Abstract
Transcranial magnetic stimulation (TMS) is a safe and easy technique for stimulating neurons in the human central nervous system. Studies combining TMS with drugs in healthy subjects and patients have advanced our knowledge of how TMS activates brain circuits and led to new techniques for evaluating the function of specific systems. For example, TMS techniques can detect effects on axon membranes, glutamatergic and GABAergic synapses and the influence of catecholaminergic systems, as well as group differences due to genetic variations in the response to drugs. With this knowledge base, TMS can now be used to explore and compare the effects of drugs on brain systems and may also serve as a surrogate for behavioral responses in clinical trials.
Keywords: Transcranial Magnetic Stimulation, Single Pulse, Transcranial Magnetic Stimulation, Paired Pulse, Drug Physiological Effects, Neurotransmitter Agents, GABA, Glutamate, Monoamines, Biogenic
Introduction
Central nervous system (CNS) pharmacology and drug discovery/development typically rely on testing in vitro or animal models to measure the effects of compounds on neuronal processes and circuits. When behavioral effects in humans are the targeted outcome, clinical effects may be very hard to measure, especially over short time spans and in small experimental samples. Therefore, reliable and well-understood physiological surrogates for effects on neurotransmitter systems and circuits in the human brain could be very useful. Non-invasive probes of brain activity, such as electroencephalography, cortical evoked potentials and functional imaging can provide information, but have generated few reliable or quantitative surrogates to measure specific effects on brain systems, so far.
Transcranial magnetic stimulation (TMS) is a safe and painless technique for evoking activity in neurons in the human brain through the intact scalp and skull [1–4]. It is well within the technical and economic reach of small laboratories. Since its introduction in the mid-1980s [3], it has evolved as a neurophysiological tool and possible therapeutic intervention [1,4–6]. A rapidly changing magnetic field, applied to the head surface using a wire coil (Fig. 1), passes unimpeded through the high resistance tissues of the head and induces electrical currents in the conductive medium of the brain. These currents can depolarize neurons, ultimately evoking readily measurable phenomena, such as muscle activation. The ability of TMS to produce discrete and graded activations in well-understood cortical circuits and thereby evoke quantifiable physiological responses provides a unique opportunity to study the effects of substances on neurons and synapses in the human brain.
Fig. 1.

The primary current in the stimulating coil induces a parallel and oppositely directed current in the cortex. From reference [1] with permission.
The physiology of TMS
While it may be tedious for non-physiologists, understanding how TMS can be used to measure drug effects in the CNS requires a somewhat detailed understanding of how it works.
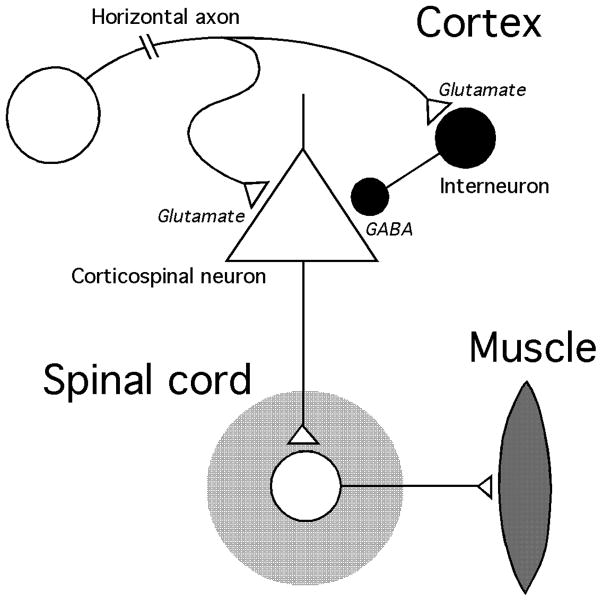
The current induced by TMS in the brain is oriented parallel to the coil and stimulates neuronal processes that lie in the same plane. Therefore, when the coil is held tangentially on the head, there is preferential depolarization of horizontally oriented axons in the cortex. These axons consist primarily of excitatory (glutamatergic) inputs to the cortex and inhibitory (GABAergic) interneurons that regulate local excitability via very short axons [7] (Fig. 2). Because of the exponential decay of the magnetic field with distance, essentially all of the axon firing produced directly by the stimulating field is thought to occur in the cortex near the surface of the brain [8]. The axons of cortical output neurons (also called pyramidal cells), including those projecting to the spinal cord, subcortical structures, and other cortical areas, are oriented radially, more or less perpendicular to the plane of the stimulating current. Therefore, pyramidal neurons are affected by TMS mainly via synaptic input from horizontally oriented elements.
Fig. 2.
Schematic representation of the motor cortex output circuit stimulated by TMS.
The measurable effects of TMS, such as the muscle twitches produced by stimulation of the motor cortex, depend on a chain of neuronal and synaptic events starting in horizontal axons and ending at an effector organ, e.g., a muscle (Fig. 2). The size of the response depends on the intrinsic excitability of the neurons in the pathway and the status of its synapses. When one component is manipulated in isolation, the effect is reflected in the overall response to stimulation.
TMS in the motor cortex
Most of the detailed physiology and virtually all the pharmacology performed with TMS have been done in the primary motor cortex (M1), where stimulation at moderate intensity produces graded and quantifiable twitches in muscles on the opposite side of the body. The threshold intensity for evoked activity is lowest in hand muscles, where the accompanying electrical activity, or motor evoked potential (MEP), is easily recorded, displayed, and analyzed with automated algorithms.
Measures of corticospinal excitation
MEP amplitude
The amplitude of the MEP (Fig. 3) reflects the status of the excitatory horizontal axons, corticospinal neurons, spinal motoneurons, and muscle fibers, as well as the efficiency of the excitatory synapses connecting them. The efficiency of each neuron in the pathway as a transmitter or amplifier of excitatory activity is a function of the tonic excitatory and inhibitory inputs impinging on it. For example, if the subject makes a small muscle contraction, driving the non-firing fraction of corticospinal and spinal motoneurons nearer their firing threshold with increased excitatory input, the MEP grows in size. The majority of pharmacological manipulations of this system operate mainly at the cortical, rather than the spinal level, but it is often impossible to exclude a small spinal contribution.
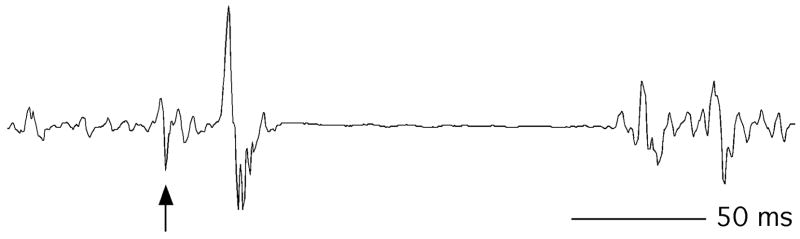
Fig. 3.
Motor evoked potentials from a single suprathreshold TMS pulse (Test alone) and subthreshold (conditioning)-suprathreshold pairs at 3 and 10 ms intervals. Arrows show stimulus artifacts.
The excitatory activity produced by M1 stimulation can be measured intra-operatively by epidural recordings from the cervical spinal cord [9]. These recordings show an initial small event (“direct” or D wave [10]) reflecting direct activation of the cortical output cells, followed by a series of larger deflections (“indirect” or I waves) representing repeated trans-synaptic activation of the same group of neurons [11]. The mechanism of this repeated firing is not completely clear, but probably results from recurrent glutamatergic excitatory activity within the cortex [10]. I waves are suppressed by benzodiazepines [12], indicating sensitivity to GABAA-mediated inhibition. D and I waves summate temporally at the spinal segment and activate spinal motor neurons which generate the MEP.
MEP threshold and recruitment
The minimum stimulus intensity needed to evoke a just-measurable MEP in a resting muscle (resting motor threshold, RMT) is a commonly used measure of excitability in the corticospinal output pathway. Axonal excitability is determined by the state of cation channels in the membrane. The excitability of neurons is set, not only by their membrane channels, but also by the balance of excitatory and inhibitory input, as discussed above. The last, but most important component, synaptic efficiency, can be altered pharmacologically, but also temporarily by exercise and motor practice [13]. Despite its many determinants, RMT is generally quite stable within individuals [14].
The stimulus-response (SR) or “recruitment” curve, plotting MEP amplitude against a range of stimulation intensities, provides additional information on the effectiveness of the recurrent excitatory process that results in I waves and large MEPs [15].
Measures of evoked inhibition and paired-pulses techniques
In addition to the excitatory mechanism that produces the MEP, TMS also activates inhibitory cortical circuits containing GABAergic interneurons (Fig. 2). This activity can be measured in several ways.
The cortical silent period and long interval intracortical inhibition
The TMS silent period is the momentary halt in muscle activity observed after a TMS pulse is delivered to M1 during a voluntary muscle contraction [16] (Fig. 4). The cortical phase of this phenomenon (cortical silent period, CSP) [17] lasts up to about 200 ms [18], depending on the intensity of the stimulating pulse, and overlaps partially with a shorter period of spinal inhibition [19]. Recruitment curves relating duration of CSP to stimulation intensity [15] can be used to further characterize the CSP. Its duration is roughly consistent with the inhibitory postsynaptic potential (IPSP) elicited by GABAB receptor activation in pyramidal neurons [20] and it is prolonged by drugs that enhance GABA action at GABAB receptors [21,22]. Therefore, it appears to be a GABAB-mediated phenomenon, especially at higher stimulus intensities [23], although GABAA inhibition coincides with its early phase (see below) and contributes to its duration at low intensities [23].
Fig. 4.
TMS silent period. Arrow shows stimulus artifact.
Essentially the same effect can be detected in resting muscles by delivering two pulses of the same intensity in short succession (≤ about 200 ms) and observing a decrease in the amplitude of the second MEP [24,25]. This effect has been referred to as long interval intracortical inhibition (LICI) and is due to GABAB-mediated diminution of I waves [16,26,27].
Short interval intracortical inhibition
Pairs of suprathreshold pulses cannot be used to study phenomena in the first few ms after the first pulse, because the MEP from the second pulse is obscured by the MEP from the first. Thus, in order to study early inhibition, “test” MEPs can be evoked a few ms after a pulse too weak to produce its own MEP. MEP amplitude is substantially reduced when the test pulse is delivered 1– 6 ms after subthreshold stimulation [28] (Fig. 3). This effect is heightened by benzodiazepines and other drugs that enhance GABAA inhibitory transmission [21,29]. This phenomenon is known as short interval intracortical inhibition (SICI) and is likely mediated by the α2- or α 3-subunit of the GABAA receptor [30–32]. The duration of the I wave inhibition produced by SICI is consistent with intracellular measurements of the inhibitory postsynaptic potential (IPSP) from stimulation of the GABAA receptor [33,34].
Short interval intracortical facilitation (ICF)
This excitatory phenomenon is grouped with SICI because it is measured using the same paradigm. At interstimulus intervals (approximately 6–20 ms)[28] slightly longer than those used to elicit SICI, the net effect of the conditioning pulse shifts from inhibition to facilitation [28,35] (Figs. 3 & 5). The physiological basis of this effect is less clear than that of ICI, but it likely involves an effect on recurrent excitatory (I wave) activity within the cortex [35,36]. Importantly, studies showing reduction of ICF by GABAA inhibition promoting drugs indicate that ICI persists through this net-facilitatory phase [21,29] and that the two processes summate at the cortical level [26,37,38].
Fig. 5.
Plot of the ratio of the motor evoked potential with vs. without subthreshold conditioning stimulation at interstimulus intervals from 2–10 ms.
Short latency afferent inhibition (SAI)
SAI [31] is a decrease in MEP amplitude occurring when the TMS pulse is preceded by electrical stimulation of a peripheral sensory nerve. SAI is produced by input from somatosensory cortex and likely mediated by excitatory cholinergic input onto GABAergic interneurons in M1 and the α 1 GABAA receptor subunit [31,39].
Pharmacology of TMS
TMS of the motor cortex has been combined extensively with drugs in healthy subjects and patients. To date, these studies have probably shed more light on the mechanisms of TMS than those of the compounds in question, but enough information is now available for the opposite to happen, particularly for drugs affecting GABAergic inhibition. This section will review a representative sample of the literature to illustrate the potential usefulness of TMS as a tool in clinical pharmacology.
Compounds acting on cation channels
Drugs that affect voltage-gated sodium channels, e.g., the antiepileptic drugs (AEDs) phenytoin [40], carbamazepine [41,42], lamotrigine [21] and valproate [43] increase MEP threshold and reduce MEP amplitude via effects on membrane excitability. Their mechanism of action does not interfere with GABAergic synaptic transmission and, therefore, these agents do not alter ICI or ICF [40,42,44].
High-frequency repetitive TMS at suprathreshold intensity, applied over an area of cortex, increases its excitability [45], probably via increased intracellular concentration of cations or increased membrane permeability to them. AEDs acting on sodium channels [46] and the local anesthetic and selective sodium channel blocker, lidocaine [47], reverse this effect. If a correlation between physiological potency and clinical efficacy can be shown, TMS may provide a useful surrogate marker of antiepileptic activity for such compounds.
Drugs affecting glutamatergic transmission
Neurotransmission in the corticospinal pathway relies on glutamatergic synapses. Therefore, drugs that affect glutamate receptors should affect single-pulse TMS measures. This principle was borne out by work with the novel agent talampanel, a selective AMPA-kainate receptor antagonist and candidate AED, which increases MEP threshold and reduces amplitude without affecting either SICI or ICF [48].
NMDA glutamate receptors modulate neurotransmission in the corticospinal pathway and may underlie the increases in MEP amplitude seen with motor practice and learning [49,50]. However, NMDA antagonists do not uniformly decrease TMS excitability. For example, ketamine may increase MEP amplitude [51], possibly by binding to presynaptic receptors and promoting the release of endogenous glutamate [51,52]. Other NMDA antagonists have no effect on MEP amplitude, but affect SICI and ICF. Memantine [53], dextromethorphan [54], and riluzole [55] all cause a dose-dependent decrease in ICF and increase in SICI in healthy subjects [56].
The AED, levetiracetam, binds to the synaptic vesicle protein SV2A [57], but how this relates to its antiepileptic effect is unclear. Levetiracetam undoubtedly decreases MEP recruitment [58,59], despite mixed results on RMT [58,59], suggesting a depressant effect on the recurrent glutamatergic excitation that produces I waves,
Glutamate agonists have rarely been given to human subjects because of their epileptogenic potential. However, experiments in healthy women show that the ovarian hormone, estradiol, which increases glutamatergic activity via several mechanisms [60,61], increases ICF and reduces SICI [62,63] during the follicular phase of the menstrual cycle, with no effect on MEP amplitude or threshold.
GABAergic agents
Benzodiazepines
As noted above, benzodiazepines, which act on GABAA receptors to reinforce the action of GABA, increase SICI [21,29,44,64], prolong the CSP [23,29], and lower ICF [21,29,44,64]. These well-established effects might provide an in vivo, human, bioassay for GABAA activity. Moreover, TMS may be able to differentiate benzodiazepines based on secondary mechanisms of actions. For example, although both lorazepam and diazepam increase SICI and decrease ICF, lorazepam markedly reduces SAI via interference with cholinergic excitatory transmission, but diazepam slightly increases it [39]. Zolpidem, which binds selectively to the benzodiazepine GABAA receptor subtype, BZ1, does not change SICI, but augments SAI [31], prolongs CSP, and enhances LICI [65]. SICI/ICF could be used as a surrogate measure for behavioral effects related to GABAA receptor function, such as antiepileptic activity, anxiolysis, and sedation.
Ethanol
Ethanol, which binds to the α6 GABAA receptor subunit [66], increases SICI, prolongs CSP, and suppresses ICF, without affecting MEP threshold [67]. While ethanol does not affect the facilitatory response to high-frequency rTMS in healthy subjects, it abolishes it in alcoholics, suggesting a cortical plastic change with chronic exposure [68].
Neurosteroids
Paired TMS can be used to detect the effects of neuroactive steroids. These substances increase GABA activity via allosteric modulation of GABAA receptors [69–71]. Elevated progesterone and its neurosteroid metabolite allopregnenolone during the luteal phase of the menstrual cycle is associated with increased SICI [62] and hormonal fluctuations may result in fluctuations of SICI [72]. Neurosteroids have a clinically relevant anticonvulsant action [73–75] and interact with GABAergic drugs [70]. TMS could serve an important role in developing neurosteroid-related pharmaceuticals.
GABAB agonists
Baclofen, a selective GABAB receptor agonist, has mixed effects on SICI [12,21,44,76], but undoubtedly increases LICI and CSP duration [44,76].
Other GABAergic AEDs
Many AEDs that appear to exert their clinical effects by blocking GABA reuptake (tiagabine), inhibiting GABA transaminase (vigabatrin) or via unknown mechanisms (gabapentin), all of these enhance TMS measures of inhibition, increasing CSP, SICI, LICI and/or decreasing ICF [17,21,44,77]. Topiramate, a drug with several putative mechanisms of action, including up-modulating GABAA receptors, also increases SICI and decreases ICF, without affecting motor threshold or the CSP [44,78]. Even when the mechanism of action is complex or unknown, increased inhibition as measured with TMS may be a useful surrogate for antiepileptic activity.
Drugs affecting monoamine systems
Catecholaminergic drugs
The effects of agents acting on dopamine (DA), norepinephrine (NE), serotonin (5-HT) systems on paired-pulse TMS measures are generally consistent with findings at the cellular level. Moreover, TMS is sensitive to genetic variations that may determine treatment response.
The primary effect of DA on cortical pyramidal neurons is inhibition via direct action [79] and through GABAergic interneurons [80–85]. These effects are mediated by a variety of DA receptors subtypes [7,79,82,85,86]. As expected, DA agonists (e.g. the ergot derivatives, bromocryptine and pergolide) increase SICI and decrease ICF, whereas DA antagonists, such as haloperidol, cause opposite effects [44,54,87].
The stimulants methylphenidate and amphetamine, which increase DA and NE transmission through action on the dopamine transporter (DAT1) [88] and other mechanisms, are used to treat attention deficit hyperactivity disorder (ADHD). Cortical inhibition is abnormal in ADHD [89] and might be implicated in its pathogenesis, since SICI correlates with the severity of ADHD symptoms (especially hyperactivity), in patients comorbid for Tourette syndrome [90,91], the only group in which this has been studied to date. Stimulants tend to decrease SICI and/or increase ICF in healthy adults [92,93], although results have been variable [93,94]. In ADHD patients, methylphenidate causes the opposite effect, enhancing ICI [89]. Interestingly, atomoxetine, a selective NE reuptake inhibitor, also used to treat ADHD, decreases SICI and increases ICF in healthy people, similarly to methylphenidate [92]. Among patients, a genetic variation in the DAT1 gene, linked to ADHD risk, modulates the effect of both atomoxetine and methylphenidate on SICI [94], despite their different mechanisms of action. If the physiological differences are pronounced enough, TMS could provide a screen for genetic variations that determine the response to treatment in ADHD and possibly other disorders (see below).
Serotonergic drugs
The 5-HT 1B/1D receptor agonist, zolmitriptan, reduces SICI without changing MEP threshold or the CSP [95], implying possible involvement of GABAA transmission in its therapeutic effect in migraine.
Changes in TMS measures produced by selective serotonin reuptake inhibitors (SSRIs) seem to depend on the individual drug and/or chronicity of use: chronic paroxetine intake does not alter either ICI or CSP but enhances ICF [96], whereas threshold, CSP and SICI increase after a single dose of citalopram [97]. Again, the physiological response may be genetically determined: only subjects with the ll-genotype of the promoter region of the serotonin transporter gene (5-HTTLPR) appear to have increased SICI with citalopram [98].
Behavioral correlates of TMS measures
There is evidence that TMS response phenotypes may serve as surrogates or endophenotypes for behavioral traits. For example, neuroticism, a dimension in the Five-Factor personality model [99] is a stable measure of anxiety and other negative emotions. Neuroticism correlates inversely with SICI [100] and is affected by 5-HTTLPR alleles [101–103] suggesting a link to serotonin and/or GABA function. Medications (such as, benzodiazepines) and substances (such as, ethanol) that reduce anxiety increase SICI [21,44], further suggesting a role for reduced cortical inhibition in anxiety. Thus, TMS could thus serve as a probe for screening novel substances for anxiolytic properties or as a surrogate endpoint in clinical trials. Psychiatric disorders, such as obsessive compulsive disorder [103] and schizophrenia [104,105] are also associated with evidence of heightened cortical excitability or deficient inhibition.
Cognitive processes underlying normal and pathological behaviors may also be amenable to study with TMS. For example, SICI increases during the expectation of money reward in a simulated gambling task [106]. This paradigm could be used to evaluate pharmaceuticals aimed at modifying the reinforcing properties of stimuli in conditions such as substance dependency.
Summary
TMS is a simple, quantitative way to measure the state of neurons and synapses in the human cerebral cortex. Responses are altered by drugs, genetic variations, neurobehavioral disorders, and behavioral traits and states. While MEPs are potentially affected by countless inputs at cortical and spinal levels, carefully designed experiments with appropriately restricted hypotheses can yield remarkably specific information. While there has been considerable work exploring the effects of drugs on TMS responses, most of it has been done by clinical neurophysiologists using TMS for other purposes and whose primary interest was in the underlying brain circuits. Now, we feel there are significant opportunities for others, particularly clinical pharmacologists, to exploit.
Acknowledgments
The authors thank Ms. Devee Schoenberg for editing the manuscript. The authors are supported by the Intramural Research Program of the National Institute of Neurological Disorders and stroke.
References
- 1.Wassermann EM, Epstein CM, Ziemann U. The Oxford handbook of transcranial stimulation. Oxford University Press; Oxford; New York: 2007. [Google Scholar]
- 2.Barker AT. J Clin Neurophysiol. 1991;8:26. doi: 10.1097/00004691-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Barker AT, Jalinous R, Freeston IL. Lancet. 1985;1:1106. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 4.Hallett M. Nature. 2000;406:147. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 5.Burt T, Lisanby SH, Sackeim HA. Int J Neuropsychopharmacol. 2002;5:73. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 6.Wassermann EM, Lisanby SH. Clin Neurophysiol. 2001;112:1367. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 7.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Nat Rev Neurosci. 2004;5:793. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 8.Epstein CM, Schwartzenberg DG, Davey KR, Sudderth DB. Neurology. 1990;40:666. doi: 10.1212/wnl.40.4.666. [DOI] [PubMed] [Google Scholar]
- 9.Fujiki M, Isono M, Hori S, Ueno S. Electroencephalogr Clin Neurophysiol. 1996;101:48. doi: 10.1016/0013-4694(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 10.Patton HD, Amassian VE. J Neurophysiol. 1954;17:345. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- 11.Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Electroencephalogr Clin Neurophysiol. 1998;109:397. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 12.Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. Electroencephalogr Clin Neurophysiol. 1998;109:321. doi: 10.1016/s0924-980x(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 13.Rioult-Pedotti MS, Friedman D, Donoghue JP. Science. 2000;290:533. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 14.Mills KR, Nithi KA. Muscle Nerve. 1997;20:570. doi: 10.1002/(sici)1097-4598(199705)20:5<570::aid-mus5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Werhahn KJ, Behrang-Nia M, Bott MC, Klimpe S. J Clin Neurophysiol. 2007;24:419. doi: 10.1097/WNP.0b013e3181379a69. [DOI] [PubMed] [Google Scholar]
- 16.Chen R, Lozano AM, Ashby P. Exp Brain Res. 1999;128:539. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- 17.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. J Physiol. 1999;517(Pt 2):591. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuhr P, Agostino R, Hallett M. Electroencephalogr Clin Neurophysiol. 1991;81:257. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- 19.Fuhr P, Cohen LG, Roth BJ, Hallett M. Electroencephalogr Clin Neurophysiol. 1991;81:81. doi: 10.1016/0168-5597(91)90001-e. [DOI] [PubMed] [Google Scholar]
- 20.Connors BW, Malenka RC, Silva LR. J Physiol. 1988;406:443. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Ann Neurol. 1996;40:367. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 22.Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Exp Brain Res. 1996;109:467. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- 23.Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G, Zara F, Kazis A, Mills KR. Exp Brain Res. 2006;173:603. doi: 10.1007/s00221-006-0402-1. [DOI] [PubMed] [Google Scholar]
- 24.Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Exp Brain Res. 1998;119:265. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- 25.Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Exp Brain Res. 1996;109:158. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. J Physiol. 1997;498(Pt 3):817. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanger TD, Garg RR, Chen R. J Physiol. 2001;530:307. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziemann U, Rothwell JC, Ridding MC. J Physiol. 1996;496:873. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Exp Brain Res. 1996;109:127. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 30.Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. J Physiol. 2006;575:721. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U. Clin Neurophysiol. 2007;118:2207. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Clin Neurophysiol. 2002;113:1673. doi: 10.1016/s1388-2457(02)00264-x. [DOI] [PubMed] [Google Scholar]
- 33.Matsunaga K, Akamatsu N, Uozumi T, Urasaki E, Tsuji S. Clin Neurophysiol. 2002;113:1099. doi: 10.1016/s1388-2457(02)00079-2. [DOI] [PubMed] [Google Scholar]
- 34.McCormick DA. J Neurophysiol. 1989;62:1018. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- 35.Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. J Neurophysiol. 2006;96:1765. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- 36.Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. J Physiol. 2002;538:253. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. J Physiol. 1998;509(Pt 2):607. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. J Physiol. 1993;471:501. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. J Physiol. 2005;569:315. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen R, Samii A, Canos M, Wassermann EM, Hallett M. Neurology. 1997;49:881. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- 41.Schulze-Bonhage A, Knott H, Ferbert A. Electroencephalogr Clin Neurophysiol. 1996;99:267. doi: 10.1016/0013-4694(96)96501-3. [DOI] [PubMed] [Google Scholar]
- 42.Turazzini M, Manganotti P, Del Colle R, Silvestri M, Fiaschi A. Neurol Sci. 2004;25:83. doi: 10.1007/s10072-004-0234-3. [DOI] [PubMed] [Google Scholar]
- 43.Reutens DC, Berkovic SF, Macdonell RA, Bladin PF. Ann Neurol. 1993;34:351. doi: 10.1002/ana.410340308. [DOI] [PubMed] [Google Scholar]
- 44.Ziemann U. In: The Oxford Handbook of Transcranial Stimulation. Wassermann EM, Ziemann U, Epstein CM, editors. Oxford University Press; Oxford; New York: 2007. pp. 135–151. [Google Scholar]
- 45.Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M. Exp Brain Res. 1998;122:79. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- 46.Inghilleri M, Conte A, Frasca V, Curra A, Gilio F, Manfredi M, Berardelli A. Exp Brain Res. 2004;154:488. doi: 10.1007/s00221-003-1685-0. [DOI] [PubMed] [Google Scholar]
- 47.Inghilleri M, Conte A, Frasca V, Gilio F, Lorenzano C, Berardelli A. Exp Brain Res. 2005;163:114. doi: 10.1007/s00221-005-2225-x. [DOI] [PubMed] [Google Scholar]
- 48.Danielsson I, Su K, Kauer L, Barnette L, Reeves-Tyer P, Kelley K, Theodore WH, Wassermann E, Rogawski MA. Epilepsia. 2004;45(Suppl 7):120. [Google Scholar]
- 49.Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Exp Brain Res. 2001;136:431. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- 50.Ziemann U. Rev Neurosci. 2004;15:253. doi: 10.1515/revneuro.2004.15.4.253. [DOI] [PubMed] [Google Scholar]
- 51.Ghaly RF, Ham JH, Lee JJ. Neurol Res. 2001;23:881. doi: 10.1179/016164101101199342. [DOI] [PubMed] [Google Scholar]
- 52.Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA. J Physiol. 2003;547:485. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M. Neurosci Lett. 1999;270:137. doi: 10.1016/s0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- 54.Ziemann U, Chen R, Cohen LG, Hallett M. Neurology. 1998;51:1320. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 55.Schwenkreis P, Liepert J, Witscher K, Fischer W, Weiller C, Malin JP, Tegenthoff M. Exp Brain Res. 2000;135:293. doi: 10.1007/s002210000532. [DOI] [PubMed] [Google Scholar]
- 56.Reis J, John D, Heimeroth A, Mueller HH, Oertel WH, Arndt T, Rosenow F. Neuropsychopharmacology. 2006;31:2758. doi: 10.1038/sj.npp.1301122. [DOI] [PubMed] [Google Scholar]
- 57.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. Proc Natl Acad Sci U S A. 2004;101:9861. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epstein CM, Girard-Siquiera L, Ehrenberg JA. Epilepsia. 2008 doi: 10.1111/j.1528-1167.2008.01562.x. in press. [DOI] [PubMed] [Google Scholar]
- 59.Sohn YH, Kaelin-Lang A, Jung HY, Hallett M. Neurology. 2001;57:858. doi: 10.1212/wnl.57.5.858. [DOI] [PubMed] [Google Scholar]
- 60.Woolley CS. Crit Rev Neurobiol. 1999;13:1. doi: 10.1615/critrevneurobiol.v13.i1.10. [DOI] [PubMed] [Google Scholar]
- 61.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. J Neurosci. 1997;17:1848. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Neurology. 1999;53:2069. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- 63.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Ann Neurol. 2002;51:599. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- 64.Ziemann U. Clin Neurophysiol. 2004;115:1717. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Mohammadi B, Krampfl K, Petri S, Bogdanova D, Kossev A, Bufler J, Dengler R. Muscle Nerve. 2006;33:778. doi: 10.1002/mus.20531. [DOI] [PubMed] [Google Scholar]
- 66.Olsen RW, Hanchar HJ, Meera P, Wallner M. Alcohol. 2007;41:201. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ziemann U, Lonnecker S, Paulus W. Brain. 1995;118:1437. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 68.Conte A, Attilia ML, Gilio F, Iacovelli E, Frasca V, Bettolo CM, Gabriele M, Giacomelli E, Prencipe M, Berardelli A, Ceccanti M, Inghilleri M. Clin Neurophysiol. 2008;119:667. doi: 10.1016/j.clinph.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Benarroch EE. Neurology. 2007;68:945. doi: 10.1212/01.wnl.0000257836.09570.e1. [DOI] [PubMed] [Google Scholar]
- 70.Belelli D, Lambert JJ. Nat Rev Neurosci. 2005;6:565. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 71.Majewska MD. Prog Neurobiol. 1992;38:379. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 72.Hattemer K, Knake S, Reis J, Rochon J, Oertel WH, Rosenow F, Hamer HM. Clin Endocrinol (Oxf) 2007;66:387. doi: 10.1111/j.1365-2265.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- 73.Kokate TG, Svensson BE, Rogawski MA. J Pharmacol Exp Ther. 1994;270:1223. [PubMed] [Google Scholar]
- 74.Backstrom T, Zetterlund B, Blom S, Romano M. Acta Neurol Scand. 1984;69:240. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- 75.Beyenburg S, Stoffel-Wagner B, Bauer J, Watzka M, Blumcke I, Bidlingmaier F, Elger CE. Epilepsy Res. 2001;44:141. doi: 10.1016/s0920-1211(01)00194-2. [DOI] [PubMed] [Google Scholar]
- 76.McDonnell MN, Orekhov Y, Ziemann U. Exp Brain Res. 2006;173:86. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 77.Pierantozzi M, Marciani MG, Palmieri MG, Brusa L, Galati S, Caramia MD, Bernardi G, Stanzione P. Brain Res. 2004;1028:1. doi: 10.1016/j.brainres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Ziemann U. Suppl Clin Neurophysiol. 2003;56:226. [PubMed] [Google Scholar]
- 79.Gao WJ, Wang Y, Goldman-Rakic PS. J Neurosci. 2003;23:1622. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Awenowicz PW, Porter LL. J Neurophysiol. 2002;88:3439. doi: 10.1152/jn.00078.2002. [DOI] [PubMed] [Google Scholar]
- 81.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. J Neurophysiol. 2006;95:1639. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 82.Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O’Donnell P. Synapse. 2006;59:412. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huda K, Salunga TL, Matsunami K. Neurosci Lett. 2001;307:175. doi: 10.1016/s0304-3940(01)01960-7. [DOI] [PubMed] [Google Scholar]
- 84.Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA. Cereb Cortex. 1998;8:614. doi: 10.1093/cercor/8.7.614. [DOI] [PubMed] [Google Scholar]
- 85.Tseng KY, O’Donnell P. J Neurosci. 2004;24:5131. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Onn SP, Wang XB, Lin M, Grace AA. Neuropsychopharmacology. 2006;31:318. doi: 10.1038/sj.npp.1300829. [DOI] [PubMed] [Google Scholar]
- 87.Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Electroencephalogr Clin Neurophysiol. 1997;105:430. doi: 10.1016/s0924-980x(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 88.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. Neurosci Lett. 2000;284:121. doi: 10.1016/s0304-3940(00)00980-0. [DOI] [PubMed] [Google Scholar]
- 90.Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T, Wassermann EM. Mov Disord. 2004;19:416. doi: 10.1002/mds.20044. [DOI] [PubMed] [Google Scholar]
- 91.Gilbert DL, Sallee FR, Zhang J, Lipps TD, Wassermann EM. Biol Psychiatry. 2005;57:1597. doi: 10.1016/j.biopsych.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 92.Gilbert DL, Ridel KR, Sallee FR, Zhang J, Lipps TD, Wassermann EM. Neuropsychopharmacology. 2006;31:442. doi: 10.1038/sj.npp.1300806. [DOI] [PubMed] [Google Scholar]
- 93.Moll GH, Heinrich H, Rothenberger A. Acta Psychiatr Scand. 2003;107:69. doi: 10.1034/j.1600-0447.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert DL, Wang Z, Sallee FR, Ridel KR, Merhar S, Zhang J, Lipps TD, White C, Badreldin N, Wassermann EM. Brain. 2006;129:2038. doi: 10.1093/brain/awl147. [DOI] [PubMed] [Google Scholar]
- 95.Werhahn KJ, Forderreuther S, Straube A. Neurology. 1998;51:896. doi: 10.1212/wnl.51.3.896. [DOI] [PubMed] [Google Scholar]
- 96.Gerdelat-Mas A, Loubinoux I, Tombari D, Rascol O, Chollet F, Simonetta-Moreau M. Neuroimage. 2005;27:314. doi: 10.1016/j.neuroimage.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 97.Robol E, Fiaschi A, Manganotti P. J Neurol Sci. 2004;221:41. doi: 10.1016/j.jns.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 98.Eichhammer P, Langguth B, Wiegand R, Kharraz A, Frick U, Hajak G. Psychopharmacology (Berl) 2003;166:294. doi: 10.1007/s00213-002-1370-1. [DOI] [PubMed] [Google Scholar]
- 99.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- 100.Wassermann EM, Greenberg BD, Nguyen MB, Murphy DL. Biol Psychiatry. 2001;50:377. doi: 10.1016/s0006-3223(01)01210-0. [DOI] [PubMed] [Google Scholar]
- 101.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Science. 1996;274:1527. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 102.Katsuragi S, Kunagi H, Sano A, Tsutsumi T, Koichi I, Shinichiro NJA. Biol Psychiatry. 1999;45:368. doi: 10.1016/s0006-3223(98)00090-0. [DOI] [PubMed] [Google Scholar]
- 103.Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, Lesch KP, Hamer D, Murphy DL. Am J Med Genet (Neuropsychiatric Genetics) 2000;96:202. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 104.Greenberg BD, Ziemann U, Harmon A, Murphy DL, Wassermann EM. Lancet. 1998;352:881. doi: 10.1016/S0140-6736(05)60009-8. [DOI] [PubMed] [Google Scholar]
- 105.Pascual-Leone A, Manoach DS, Birnbaum R, Goff DC. Biol Psychiatry. 2002;52:24. doi: 10.1016/s0006-3223(02)01317-3. [DOI] [PubMed] [Google Scholar]
- 106.Kapogiannis D, Campion P, Grafman J, Wassermann EM. Eur J Neurosci. 2008;27:1836. doi: 10.1111/j.1460-9568.2008.06147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]