, functional information about the taste pathway is available for single neurons. The neurophysiological responses of individual cells to taste stimulation have been reported for single taste bud cells, for single taste ganglion cells, and for single taste neurons of the NST and related brain regions. These single-cell responses have been most extensively studied for geniculate ganglion neurons owing to the accessibility of their single fibers for recording and the accessibility of fungi-form taste buds of the anterior tongue to applied stimuli. Physiological responses of single geniculate neurons to taste stimuli indicate clearly that taste ganglion cells are classifiable into various physiological classes. Some geniculate ganglion cells are relatively specifically tuned to one taste quality (e.g., respond best to sucrose and other sweet stimuli or to NaCl and other salty stimuli). Other geniculate cells are “generalists” (responding to multiple stimuli, e.g., to HCl, NaCl and to ionic bitter stimuli such as quinine hydrochloride; see, e.g., Pfaffann, 1955; Fishman, 1957; Frank, 1973; Formaker and Hill, 1988; Frank et al., 1988; Lundy and Contreras, 1999; Frank and Hettinger, 2005; Spector and Travers, 2005). Whether this diversity of physiological classes of ganglion cells may correlate with differential connections of anatomical classes of ganglion cells is unknown. In other sensory systems, e.g., vision, different classes of ganglion cells have been characterized physiologically and shown anatomically to project differentially in the various visual pathways (for review see Stone, 1983). In taste, parallel processing has been shown for projections of NST neurons; i.e., neurons projecting to the pons are distinct from those that project to the medullary reticular formation (Halsell et al., 1996). At the level of entire taste nerves and their differential central projections, parallel processing involving different functions has been demonstrated in fish (Atema, 1971) and mammals (for review see Spector and Travers, 2005). Conceivably, similar “parallel pathways,” beginning with individual ganglion cells, exist in the taste system. The present study evaluates the central circuits that are synaptically engaged by very small subsets of the total population of geniculate ganglion cells to test the hypothesis that taste ganglion cells are heterogeneous in terms of their central connections. Our approach is to dissect the peripheral and brainstem taste system into component parts (i.e., circuits of small groups of synaptically connected neurons) to determine whether in taste, as in other sensory systems, the ganglion cells have differential central connections.
We used a new recombinant strain of pseudorabies virus (PRV 823) to label ganglion cells and the cells with which they synapse in the brain of the mouse. This virus appears to label small sets of neurons transsynaptically and only in the anterograde direction. Thus, the viral labeling allows for analysis of the taste system anatomically in terms of subsystems of synaptically connected neurons. PRV 823-infected cells express an mRFP-VP26 (red fluorescent) fusion protein that labels the nuclei of infected cells and also is assembled into viral capsids (Olsen at al., 2006). The PRV 823 was injected into mouse fungiform taste buds on the anterior tongue in order to infect either single geniculate ganglion cells or just a few ganglion cells, together with the neurons in the brain with which they are synaptically linked. The method avoids the artefacts and imprecision associated with the “bulk” labeling that attends traditional neuroanatomical marker injections. The virus injection sites were confined to fungi-form papillae and included both their taste bud (geniculate) innervation and their general sensory peribud (trigeminal) innervation (Whitehead et al., 1985). Therefore, neurons in both the geniculate and the trigeminal ganglia were labeled in various numbers in all cases. In addition, first-order central neurons contacted by the infected ganglion cell axon endings invariably were labeled, as were synaptically linked higher order neurons in the taste and trigeminal pathways. A comparison of cases, each characterized by a small, random subset of labeled geniculate ganglion cells, revealed differential patterns of synaptically linked neurons in the taste pathway. Analysis of the central labeling patterns emphasized careful plotting and counting of labeled neurons within the nucleus of the solitary tract (NST), the first central nucleus for taste information processing, and within synaptically related brainstem nuclei.
Virus-labeled cells in the brain were localized relative to and predominated within known gustatory subdivisions in the rostral NST. Labeled cells were also located and analyzed in higher order nuclei, e.g., those known to receive synapses from the first-order sensory neurons in the NST; i.e., the virus crossed at least two synapses within the brain. Thus, cells in the various cases were identified in caudal subdivisions in the NST (i.e., in subdivisions that do not receive direct taste primary inputs), in the parabrachial nucleus (PBN) of the pons, or in the medullary reticular formation. These latter two sites figure prominently in the ascending and in the brainstem oromotor reflex gustatory pathways, respectively (for review see Whitehead and Finger, 2008). Comparisons between cases, each with a random, very small subset of labeled geniculate neurons, including correlations between labeling of cells in different brain regions, revealed at least two “types” of central neural circuits consistent with a differential engagement of the ascending or the local, intramedullary pathways by different anatomical classes of ganglion cells.
MATERIALS AND METHODS
Animals
One hundred C57/bl6 mice aged 6–16 weeks were used. All laboratory procedures were approved by the University of California at San Diego’s Laboratory Animal Care and Use Committee and followed the NIH Guide for the care and use of laboratory animals.
Virus description and production
PRV 823 is a double mutant of PRV Becker (Olsen et al., 2006). The genome harbours a nonsense mutation in the Us3 gene, which reduces virulence but has no effect on transneuronal spread of infection. In addition, PRV 823 expresses an mRFP-VP26 fusion protein that is assembled into capsids and labels infected cell nuclei intensely. PRV 823 was propagated in PK15 cells, a transformed, adherent epithelial pig kidney cell line (ATCC No. CCL-33) as described by Olsen et al. (2006). Viral stocks were prepared for injection as described by Card and Enquist (1999).
Taste papillae injections
Animals were anesthetized by intraperitoneal injection with pentobarbital, 40 mg/kg, after calming with metofane vapors. Additional injections of pentobarbital (10 mg/kg) were given as needed (approximately every 30 minutes) to maintain deep surgical anesthesia. The animal was maintained on a heating pad connected to a heat controlling unit (Microcal) and kept warm until it recovered from anesthesia. The dorsal half of the tongue was gently pulled out of the mouth and immobilized by pressing against the sticky surface of a double-sided tape attached to a tongue platform. Fungiform papillae on the anterior one-third (tip) of the tongue (near the midline) were visualized with the aid of 0.5% methylene blue solution (Fischer Scientific, Pittsburgh, PA). With a surgical microscope (Zeiss, Oberkochen, Germany) and micropipette manipulator (Fine Science Tools), taste papillae were identified, and the taste pore region was probed with a PRV 823-filled 26-g needle tip. Care was taken not to puncture too deeply into the bud. Similarly, 10–12 more buds per side of the tongue and in the immediate vicinity were injected. After the PRV tongue injections, animals were allowed to recover and were kept alive in a heated chamber. Two milliliters of 5% dextrose saline was given subcutaneously every 6 hours postsurgically. After a postinjection survival interval of 4 days, animals were anesthetized with a lethal injection of sodium pentobarbital and immediately perfused (transcardially) with 4% paraformaldehyde in normal saline. Survival times were dictated by the health of animals, which always deteriorated rapidly after 4 days, presumably owing to a general immunological reaction to infection by PRV Becker (Brittle et al., 2004). Thus, all animals were monitored very closely and euthanized during the fourth day at the first signs of deterioration but before they became moribund.
CT nerve transection
Twelve to sixteen hours after virus injection, animals were anesthetized (as described above), and the tympanic bulla and the tympanic membrane were exposed through external auditory meatus. The tympanic membrane was incised and lifted, and the chorda tympani nerve was identified, cut, and avulsed to interrupt continuous transport of the virus from tongue to geniculate ganglion (i.e., to effect a limited centrally directed “pulse” of viral transport). In most cases, the auditory ossicles were not disturbed. Similarly, in the trigeminal control experiments, the CT nerve was transected in the middle ear prior to viral injection in the tongue.
Histology and microscopy
After a postinjection survival time of 4 days, the animals were anesthetized with sodium pentobarbital and perfused through the heart with 4% paraformaldehyde in normal saline. Dissection of ganglia included the geniculate and trigeminal ganglia bilaterally. In addition, for all cases, the glossopharyngeal and vagal ganglia were harvested bilaterally as a control for spread of injected virus to the caudal tongue or, by ingestion, to the pharynx, esophagus, or gastrointestinal tract. Cases with any cell labeling in ganglia other than the geniculate or trigeminal ganglia were excluded from analysis, because any central labeling would be impossible to attribute to specific ganglia and thus uninterpretable. Tongues, brains, and ganglia were removed and left in 4% paraformaldehyde for 1–3 hours, followed by 30% sucrose incubation overnight. The posterior brain, including medulla, pons, midbrain, thalamus, and caudal two-thirds of the cerebral cortex, was blocked, frozen (using dry ice), sectioned into 40-µm slices, and mounted with Aqua-Mount (Lerner Laboratories). For most cases, every fourth section (i.e., the first, skip two, the fourth, skip two, the seventh, etc.) was analyzed with an epiflourescent microscope (Nikon Eclipse E800) for the distribution and number of virally labeled (RFP-positive) neurons. Likewise, the whole ganglion was imaged. Optical images thus obtained were exported into Adobe Photoshop for detailed analysis (see below). Modifications of the images were limited to adjustments of brightness and contrast. A few of the analyzed sections of medulla and most of the sections adjacent to those analyzed for infected neurons were counterstained with the Nissl green reagent (Molecular Probes, Eugene, OR; a neuronal cell marker) to confirm the cytoarchitectonic subdivisions containing the filled cells (Ganchrow et al., 2007; see, e.g., Fig. 3D). Once the labeled cells were localized relative to the NST and its subdivisions or to the PBN and its divisions medial, lateral, or ventrolateral to the brachium conjunctivum, they were plotted on standard schematic drawings. All labeled cells were found to be the neurons and not glia.
Fig. 3.
A,C: Labeled cells in the caudal NST (arrows) located in the caudal central (CC) subdivision. B: Nissl-stained section of the caudal NST comparable to that in A. Cytoarchitectonic features (Ganchrow et al., 2007) include dense cellularity of CC medial to the solitary tract (T), sparse cellularity of the medial (M) subdivision, and large cells of the ventrolateral (VL) subdivision (from the Allen Brain Atlas, Brain-Maps.org, Mus musculus, Nissl, coronal, data set 43, C57-C2a, section m18e). D: Fluorescent “Nissl green” counterstain of the same section as in C showing the location of T and the boundaries of CC, M, and area postrema (AP). E,F: Labeled cells (arrows) in the pontine para-brachial nucleus comlex are located medial (E) or ventrolateral (F) to the brachium conjunctivum (BC, dashed outline). The medial (M) and lateral (L) divisions of the PBN are indicated. Cells in the locus ceruleus (LC) are autofluorescent. For letters not defined see list of Abbreviations. Scale bars = 100 µm in B (applies to A–D); 100 µm in E,F.
Lucifer yellow injection of PRV-labeled neurons in the NST
In four animals, 200-µm-thick slices of 4% paraformaldehyde-fixed brains, 2–12 hr postfixation, were examined on the stage of an Axioskop 2 compound microscope (Zeiss) equipped for both light and fluorescence microscopy. Cells were illuminated briefly with fluorescent light, which revealed and allowed targetting of the PRV-mRFP labeled neurons. Individual neurons were impaled with a sharp microelectrode (60–80 MOhms) loaded with 5% Lucifer yellow (LY) CH (absorption 425 nm, emission 528 nm; Invitrogen, Carlsbad, CA) in deionized water. Iontophoretic injections, 1–3 minutes in duration with 5-second pulses of hyperpolarizing current ~1.5 nA from an AxoC-lamp 2B amplifier (Molecular Devices, Sunnyvale, CA) were made. Only PRV 823-infected cells (red) near the surface of the 200-µm slices within the rostral central (RC) subdivision of the NST (Whitehead, 1988; Ganchrow et al., 2007) were targeted and injected. The injection progress was monitored by watching the Lucifer yellow fluorescence, and final images (50 optical slices) were captured using a confocal fluorescent microscope (Zeiss LSM510 Meta with the confocal scan head inverted). All x–y optical images (corresponding to LY fluorescence) were superimposed and flattened (in the z axis) into one final image. This merged image was pseudocolored as green (LY) and overlapped (merged transparent) with red images (viral mRFP-VP26 fusion protein).
Brain mapping and neuron counting
Brains were sectioned in a standard plane, perpendicular to the long axis of the excised brain as it rests on the pons and temporal lobes. This plane is an established transverse neuroanatomical plane of section for rodents; it is identical to that employed in the preparation of a cyto-architectonic atlas of the nucleus of the solitary tract (NST) in hamster (Whitehead, 1986) and in the mouse (Paxinos and Franklin, 2001; Ganchrow et al., 2007). Serial sections (40 µm transverse) of each brain were examined for mRFP-labeled neurons at every rostrocaudal level of the medulla, the pons, the ventromedial thalamus and of the gustatory region of the cerebral cortex.
Labeled cells were counted and plotted relative to the cytoarchitectonic subdivisions of the NST at six standard, representative rostrocaudal levels of the nucleus. Sections of the most rostral three levels selected as representative through the NST were considered as encompassing the “rostral” NST; i.e., those levels receiving direct taste primary afferent input, and rostral to where the medial border of the NST reaches the wall of the fourth ventricle. The “caudal” NST was captured in the last three sections that represent the caudalmost medullary region containing labeled cells (excepting the trigeminal sensory column). These representative levels correspond, in terms of plane of section and rostrocaudal location, to those in a published mouse brain atlas (Paxinos and Franklin, 2001). Thus, the three rostral levels are 1.0 mm, 0.8 mm, and 0.5 mm rostral to the obex (see similar Paxinos and Franklin Atlas Figs. 83, 85, and 88, respectively). The three caudal levels are 0.25 mm rostral, 0.12 mm caudal, and 0.72 mm caudal to the obex (see Paxinos and Franklin Atlas Figs. 83, 85, and 88, respectively).
Certain subdivisions of the NST figure prominently in the taste pathway of rodents (Whitehead, 1988, in hamster; Harrer and Travers, 1996, in rat), including mouse (Ganchrow et al., 2007). Thus, particular attention was paid to localizing cells in the rostral central subdivision (NST-RC), where most taste primary afferent endings synapse (this region was arbitrarily divided in half medio-laterally for plotting purposes), and the rostral lateral subdivision (NST-RL), where trigeminal afferent endings that reach the NST are concentrated. Cells in other regions of the medulla, e.g., reticular formation and spinal trigeminal nucleus were plotted schematically, i.e., at the representative rostrocaudal level, but not in detail topo-graphically nor in relation to specific subdivisions. Labeled cells, when present in the pons, were plotted relative to the medial or lateral divisions of the parabrachial nucleus, i.e., on either side of the centerline of the brachium conjuntivum (PBN-M and PBN-L, respectively), or ventro-lateral to the brachium, at three representative rostrocaudal levels of the PBN complex (see similar Paxinos and Franklin Atlas Figs. 73, 75, and 78). More detailed localization, e.g., in relation to the Kolliker-fuse nucleus, or the external medial nucleus, etc., is not shown, because no thoroughgoing cytoarchitectonic atlas that correlates sub-divisions with connections is available for the mouse.
In most cases, the plots localized and counted every labeled cell in a systematic sampling of sections (every fourth, skipping 80 µm between analyzed sections; see Histology and microscopy above), and the number of cells was tallied for each region or subdivision and multiplied by 3 to obtain an estimate of total cells. Sections that intervened between those counted were examined and found to be consistent with neighboring sections in terms of the degree of labeling in the various regions, especially with regard to labeling density (or dearth) in the reticular formation and PBN. In the cases with only one or two labeled geniculate neurons, all of the fluorescent neurons in every section were plotted and counted. For every case, the resulting raw numbers were adjusted to reduce the error from “overcounting” of split cells by applying the Abercrombie factor (Guillery, 2002): T/T + h, where T = the section thickness, h = the average size of the counted objects (fluorescent nuclei) in the z axis (focal plane of the microscope). T was determined to be 47.5 µm, ave., using a recently calibrated confocal microscope (MRC1024; Bio-Rad Microscience, Cambridge, MA). The average value of h for labeled nuclei, 9.8 µm, was similar for all regions containing labeled cells. The resulting correction factor of 0.82 was multiplied times all raw estimates to obtain the values presented for each brain region in every case.
RESULTS
Fluorescent, PRV 823-infected neurons were present in the geniculate and trigeminal ganglia in all cases when the virus was injected into fungiform papillae of animals with intact innervation (Fig. 1). In addition, neurons in the brain were also labeled in all cases (Fig. 2, Fig. 3). The labeling appeared as intense fluorescence filling with bright dots the nuclei of the infected neurons as expected for PRV 823-infected cells (Olsen et al., 2006). The number of labeled cells in the ganglia was highly variable and random; i.e., the number varied (from 1 to 50+ cells) despite a consistent taste bud/papilla injection protocol. Regarding the geniculate ganglion, in rare cases, only one cell was labeled per animal (n = 3; Fig. 1A–C). Most cases analyzed had two to nine geniculate cells labeled per animal (n = 6) or 11–20 cells labeled (n = 5; Fig. 1D–F). Cases with 41–45 labeled geniculate cells were also analyzed (n = 3; Fig. 1G); Cases with 45–100+ labeled geniculate cells (n = 29) were not analyzed. With regard to the trigeminal ganglion, in all cases, cells were labeled in the mandibular division. Most cases had 10–50 labeled trigeminal ganglion cells; three cases had only three to five cells; a few cases had 50–100 labeled cells. In “trigeminal control cases” (n = 4) where the chorda tympani was cut prior to injecting the buds, there were labeled trigeminal ganglion cells in varying numbers but no labeled geniculate cells (see, e.g., Fig. 5, left column; Fig. 7A).
Fig. 1.
Numbers of virus-labeled ganglion cells differ from case to case. A–C: Cases with a single cell labeled in the geniculate ganglion (some outlined by dashes). D–G: Increasing numbers of labeled geniculate neurons in other cases. The greater petrosal nerve in A–G is indicated (asterisks). H,I: Labeled trigeminal ganglion cells in the mandibular division (asterisks). Scale bars = 50 µm.
Fig. 2.
Virus-labeled neurons in taste-related areas of the rostral medulla in cases with labeled geniculate and trigeminal ganglion cells. A: Labeled cells are concentrated in the nucleus of the solitary tract (NST; oval outline). Labeled cells are also present in the reticular formation (dashed square) and spinal trigeminal sensory nucleus, including the paratrigeminal islands (dashed circle). B: High-magnification view of labeled cells in the rostral NST concentrated in the rostral central (RC) and rostral lateral (RL) subdivisions. C: The rostral NST from a case different from that depicted in B showing many labeled cells in the lateral (RCL) and medial (RCM) halves of the rostral central subdivision. Fewer labeled cells are present in the rostral lateral (RL) and ventral (V) subdivisions; only two cells are labeled in the medial (M) subdivision. D: Nissl-stained section of the rostral NST, level comparable to that depicted in C. Cytoarchitectonic features include desnsely packed small cells in RC, sparse cellularity in M and RL, larger cells in V (Ganchrow et al., 2007; image from the Allen Brain Atlas, BrainMaps.org, Mus musculus, Nissl, coronal, data set 43, C57-C2a, section m17c). For letters not defined see list of Abbreviations. Scale bars = 100 µm.
Fig. 5.
Left: Case 4. Trigeminal ganglion labeling of many cells (but none in the geniculate ganglion) results in NST labeling primarily in RL, with no PBN labeling. Right: Case 5. Geniculate ganglion labeling of many cells results in labeling in NST (RC, especially), in RF, and in the PBN.
Fig. 7.
Summary of all cases grouped according to their “types” of central circuits labeled. A: Trigeminal ganglion cell cases: central NST labeling, when present, includes cells in RL and V, but not the pons (dashed column, all cases). B: Geniculate ganglion cell cases with central, multisynaptic labeling leading predominantly to the RF. C: Geniculate ganglion cell cases with central, multisynaptic labeling leading predominantly to the pons. D: Geniculate ganglion cell cases with mixed pontine and RF circuits. E: Single-labeled geniculate ganglion cell cases (enclosed by heavy lines), compared with pontine-and mixed-projection multiple geniculate ganglion cases. Note: Case 22 is positioned below the RF cases for layout purposes; it resembles the pontine-projection cases.
Labeled neurons in the brain were most heavily concentrated in the NST in every case with geniculate ganglion labeling (Fig. 2A–C). In addition, beyond the NST, many, but not all, of these cases exhibited labeled cells in the parabrachial nucleus of the pons (Fig. 3E,F). Cells were also labeled, to varying degrees from case to case, in the medullary reticular formation (Fig. 2A, dashed box). Labeled cells in the spinal trigeminal nucleus were localized dorsally, some in the nucleus proper (not shown) and some in the paratrigeminal islands (Fig. 2A, dashed circle); this dorsolateral topographic location was consistent across all animals. Fluorescent cells were also present lateral to the NST and laterally in the rostral NST; their numbers varied from case to case and were correlated with the degree of trigeminal ganglion cell labeling (see below). No cells were labeled rostral to the pons except for one case with a few viral-infected cells in the lateral hypothalamus. No labeled cells were seen in the thalamus or cerebral cortex in any of the cases.
Single geniculate ganglion cell connections
In three cases, only a single geniculate neuron was virally labeled (Fig. 4). The central labeling associated with those single cells was generally similar across the cases inasmuch as most of the cells labeled were located in the rostral NST and, with fewer cells, in the PBN. However, details of central labeling, e.g., in relation to the cytoarchitectonic subdivisions of the NST, differed from case to case, both topographically and quantitatively. Thus, although each case had labeled cells in the rostral central (RC) subdivision of the NST and in the medial (M) PBN, there were differences between cases in labeling of other regions: Cases 1 and 2 had labeled cells in the reticular formation; case 3 did not. Cases 2 and 3 had labeled cells in the ventral NST subdivision (V); case 1 did not. Case 2 was the only one of the three to have labeled cells in the caudal NST, in its central subdivision (CC; see also Fig. 3A–D).
Fig. 4.
Brain cells labeled in three cases, each with a singlegeniculate ganglion cell labeled. Each case has labeled cells in the NST-RC, and in the PBN-M (see list of Abbreviations). Differences between cases: cases 1 and 2 have cells in RF; cases 2 and 3 have cells in V; case 2, only, has cells in the caudal NST-CC. Case 2 has the greatest number of labeled cells in the trigeminal ganglion and in NST-RL.
With regard to differences among the cases in the numbers of central neurons labeled after single geniculate ganglion cell infection, case 2 had more cells in the rostral NST than the other cases. Case 2, with the greatest number of labeled trigeminal ganglion cells, had the greatest number of labeled cells laterally in the NST (in NST-RL).
Geniculate and trigeminal central connections compared
Trigeminal ganglion cell labeling inevitably accompanied geniculate ganglion cell labeling consequent to taste papilla virus injection. Therefore, labeled central circuits included both taste- and trigeminal-infected neurons. This mixed primary afferent nerve labeling complicated interpretation of the central labeling patterns attributable to taste pathways, because, although most trigeminal input synapses in the medullary trigeminal nucleus (not plotted in the present study), some input is known to reach lateral portions of the rostral NST in rodents (Whitehead and Frank, 1983). Eliminating trigeminal inputs by cutting the lingual (trigeminal) nerve central to its union with the chorda tympani (containing geniculate fibers that innervate fungiform taste buds) would differentiate between the two functionally distinct inputs by eliminating the former. However, this proved infeasible surgically; animals were not viable owing to the anatomical inaccessibility of the lingual nerve proper. Therefore, to control for the trigeminal contribution to the labeled central circuits in the region of the NST, four animals had their fungiform buds and papillae injected with virus immediately after cutting the chorda tympani. One such “trigeminal control” case (case 4), with heavy labeling of the trigeminal ganglion but none of the geniculate ganglion, is representative (Fig. 5). The central trigeminal labeling is heavy in the dorsal spinal trigeminal nucleus (not shown); in the NST, it is confined laterally in the nucleus, most heavily in the rostrolateral subdivision (RL), and ventrally. This distribution only overlaps laterally with that resulting from heavy geniculate (but light trigeminal) labeling (case 5; Fig. 5).
Detailed comparison of the NST-related trigeminal central labeling with predominately geniculate labeling shows significant and consistent differences. None of the trigeminal cases had labeled cells in the PBN, and none had heavy labeling in RC, which was the site of the highest concentration of labeled cells in the geniculate cases. These differences are clearly evident in comparing cases 4 and 5 (Fig. 5), notwithstanding the fact that there were twice the number of labeled trigeminal ganglion cells (in case 4) as geniculate ones (in case 5). Trigeminal labeling in RC that was present was relatively light and localized in the lateral half of the subdivision, never the medial (Fig. 5, case 4, D). No trigeminal labeling reached the medial NST subdivision. In other regions, differences were quantitative, e.g., geniculate central labeling was much heavier than trigeminal labeling in the ventral subdivision and in the reticular formation.
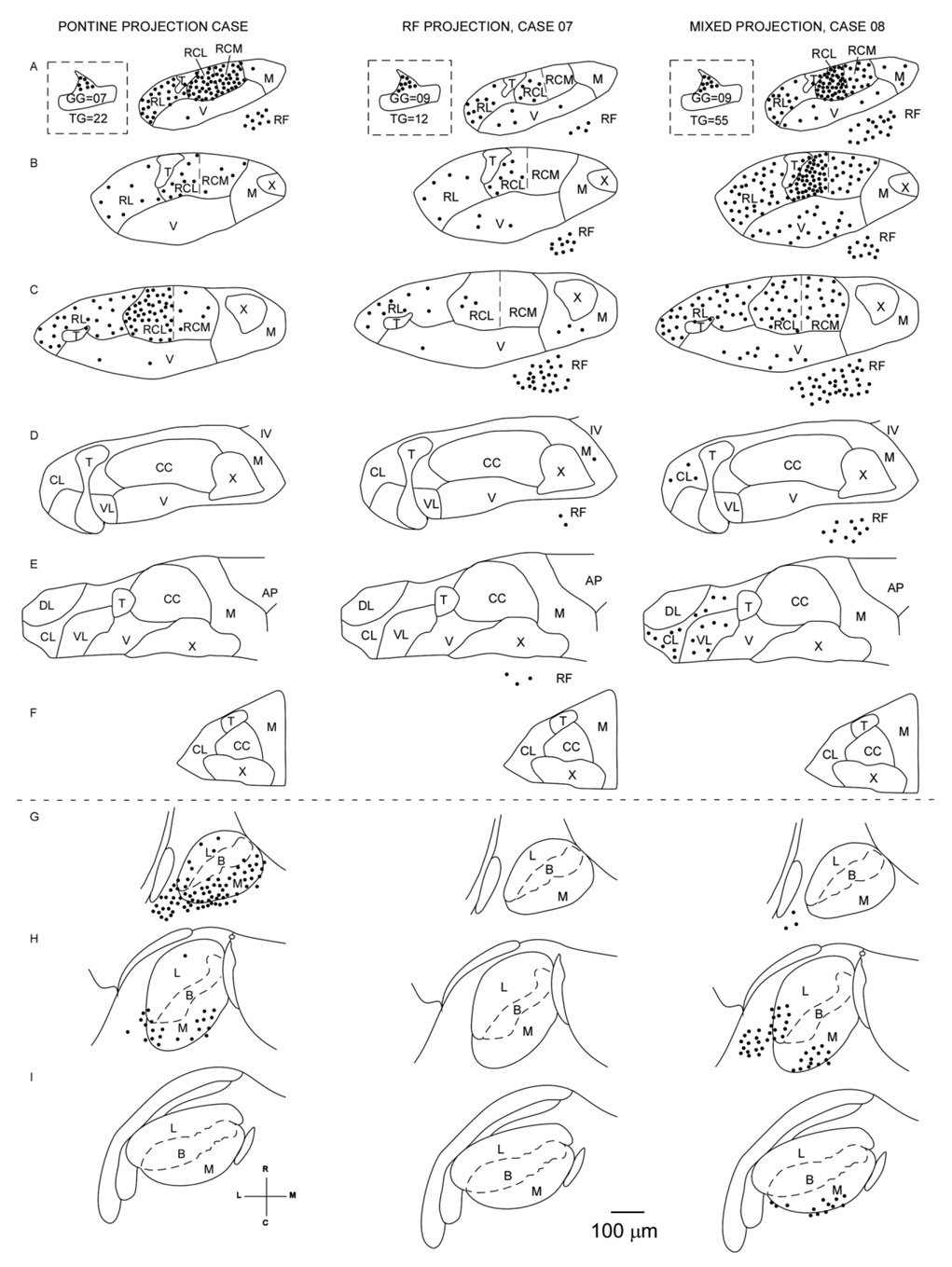
Types of central geniculate circuits
Comparing cases with similar small numbers of labeled geniculate ganglion cells revealed differential patterns of labeled central circuits within established taste pathways. This is illustrated by two cases (7 and 8), each with nine labeled geniculate ganglion cells (Fig. 6). Although in both cases the rostral central subdivision of the NST was labeled, only case 8 had heavy labeling in the pons. Pontine labeling in case 8 was concentrated in the medial subdivision of the PBN and in the lateral part of its lateral division and ventrolateral to the brachium conjunctivum. Additionally, case 8 had significant labeling of cells in the caudal NST, particularly the VL and CL subdivisions. By contrast, case 7 was entirely devoid of pontine labeling, and, with the exception of a single cell in the M subdivision, no labeling was present in the caudal NST. Both cases had a number of labeled cells in RF, but, within the rostral NST, there were qualitative and quantitative differences in labeling. In RC of case 7, cells were concentrated laterally (in RC-L) and were fewer in RC-M. In RC of case 8, the only cells present were in RCL. Both cases had infected cells in RL and V, but they were much more numerous in case 8, consistent with the greater number of labeled trigeminal ganglion cells in this case and their identified central connections (see previous section).
Fig. 6.
Three different cases (6, 7, and 8), each with a similar small population of geniculate ganglion cells labeled, differ in the extent of local (NST and RF) and lemniscal (PBN) central circuits labeled. Case 6 had many cells labeled in the medial PBN. Case 7 had considerable RF labeling but no cells labeled in the PBN. Case 8 had significant labeling in both the RF and PBN, and, uniquely, labeled cells in the caudal NST (E).
Cases 6, 7, and 8, each with a small population of infected geniculate and trigeminal ganglion cells, differed in the extent of labeling of local medullary (NST and RF) circuits or labeling of higher level pontine (PBN) central circuits (Fig. 6). Case 6, with seven labeled geniculate ganglion cells, exhibited heavy labeling of cells in the rostral central NST subdivision and in the medial PBN. Case 7, with nine labeled geniculate cells, had relatively light labeling in the rostral central NST subdivision, all in its lateral half, moderate labeling in the RF, and no cells labeled in the PBN. Thus, these two cases exemplify very different central circuits, both qualitatively and quantitatively. Case 6 involves predominately pontine projection circuits; case 7 involves exclusively local medullary circuits. In terms of numbers of labeled central neurons, case 6 exhibited many more labeled cells in the rostral central subdivision; case 7 had more labeled cells in the ventral subdivision and in the RF, the latter distributed more broadly from rostral to caudal. Virus-infected cells were more numerous in RL of case 6, consistent with its greater number of infected trigeminal ganglion cells.
To compare the differential topography of the labeled central circuits across all 22 of the cases analyzed, each case was summarized as a histogram showing the proportion of total labeled cells in the taste system that were located in each region. In addition, the cases thus summarized were classified as “trigeminal,” i.e., control cases with only ganglionic trigeminal labeling; “pontine-projection,” i.e., cases with significant PBN labeling and light RF labeling; “RF projection,” i.e., cases with significant RF labeling but little or no PBN labeling; and “mixed (RF and PBN) projection” cases (Fig. 7A–D). The three cases with labeling of single geniculate cells were similarly summarized, and their histograms are arranged in Figure 7 for comparison with the more common cases involving small sets of labeled geniculate cells (Fig. 7E). Thus, single geniculate cell case 3 exhibited multisynaptic labeling leading predominantly to the pons. By contrast, case 2 exhibited local labeling, i.e., confined primarily to the NST, uniquely (among the single geniculate cell cases) involving its caudal subdivisions (cNST).
The “pontine-projection” cases number six (Fig. 7); they exhibit similar, significant third-order neuronal labeling in the PBN subsequent to infection of a few (13 ave.) first-order (ganglion) cells. The “RF-projection” cases number four; they exhibit predominant third-order neuronal labeling in the medullary reticular formation subsequent to infection of a few (8 ave.) first-order (ganglion) cells. Labeled reticular neurons, though plotted only semi-schematically (e.g., Fig. 6), were found in several medullary regions. Most were ventral to the rostral or intermediate NST in the parvicellular and intermediate reticular nuclei. Some few were ventral to the caudal NST, lateral to the hypoglossal motor nucleus. Some, fewer, were immediately ventral to the medial border of the rostral pole of the NST. No labeled cells were located in nucleus ambiguus or the hypoglossal nucleus.
Numbers of central neurons synaptically related to geniculate neurons
PRV 823 infection of small numbers of geniculate ganglion cells always resulted in larger numbers of labeled neurons in the brain. The ratio of labeled central neurons to geniculate neurons across all 21 cases was 27:1 (26.81 ± 19.45 SD) for the medulla and 5:1 (5.0 ± 4.28 SD) for the pons (Fig. 8A), albeit with considerable variability (from three to more than 80 medullary cells per single geniculate neuron) for the few cases with one, two, or five infected GG cells. Within the medulla, the ratio of labeled cells in the RC subdivision of the NST to labeled geniculate neurons was 12:1 (12.25 ± 9.55 SD; Fig. 8B), the largest such central/peripheral ratio for any brain region or NST subdivision across all cases. Increases in GG cell numbers related systematically to increases in labeled cells in the RC subdivision of the NST and the PBN (Fig. 8C) and to increases in cells labeled in the medulla and pons generally (Fig. 8D). In contrast, labeling of GG cells did not increase systematically with labeling of trigeminal ganglion cells or of cells in trigeminal-related brain regions (i.e., RL and CL within the NST; Fig. 8E). Neither were increases in GG labeling related to increases in numbers of labeled TG or caudal NST cells. Increases in TG cells were, however, related to NST-RL, NST-CL, and V increases (Fig. 8F).
Fig. 8.
Relationship between the number of labeled ganglion cells and the number of labeled brain cells, by brain region and by prominent NST subdivisions. A,B: On average, for each GG cell labeled, 32 cells are labeled in the medulla (14 in the NST-RC), six in the pons. C,D: Increases in GG cell numbers relate systematically to increases in NST-RC, PBN, and all cells in the medulla and pons. E: Increases in labeled GG cells are not related to increases in numbers of labeled TG or caudal NST cells. F: Increases in TG cells are related to NST-RL and NST-CL increases.
Locations of central neurons related to geniculate neurons
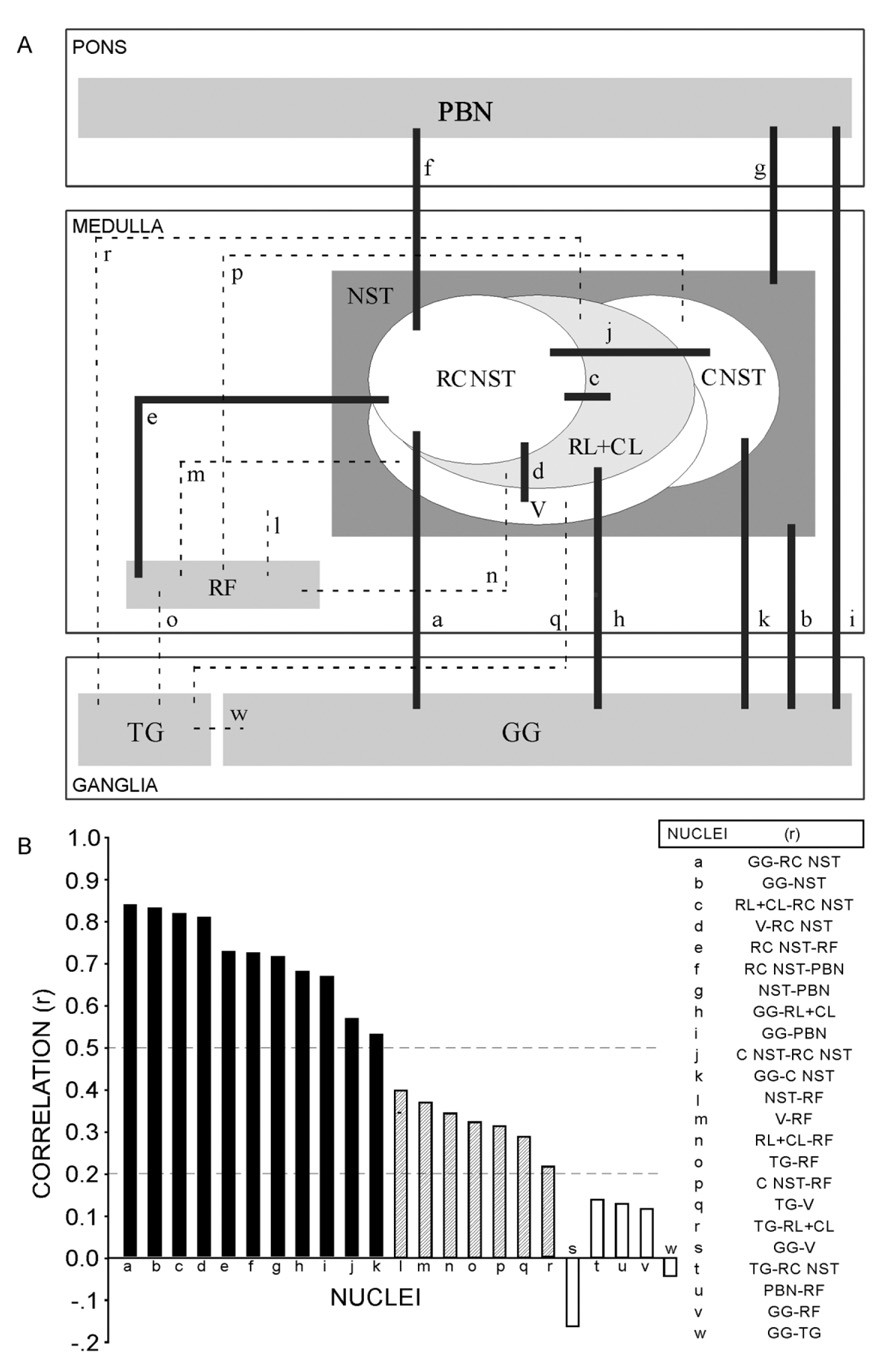
Labeling of geniculate ganglion cells is correlated most strongly with central labeling in specific regions of the dorsal medulla. Strongest correlations (Fig. 9A, heavy lines; see corresponding dark bars and r values > 0.5 in Fig. 9B) are between GG labeling and labeling of certain rostral NST subdivisions, especially RC. Within the NST, RC labeling is strongly correlated with labeling of V. RC labeling is also highly correlated with RF and caudal NST labeling. PBN labeling is strongly correlated with labeling of NST-RC, with the NST generally, and with GG. Modest correlations were between labeling of the NST generally or its ventral (V), lateral (RL and CL), and caudal (C) subdivisions and labeling in the reticular formation (RF; Fig. 9, striped bars).
Fig. 9.
Strong correlations (heavy lines in A, see corresponding r values in B) are between GG labeling and labeling of several NST subdivisions, especially RC. RC labeling is strongly correlated with labeling of V, RF, and caudal NST. PBN labeling is strongly correlated with labeling of NST-RC, of NST generally, and of GG.
Labeling of trigeminal ganglion cells was only modestly correlated with labeling in the NST (the RL, CL, and V subdivisions). Weakest correlations were between TG and NST-RC labeling, between GG and RF labeling, between RF and PBN labeling, and between GG and TG labeling (Fig. 9, open bars).
Identity of labeled neurons in the rostral NST
To provide morphological evidence that labeled cells located in the rostral NST were part of the taste pathway, their dendritic morphologies were visualized by injecting virus-containing cells with a second, contrasting marker in slices of paraformaldehyde-fixed brain. The injections of Lucifer yellow were confined to the RC subdivision of the NST, the subnuclear domain that contained the greatest number of cells across cases. This subdivision receives direct input from GG cells, and it projects most heavily to the PBN; it also projects to the RF (Ganchrow et al., 2007, in mouse). The dendritic morphologies of cells in the RC subdivision in the rodent species studied (e.g., hamster, Whitehead, 1988; Davis, 1988; Whitehead et al., 1993; and rat, Renehan et al., 1994) are characterized by neurons with elongate, stellate, and tufted dendrites. Virus-infected cells in the mouse NST-RC, when relabeled with Lucifer yellow, exhibited elongate, stellate, and tufted morphologies (Fig. 10). The filling of the cells was incomplete; i.e., tertiary dendrites and dendritic appendages (e.g., spines) were rarely filled, and the dendrites appeared thinner than they appear when seen with traditional stains (see, e.g., the Golgi stain;Whitehead, 1988). Nevertheless, the number and orientation of primary dendrites (e.g., of two dendrites aligned in the mediolateral plane of elongate cells) allowed unequivocal identification of virus-infected cells as including the three morphological types that characterize the taste afferent zone of the NST. Stellate and elongate neurons were more numerous than tufted neurons in the population of virus-containing cells successfully relabeled with Lucifer yellow.
Fig. 10.
A,B: Cells virally labeled in NST-RC (pseudocolored magenta) and subsequently filled with Lucifer yellow (pseudocolored green) are of three types. D–F: Elongate morphological type (e). G–I: Stellate type (s). J–L: Tufted type (t). Similar to cells in NST-RC of hamster (Whitehead, 1988) and rat (Renehan et al., 1994). B: Several cells of each type were doubly labeled. C: Proportion of cell types sampled. Scale bars = 10 µm.
DISCUSSION
Infection of geniculate ganglion cells with PRV 823 always resulted in labeling of brain neurons. The labeled central neurons populated caudal brainstem regions that figure prominently in the taste pathways. Compared with known connections, the patterns of labeling indicate that the PRV 823, used in this mapping of a sensory system for the first time, crosses two central synapses in the anterograde direction with the present virus application method and survival time in mouse. Circuits linked to small groups of ganglion cells differ from case to case, e.g., leading to the reticular formation, to the PBN, or to both. The data suggest that there are “types” of ganglion cells that differ in the central circuits to which they connect.
Specific anterograde viral labeling of the taste pathway
Injection of PRV 823 into anterior tongue taste papillae infected and labeled geniculate ganglion neurons; it also labeled, by central anterograde transport, neurons in regions known to receive input from geniculate primary afferent axons. Specifically, the heaviest and invariant central labeling was of neurons in the rostral central (RC) subdivision of the NST. This subnuclear area, compared with all others in the NST, is characterized by the densest concentration of synaptic endings from taste primary afferent axons; it is the major termination zone of geniculate ganglion cells (Whitehead and Frank, 1983; Whitehead, 1988, in hamster; May and Hill, 2006, in rat; Ganchrow et al., 2007, in mouse), and it is the site where taste-responsive neurons have been localized in electrophysiological (Travers and Norgren, 1986, 1995; McPheeters et al., 1990) or functional (cfos; Harrer and Travers, 1996; King et al., 1999; Travers, 2002) studies. Consistently with this, the present viral labeling of neurons in the RC subdivision was more highly correlated with geniculate ganglion cell labeling than labeling in any other NST subdivision or brain region. Moreover, the RC subdivision consistently contained more labeled cells than other areas in the brain.
Labeling of neurons in central sites beyond the NST RC subdivision involved predominantly nuclei or NST subdivisions known to receive axonal input from RC. Thus, cells were labeled in the ventral (V) subdivision and in the reticular formation (RF) ventral to the rostral NST. Both of these regions are components of the pathway leading to oromotor nuclei (e.g., the trigeminal, facial, and hypoglossal motor nuclei). Neuroanatomical tract tracing has established for rodents that RC projects to the RF directly and to the V subdivision, which, in turn, projects to the RF (Travers, 1988; Beckman and Whitehead, 1991; Ganchrow et al., 2007). The RF projects to the oromotor nuclei, but the motor neurons do not receive direct input from the NST (Travers and Norgren, 1983); neither were motor neurons labeled in the present study. This multisynaptic pathway is regarded as a “local,” intramedullary “reflex” circuit that mediates ingestion and rejection oromotor behaviors depending on the taste quality of chemicals stimulating the animal’s taste buds (see, e.g., Grill and Norgren, 1978a,b; Whitehead and Finger, 2008). Some of the labeled cells in the RF near the rostral pole of the NST could, based on their location ventral to the medial NST, include preganglionic parasympathetic neurons of the superior salivatory nucleus (Ganchrow et al., 2007). If so, they too could have been labeled by transsynaptic anterograde transport of the virus from local NST output neurons (Bradley et al., 2005). It is unlikely that preganglionic parasympathetic neurons were labeled by retrograde viral transport from the periphery unless the injected PRV spread deep into the tongue (see below).
Central labeling associated with geniculate labeling was also present rostrally in the pons. Pontine labeling was found medial, ventrolateral, and lateral or dosolateral to the brachium conjunctivum. The presence of many cells lateral in the PBN is consistent with a recent description of cells projecting to forebrain gustatory regions as localized both medial and lateral to the brachium in mouse (Tokita and Boughter, 2008). In other previously studied rodents, the medial PBN and, to a lesser extent, the lateral PBN were shown to receive direct input from cells in the rostral NST (Karimnamzi et al., 2002). The RC subdivision is the site of the majority of NST-PBN projection neurons (Whitehead, 1990; Cho et al., 2002; Ganchrow et al., 2007). Study of RC NST-PBN projection neurons indicates that they receive direct synaptic input from geniculate primary afferent taste axons and that they morphologically resemble elongate and stellate cell types that characterize the RC subdivision (Whitehead, 1990, 1993, in hamster). Thus, the viral labeling in the NST RC and in the PBN likely represents selective, transneuronal anterograde transport of virus across one and two synapses, respectively, between connected neurons within the ascending, i.e., “lemniscal,” taste pathway, a circuit associated with higher order discriminative and homeostatic functions (for review see Whitehead and Finger, 2008).
Although the patterns of central labeling with PRV 823 in the present study can be explained entirely by anterograde transsynaptic transport, we cannot definitively rule out retrograde transport with the available data. Indeed, PRV 823 is capable of retrograde spread of infection after eye injections (Olsen et al., 2006). In the present study, retrograde transport of PRV between nuclei within the brain appears unlikely. For example, no cells were labeled in agranular insular (gustatory) cortex or the amygdala. These areas project axons heavily to the rostral NST, particularly RC and V (Whitehead et al., 2000; Cho et al., 2003; Spray and Bernstein, 2004), yet they were unlabeled. Possible retrograde entry into the brain is similarly unlikely. This possible route would involve the hypoglossal or autonomic nerves. Our study evaluated the brains of the mice and all of the sensory ganglia innervating the tongue. We did not harvest the sympathetic ganglia, the thoracic spinal cord, or the submandibular parasympathetic ganglion. The latter, in mouse, presumably are located somewhere near the salivary gland ducts and the chorda lingual nerve (Ng et al., 1992, in rat). In this location, the parasympathetic ganglion, its axonal inputs, and its endings are likely located deep in the ventrolateral tongue, far from the dorsal epithelium, which was point infected. For the infection to reach the endings associated with parasympathetic neurons, the injected virus would have had to extend far from the taste buds, which were discretely probed with a fine-gauge hypodermic needle tip wet with virus, not pressure injected. Hypoglossal motor neurons innervating muscles immediately below the taste papillae were unlabeled. Given that these hypoglossal endings and tongue muscles intervene between the labeled buds and the autonomic endings related to the salivary glands (except for von Ebner’s glands, which are far posterior and ruled out insofar as the glossopharyngeal ganglia were negative), it is unlikely that the latter would be labeled when the former were not. However, we cannot rule out possible retrograde labeling of sympathetic post-ganglionics that may innervate blood vessels deep in or below the fungiform papillae below the injected bud (Wang and Chiou, 2004, in rat), This, too, is unlikely unless many synapses are crossed by the virus retrogradely: from postganglionic neuron to preganglionic in the intermediolateral horn of the thoracic cord, then to cells in the ventral medulla, ventral pons, or dorsal para-ventricular hypothalamus (see, e.g., Loewy, 1990). All of these latter regions lacked labeled cells.
Transneuronal virus transport across two central synapses
The locations of labeled cells in the brain, in all cases, are consistent with known connections of the brainstem taste pathways (see, e.g., Norgren, 1985; Whitehead and Finger, 2008). Moreover, the locations are consistent with viral transport across only two central synapses: the first between geniculate axons and RC NST neurons, the second between the RC neurons and their synaptic targets. These second-order central targets include other NST subdivisions (e.g., such caudal ones as the CC subdivision), the reticular formation, and the PBN. There is no evidence for the virus crossing a third synapse, in that anterograde labeling was never seen in the thalamus, in cerebral cortex, or in oromotor neurons. These regions are known to be at least one synapse removed from regions containing labeled cells in all of the present cases (for review see Whitehead and Finger, 2008). Conceivably, for PRV 823 to travel a longer distance in the brain to reach these sites, or to transport across an additional synapse, a survival time longer than the 4 days of the present study might be required. This was not possible owing to the poor health of the animals; they do not survive for even 5 days after tongue injection. Remarkably, despite the invariable poor health of the animals at the time of death 4 days postinjection, the infected neurons showed no histological signs of pathology. Labeling in the cells was discrete and confined to their nuclei. There was never fluorescent evidence of swollen or fragmented cells and never evidence of free virus or fluorescence anywhere but within the nuclei of neurons, the locations of which were invariably explicable based on known connections. Thus, the illness of the animals appears to be one of general immunophysiology, not neuropathology (see also Brittle et al., 2004); it may be a limitation to how extensively PRV can label a pathway. A further technical limitation that might account for the lack of labeling in the thalamus, hypothalamus, and cerebral cortex is that PRV 823 may be unable to infect the types of neurons in those regions owing to cell-selective features of the virus or variability in transport by different neurons.
Although the patterns of central labeling can be explained as the virus crossing one or two central synapses, it is not always possible to identify any particular labeled neuron as a first- or second-order central. Based on known connections, it is certain that cells in the PBN are not first-order central. Therefore, it is certain that virus-labeled PBN cells were infected by PRV 823 crossing more than one central synapse. However, among the many cells labeled in the RC subdivision of the NST, it cannot be determined which cells are first-order, i.e., receive direct input from primary afferent axons, and which might be receiving input secondarily, i.e., via synaptic endings from nearby first-order cells. The identity of labeled medullary neurons, defined as their position in the sequences of synapses of the taste pathway, can reasonably be inferred by comparison with published descriptions of the principal connections of the rodent taste system. Thus, for example, whereas some viral labeled cells in the rostral NST receive direct geniculate input and project outside the NST to the PBN, other labeled cells could be interneurons, labeled by intra-NST short axon connections (Travers and Hu, 2000). Nevertheless, It is likely that many of the labeled cells of RC NST are first-order central pontine-projection cells. This conclusion is based on the morphological resemblance of the double-labeled (virus and Lucifer yellow) RC neurons to elongate and stellate PBN projection neurons and on previous evidence that these morphological cell types receive direct input from geniculate axons (Whitehead, 1986, 1993). Moreover, a high proportion of taste-responsive cells in the rostral NST can be activated anti-dromically by stimulating the PBN (Cho et al., 2002, in hamster). However, we cannot rule out the possibility that some labeled neurons in RC are, instead, engaged in short, intranuclear connections. Additionally, we cannot rule out possible transfer of the virus across more than two central synapses over short distances, e.g., within the confines of the NST, even with the present 4-day survival. Nevertheless, the pattern and quality of central labeling indicatesd that PRV 823 is well confined in every case to a discrete subset of the neurons that are synaptically linked as part of the taste pathway.
Numbers of central neurons connected to a taste ganglion cell
Comparing the numbers of neurons labeled in the geniculate ganglia with the numbers of neurons labeled in the brain provides clear evidence of the degree to which taste information is disseminated in central circuits in the mouse. In general, there is considerable, if variable, divergence from relatively few labeled peripheral neurons to many central neurons. The single-cell cases illustrate this. Leaving aside central labeling in the RL NST subdivision because that labeling is dominated by trigeminal labeling, the three single-labeled geniculate cell cases have 4, 10, and 35 neurons labeled in the brain. Across all cases, the ratio of central to geniculate neurons was 32:1 (±23.52 SD). Although this latter ratio includes some central cells labeled via infected trigeminal inputs, some of the large variability relates to the large number of labeled medullary cells per GG cells in the few cases with only one, two, or five labeled GG cells (open circles at the left in Fig. 8A). Conceivably, whereas axon endings of individual GG cells may diverge widely in the NST, for cases with many labeled GG cells many may converge onto the same postsynaptic neuron, resulting in fewer brain neurons labeled per GG cell than for those cases with just a few GG cells labeled.
Within the RC subdivision where taste primary afferent axons synapse, the ratio of labeled cells to labeled geniculate neurons is 12:1 (12.25 ± 9.55 SD). Even with the acknowledgment that some labeled cells in RC may be one synapse beyond the primary afferent synapse, there is clear evidence for substantial divergence of the inputs provided to the NST by peripheral ganglion cells. It is likely that a single geniculate ganglion cell provides input directly to 10 or more NST neurons. This conclusion fits with previous, more traditional neuroanatomical labeling (HRP, Golgi staining) of individual geniculate axons, which in the rodent were shown to ramify extensively in the NST RC; they bore many swellings suggestive of multiple synaptic endings (Whitehead and Frank, 1983). The swellings were shown by electron microscopy to be axonal endings synapsing with NST neurons (Whitehead, 1986). Such divergence of primary inputs and possible convergence of some primary inputs onto second-order neurons, suggested by the present counts, may underlie the broader specificity of central vs. peripheral taste neurons (Lemon and Smith, 2005).
The spread of information from single ganglion cells to brain, demonstrated here with spread of virus, reflects a very specific input from the lingual receptive field. In the mouse, each anterior tongue (fungiform) taste bud is innervated by only three to five geniculate ganglion cells (Zaidi and Whitehead, 2006). Moreover, with rare exceptions, each single ganglion cell innervates only a single taste bud. Consistently with this numeric relationship between ganglion cells and buds, in the cases analyzed here the numbers of ganglion cells labeled after point infecting 11–13 buds did not exceed 50. The specific number of labeled ganglion cells in each case, however, was typically fewer than 50, sometimes only one, notwith-standing a consistent protocol of point injecting 11–13 buds. This variability reflects unknown factors at the initial site of infection that influence the propensity of neurotrophic viruses to invade the peripheral fibers of the ganglion cells. Nevertheless, the high correlation between numbers of infected ganglion cells and numbers of infected NST neurons (e.g., Fig. 8 C, Fig. 9, GG-RC NST) suggests that, although the infection of peripheral neurons is random and variable, the infection of central neurons is a consistent quantitative reflection of the degree of ganglion cell labeling. Across all cases, numbers of infected central neurons markedly exceed numbers of infected peripheral neurons. Therefore, the peripheral to central taste circuitry that defines the anterior tongue taste system is characterized by ganglion cells with extensive central spread of information, derived from the activity of a single taste bud, disseminated to many NST neurons. The purpose of this arrangement is unclear, but the picture that emerges of the geniculate ganglion cell’s wiring is that it has a peripheral fiber that is unbranched but a central axon that branches extensively. As a consequence, a single taste bud has multiple representations in the NST of the brain.
Types of taste circuits
Differences in the patterns of central labeling indicate that geniculate ganglion cells are heterogeneous in terms of the circuits with which they synapse in the brain. The single ganglion cell cases and those with small numbers of labeled ganglion cells alike showed differences in the distributions of the virus-containing central neurons both topographically and in terms of the sites with the greatest proportions of labeled neurons. At the same time, although these differences between cases were apparent, all cases labeled cells that, with the exception of the trigeminal cells, were invariably located somewhere within the taste pathway, generally defined. Thus, for example, some cases with a few labeled geniculate ganglion cells had labeled cells in the NST and PBN, whereas others had labeled cells in the NST and RF (Fig. 11). Some cases labeled cells in the caudal NST; others did not. Cases differed and were classifiable regarding type based on the density or numeric proportions of neurons in either the PBN or the RF. If ganglion cells were not of different types, in terms of their central connections and circuits across at least two synapses, then all the data on transneuronal transport of PRV would be identical across animals. This was not the case. In terms of the central connectivity, the present data suggest anatomical heterogeneity of taste ganglion cells.
Fig. 11.
Two different circuits synaptically engaged by ganglion cell inputs. Large circles represent the locations and relative numbers of virus-labeled cells in the circuit that predominantly contributes either to the “ascending lemniscal” taste pathway (red) or to the “local reflex” pathway (blue). Geniculate ganglion cells in mouse innervate a single taste bud in the periphery. Centrally, some ganglion cells synapse (small circles at ends of axon branches) in the rostral central subdivision (RC) of the NST with many PBN-projection neurons, the axons of which, in turn, synapse with somewhat fewer cells in the parabrachial nucleus. Other ganglion cells synapse in RC-NST with intramedullary projection neurons, the axons of which synapse with neurons in the preoromotor brainstem reticular formation.
ACKNOWLEDGMENTS
L.W.E. acknowledges support from the Center for Behavioral Neuroscience Viral Tract Tracing Core at Georgia State University through the STC Program of the National Science Foundation under agreement No. IBN-9876754 to L.W.E. and Tim Bartness. We also gratefully acknowledge statistical guidance provided by Dr. Judith Ganchrow and graphic and analytical support provided by Nicholas Warner.
Grant sponsor: National Institutes of Health; Grant number: RO1 DC01091 (to M.C.W.); Grant number: R01 33506 (to L.W.E.); Grant number: NCRR P40 RR0118604 (to L.W.E.).
Abbreviations
- CC
caudal central subdivision of NST
- CL
caudal lateral subdivision of NST
- CT
chorda tympani nerve
- GG
geniculate ganglion
- K
Kolliker Fuse nucleus (of pons)
- M
medial subdivision of NST
- NST
nucleus of the solitary tract
- PBN
parabrachial nucleus (of pons)
- PBN-L
parabrachial nucleus, lateral division
- PBN-M
parabrachial nucleus, medial division
- RC
rostral central subdivision of NST
- RC-L
rostral central subdivision, lateral half
- RC-M
rostral central subdivision, medial half
- RF
reticular formation (of medulla)
- RL
rostral lateral subdivision of NST
- TG
trigeminal ganglion
- V
ventral subdivision of NST
- X
vagal motor nucleus
- XII
hypoglossal nucleus
LITERATURE CITED
- Atema J. Structures and functions of the sense of taste in the catfish (Ictalurus natalis) Brain Behav Evol. 1971;4:273–294. doi: 10.1159/000125438. [DOI] [PubMed] [Google Scholar]
- Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res. 1991;557:265–279. doi: 10.1016/0006-8993(91)90143-j. [DOI] [PubMed] [Google Scholar]
- Bradley RM, Fukami H, Suwabe T. Neurobiology of the gustatory-salivary reflex. Chem Senses. 2005;30:70–71. doi: 10.1093/chemse/bjh118. [DOI] [PubMed] [Google Scholar]
- Brittle EE, Reynolds AE, Enquist LW. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol. 2004;78:12951–12963. doi: 10.1128/JVI.78.23.12951-12963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Enquist CW. Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci. 1999 Suppl 9:1–27. doi: 10.1002/0471142301.ns0105s68. Unit 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YK, Cheng-Shu L, Smith DV. Gustatory projections from the nucleus of the solitary tract to the parabrachial nuclei in the hamster. Chem Senses. 2002;27:81–90. doi: 10.1093/chemse/27.1.81. [DOI] [PubMed] [Google Scholar]
- Cho YK, Cheng-Shu L, Smith DV. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem Senses. 2003;28:155–171. doi: 10.1093/chemse/28.2.155. [DOI] [PubMed] [Google Scholar]
- Davis BJ. Computer-generated rotation analyses reveal a key three-dimensional feature of the nucleus of the solitary tract. Brain Res Bull. 1988;20:545–548. doi: 10.1016/0361-9230(88)90213-4. [DOI] [PubMed] [Google Scholar]
- DeFalco F, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Frank ME. Analysis of hamster afferent taste nerve response functions. J Gen Physiol. 1973;61:588–618. doi: 10.1085/jgp.61.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Bieber SL, Smith DV. The organization of taste sensibilities in hamster chorda tympani nerve fibers. J Gen Physiol. 1988;91:861–895. doi: 10.1085/jgp.91.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchrow D, Ganchrow J, Warner N, Whitehead MC. The mouse NST: a cytoarchitectonic atlas. AchemS Abstr. 2007;512 [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978a;143:263–279. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978b;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Halpern BP, Nelson LM. Bulbar gustatory responses to anterior and to posterior tongue stimulation in the rat. Am J Physiol. 1965;209:105–110. doi: 10.1152/ajplegacy.1965.209.1.105. [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience. 1996;72:185–197. doi: 10.1016/0306-4522(95)00528-5. [DOI] [PubMed] [Google Scholar]
- Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 1996;711:125–137. doi: 10.1016/0006-8993(95)01410-1. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Karimnamizi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- King CT, Travers SP, Rowland NE, Garcea M, Spector AC. Glosso-pharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci. 1999;19:3107–3121. doi: 10.1523/JNEUROSCI.19-08-03107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol. 2005;94:3719–3729. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. J Comp Neurol. 2006;497:658–669. doi: 10.1002/cne.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters M, Hettinger T, Nuding S, Savoy L, Whitehead MC, Frank M. Taste-responsive neurons and their locations in the solitary nucleus of the hamster. Neuroscience. 1990;34:745–758. doi: 10.1016/0306-4522(90)90179-8. [DOI] [PubMed] [Google Scholar]
- Morganti JM, Odegard AK, King MS. The number and location of fos-like immunoreactive neurons in the central gustatory system following electrical stimulation of the parabrachial nucleus in conscious rats. Chem Senses. 2007;32:543–555. doi: 10.1093/chemse/bjm023. [DOI] [PubMed] [Google Scholar]
- Ng YK, Wong WC, Ling EA. The intraglandular submandibular ganglion of postnatal and adult rats I. A light and electron microscope study. J Anat. 1992;180:305–314. [PMC free article] [PubMed] [Google Scholar]
- Norgren R. Taste and the autonomic nervous system. Chem Senses. 1985;10:143–161. [Google Scholar]
- Olsen LM, Ch’ng TH, Card JP, Enquist LW. Role of pseudorabies virus Us3 protein kinase during neuronal infection. J Virol. 2006;80:6387–6398. doi: 10.1128/JVI.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in sterotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Pfaffman C, Erickson RO, Frommer GP, Halpern BP. Gustatory discharges in the rat medulla and thalamus. In: Rosenblith WA, editor. Sensory communication. New York: Wiley; 1961. pp. 445–473. [Google Scholar]
- Pfaffman C, Frank M, Norgren R. Neural mechanisms and behavioral aspects of taste. Annu Rev Psychol. 1979;30:283–325. doi: 10.1146/annurev.ps.30.020179.001435. [DOI] [PubMed] [Google Scholar]
- Renehan WE, Jin A, Ahang X, Schweitzer L. Structure and function of gustatory neurons in the nucleus of the solitary tract. I. A classification of neurons based on morphological features. J Comp Neurol. 1994;347:531–544. doi: 10.1002/cne.903470405. [DOI] [PubMed] [Google Scholar]
- Smith GA, Gross SP, Enquist LW. Herpes viruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A. 2001;98:3466–3470. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4:143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- Spray KJ, Bernstein IL. Afferent and efferent connections of the parvicellular subdivision of iNST: defining a circuit involved in taste aversion learning. Behav Brain Res. 2004;154:85–97. doi: 10.1016/j.bbr.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Stone J. The classification of retinal ganglion cells and its impact on the neurobiology of vision. New York: Plenum Press; 1983. Parallel processing in the visual system. [Google Scholar]
- Tokita K, Boughter JD. Afferent and efferent connections of the parabrachial nucleus in the c57BL/6J mouse. International Symposium on Olfaction and Taste. 2008 Abstr 456. [Google Scholar]
- Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res. 1988;457:1–11. doi: 10.1016/0006-8993(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci. 1986;4:544–555. doi: 10.1037//0735-7044.100.4.544. [DOI] [PubMed] [Google Scholar]
- Travers SP. Quinine and citric acid elicit distinctive Fos-like immunoreactivity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1798–R1810. doi: 10.1152/ajpregu.00590.2001. [DOI] [PubMed] [Google Scholar]
- Travers SP, Hu H. Extranuclear projections of rNST neurons expressing gustatory-elicited fos. J Comp Neurol. 2000;427:124–138. [PubMed] [Google Scholar]
- Wang H-W, Chiou W-Y. Sympathetic innervation of the tongue in rats. J. Otorhinolaryngol Rel Spec. 2004;66:16–20. doi: 10.1159/000077228. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Anatomy of the gustatory system in the hamster: synaptology of facial afferent terminals in the solitary nucleus. J Comp Neurol. 1986;224:72–85. doi: 10.1002/cne.902440106. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol. 1988;276:547–572. doi: 10.1002/cne.902760409. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Subdivisions and neuron types of the nucleus of the solitary tract that project to the parabrachial nucleus in the hamster. J Comp Neurol. 1990;301:554–574. doi: 10.1002/cne.903010406. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Distribution of synapses on identified cell types in a gustatory subdivision of the nucleus of the solitary tract. J Comp Neurol. 1993;332:326–340. doi: 10.1002/cne.903320306. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Finger TE. Gustatory pathways in fish and mammals. In: Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, editors. The senses: a comprehensive reference, vol 4, olfaction and taste. San Diego: Academic Press; 2008. pp. 237–260. [Google Scholar]
- Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol. 1983;220:378–395. doi: 10.1002/cne.902200403. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Beeman C, Kinsella B. Sensory endings of taste and general sensory nerves in fungiform papillae of the hamster. Am J Anat. 1985;173:185–201. doi: 10.1002/aja.1001730304. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, McPheeters M, Savoy LD, Frank ME. Morphological types of neurons in taste-responsive sites in the nucleus of the solitary tract in the hamster. Microsc Res Techniq. 1993;26:245–259. doi: 10.1002/jemt.1070260307. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Bergula A, Holliday K. Forebrain projections to the rostral nucleus of the solitary tract in the hamster. J Comp Neurol. 2000;422:429–447. [PubMed] [Google Scholar]
- Zaidi F, Whitehead MC. Discrete innervation of murine taste buds by peripheral taste neurons. J Neurosci. 2006;26:8243–8253. doi: 10.1523/JNEUROSCI.5142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]