Abstract
While progress has been made in determining the molecular basis for the circadian clock, the mechanism by which mammalian brains time intervals measured in seconds to minutes remains a mystery. An obvious question is whether the interval timing mechanism shares molecular machinery with the circadian timing mechanism. In the current study, we trained circadian CLOCK +/− and −/− mutant male mice in a peak-interval procedure with 10 and 20-s criteria. The mutant mice were more active than their wild-type littermates, but there were no reliable deficits in the accuracy or precision of their timing as compared with wild-type littermates. This suggests that expression of the CLOCK protein is not necessary for normal interval timing.
Keywords: Biological rhythms, Time perception, Peak-interval procedure, Weber’s law, Dopamine, Clock gene
1. Introduction
Much is known about the psychophysics of interval timing (i.e., timing of intervals in the seconds to minutes range) in human and nonhuman animal subjects. In particular, the variability in timed responding is known to be approximately scalar; it increases in proportion to the duration of the interval timed, such that the coefficient of variation (the ratio of the standard deviation to the mean response) is a constant. For example, in the peak-interval (PI) procedure (Church, Meck, & Gibbon, 1994; King, McDonald, and Gallistel, 2001; Roberts, 1981), an animal is trained that food will be available after a fixed interval (FI) following onset of a signal (e.g., light in hopper). On some trials (probe trials), responses are not reinforced. On these trials, the animal responds as usual, eventually giving up once they are certain the FI has passed and food has not been made available. When the probability of a response is plotted as a function of the time since the onset of the signal, the function is an inverted U shape, with the maximum probability of responding occurring at approximately the FI value. When these same functions are plotted as a function of relative time (time since signal onset / FI value), the data from different FI values superimpose, to reveal the scalar property of the timing mechanism.
Fine-grained determinations of timing at a series of closely spaced intervals reveals systematic small departures from linearity at certain intervals, which has led to the suggestion that the interval timing mechanism depends not on a linear accumulator, but rather on a series of biological oscillators with different characteristic periods (Crystal, 1999; 2003). If interval timing is mediated by oscillators with non-circadian periods, then the mechanism underlying these oscillators might share components with the circadian oscillator. That consideration prompted this research.
There are a number of reasons to suspect a shared molecular basis for the circadian clock and interval timer. The circadian clock and the interval timer are used in complementary ways to time the anticipation of the next feeding opportunity (e.g., Crystal, 2001, 2003, 2006; Crystal and Baramidze, 2007; Terman et al., 1984) as well as the amount of time that a female ringdove spends sitting on its nest and when it is time for the male ringdove to take over (Gibbon, Morrell, and Silver, 1984; Silver and Bittman, 1984). The duration with which both rats and humans reproduce short temporal intervals (seconds to minutes) varies with the circadian cycle (Aschoff, 1998; Pati and Gupta, 1994; Shurtleff, Raslear, & Simmons, 1990) while the allocation of attentional control to the durations of auditory and visual stimuli covary as a function of the age of the subjects and circadian phase (Lustig and Meck, 2001 – see also Meck, 1991). Temporal memory for the delivery of food following signal durations between 20 and 60 s are sensitive to photoperiodic variation in laboratory rats in a manner similar to that previously observed for reproductive function (MacDonald et al., 2007). In addition, when humans live in isolation with no external time cues, their perception of the duration of an hour is highly correlated with τ (tau), their mean circadian period (Aschoff, 1984).
These behavioral correlations may be indicative of a deeper, biological relationship. Diverse lines of evidence suggest that dopaminergic pathways in the basal ganglia are important for interval timing (Abner et al., 2001; Buhusi and Meck, 2005; Hinton and Meck, 2004; MacDonald and Meck, 2004; Malapani et al., 1998; Matell and Meck, 2004; Meck, 1996, 2006a, b). Evidence suggests dopamine (DA) levels regulate the speed of the interval timer, as administration of cocaine and methamphetamine (indirect DA agonists) produce a leftward shift of interval timing functions (e.g., Matell et al., 2004, 2006), while DA receptor blockers such as haloperidol produce the opposite effect (e.g., Buhusi and Meck, 2002; Drew et al., 2003; MacDonald and Meck, 2005; Maricq and Church, 1983; Meck, 1983, 1986, 1996; Ohyama et al., 2001).
DA is also involved in the regulation of circadian rhythms. The rate of DA release and metabolism varies throughout the day and is reinforced by light in the mammalian retina (Witkovsky, 2004). This daily DA cycling is even apparent in the striatum, the neural substrate implicated in interval timing (Sleipness, Sorg, and Jansen, 2006). Administration of haloperidol has been found to increase expression levels of one of the genes involved in the transcriptional feedback loop responsible for circadian rhythms, both in vivo and in cultured suprachiasmatic nucleus (SCN) cells (Viyoch et al., 2005). McClung et al. (2005) reported that CLOCK mice, which have a point mutation in the Clock gene leading to inactive CLOCK proteins and impaired circadian timing, reveal increased dopaminergic function, suggesting that the CLOCK protein plays a part in regulating the transmission of DA in the brain. If this is the case, then CLOCK may be responsible for regulating the speed of the pacemaker in the interval timer as well.
These CLOCK mice are ideal candidates for investigating the genetic basis for this relationship. When housed in a 12:12 hour light-dark (LD) cycle, CLOCK mice entrain to the light cycle and maintain rhythmicity like their wild-type littermates. In complete darkness, however, heterozygotes have a longer rhythm than wild types (~24.4 hours, as compared with ~23.3 hours) while homozygotes maintain an even longer period (~27.3 hours), before losing rhythmicity within the first 5–15 cycles (Vitaterna et al., 1994).
In the current study, we trained CLOCK mutant mice and controls in a peak-interval (PI) procedure which is a simple timing task in order to determine if expression of the Clock gene was necessary for normal interval timing. To our knowledge, this is the first attempt to determine whether the circadian clock and the interval timer share a genetic basis.
2. Results
2.1. Trial initiation and response levels
The homozygous mice were clearly more active, consistent with other reports (Easton et al., 2003). On average, they initiated about three times as many trials (Phase 1: M=453, Phase 2: M=227) as did wild types (Phase 1: M=128, Phase 2: M=68; p < 0.001) and heterozygotes (Phase 1: M=136, Phase 2: M=81; p < 0.001; (F(2,35)=26.48, p < 0.001).
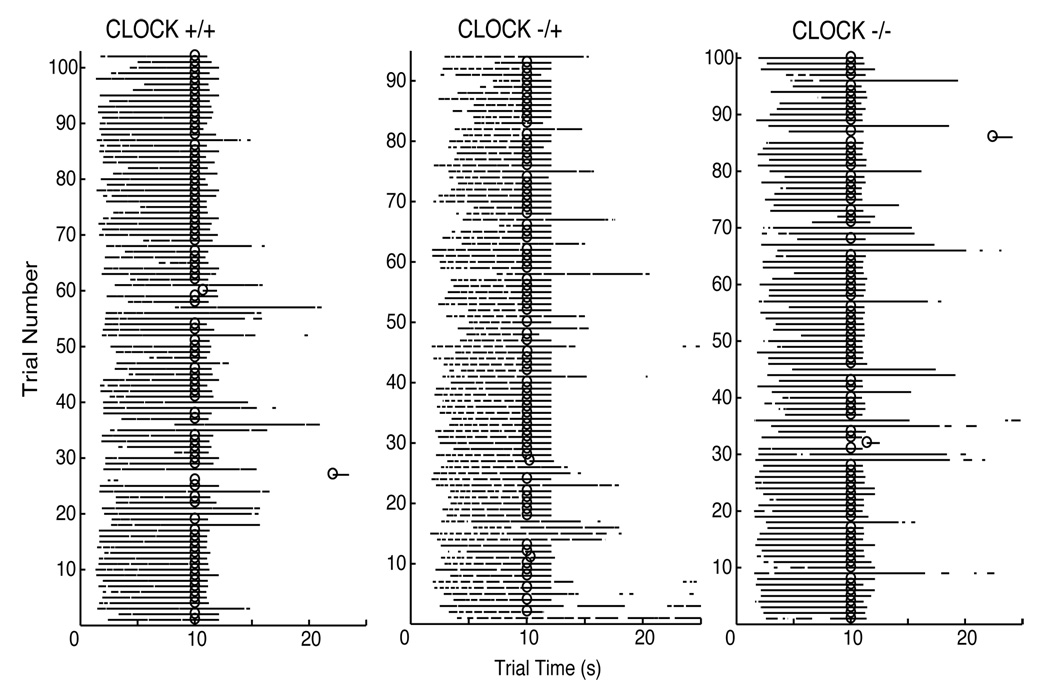
Raster plots of the raw data from representative wild type, heterozygous, and homozygous mice from Phase 1 (same session) are presented in Figure 1. As can be seen in these plots, the mice typically poked their head in the target hopper prior to the target time and, on most trials, kept their head in the hopper until the target interval elapsed and a food pellet was delivered or until long after the target interval had elapsed (on probe trials). This pattern of responding is typical of the PI procedure data. Most notable, however, is that there are not any obvious differences in responding between the wild type, heterozygous, and homozygous mice. Further analyses confirmed this impression.
Figure 1.
Raster plots of the raw data for representative Wild Type, Heterozygous, and Homozygous mice from Session 9, Phase 1 of the 10-s PI training. Each horizontal row corresponds to a trial, with the black line denoting when the mouse had its head in the target hopper. A trial time of zero corresponds to when the light turned on in the target hopper, signaling the start of the target interval. The circles denote when a food pellet was delivered. Probe trials are those in which a food pellet was not delivered.
For purposes of data analyses, data from sessions 9–16 for Phase 1, and sessions 19–26 for Phase 2 were used. Analyses of interval-timing behavior were performed only on probe trials. Because the duration of probe trials was variable, analyses were limited by the duration of the shortest probe trial per phase. This way, responses during all probe trials were treated equally. Thus, Phase 1 analyses looked only at the first 32.1 s of the trial, and Phase 2 analyses included the first 62.1 s. Trials in which the mouse failed to poke in the target hopper in the first 32.1 s (or 62.1 s) of the trial were excluded from analyses. The percentage of trials excluded due to a failure to poke in the target hopper during the allotted time varied as a function of genotype and phase, as revealed by a 2 (Phase) × 3 (Genotype) repeated measures ANOVA. A greater percentage of trials were excluded in Phase 1 (7.5%) compared with Phase 2 (1.7%); greater percentage of trials from the wild type mice (10.4%) were excluded than from the homozygotes (0.7%), with the percentage of excluded heterozygote trials falling somewhere in between (2.5%). Significant main effects were observed for both Phase and Genotype; F(1,35) = 9.26, p < 0.001 and F(2, 35) = 4.68, p = < 0.05, respectively. Oddly, while both the wild types and heterozygotes had fewer excluded trials in Phase 2 of the experiment (as compared with Phase 1), the homozygotes had an increase in the percentage of excluded trials from Phase 1 to Phase 2; interaction F(2, 35) = 4.53, p < 0.05. In sum, because they were more active, the homozygotic mice were better subjects, initiating more trials and failing to make anticipatory pokes on a smaller proportion of those trials,
2.2. Peak-Interval (PI) timing performance as a function of individual trials
Previous research using the PI procedure with rats and pigeons has shown that responding on individual trials follows a break-run-break pattern (e.g., Cheng and Westwood, 1993; Church et al., 1994), in which there is a step-like onset of an increased rate of responding some while before the expected time of food delivery and step-like offset some while after that time. This pattern of responding was evident in the present data as well, but the algorithmic identification of the Starts (onsets) and Stops (offsets) was complicated by between-mice differences in mode of responding. Some mice kept their head in the hopper for long intervals around the expected time of food delivery, while others rapidly poked in and out, never keeping their head in the hopper for more than a fraction of a second (neither behavior was specific to a particular genotype). The algorithm we used to determine the high-response interval found the longest sequence of pokes in which the interval between two pokes was no longer than 1.25 s1. Given the erratic poking behavior we observed, for purposes of this analysis, only trials in which the total duration of nose pokes exceeded 1.5 s were included. The proportion of trials excluded based on this criteria did not vary as a function of genotype; F(2,35) = 0.30, p > 0.05).
From the run intervals thus estimated, we determined four run-describing parameters for each trial: (1) Starts: trial time at which the mouse began to poke in the target hopper; (2) Stops: trial time when the mouse stopped poking; (3) Middles: the midpoint between the Start and Stop for each trial; and (4) Durations: the duration of the mouse's poke in the target hopper (Stop – Start). Repeated-measures ANOVAs were carried out on the coefficient of variation (CV – a measure of relative variability = the ratio of the standard deviation to the mean) and the mean of each of these four parameters for both phases (see Table 1 for means). All tests revealed a significant main effect of experimental phase (p < .01), an expected result given the change in target time from Phase 1 to Phase 2. This effect will not be discussed further. Other significant interactions and main effects were followed up by additional one-way ANOVAs and follow-up Tukey HSD post-hoc tests.
Table 1.
Summary of the mean values (and standard errors) for each genotype.
| Phase | Cl +/+ | Cl +/− | Cl −/− | |
|---|---|---|---|---|
| Mean Starts | 1 | 8.0 (.97) | 6.5 (.79) | 4.8 (.66) |
| 2 | 12.4 (1.31) | 9.0 (1.07) | 15.6 (2.9) | |
| Mean Stops | 1 | 19.1 (.66) | 17.8 (.32) | 16.7 (.35) |
| 2 | 34.2 (1.22) | 32.0 (0.94) | 30.8 (0.88) | |
| Stop CV | 1 | .26 (.011) | .28 (.019) | .25 (.017) |
| 2 | .32 (.024) | .26 (.022) | .35 (.047) | |
| Mean Dur. | 1 | 11.1 (.53) | 11.4 (.60) | 11.9 (.58) |
| 2 | 21.8 (1.88) | 22.9 (1.14) | 15.2 (3.12) | |
| Mean Mid. | 1 | 13.5 (.78) | 12.1 (.52) | 10.7 (.44) |
| 2 | 23.3 (.85) | 20.5 (.83) | 23.2 (1.44) |
2.2.1 Analysis of Starts
There was no significant effect of genotype (p > .05) on start times, but there was a genotype x phase interaction (repeated measures F (2,35)=6.94, p < .01): The homozygous mice started poking earlier in the trial than wild type mice (p< .05) in Phase 1, whereas in Phase 2, they started poking later than the other mice.
2.2.2 Analysis of Stops
There was a significant effect of genotype on stop times (repeated measures F(2, 35)=3.74, p<.05): Across both phases, the homozygotes stopped poking significantly earlier (23.8 s ± .54) than wild types (26.6 s ± .88; p < .05), with the heterozygotes falling in-between (24.9 s ± .60).
2.2.3 Analysis of Durations
There was no significant effect of genotype on the duration of poking, but there was a significant genotype x phase interaction (F(2, 35)=5.04, p<.05): Although the genotypes did not differ in the average duration of poking for Phase 1, the homozygotes’ significantly later starts and earlier stops in Phase 2 reduced their average run time (15.2 s ± 3.1) relative to the heterozygotes (22.9 s ± 1.1; p < .05) and the wild types (21.8 s ± 1.9; p > .05).
2.2.4 Analysis of Middles
There was no significant main effect of genotype on the average midpoint of a run, but there was a significant phase x genotype interaction (F (2, 35) = 6.2, p < .01). The mean midpoint of a homozygotes’ run did not differ significantly from the target in either phase (single sample t tests: t(9) = 1.67, p > .05). In Phase 1, both the mean midpoints of both wild types (t(14) = 4.53, p < .01) and the heterozygotes (t(12) = 4.10, p < .01) were significantly longer than the 10 second target time. In Phase 2, only the mean of the wild types was significantly longer than the 20-second target time (+/+ t(14) = 3.88, p < .01; +/− t(12) = 0.62, p > .05; −/− t(9) = 2.25, p > .05).
In sum, by these measures, mice homozygous for the clock mutation do not have an impaired interval timer. If anything, they were more accurate in the placement of their peak times than their wild type counterparts. Group plots of the raw data are presented in Figure 2.
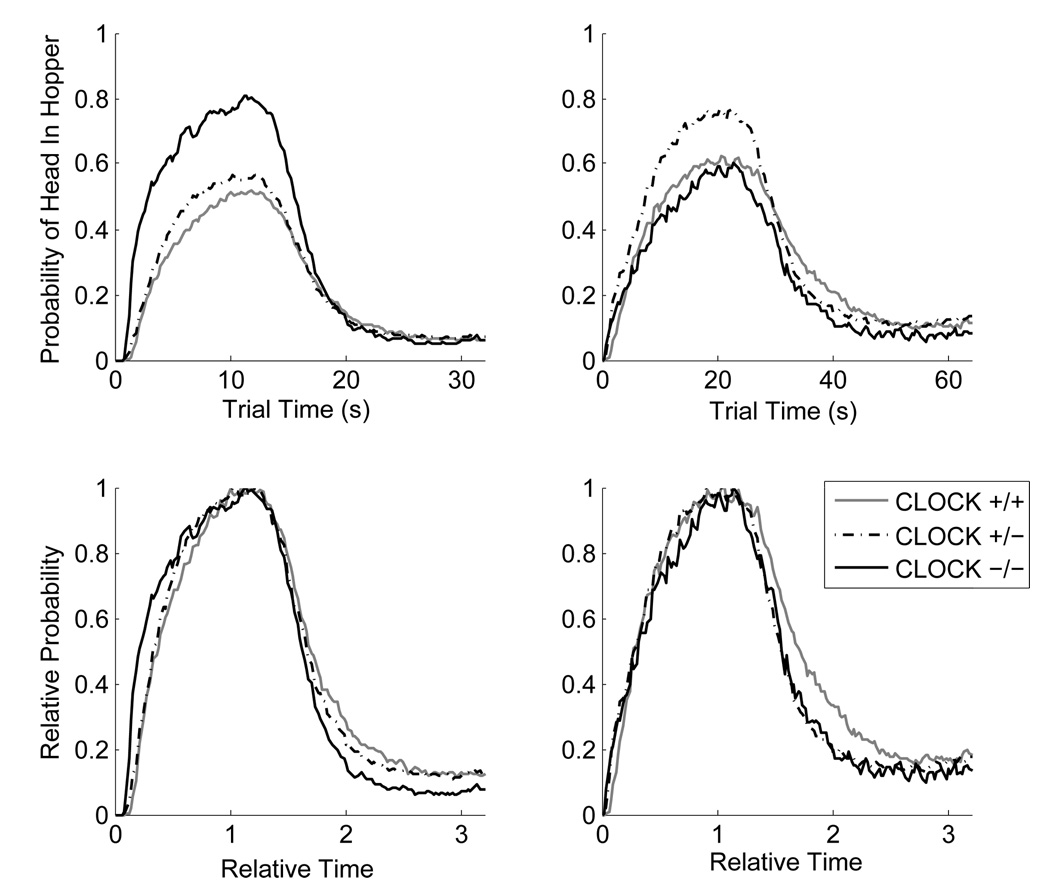
Figure 2.
Top row: Group plots of the probability of the mouse’s head being in the target hopper as a function of the trial time for Phase 1 (left panel) and Phase 2 (right panel) for mice trained on the 10-s PI and 20-s PI procedure, respectively. Bottom row: Group plots of the proportion of maximum responding (relative probability of mouse's head in target hopper) as a function of relative time (trial time / target time) for Phase 1 (left panel) and Phase 2 (right panel).
2.2.5 Analysis of Stop CV
Precision in responding (variability about the mean) was assessed in addition to timing accuracy (the difference between the mean midpoint of a run and the target time). If the interval timer was impaired but still operative, we might expect greater trial-to-trial variability in the timing of the Starts and Stops. Because the variability associated with interval timing is known to follow Weber’s law and to exhibit the scalar property (i.e., variability grows proportional to the mean of the interval being timed – see Gibbon, 1977; Gibbon, Church, and Meck, 1984), the appropriate measure of precision is the CV, which is the ratio between the standard deviation and the mean of a distribution.
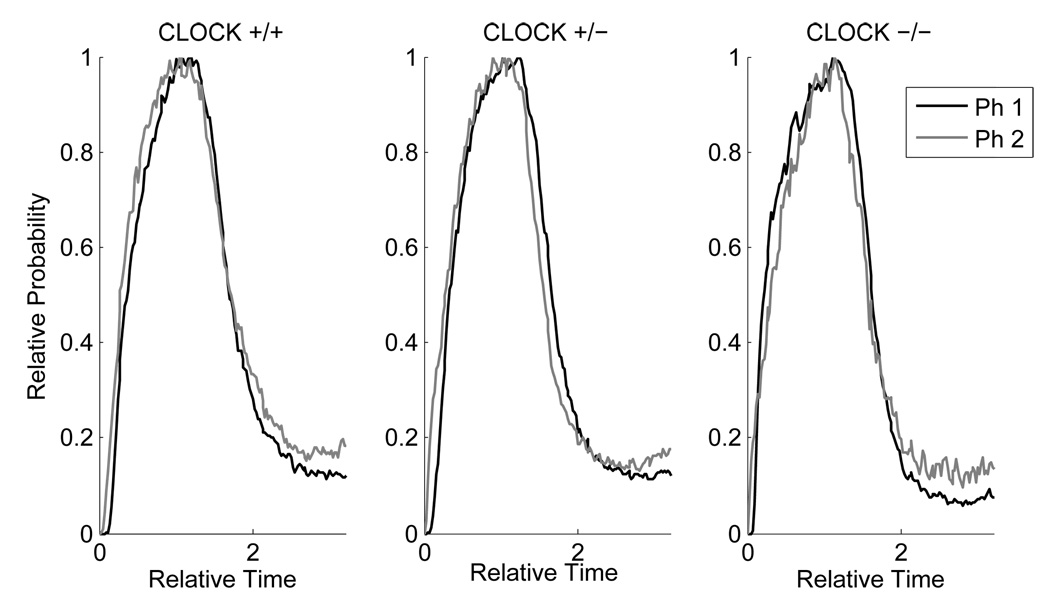
The distribution of Start times is significantly more variable than that of Stop times, it is positively skewed, and appears to be a mixture distribution from two different response strategies (timed Starts on some trials, untimed Starts on others – see King et al., 2004). In contrast, the distribution of Stop times is much more precise and symmetrical (e.g., Cheng and Meck, 2007; Gallistel et al., 2004; Matell et al., 2006). The distribution of Stop times is where one typically finds the scalar property to be the most robust, yielding a constant CV across a variety of target times. There was a small (from 0.26 to 0.31), but statistically significant increase in the Stop CV from Phase 1 to Phase 2; F(1,35) = 7.51, p < .01, the interpretation of which is complicated by an uninterpretable significant interaction between Genotype and Phase; F(2,35) = 5.57, p < .01, in which the homozygotes and the wild types showed the effect (increase in CV), but the heterozygotes appeared to become slightly more precise with the longer target time. Moreover, the increase in Stop CV was not seen in all subjects within either of the first two groups: 11 out of 15 of the wild types showed it and 7 out of 10 of the homozygotes. For these reasons, we conclude that the violations of the scalar property were small and inconsistent as illustrated in Figure 2 (bottom) and in Figure 3 (in which the data from the bottom row of Figure 2 are replotted as a function of genotype).
Figure 3.
Group plots of the proportion of maximum responding (relative probability of mouse's head in target hopper) as a function of relative time (trial time / target time) for each genotype trained in the PI procedure. The solid grey line corresponds to the data from Phase 1 in which the temporal criterion was 10 s, and the dotted black line corresponds to data from Phase 2 in which the temporal criterion was 20 s.
3. Discussion
There are no clear differences in interval-timing behavior across the three Clock genotypes. If anything, the data suggest that homozygous Clock mice are both more accurate and more precise in timing short intervals as compared with their wild-type littermates. Most importantly, results of the current study do not suggest that expression of the Clock gene is necessary for normal interval timing in the mouse. Our findings corroborate and extend previous work suggesting the neurobiological pathways responsible for circadian timing and interval timing may be independent. Lewis et al. (2003) trained C57/Bl6 male mice with lesions aimed at the SCN, the brain's master circadian clock, using a similar 10-s PI procedure. The mice were subsequently divided into two groups: those that maintained behavioral rhythmicity in DD (complete darkness) following the lesion, and those that did not. Mice were tested in both LD (12:12 light/dark conditions) and DD. As in our current study, results revealed no difference in mean, standard deviation, or amplitude of the peak times for rhythmic or arrhythmic groups, suggesting the timing of a short interval does not require an intact SCN or light-entrained behavioral rhythmicity.
There are two major caveats to the Lewis et al. (2003) study. First, the results can only speak to a putative relationship between interval and circadian timing modulated by the SCN (i.e., results do not address other circadian oscillators such as the food-entrainable one(s), possibly located in the dorsomedial hypothalamic nucleus; Mieda, Williams, Richardson, Tanaka, and Yanagisawa, 2006). Thus, although the Lewis et al. findings agree with ours by failing to find a direct physiological link between circadian and interval timing, it should be cautioned that their findings only speak to the role of the SCN in interval timing. Similarly, although CLOCK is expressed throughout the brain and periphery, it is specifically implicated in the light-entrained circadian oscillator. The food-entrainable circadian oscillator(s) does not require the CLOCK protein for normal functioning as arrhythmic Clock mice continue to display food anticipatory activity (FAA; Pitts, Perone, and Silver, 2003). Thus, our results, like those of Lewis et al., can only address the putative relationship between the light-entrained circadian oscillator and interval timing.
Secondly, and more importantly, none of the mice in Lewis et al. demonstrated very precise timing functions either before or following the lesions, thus leaving open the possibility of a "floor effect" in performance making any interval-timing impairments following successful SCN lesions difficult to detect. The failure to establish smooth timing functions prior to the lesions limits the sensitivity of their behavioral measures to changes that may have arisen due to the lesions. For this reason in particular, results of this study should be interpreted with caution.
Although the Lewis et al. (2003) study addressed questions regarding the role of the SCN in interval timing, it left open questions regarding the nature of the relationship between the circadian clock and the interval timer at the molecular level. It is entirely possible that the molecular machinery of interval timing has elements in common with the molecular machinery of the circadian clock, even though the SCN is not involved in interval timing. Our current study was a first glimpse at this relationship, revealing that expression of the Clock gene is not necessary for interval timing – at least under conditions of 12:12 LD entrainment.
It is important to note that while there appears to be no deleterious impact of the CLOCK mutation on timing of these short intervals, it is not necessarily the case that there is no impact of the circadian oscillator on longer durations. For example, in the timing model of Church and Broadbent (1990), a full range of periodic oscillators are involved in interval timing for every duration, but their influence is proportional to the extent to which their period matches the duration being timed. As the 20-s interval used here is only 0.023% of the circadian period, it may be that the interval being tested is too short to detect an impact.
An interesting finding of this study is that while their wild-type littermates centered their behavior slightly later than the 10 and 20-s target times, the CLOCK mutant mice timed their behavior earlier, closer to the target time with greater precision than their wild type counterparts. These findings are consistent with an increase in the speed of the interval timer in these mutant mice (Meck, 1996, 2001; Meck and Benson, 2002), possibly related to the heightened DA levels in these animals as reported by McClung et al. (2005). It may also simply be the case that, given a purported greater reliance upon environmental cues to maintain circadian rhythmicity (i.e., they fail to maintain rhythmicity in DD; Vitaterna et al., 1994), these animals are more reliant on their interval timers for tracking regularities in their environment and thus have more fine-tuned interval-timing mechanisms. These two explanations are clearly not mutually exclusive.
Although findings of the current study suggest distinct molecular foundations for interval timing and the circadian clock, a number of open questions remain. For example, animals in the current study were entrained to a 12:12 light/dark cycle. While the current study examined whether expression of the Clock gene is necessary for interval timing, it did not address whether rhythmicity in the circadian clock is necessary. Although our findings do not suggest a direct molecular link between circadian and interval timing, a number of studies reveal interval timing is modulated by circadian timing (e.g., Aschoff, 1984, 1998; Meck, 1991; Pati and Gupta, 1994; Shurtleff et al., 1990). Future studies may wish to look at interval-timing behavior in these animals under arrhythmic conditions to examine the relationship between circadian rhythmicity and regular interval timing. In addition, there are a number of other genes implicated in the circadian clock. Investigations of the role of those genes in the interval timer are clearly necessary before one can conclude that there is no shared molecular basis.
In conclusion, results of the current study do not suggest a shared molecular basis for the circadian clock and the interval timer. Despite both behavioral and physiological evidence suggesting potential relationships between the two, our data support and extend previous findings suggesting that circadian and interval timing involve two distinct biological mechanisms. Mice lacking expression of a circadian clock gene performed just as well, if not better, than their wild type littermates in an interval timing task, suggesting distinct molecular bases for circadian and interval timing.
4. Experimental procedures
4.1. Animals and housing
We trained three groups of male albino CLOCK mice: 10 homozygous CLOCK mutants (−/−), 13 heterozygous CLOCK mutants (+/−), and 15 wild type mice (+/+). The animals were obtained from a breeding colony from the lab of Joseph Takahashi at Northwestern University (derived and characterized as in Vitaterna et al., 1994). They were housed individually with a 12:12 LD cycle and given daily rations of food to maintain weights at 90% of their free feeding weight. The experiment was conducted during their dark cycle at approximately the same time everyday, zeitgeber time (ZT) 14–17.
4.2 Apparatus
Mice were trained in Med-Associates operant chambers, equipped with a tone generator and four hoppers, and housed in sound attenuating boxes. Water was available throughout experimental sessions. The hoppers were equipped with a standard-issue LED light (Med Associates ENV-321W) to illuminate the hopper and an infrared beam to detect head entries. The light from the hopper LED was 3 lux at 2 cm in front of the hopper and below 1 lux in the center of the box. Given the low level of highly localized light and its aperiodic appearance, we do not believe that it interfered with the animals’ normal circadian timing. One hopper (the control hopper) was on the opposite side of the chamber from the other three. Only the control hopper and the middle of the three hoppers opposite it (the target hopper) were used in this experiment. The target hopper was connected to a feeder that delivered one 20 mg grain-based food pellet (A.J. Noyes, #PJA/100020) per feeding. Operant chambers were controlled by a Med-Associates interface, and experiments were programmed in Med-PC code.
4.3 Behavioral Procedures
4.3.1. Peak-interval (PI) 10s (Sessions and 20s Training (Sessions 1–27)
We trained mice on a modified version of the peak-interval (PI) procedure (adapted from Church, Meck, and Gibbon, 1994; Paule et al., 1999). Mice participated in a 2 hr session at approximately the same time every day, 7 days a week. There were two phases to the experiment. In Phase 1 (the first 16 sessions), the target interval (T) was 10 s. In Phase 2, which lasted 11 sessions, the target interval was 20 s.
Responding in our timing task required animals to poke their head into a hopper in anticipation of feeding – a very natural behavior for these animals. Thus, pre-training (hopper training) in the experimental procedure was not necessary. Instead, following three days of acclimatization to the experimental chambers during which the animals were placed inside the chambers for 15 minutes each day, on the fourth day, the full experimental procedure was instituted.
Chambers were dark during the session. Trials began with the light on in the control hopper. Mice had to poke their head into this hopper far enough to break the infrared beam in order to start the interval. Once this happened, the light in the control hopper turned off, the light in the target hopper illuminated and a tone came on signaling the elapsing interval (both auditory and visual stimuli signaled the interval). There were three kinds of trials:
Fixed-time trials
(1/5 of all trials) In fixed-time (FT) trials, the tone turned off and food was delivered once the target interval elapsed, independent of the mouse's behavior. The light in the target hopper turned off 2 s after the food was delivered and the trial ended. These FT trials were included to ensure that subjects repeatedly observed the true target interval regardless of how appropriately or inappropriately they timed their anticipatory poking.
Fixed-interval trials
(3/5 of all trials) In fixed-interval (FI) trials, the feeder was armed once the target interval elapsed. The tone turned off and reward was delivered on the first break in the hopper entry beam (signaling a head poke in that hopper). The light in the target hopper turned off 2 s after the food was delivered and the trial ended. On these FI trials, the delay in feeding was jointly determined by the target interval and the subject’s behavior.
Probe trials
(1/5 of all trials) Responding on probe trials was not reinforced. The target hopper remained illuminated and the tone stayed on long past the target interval. The duration of a probe trial was three times the target interval (T), plus an additional duration chosen from an exponential distribution with an expectation of the target interval (probe window = 3T +E(T)). The tone and target hopper light turned off at the end of the probe window.
All trials were followed by a variable inter-trial interval (ITI) equivalent to 3T+E(2T). Following all behavioral testing, the mice were sacrificed and DNA-typed to verify their genotypes.
Acknowledgments
We would like to thank Martha Vitaterna for providing us with the CLOCK mice and for DNA-analyses of the animals and Warren Meck for comments on an earlier draft of this paper. This work was supported by Grant R21 MH63866 from the NIMH to CRG and by an NSF pre-doctoral fellowship and an NIH NRSA post-doctoral fellowship to SC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This criterion of 1.25 s was an arbitrary value chosen so as to best describe the poking behavior of the mice.
References
- Abner RT, Edwards T, Douglas A, Brunner D. Pharmacology of temporal cognition in two mouse strains. Inter. J. Comp. Psychol. 2001;14:189–210. [Google Scholar]
- Aschoff J. Circadian Timing. Ann. NY Acad. Sci. 1984;423:442–468. doi: 10.1111/j.1749-6632.1984.tb23452.x. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Human perception of short and long time intervals: Its correlation with body temperature and the duration of wake time. J. Biol. Rhythms. 1998;13:437–442. doi: 10.1177/074873098129000264. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav. Neurosci. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Cheng K, Westwood R. Analysis of single trials in pigeons timing performance. J. Exp. Psychol. Anim. Behav. Process. 1993;19:56–67. [Google Scholar]
- Cheng RK, Meck WH. Prenatal choline supplementation increases sensitivity to time by reducing non-scalar sources of variance in adult temporal processing. Brain Res. 2007;1186:242–254. doi: 10.1016/j.brainres.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM, Broadbent HA. Alternative representations of time, number, and rate. Cogn. 1990;37:55–81. doi: 10.1016/0010-0277(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Church RM, Meck WH, Gibbon J. Application of scalar timing theory to individual trials. J. Exp. Psychol. Anim. Behav. Process. 1994;20:135–155. doi: 10.1037//0097-7403.20.2.135. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Systematic nonlinearities in the perception of temporal intervals. J. of Exper. Psych.: Animal Behav. Proc. 1999;25(1):3–17. [PubMed] [Google Scholar]
- Crystal JD. Circadian time perception. J. Exp. Psychol. Anim. Behav. Process. 2001;27:68–78. [PubMed] [Google Scholar]
- Crystal JD. Nonlinearities in sensitivity to time: Implications for oscillator-based representations of interval and circadian clocks. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 61–75. [Google Scholar]
- Crystal JD. Long-interval timing is based on a self-sustaining endogenous oscillator. Behav. Process. 2006;72:149–160. doi: 10.1016/j.beproc.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Crystal JD, Baramidze GT. Endogenous oscillations in short-interval timing. Behav. Processes. 2007;74(2):152–158. doi: 10.1016/j.beproc.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD. Effects of dopamine antagonists on the timing of two intervals. Pharm. Biochem. Behav. 2003;75:9–15. doi: 10.1016/s0091-3057(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes, Brain, Behav. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, King AP, McDonald R. Sources of variability and systematic error in mouse timing behavior. J. Exp. Psychol. Anim. Behav. Process. 2004;30:3–16. doi: 10.1037/0097-7403.30.1.3. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s Law in animal timing. Psychol. Rev. 1977;84:279–325. [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann. NY Acad. Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Morrell M, Silver R. Two kinds of timing in circadian incubation rhythm of ring doves. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984;247:R1083–R1087. doi: 10.1152/ajpregu.1984.247.6.R1083. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Cogn. Brain Res. 2004;21:171–182. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- King AP, McDonald RV, Gallistel CR. Screening for mice that remember incorrectly. Inter. J. Comp. Psychol. 2001;14:232–257. [Google Scholar]
- Lewis PA, Miall RC, Daan S, Kacelnik A. Interval timing in mice does not rely upon the circadian pacemaker. Neurosci. Let. 2003;348:131–134. doi: 10.1016/s0304-3940(03)00521-4. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Paying attention to time as one gets older. Psychol. Sci. 2001;12:478–484. doi: 10.1111/1467-9280.00389. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Systems-level integration of interval timing and reaction time. Neurosci. Biobehav. Rev. 2004;28:747–769. doi: 10.1016/j.neubiorev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology. 2005;182:232–244. doi: 10.1007/s00213-005-0074-8. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Cheng RK, Williams CL, Meck WH. Combined organizational and activational effects of short and long photoperiods on spatial and temporal memory in rats. Behav. Process. 2007;74:226–233. doi: 10.1016/j.beproc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson’s Disease: A dopamine-related dysfunction. J. Cog. Neurosci. 1998;10:316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology. 1983;79:10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Matell MS, King GR, Meck WH. Differential adjustment of interval timing by the chronic administation of intermittent or continuous cocaine. Behav. Neurosci. 2004;118:150–156. doi: 10.1037/0735-7044.118.1.150. [DOI] [PubMed] [Google Scholar]
- Matell MS, Bateson M, Meck WH. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology. 2006;188:201–212. doi: 10.1007/s00213-006-0489-x. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence-detection of oscillatory processes. Cog. Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceed. Nat. Acad. Sci. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. J. Exp. Psychol. Anim. Behav. Process. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharm. Biochem. Behav. 1986;25:1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Modality-specific circadian rhythmicities influence mechanisms of attention and memory for interval timing. Learn. Motiv. 1991;22:153–179. [Google Scholar]
- Meck WH. The neuropharmacology of interval timing. Cog. Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Interval timing and genomics: What makes mutant mice tick? Inter. J. Comp. Psychol. 2001;14:211–231. [Google Scholar]
- Meck WH. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006a;1108:157–167. doi: 10.1016/j.brainres.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006b;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain's internal clock: How frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proceed. Natl. Acad. Sci. 2006;103(2):12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Horvitz JC, Kitsos E, Balsam P. The role of dopamine in the timing of Pavlovian conditioned keypecking in ring doves. Pharm. Biochem. Behav. 2001;69:617–627. doi: 10.1016/s0091-3057(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Pati AK, Gupta S. Time estimation circadian rhythm in shift workers and diurnally active humans. J. Biosci. 1994;19(3):325–330. [Google Scholar]
- Paule MG, Meck WH, McMillan DE, Bateson M, Popke EJ, Chelonis JJ, Hinton SC. The use of timing behaviors in animals and humans to detect drug and/or toxicant effects. Neurotoxicol. Teratol. 1999;21:491–502. doi: 10.1016/s0892-0362(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Pitts S, Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R57–R67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. J. of Exper. Psych.: Animal Behav. Proc. 1981;7(3):242–268. [PubMed] [Google Scholar]
- Shurtleff D, Rawslear TG, Simmons L. Circadian variations in time perception in rats. Physiology & Behavior. 1990;47:931–939. doi: 10.1016/0031-9384(90)90021-u. [DOI] [PubMed] [Google Scholar]
- Silver R, Bittman E. Reproductive mechanisms: Interaction of circadian and interval timing. Ann. NY Acad. Sci. 1984;423:488–514. doi: 10.1111/j.1749-6632.1984.tb23455.x. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: Dependence on the suprachiasmatic nucleus. Brain Research. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Terman M, Gibbon J, Fairhurst S, Waring A. Daily meal anticipation: Interaction of circadian and interval timing. Ann. NY Acad. Sci. 1984;423:470–487. doi: 10.1111/j.1749-6632.1984.tb23454.x. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowren PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viyoch J, Matsunaga N, Yoshida M, To H, Higuchi S, Ohdo S. Effect of haloperidol on mPer1 gene expression in mouse suprachiasmatic nuclei. J. of Biological Chemistry. 2005;280(8):6309–6315. doi: 10.1074/jbc.M411704200. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Documenta Ophthalmologica. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]