Abstract
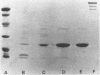
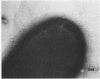
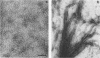
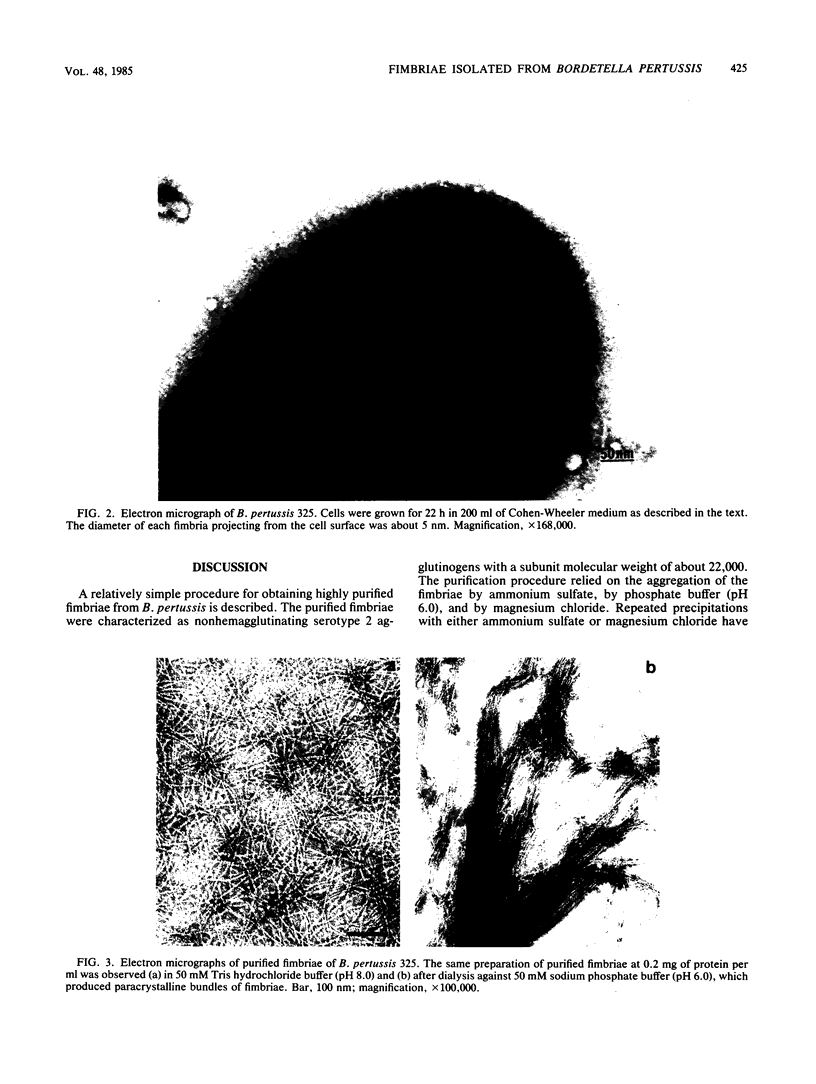
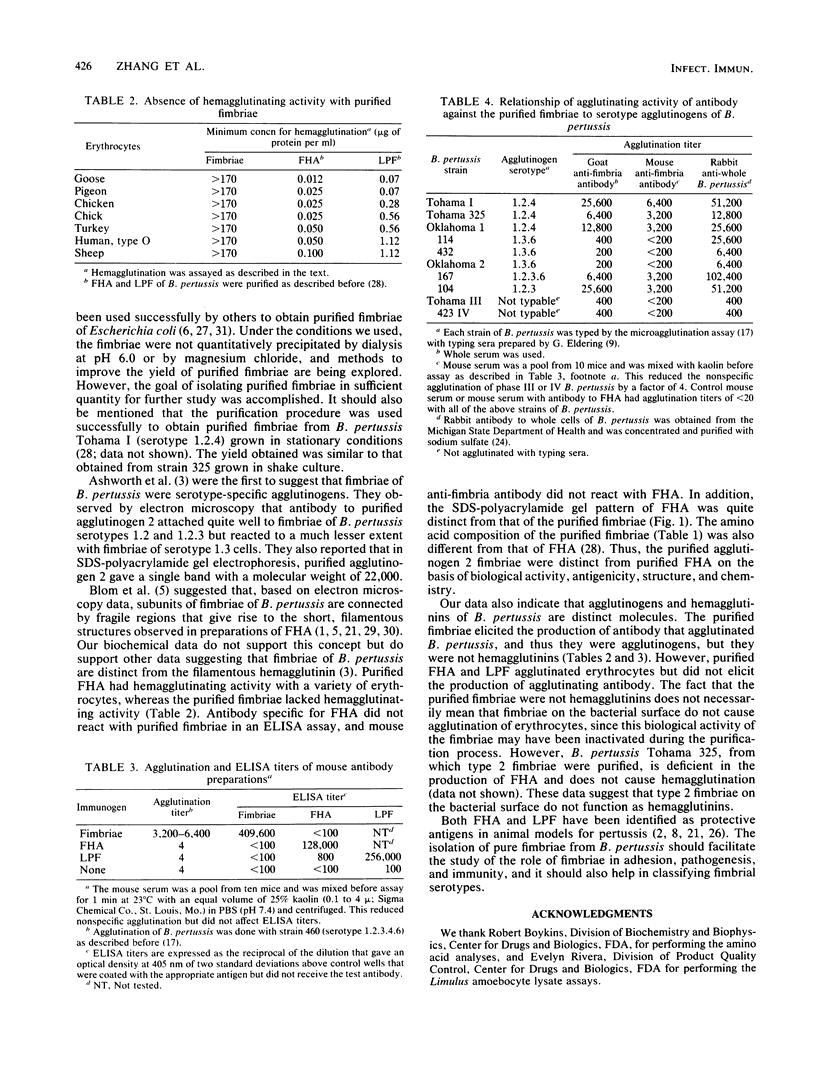
Fimbriae were detached from Bordetella pertussis by mechanical shearing and purified by successive precipitations with ammonium sulfate, phosphate buffer (pH 6.0), and magnesium chloride. In each of these purification steps, the fimbriae aggregated into bundles as seen by electron microscopy. These aggregates could be disaggregated at pH 9.5. By electron microscopy, the purified fimbriae appeared as long filaments with a diameter of 5 nm. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified fimbriae showed a single protein subunit with a molecular weight of 22,000. The purified fimbriae did not have hemagglutinating activity when assayed with several types of erythrocytes, and they were antigenically, chemically, and structurally distinct from the filamentous hemagglutinin of B. pertussis. The purified fimbriae were also identified as serotype 2 agglutinogens, since antibody to the purified fimbriae agglutinated B. pertussis strains serotyped as 1.2.4, 1.2.3, or 1.2.3.6 but did not agglutinate those serotyped as 1.3.6.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Sato Y. Separation and characterization of two distinct hemagglutinins contained in purified leukocytosis-promoting factor from Bordetella pertussis. Biochim Biophys Acta. 1976 Oct 22;444(3):765–782. doi: 10.1016/0304-4165(76)90323-8. [DOI] [PubMed] [Google Scholar]
- Ashworth L. A., Fitzgeorge R. B., Irons L. I., Morgan C. P., Robinson A. Rabbit nasopharyngeal colonization by Bordetella pertussis: the effects of immunization on clearance and on serum and nasal antibody levels. J Hyg (Lond) 1982 Jun;88(3):475–486. doi: 10.1017/s0022172400070339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth L. A., Irons L. I., Dowsett A. B. Antigenic relationship between serotype-specific agglutinogen and fimbriae of Bordetella pertussis. Infect Immun. 1982 Sep;37(3):1278–1281. doi: 10.1128/iai.37.3.1278-1281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Blom J., Hansen G. A., Poulsen F. M. Morphology of cells and hemagglutinogens of Bordetella species: resolution of substructural units in fimbriae of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):308–317. doi: 10.1128/iai.42.1.308-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Wheeler M. W. Pertussis Vaccine Prepared with Phase-I Cultures Grown in Fluid Medium. Am J Public Health Nations Health. 1946 Apr;36(4):371–376. [PMC free article] [PubMed] [Google Scholar]
- Eldering G., Holwerda J., Davis A., Baker J. Bordetella pertussis serotypes in the United States. Appl Microbiol. 1969 Oct;18(4):618–621. doi: 10.1128/am.18.4.618-621.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hochstein H. D. Review of the Bureau of Biologic's experience with Limulus amebocyte lysate and endotoxin. Prog Clin Biol Res. 1982;93:141–151. [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Kallio P., Nurmiaho-Lassila E. L., Ranta H., Siitonen A., Elo J., Svenson S. B., Svanborg-Edén C. The role of pili in the adhesion of Escherichia coli to human urinary tract epithelial cells. Scand J Infect Dis Suppl. 1982;33:26–31. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labaw L. W., Padlan E. A., Segal D. M., Davies D. R. An em study of phosphorylcholine-binding fab immunoglobulin fragment crystals. J Ultrastruct Res. 1975 Jun;51(3):326–339. doi: 10.1016/s0022-5320(75)80097-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Stacy T., Krous H. F. Progressive cough associated with lymphocytic leukemoid reaction in an infant. J Pediatr. 1980 Jul;97(1):156–160. [PubMed] [Google Scholar]
- Morse J. H., Morse S. I. Studies on the ultrastructure of Bordetella pertussis. I. Morphology, origin, and biological activity of structures present in the extracellular fluid of liquid cultures of Bordetella pertussis. J Exp Med. 1970 Jun 1;131(6):1342–1357. doi: 10.1084/jem.131.6.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Morse J. H. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J Exp Med. 1976 Jun 1;143(6):1483–1502. doi: 10.1084/jem.143.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M., Cowell J. L., Burstyn D. G., Manclark C. R. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J Infect Dis. 1984 Dec;150(6):823–833. doi: 10.1093/infdis/150.6.823. [DOI] [PubMed] [Google Scholar]
- Oliveira E. B., Gotschlich C., Liu T. Y. Primary structure of human C-reactive protein. J Biol Chem. 1979 Jan 25;254(2):489–502. [PubMed] [Google Scholar]
- Pittman M., Gardner R. A., Marshall J. F. Evaulation of the potency of antipertussis serum products. J Biol Stand. 1979 Jul;7(3):263–273. doi: 10.1016/s0092-1157(79)80030-x. [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Dainelli B., Ricchetti M. Piliated Bacteroides fragilis strains adhere to epithelial cells and are more sensitive to phagocytosis by human neutrophils than nonpiliated strains. Infect Immun. 1984 Jan;43(1):189–194. doi: 10.1128/iai.43.1.189-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Irons L. I. Synergistic effect of Bordetella pertussis lymphocytosis-promoting factor on protective activities of isolated Bordetella antigens in mice. Infect Immun. 1983 May;40(2):523–528. doi: 10.1128/iai.40.2.523-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect Immun. 1983 Jul;41(1):313–320. doi: 10.1128/iai.41.1.313-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Ultraviolet irradiation disrupts somatic pili structure and function. Infect Immun. 1979 Sep;25(3):1060–1065. doi: 10.1128/iai.25.3.1060-1065.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Steven A. C., Serwer P., Bisher M. E., Trus B. L. Molecular architecture of bacteriophage T7 capsid. Virology. 1983 Jan 15;124(1):109–120. doi: 10.1016/0042-6822(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. I., Hendley J. O. Adherence of Bordetella pertussis to human respiratory epithelial cells. J Infect Dis. 1983 Jul;148(1):125–130. doi: 10.1093/infdis/148.1.125. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]