Abstract
We have previously shown that administration of orphanin FQ/nociceptin (OFQ/N), the endogenous ligand of the opioid receptor-like (ORL-1) receptor, into the lateral ventricles or VTA blocked cocaine sensitization. In the present study, we determined the effect of acute and chronic cocaine treatment on the level of endogenous OFQ/N in rat brain regions. Male Sprague Dawley rats were tested for motor activity in response to saline or cocaine (20 mg/kg) injection once daily for three consecutive days. To determine the effect of single or repeated cocaine administration on the level of OFQ/N, rats were sacrificed one hour following saline or cocaine injection either on day 1 or 3, respectively. Additional groups of rats were treated similarly with saline or cocaine on days 1–3 and sacrificed or tested for locomotor sensitization on day 8. Consistent with previous studies, repeated cocaine administration induced locomotor sensitization to a challenge dose of cocaine (7.5 mg/kg) given on day 8. Measurements of tissue content of OFQ/N-IR using radioimmunoassay indicated that the rat hypothalamus and striatum respectively contained the highest and lowest level of the peptide among the brain regions tested. Acute cocaine decreased the level of OFQ-IR in the rat midbrain and to a lesser extent in the striatum. On the other hand, the level of OFQ/N was higher in rats treated with cocaine on days 1–3 and sacrificed on day 8. These findings suggest that endogenous OFQ/N may be involved in the actions of cocaine and possibly in cocaine-induced motor stimulation and locomotor sensitization.
Keywords: Cocaine, Orphanin FQ/Nociceptin (OFQ/N), Opioid receptor-like (ORL-1) receptor, Radioimmunoassay (RIA), Sprague Dawley rats, Behavioral sensitization
Introduction
Drug addiction is a chronic relapsing brain disorder that involves neuroadaptive alterations in numerous neuronal circuits leading to compulsive drug seeking and drug taking behaviors despite catastrophic consequences associated with continued drug use/abuse. Research in laboratory animals has also revealed neuroadaptions following administration of cocaine and other drugs of abuse in different brain circuits. Behavioral changes which accompany these neuroadaptive changes mimic some aspects of addictive behaviors in humans.
In rodents, repeated intermittent cocaine administration has been shown to induce a progressive and enduring increase in motor activity, a phenomenon referred to as locomotor sensitization (Kalivas and Weber 1988; Post and Rose 1976; Robinson and Becker 1986; Stripling and Ellinwood, Jr. 1977). This phenomenon is thought to play an important role in the development and maintenance of drug dependency through an increase in drug "wanting" upon repeated administration such that the urge to take the drug becomes irresistible, i.e., drug craving. Thus, behavioral sensitization is considered as an animal model of some aspects of addiction, particularly craving (Robinson and Berridge 1993; Robinson and Berridge 2000).
The phenomenon of behavioral sensitization is believed to be due to numerous changes that occur along the mesolimbic dopaminergic neurons following repeated drug administration (Anderson and Pierce 2005; Everitt and Wolf 2002; Vanderschuren and Kalivas 2000; White and Kalivas 1998; Woolverton and Johnson 1992). In particular, changes in the dynamics of dopaminergic neurotransmission, and dopamine receptor number and signaling have been reported (Kalivas and Duffy 1990; Pierce and Kalivas 1995; Pierce et al. 1995; Zahniser et al. 1988; Anderson and Pierce 2005). Furthermore, alterations in the function of the guanine regulatory binding proteins have been implicated in the phenomenon of sensitization (Cunningham and Kelley 1993; Hummel and Unterwald 2003; Nestler et al. 1990). Hyperactivity of the glutamatergic system is another hallmark of behavioral sensitization (for reviews, see Carlezon, Jr. and Nestler 2002; Everitt and Wolf 2002; Vanderschuren and Kalivas 2000). Recent evidence has also implicated protein kinase A (for review, see Anderson and Pierce 2005) as well as extracellular signal-regulated kinase (for review, see Girault et al. 2007) in the phenomenon of locomotor sensitization.
The endogenous opioid system has long been known to modulate the function of the mesolimbic dopaminergic neurons. Thus, while mu and delta receptor agonists increase, kappa receptor agonists decrease the function of the mesolimbic dopaminergic neurons (Di Chiara and Imperato 1988; Herz 1997). The endogenous opioid system may also be involved in the phenomenon of locomotor sensitization. For example, drugs that block the mu and delta opioid receptors (Hummel et al. 2004; Hummel et al. 2006; Kim et al. 1997; Schroeder et al. 2007) or activate the kappa opioid receptor (for review, see Shippenberg and Rea 1997) have been shown to attenuate the development of psychostimulant-induced locomotor sensitization. Repeated intermittent cocaine treatment has also been shown to modify the level of endogenous opioid peptides (Hurd and Herkenham 1992; Hurd et al. 1992; Hurd 1996; Hurd et al. 1999; Sivam 1989) and receptors (Hammer, Jr. 1989; Izenwasser et al. 1996; Unterwald et al. 1994).
In 1994, several laboratories cloned a receptor that showed approximately 65% homology to the classical (mu, delta and kappa) opioid receptors (Bunzow et al. 1994; Chen et al. 1994; Fukuda et al. 1994; Hammer, Jr. 1989; Mollereau et al. 1994). This receptor was termed as the opioid receptor-like (ORL-1) receptor. A year later, two independent laboratories isolated orphanin FQ/nociceptin (OFQ/N) as the endogenous ligand of the ORL-1 receptor (Meunier et al. 1995; Reinscheid et al. 1995). OFQ/N, a 17 amino acid peptide, is structurally similar to the endogenous opioid peptides, in particular to dynorphin A (1–17) (Julius 1995; Meunier et al. 1995). However, OFQ/N does not display appreciable affinity for the classical opioid receptors and the endogenous opioid peptides do not bind to the ORL-1 receptor. Thus, the endogenous OFQ/N/ORL-1 receptor system has its unique pharmacology.
The OFQ/N/ORL-1 receptor system regulates the function of the mesolimbic dopaminergic neurons and attenuates the rewarding and addictive effects of abused drugs. Thus, intracerebroventricular OFQ/N administration has been reported to attenuate elevations in accumbal dopamine induced by morphine (Di Chiara et al. 1999) or cocaine (Lutfy et al. 2001). Furthermore, OFQ/N has been shown to block the development of behavioral sensitization (Lutfy et al. 2002), raising the possibility that the endogenous OFQ/N/ORL-1 receptor system may be involved in the phenomenon of locomotor sensitization. Thus, the present study was designed to determine whether repeated cocaine treatment that induces locomotor sensitization would alter the level of endogenous OFQ/N in various brain regions in rats. We also determined the effect of acute cocaine on the level of OFQ/N-immunoreactivity (OFQ/N-IR) in rat brain regions.
Materials and Methods
Subjects
Male Sprague Dawley rats, weighing 200–250 g, were obtained from Harlan Laboratories (San Diego, California, USA) and used in all experiments. Animals were maintained under a 12 h light/12 h dark cycle (light on at 7:00 AM) with free access to water and food in a humidity-and temperature-controlled room. All experiments were conducted according to the NIH guideline and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences (Pomona, California, USA).
Experimental Procedure
Measurement of OFQ/N-immunoreactivity (OFQ/N-IR) in rat brain regions
Rats (n = 6) were deeply anesthetized, their brains removed and placed in ice-cold buffer. Different brain regions (brain stem (mainly pons), cerebellum, midbrain (mainly VTA), thalamus, hypothalamus, hippocampus, striatum, and prefrontal and parietal cortices) were isolated and sonicated in 40 volumes of ice-cold acid acetone. The tissue was spun, the supernatant collected and lyophilized. A 1:10 dilution of each sample was prepared in artificial cerebrospinal fluid and assayed in triplicates for the measurement of OFQ-IR using a commercially available radioimmunoassay kit (Phoenix Pharmaceuticals, Belmont, CA). The sensitivity of the assay was 0.1 fmol and there was no cross-reactivity with opioid peptides, dynorphins A (1–17), enkephalins, beta-endorphin, endomorphin-1 and endomorphin-2. Total and non-specific bindings were 50% and 3%, respectively.
Measurement of OFQ/N-IR in rat brain regions following single cocaine administration
Rats were habituated to motor activity chambers for one hour, then injected with saline (n = 6) or cocaine (20 mg/kg, i.p.; n = 6) and motor activity was recorded for one hour (4 × 15-min epochs) using a Videomex-V system (Columbus Instruments, Inc; Columbus, Ohio, USA). Rats were then immediately sacrificed and the level of OFQ/N-IR was measured in different brain regions, as described above.
Measurement of OFQ/N-IR in rat brain regions following repeated cocaine administration
Rats were habituated to the motor activity chambers for one hour, then injected with saline (n = 6) or cocaine (20 mg/kg, i.p.; n = 6) and motor activity was recorded for one hour (4 × 15-min epochs). The same treatment was given once daily for three consecutive days. On day 3, rats were sacrificed one hour after the last cocaine or saline injection and their brains processed for the measurement of OFQ/N-IR, as described above.
Measurement of OFQ-IR in brain regions of rats sensitized to cocaine
Rats were habituated to the motor activity chambers, treated with saline (n = 6) or cocaine (20 mg/kg; n = 6) once daily for three consecutive days, as described above. Rats were then left untreated until day 8. On this day, rats were either sacrificed and their brains processed for the measurement of OFQ/N-IR, as described above, or tested for locomotor sensitization. To confirm that the current cocaine treatment paradigm indeed induces locomotor sensitization, rats were habituated to the motor activity chambers, injected with saline or cocaine (7.5 mg/kg) and motor activity was recorded for one hour (4 × 15-min epochs).
Data Analysis
Data are presented as means (±SEM). Behavioral data (the first 15-min epoch following cocaine administration) were analyzed using one-way analysis of variance (ANOVA) followed by the post-hoc Dunnet’s test. Neurochemical data were analyzed by one-way ANOVA or unpaired student’s t test. A p<0.05 was considered statistically significant.
Results
Hypothalamus contained the highest level of OFQ/N-IR in rat brain
Our pilot studies showed that the level of OFQ/N-IR in some brain regions did not fall on the linear portion of the standard curve. To mitigate this problem, samples were diluted 10 times and 50 µL of the samples yielded values falling on the linear portion of the standard curve. Additionally, the level of OFQ/N-IR in the diluted samples was above the level of detection (0.1 fmol) for each brain region (Table 1). A one-way ANOVA revealed a significant effect of brain region (F8,41 = 27.32; p<0.001). The post-hoc test showed that the rat hypothalamus, as compared to the other brain regions tested in the current study, contained the highest amount of OFQ/N-IR (p<0.05). Midbrain, thalamus, brain stem, cortical regions and hippocampus contained high to moderate levels of OFQ/N-IR. The lowest level of OFQ/N-IR was detected in the cerebellum and striatum (Table 1).
Table 1.
The level of OFQ/N-IR in different brain regions of naïve rats measured by RIA.
| Brain region | Level of OFQ/N-IR ± S.E.M. |
|---|---|
| Hypothalamus | 29.59 ± 3.76* |
| Midbrain | 15.17 ± 2.16 |
| Thalamus | 12.65 ± 0.84 |
| Brainstem | 9.25 ± 0.60 |
| Prefrontal Cortex | 6.46 ± 0.48 |
| Parietal cortex | 6.42 ± 0.62 |
| Hippocampus | 5.88 ± 0.58 |
| Striatum | 3.09 ± 0.28 |
| Cerebellum | 2.46 ± 0.32 |
Rats (n = 6) were deeply anesthetized with isoflurane, then sacrificed and their brain regions isolated and processed for the measurement of OFQ/NIR using a commercially available RIA kit (Phoenix Pharmaceuticals, Inc; Belmont, California, USA).
indicates a significantly greater level of OFQ/N-IR as compared to other brain regions (p<0.05).
Acute cocaine treatment decreased the level of OFQ/N-IR in midbrain region
The level of OFQ/N-IR in brain regions of rats sacrificed one hour following saline or cocaine (20 mg/kg) treatment is shown in figure 1. Once again, analysis of the data using one-way ANOVA in the control group (saline-treated rats) revealed a significant effect of brain region (F7,36 = 23.93; p<0.001). The post-hoc analysis revealed the highest level of OFQ/N-IR in the hypothalamus (p<0.05 as compared to other brain regions) followed by midbrain, thalamus and brain stem (Fig. 1A). The lowest amount of OFQ-N-IR was observed in the striatum (Fig. 1B). Acute cocaine, as compared to saline, treatment induced a modest but significant reduction in the level of OFQ/N-IR in rat midbrain (p<0.05). Also, cocaine produced a 20–40% reduction in OFQ/N-IR in the striatum and thalamic regions, but those changes were not statistically significant (p>0.05). However, the level of OFQ/N-IR was comparable in the hypothalamus, brain stem, cortical regions and hippocampus of rats treated with cocaine or saline (p>0.05).
Fig. 1. Acute cocaine treatment reduced the level of OFQ/N-IR in the rat midbrain.
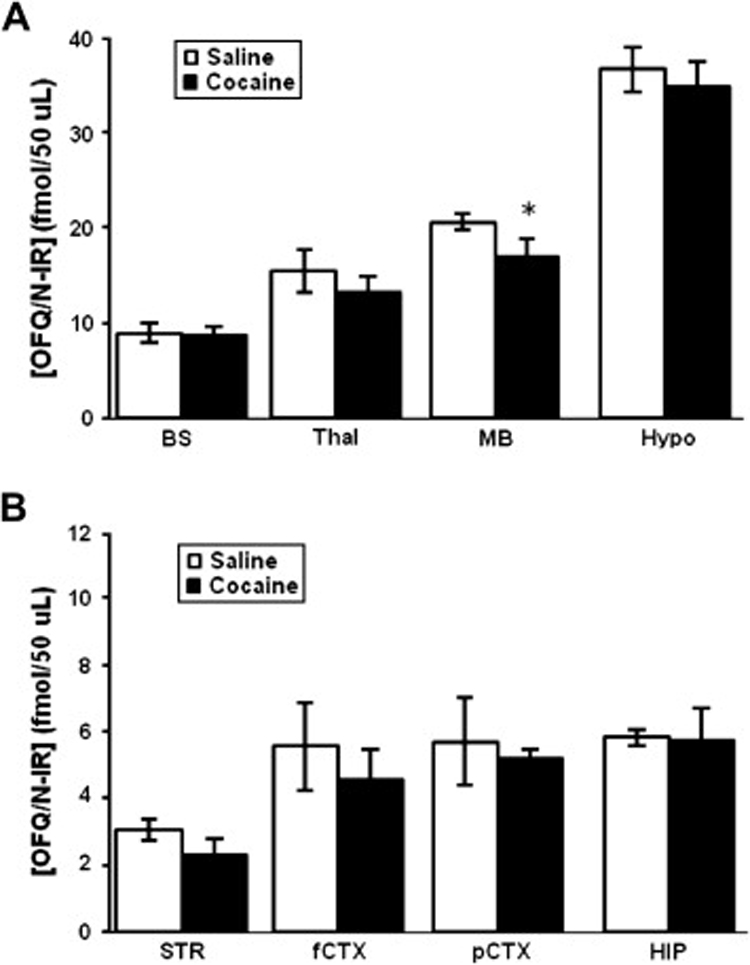
Rats were habituated to the motor activity tests, injected with saline or cocaine (20 mg/kg, i.p.) and sacrificed one hour later. Brain regions were isolated and homogenized in acid acetone solution by means of sonication. Samples were lyophilized, resuspended in RIA buffer and 50 µl of a 1:10 dilution of each sample were assayed in triplicates for the measurement of OFQ/N-IR using a commercially available RIA kit (Phoenix Pharmaceuticals; Belmont, CA). Data are mean (±SEM) of 6 rats/group. *indicates a significant reduction as compared to saline group (p<0.05).
Repeated cocaine administration (once daily for three consecutive days) failed to reduce the level of OFQ/N-IR in the midbrain
We have previously shown that rats treated with cocaine (20 mg/kg) once daily for three consecutive days express locomotor sensitization (Lutfy et al. 2002a). Therefore, we used a similar paradigm to determine whether the level of OFQ/N-IR would be altered in rat brain regions during the development of sensitization. Figure 2 depicts the level of OFQ/N-IR in rats treated with saline or cocaine once daily for three consecutive days and sacrificed one hour following the last saline or cocaine injection. Once again, the highest and lowest level of OFQ/N-IR was found in the hypothalamus (Fig. 2A) and striatum (Fig. 2B), respectively. Although a pattern toward reduction was observed in the hypothalamus, midbrain, and cortical regions in cocaine-treated rats, none of the changes were statistically significant in the cocaine-treated rats as compared to their saline-treated controls (p>0.05). However, the reduction in OFQ-IR in the parietal cortex of cocaine treated rats approached a significant level as compared to their controls (Fig. 2B; t1,10 = 1.99; p<0.04; one-tailed).
Fig. 2. The level of OFQ/N-IR was not altered in the midbrain in rats repeatedly treated with cocaine.
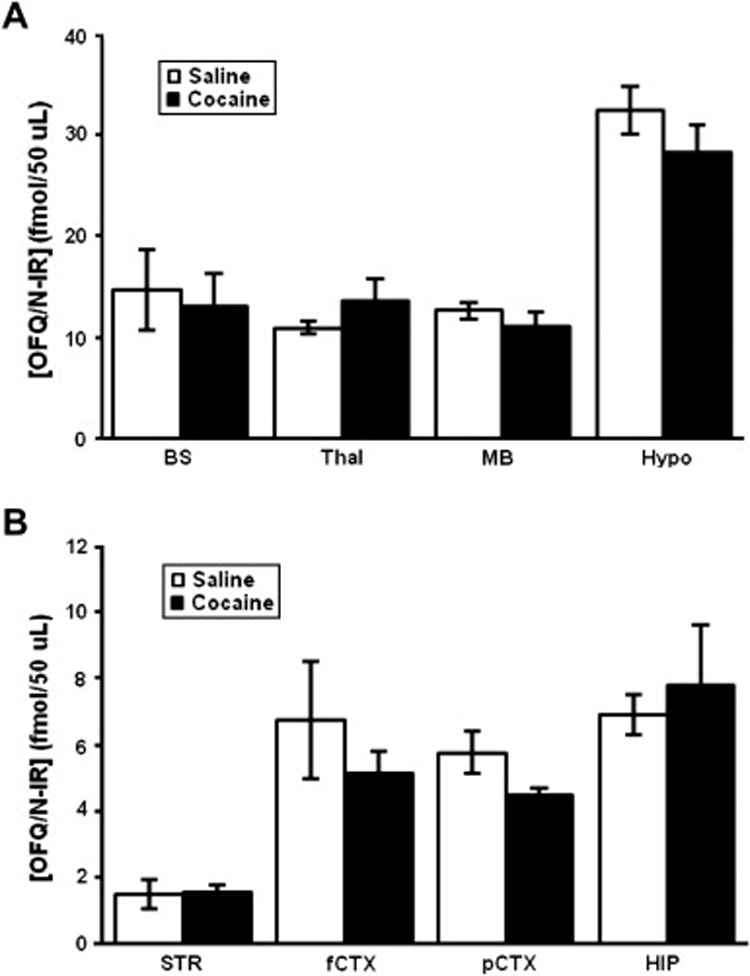
Rats were habituated to the motor activity tests, injected with saline or cocaine (20 mg/kg, i.p.) and motor activity was recorded for one hour. The same treatment was given once daily for three days and rats were sacrificed one hour following the last saline or cocaine injection. Brain regions were isolated, homogenized and assayed as described under legend to figure 1. Data are mean (±SEM) of 6 rats/group.
Repeated intermittent cocaine administration induced locomotor sensitization and increased the level of OFQ/N-IR in the hippocampus
Consistent with our previous results (Lutfy et al. 2002f), rats treated with cocaine on days 1–3 expressed locomotor sensitization following a challenge dose of cocaine (7.5 mg/kg) given on day 8 (Fig. 3). One-way ANOVA of the behavioral data (the first 15-min time point following saline or cocaine injection on day 8, when the action of cocaine was maximal) revealed a significant effect of treatment (F3,20 = 6.72; p<0.001). The post-hoc test revealed that a challenge dose of cocaine, as compared to saline, injected on day 8, increased motor activity (p<0.05; compare SAL-Coc versus SAL-Sal group). However, the magnitude of this response was significantly greater in rats treated with cocaine on days 1–3 as compared to their saline-treated control rats (p<0.05; compare COC-Coc versus SAL-Coc group). This result demonstrates that sensitization developed to the motor stimulatory action of cocaine. To determine whether the level of OFQ/N-IR would be altered during the expression of sensitization, we determined the level of OFQ/N-IR in rats treated with saline or cocaine (20 mg/kg) once daily for three consecutive days and sacrificed on day 8 (Fig. 4). Once again, a similar pattern of OFQ/N-IR was detected in different brain regions, i.e., the highest and lowest level of OFQ/N-IR were detected in the hypothalamus (Fig. 4A) and striatum (Fig. 4B), respectively. The level of OFQ/N-IR was increased in the hippocampus of rats sensitized to cocaine as compared to their saline-treated controls (p<0.05; Fig. 4B). However, the level of OFQ/N-IR was not different in other brain regions studied between the cocaine sensitized and control rats (p>0.05; Fig. 4).
Fig. 3. Locomotor sensitization developed to the motor stimulatory action of cocaine in rats.
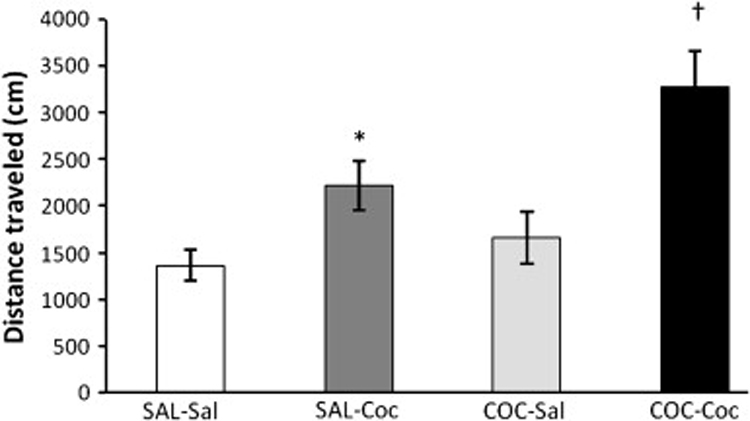
Rats were treated with saline (SAL) or cocaine (COC; 20 mg/kg, i.p.) once daily for three consecutive days and tested for locomotor sensitization on day 8. On this day, rats were habituated to the testing chambers for one hour, injected with saline (Sal) or cocaine (Coc; 7.5 mg/kg, i.p.) and motor activity was recorded for one hour (4 × 15 min epochs). Data are mean (±SEM) of distance traveled during the first 15-min epoch following saline or cocaine administration on day 8. *p<0.05 as compared to the rats treated with saline on days 1–3 and injected again with saline on day 8 (SAL-Sal group); †p<0.05 as compared to all other groups.
Fig. 4. The level of OFQ/N-IR was increased in the hippocampus of rats sensitized to cocaine.
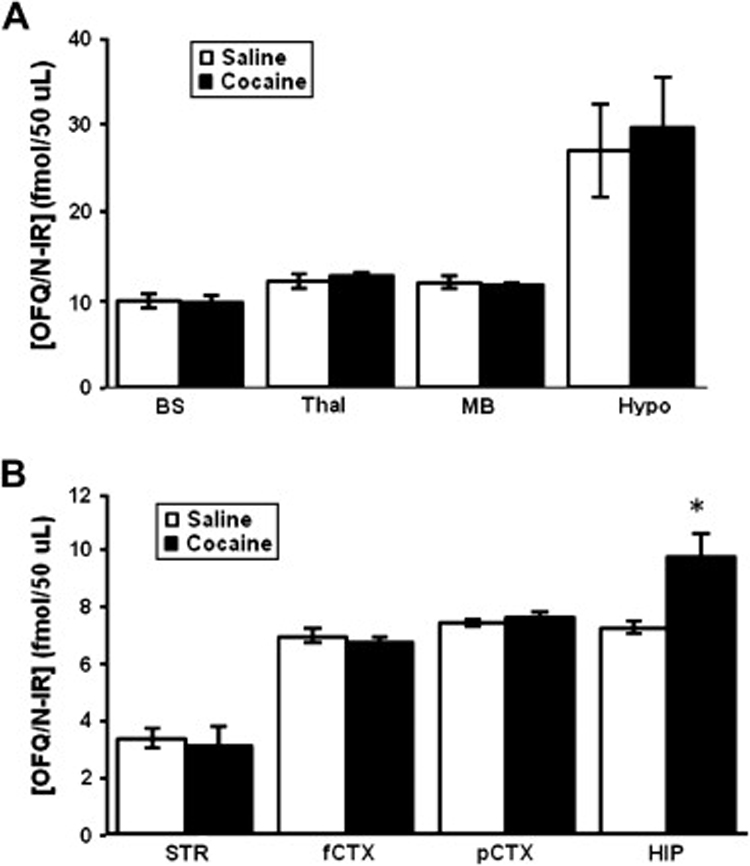
Rats were injected with saline or cocaine (20 mg/kg, i.p.) once daily for three days and sacrificed on day 8. Brain regions were isolated, homogenized and assayed for the level of OFQ/N-IR as described under legend to figure 2. Data are mean (±SEM) of 6 rats/group. *indicates a significant increase as compared to saline group (p<0.05).
Discussion
Previous studies have shown that the neuropeptide OFQ/N is widely distributed throughout the central nervous system (CNS), particularly in brain regions involved in emotional and motivational behaviors (Neal, Jr. et al. 1999). Consistent with its central distribution, endogenous OFQ/N and its receptor have been implicated in learning and memory, stress response, motivational and emotional behaviors as well as in the rewarding and addictive properties of cocaine and other drugs of abuse (for review, see Calo et al. 2005; Meis 2003; Mogil and Pasternak 2001). In the current study, we report alterations in the level of OFQ/N-IR following single as well as repeated cocaine treatment in rat brains regions, showing that the endogenous OFQ/N/ORL-1 receptor system may be involved in the actions of cocaine.
Our neurochemical studies showed that the hypothalamus contained the highest level of OFQ/N-IR followed by midbrain, thalamus, brain stem, hippocampus and cortical regions. The lowest amount of OFQ/N-IR was detected in the cerebellum and striatum. The regional distribution of OFQ/N-IR in the present study is consistent with previous observations in rat (Lindholm et al. 2002), mouse (Ploj et al. 2000) or human (Witta et al. 2004) brain. The novelty of our study is that a single cocaine administration, as compared to saline, decreased the level of OFQ/N-IR in the midbrain and to a lesser extent in the striatum and cortical regions in rats, showing that cocaine could reduce tissue content of OFQ/N-IR, a response that we interpreted as an increase in the release or degradation of endogenous OFQ/N. Although the response was modest, it is of interest to note that the level of OFQ/N-IR was measured one hour following cocaine administration. It may be that more robust reduction could have been detected, if the levels had been measured at earlier time points.
Previous neurochemical (Murphy and Maidment 1999; Murphy et al. 2004), dual in situ hybridization (Norton et al. 2002), electrophysiological (Zheng et al. 2002) and behavioral (Lutfy et al. 2002; Narayanan et al. 2004) studies have demonstrated that VTA could be one of the brain regions where OFQ/N exerts its modulatory actions on mesolimbic dopaminergic neurons and alters the actions of cocaine and other drugs of abuse. For example, we have shown that intra-VTA administration of OFQ/N reduced cocaine-induced motor stimulation and also blocked locomotor sensitization induced by repeated intermittent cocaine administration (Lutfy et al. 2002). The current neurochemical data demonstrating that the level of OFQ/N-IR was reduced by acute cocaine treatment raises the possibility that cocaine may cause the release of OFQ/N along the mesocorticolimbic dopaminergic pathway and might be a means through which the action of cocaine can be auto-regulated. Interestingly, we have recently demonstrated that the reinforcing effect of acute cocaine was enhanced in mice lacking the ORL-1 receptor or in wild-type mice treated with an ORL-1 receptor antagonist (Marquez et al. 2008).
Previous studies have shown changes in the level of endogenous opioid peptides following cocaine treatment (Hurd and Herkenham 1992; Hurd et al. 1992; Hurd 1996; Hurd et al. 1999; Sivam 1989). In the present study, we examined whether the level of endogenous OFQ/N, an anti-opioid peptide in the brain (Mogil and Pasternak 2001), would be altered in various brain regions of rats sensitized to cocaine. A recent report showed reduced levels of OFQ/N-IR in the substantia nigra and caudate putamen following continuous cocaine infusion. The level of OFQ/N was reduced in several other regions using immunoautoradiography but not radioimmunoassay (Romualdi et al. 2007). However, in the present study, the level of OFQ/N-IR was not reduced in midbrain or striatum of rats following repeated (once daily for three consecutive days) cocaine treatment (Fig. 2). Likewise, the level of OFQ/N-IR was not altered in midbrain or striatum of rats treated with cocaine once daily for three consecutive days and sacrificed on day 8 (Fig. 4). The previous study used a protocol of chronic cocaine infusion known to produce tolerance to the motor stimulatory action of cocaine (King et al. 1992; Reith et al. 1987). On the other hand, the present study determined the level of OFQ/N-IR under a repeated cocaine treatment paradigm that induces sensitization to the locomotor stimulatory effects of cocaine (Lutfy et al. 2002), which was confirmed in the current study (Fig. 3). Also, the dose of cocaine was higher (50 mg/kg/day) in the previous as compared to the current study. Lastly, in the previous study, rats were sacrificed while being infused with cocaine (Romualdi et al. 2007). Thus, the inconsistency in the results of the two studies could be due to the use of different experimental paradigms. However, we observed reduction in the level of OFQ/N-IR following acute cocaine administration in some brain regions (Fig. 1) that was consistent with the previous study (Romualdi et al. 2007).
Previous studies have shown that OFQ/N inhibits long-term potentiation (LTP) in the hippocampus (Yu et al. 1997) and therefore impairs spatial learning in rats (Sandin et al. 1997). Moreover, enhancement of LTP and spatial attention has been reported in mice lacking the ORL-1 receptor (Mamiya et al. 1998; Manabe et al. 1998), suggesting that the endogenous OFQ/N/ORL-1 receptor system is involved in synaptic plasticity. As cocaine sensitization involves conditioned learning and the hippocampus is involved in this process, our novel finding showing the level of OFQ/N-IR was increased in the hippocampus of cocaine sensitized rats (Fig. 4) could be important. As tissue content may represent the level of endogenous OFQ/N under the steady-state condition, the enhanced OFQ/N-IR in the hippocampus could be due to a decrease in the release or increase in the synthesis of endogenous OFQ/N. We interpreted this novel finding as an attempt of the internal milieu to reduce the strength of the conditioned response or to facilitate its extinction. However, the enhanced OFQ/N-IR in rats sensitized to cocaine may have been due to withdrawal from cocaine since these rats were sacrificed five days following the last cocaine treatment. Notably, we did not observe such changes in rats treated with cocaine for three days and sacrificed one hour following the last cocaine injection (Fig. 2). Thus, further studies are needed to shed more lights on the involvement of the endogenous OFQ/N in cocaine-induced locomotor sensitization. For example, dose-response and time-course data would provide important information about the dynamics of OFQ/N regulation following cocaine treatment in naïve and cocaine-sensitized rats. Further, while RIAs are more quantitative, anatomical resolution is lost with this method. Thus, inclusion of other approaches such as in situ immunohistochemical or immunoautoradiographic studies will provide useful information in this regard.
In summary, the level of OFQ/N-IR was reduced in the midbrain regions in response to acute but not repeated cocaine administration. On the other hand, the level of OFQ/N-IR was increased in the hippocampus of rats sensitized to cocaine as compared to their controls. Together, these results suggest that the endogenous OFQ/N/ORL-1 receptor system may play a functional role in the acute and chronic actions of cocaine.
Acknowledgments
The authors wish to thank Dr. Arbi Nazarian for his suggestions and comments. The present study was supported in part by the NIDA Grant DA016682 to KL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol. Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Calo G, Guerrini R, Rizzi A, Salvadori S, Burmeister M, Kapusta DR, Lambert DG, Regoli D. UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS. Drug Rev. 2005;11:97–112. doi: 10.1111/j.1527-3458.2005.tb00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE. Hyperactivity and sensitization to psychostimulants following cholera toxin infusion into the nucleus accumbens. J. Neurosci. 1993;13:2342–2350. doi: 10.1523/JNEUROSCI.13-06-02342.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci. Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin. Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hummel M, Ansonoff MA, Pintar JE, Unterwald EM. Genetic and pharmacological manipulation of mu opioid receptors in mice reveals a differential effect on behavioral sensitization to cocaine. Neuroscience. 2004;125:211–220. doi: 10.1016/j.neuroscience.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Hummel M, Schroeder J, Liu-Chen LY, Cowan A, Unterwald EM. An antisense oligodeoxynucleotide to the mu opioid receptor attenuates cocaine-induced behavioral sensitization and reward in mice. Neuroscience. 2006;142:481–491. doi: 10.1016/j.neuroscience.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. Intra-accumbens pertussis toxin sensitizes rats to the locomotor activating effects of a single cocaine challenge. Brain Res. 2003;965:100–107. doi: 10.1016/s0006-8993(02)04142-2. [DOI] [PubMed] [Google Scholar]
- Hurd YL. Cocaine effects on dopamine and opioid peptide neural systems: implications for human cocaine abuse. NIDA Res. Monogr. 1996;163:94–116. [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res. Mol. Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Influence of a single injection of cocaine, amphetamine or GBR 12909 on mRNA expression of striatal neuropeptides. Brain Res. Mol. Brain Res. 1992;16:97–104. doi: 10.1016/0169-328x(92)90198-k. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Svensson P, Ponten M. The role of dopamine, dynorphin, and CART systems in the ventral striatum and amygdala in cocaine abuse. Ann. N. Y. Acad. Sci. 1999;877:499–506. doi: 10.1111/j.1749-6632.1999.tb09285.x. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Heller B, Cox BM. Continuous cocaine administration enhances mu- but not delta-opioid receptor-mediated inhibition of adenylyl cyclase activity in nucleus accumbens. Eur. J . Pharmacol. 1996;297:187–191. doi: 10.1016/0014-2999(95)00828-4. [DOI] [PubMed] [Google Scholar]
- Julius D. Pharmacology. Home for an orphan endorphin. Nature. 1995;377:476. doi: 10.1038/377476a0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J. Pharmacol. Exp. Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- Kim HS, Park WK, Jang CG, Oh KW, Kong JY, Oh S, Rheu HM, Cho DH, Kang SY. Blockade by naloxone of cocaine-induced hyperactivity, reverse tolerance and conditioned place preference in mice. Behav. Brain Res. 1997;85:37–46. doi: 10.1016/s0166-4328(96)00162-3. [DOI] [PubMed] [Google Scholar]
- King GR, Joyner C, Lee T, Kuhn C, Ellinwood EH., Jr Intermittent and continuous cocaine administration: residual behavioral states during withdrawal. Pharmacol. Biochem. Behav. 1992;43:243–248. doi: 10.1016/0091-3057(92)90664-2. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Nociceptin/orphanin FQ tissue concentration in the rat brain. Effects of repeated ethanol administration at various post-treatment intervals. Prog. Neuropsychopharmacol Biol. Psychiatry. 2002;26:303–306. doi: 10.1016/s0278-5846(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Nishi M, Takeshima H, Nabeshima T. Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res. 1998;783:236–240. doi: 10.1016/s0006-8993(97)01406-6. [DOI] [PubMed] [Google Scholar]
- Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T, Takeshima H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008;54:564–568. doi: 10.1016/j.neuropharm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S. Nociceptin/orphanin FQ: actions within the brain. Neuroscientist. 2003;9:158–168. doi: 10.1177/1073858403252231. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Tan AM, Lam HA, Maidment NT. Nociceptin/orphanin FQ modulation of rat midbrain dopamine neurons in primary culture. Neuroscience. 2004;127:929–940. doi: 10.1016/j.neuroscience.2004.05.055. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Lam H, Carroll FI, Lutfy K. Orphanin FQ/nociceptin suppresses motor activity through an action along the mesoaccumbens axis in rats. J. Psychiatry Neurosci. 2004;29:116–123. [PMC free article] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J. Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J. Comp Neurol. 2002;444:358–368. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Duffy P, Kalivas PW. Sensitization to cocaine and dopamine autoreceptor subsensitivity in the nucleus accumbens. Synapse. 1995;20:33–36. doi: 10.1002/syn.890200106. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J. Pharmacol. Exp. Ther. 1995;275:1019–1029. [PubMed] [Google Scholar]
- Ploj K, Roman E, Gustavsson L, Nylander I. Basal levels and alcohol-induced changes in nociceptin/orphanin FQ, dynorphin, and enkephalin levels in C57BL/6J mice. Brain Res. Bull. 2000;53:219–226. doi: 10.1016/s0361-9230(00)00328-2. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reith ME, Benuck M, Lajtha A. Cocaine disposition in the brain after continuous or intermittent treatment and locomotor stimulation in mice. J. Pharmacol. Exp. Ther. 1987;243:281–287. [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Romualdi P, Di BM, D’Addario C, Collins SL, Wade D, Candeletti S, Izenwasser S. Chronic cocaine produces decreases in N/OFQ peptide levels in select rat brain regions. J. Mol. Neurosci. 2007;31:159–164. doi: 10.1385/jmn/31:02:159. [DOI] [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur. J. Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Unterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology (Berl) 2007;195:265–272. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Rea W. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol. Biochem. Behav. 1997;57:449–455. doi: 10.1016/s0091-3057(96)00450-9. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J. Pharmacol. Exp. Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- Stripling JS, Ellinwood EH., Jr Augmentation of the behavioral and electrophysiologic response to cocaine by chronic administration in the rat. Exp. Neurol. 1977;54:546–564. doi: 10.1016/0014-4886(77)90256-4. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Witta J, Palkovits M, Rosenberger J, Cox BM. Distribution of nociceptin/orphanin FQ in adult human brain. Brain Res. 2004;997:24–29. doi: 10.1016/j.brainres.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol. Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Yu TP, Fein J, Phan T, Evans CJ, Xie CW. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Peris J, Dwoskin LP, Curella P, Yasuda RP, O'Keefe L, Boyson SJ. Sensitization to cocaine in the nigrostriatal dopamine system. NIDA Res. Monogr. 1988;88:55–77. [PubMed] [Google Scholar]
- Zheng F, Grandy DK, Johnson SW. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br. J. Pharmacol. 2002;136:1065–1071. doi: 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]


