Abstract
Dopaminergic afferents from the ventral tegmental area (VTA) modulate information processing in the nucleus accumbens (NA), a brain region critical for motivation and reward mechanisms. In NA medium spiny neurons (MSNs) from young rats, D2 agonists have been shown to decrease the amplitude of corticoaccumbens synaptic responses. As several dopamine-related functions change during adolescence, we assessed the D2 modulation of cortical inputs with whole-cell recordings in slices obtained from adult and preadolescent rats. The D2 agonist quinpirole (5 μM) decreased synaptic response of NA MSNs to electrical cortical stimulation in slices from preadolescent rats. In slices from adult rats, however, quinpirole increased both the amplitude of evoked synaptic responses and the frequency of spontaneous synaptic events. These effects were blocked by the GABA-A antagonist picrotoxin (50 μM), revealing a D2-mediated decrease These results suggest that D2 receptors modulate NA neurons differently in young and adult rats, due to the emergence of a D2-facilitated GABA component in corticoaccumbens responses during adolescence.
Keywords: electrophysiology, GABA, glutamate, interneuron, rat, striatum
Introduction
The nucleus accumbens (NA) is a key brain region in which cortical and limbic inputs are integrated (Sesack & Pickel, 1990; O’Donnell et al., 1999). The NA receives glutamatergic afferents from the prefrontal cortex (PFC), hippocampus and amygdala (Kelley & Domesick, 1982; Brog et al., 1993; Groenewegen et al., 1999), and these inputs converge on individual medium spiny neurons (MSNs; O’Donnell & Grace, 1995; French & Totterdell, 2002, 2003, 2004). In addition to its glutamatergic afferents, the NA receives a dense dopamine (DA) innervation originating in the ventral tegmental area (VTA; Groenewegen et al., 1980) with terminals in close apposition to glutamatergic afferents (Sesack & Pickel, 1990). The interactions among all these inputs may provide a substrate for the ‘linking motivation to action’ role attributed to the NA (Mogenson et al., 1980).
DA has a complex set of postsynaptic effects on NA neurons. These include depolarization and changes in cell excitability (Akaike et al., 1987; Uchimura et al., 1989; O’Donnell & Grace, 1996), as well as modulation of glutamatergic afferents. DA consistently reduces the amplitude of corticoaccumbens excitatory postsynaptic potentials (EPSPs), both in slices (O’Donnell & Grace, 1994; Harvey & Lacey, 1996; Nicola et al., 1996; Brady & O’Donnell, 2004) and in vivo (Brady & O’Donnell, 2004). Our in vivo study revealed that a D2, not D1, antagonist blocked the attenuation of corticoaccumbens EPSPs by VTA stimulation in adult animals, but this was evident only during MSN up-states (Brady & O’Donnell, 2004). This suggests that at the negative membrane potential typically observed in vitro (equivalent to the in vivo down-state in which no effect of VTA stimulation was observed), D2 modulation would not be expected in slices from adult animals. Also, as previous in vitro studies have been conducted in preadolescent animals, it is possible that the D2 modulation of corticoaccumbens responses changes during adolescence. Indeed, the NA is not completely mature before five or more weeks of age in rats. Electrophysiological properties of striatal MSNs become stable by postnatal day (PD) 40 in rats (Tepper et al., 1998), and the levels of D1 and D2 receptors determined with binding assays increase until PD 28 in the NA (Teicher et al., 1995; Tarazi et al., 1998). Recordings from the PFC have shown that cortical DA actions continue to mature during adolescence (Tseng & O’Donnell, 2005). As puberty in rats typically occurs at PD 35–40 and the subsequent couple of weeks have been defined as adolescence (Spear, 2000), we sought to explore the nature of D2 modulation of corticoaccumbens synaptic responses before and after this age range. We performed whole-cell recordings from NA MSNs in brain slices obtained from preadolescent (PD 23–38) and developmentally mature (PD 50–63) rats, examining the D2 modulation of MSN corticoaccumbens synaptic responses.
Materials and methods
All experimental procedures were performed according to the USPHS Guide for Care and Use of Laboratory Animals and were approved by the Albany Medical College and the University of Maryland School of Medicine Institutional Animal Care and Use Committees. Young adult (PD 50–63) and preadolescent (PD 23–38) male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were maintained on a 12-h light–dark cycle with food and water available ad libitum until the time of recording. Rats were deeply anaesthetized with chloral hydrate (400 mg/kg, i.p.) before decapitation. Brains were removed into ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl, 125; NaHCO3, 25; glucose, 10; KCl, 3.5; NaH2PO4, 1.25; CaCl2, 0.5; and MgCl2, 3; pH, 7.45; osmolarity 295 mOsm. Parasagittal slices (350 μm thick) with a near 10° angle that preserves corticoaccumbens fibers (O’Donnell & Grace, 1994) were cut on a Vibratome in ice-cold ACSF. Slices were transferred and incubated in warm (35 °C) ACSF solution constantly oxygenated with 95% O2 and 5% CO2 for at least 1 h before recording. Slices were then transferred to a submersion-type recording chamber maintained at 33–35 °C and superfused with oxygenated ACSF at a flow rate of 2 mL/min. In the recording ACSF, CaCl2 was increased to 2 mM and MgCl2 was reduced to 1 mM.
Whole-cell current- and voltage-clamp recordings were performed from MSNs within the core region of the NA in adult and preadolescent rat brain slices. Patch pipettes (5–10 MΩ) were filled with (in mM): K-gluconate, 115; HEPES, 10; MgCl2, 2; KCl, 20; Mg-ATP, 2; Na2-ATP, 2; and GTP, 0.3; pH 7.3, 280 mOsm. Neurobiotin (0.125%) was added to the internal solution for labelling of the recorded cell. All drugs were mixed into oxygenated ACSF and applied to the recording solution in known concentrations. Both control and drug-containing ACSF were oxygenated continuously throughout the experiments. Quinpirole, eticlopride, picrotoxin and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were purchased from Sigma (St Louis, MO, USA).
Nucleus accumbens MSNs were identified under visual guidance using infrared differential interference contrast (IR-DIC) video microscopy (Olympus BX50-WI). The image was detected with an IR-sensitive CCD camera (DAGE-MTI) and displayed on a monitor. Whole-cell current-clamp and voltage-clamp signals were acquired with an Axoclamp-2A amplifier (Axon Instruments, Foster city, CA, USA), digitized with an A/D converter (Digidata, Axon Instruments) and sampled with Axoscope 8.1 (Axon Instruments) at a rate of 10 kHz. Electrode potentials were adjusted to zero before recording without correcting the liquid junction potential (estimated at 10 mV). Postsynaptic potentials were evoked in MSNs by electrical stimulation of corticoaccumbens fibers in the white matter between the rostral PFC and the NA with 0.2–0.9 mA, 0.5 ms current pulses.
Membrane potential, input resistance (measured with the slope of a current–voltage (I/V) plot obtained with 500-ms-duration depolarizing and hyperpolarizing pulses) and synaptic potentials evoked by cortical stimulation were measured before and after applying drugs to the bath. Neurons that exhibited a resting membrane potential more depolarized than −70 mV or an input resistance < 60 MΩ were excluded from the study. Typically, baseline activity was recorded for 10 min before drug application. Drug-containing solutions were superfused for 5 min. Baseline data were calculated from the average values in the 3-min period preceding drug superfusion and, for the analysis of drug effects, average values were calculated from a 3-min period starting 3 min after administration onset, to allow stabilization of drug levels. Data are expressed as mean ± SD. The statistical significance of the results was assessed by using a Wilcoxon test or a Mann–Whitney test (for paired and unpaired observations, respectively) and repeated-measures Anova.
At the end of each experiment, the slices were placed overnight in 4% paraformaldehyde, washed in 0.1 M PBS (pH 7.4), incubated with 0.3% Triton X-100 in PBS, and then incubated with 0.3% hydrogen peroxide for 15 min to block endogenous peroxidases. The avidin–biotin complex method was used to detect Neurobiotin-injected cells (ABC peroxidase kit; Vector Laboratories, Burlingame, CA, USA) and the reaction product was visualized with 3,3′-diaminobenzidine tetrachloride (DAB; Sigma). To visualize tyrosine hydroxylase-containing structures, some slices were re-sectioned to 30 μm using a freezing microtome. Sections were also washed in 0.1 M PBS (pH 7.4) and incubated with 0.3% hydrogen peroxide for 15 min. After washing in PBS, sections were incubated for 1 h in 10% normal horse serum and 0.3% Triton X-100 in PBS, then incubated overnight at 4 °C in mouse monoclonal antityrosine hydroxylase (1 : 10 000; Sigma-T1299) with 1% normal horse serum. After washing in PBS, sections were incubated with the secondary antibody (biotinylated horse antimouse IgG, 1 : 800; Vector laboratories, Burlingame, CA) at room temperature for 1 h. The avidin-biotin complex method was used to detect the secondary antibody (ABC peroxidase kit, Vector Laboratories) and the reaction product was visualized with DAB.
Results
Passive membrane properties of MSNs in the NA of preadolescent and adult rats
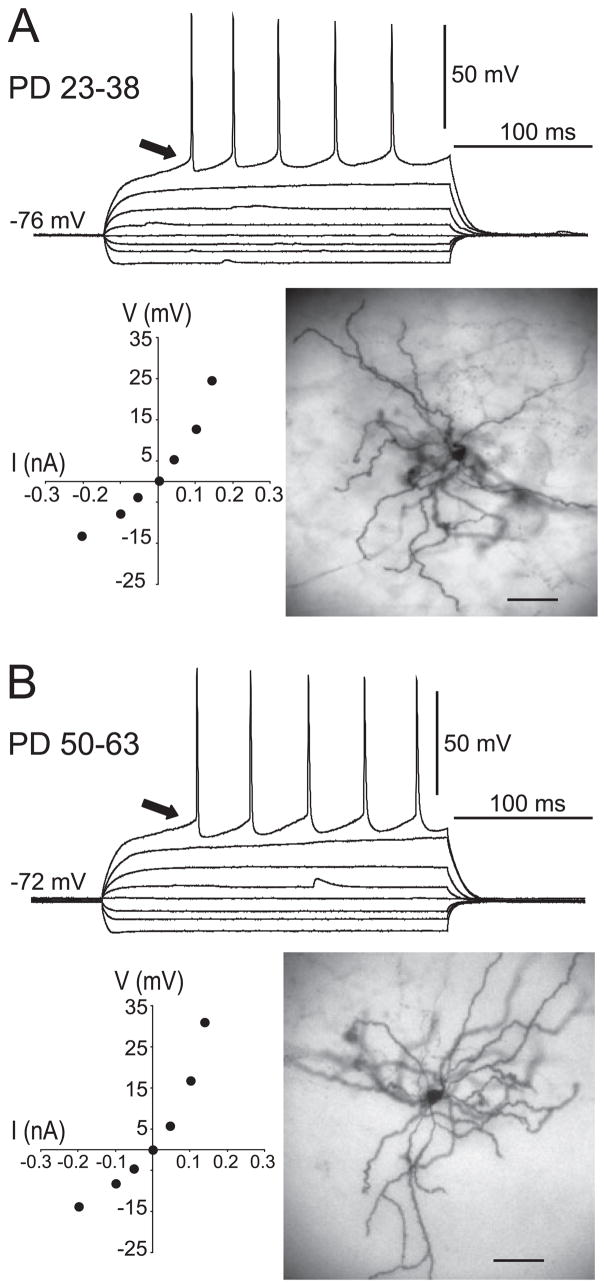
Ninety-three MSNs from preadolescent rats and 129 MSNs from adult rats were recorded in NA slices. The resting membrane potential was −76.7 ± 3.8 mV (mean ± SD) in preadolescent rats and −76.3 ± 4.0 mV in adult rats; input resistance was 97.1 ± 31 MΩ in preadolescent rats and 99.9 ± 45 MΩ in adult rats (Fig. 1A and B). No evident statistical differences between adult and preadolescent animals could be found for basic membrane properties.
Fig. 1.
Basic membrane properties of preadolescent and adult MSNs recorded in the NA. Traces show overlays of whole-cell current-clamp responses to depolarizing and hyperpolarizing current pulses (50-pA steps from −200 to +300 pA, 500 ms duration) in (A) preadolescent and (B) adult rats. Arrows point to the ramp depolarization preceding spikes in both age groups. Bottom left on both panels: I/V plots revealing an inward rectification with depolarizing current pulses, which corresponds to the ramp depolarization indicated by the arrow. Bottom right on both panels: DAB staining of representative MSNs filled with Neurobiotin during recording. Calibration bars, 50 μm.
Action potential firing in the NA of adult and preadolescent rats
Firing properties were assessed with 500-ms depolarizing current pulses. In both preadolescent and adult rats, the first action potential (AP) was preceded by a ramp depolarization (Fig. 1A and B), as previously reported (O’Donnell & Grace, 1993). The thresholds for AP firing were −36.9 ± 3.9 and −37.9 ± 4.0 mV in preadolescent and adult rats, respectively. AP amplitude (measured from threshold) was 76.2 ± 6.6 mV in preadolescent and 75.8 ± 5.9 mV in adult rats. AP half-widths were 1.7 ± 0.4 and 1.6 ± 0.4 ms in preadolescent and adult rats, respectively. As the amplitude of depolarizing current injections increased, more APs were evoked. Firing-frequency adaptation was observed as increased interspike intervals between consecutive APs. Instantaneous frequency was calculated from each interspike interval and plotted against the amplitude of depolarizing current injected. As previously described in young rats (O’Donnell & Grace, 1993), firing frequency adaptation was proportional to the current injected in both preadolescent and adult rats (data not shown). Thus, firing properties of NA MSNs did not differ between preadolescent and adult rats.
Synaptic responses of NA MSNs to cortical stimulation in preadolescent and adult rats
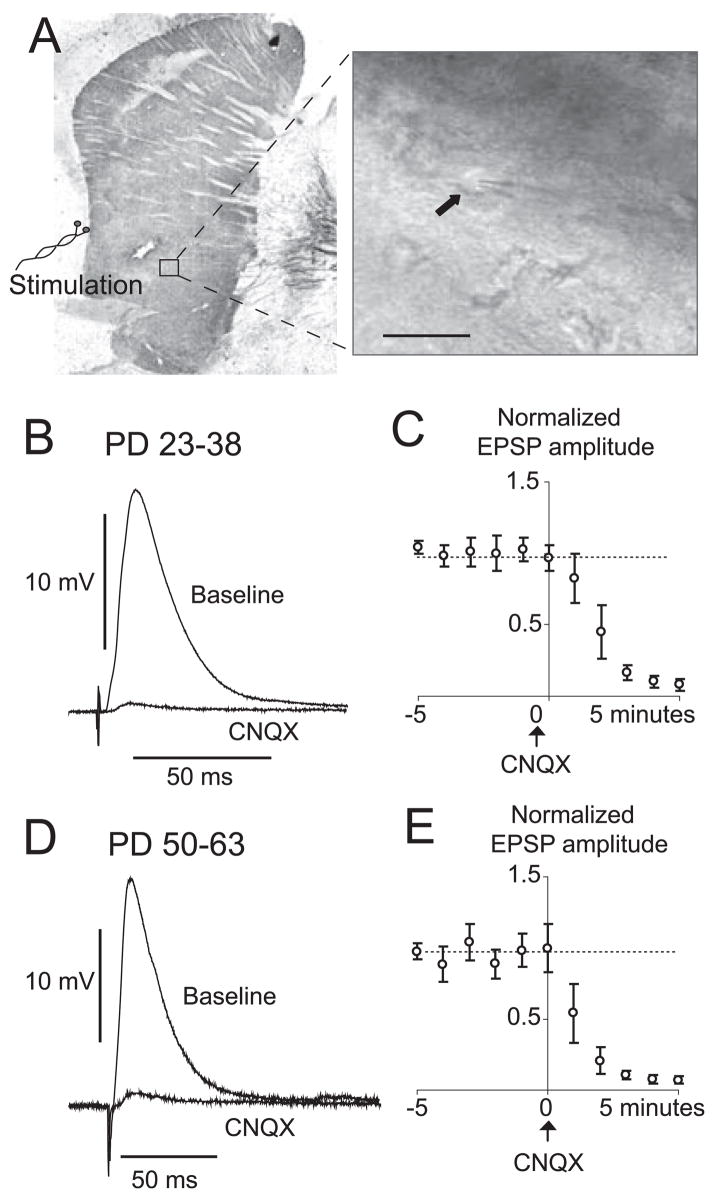
EPSPs were evoked by electrical stimulation of the white matter adjacent to the NA and carrying cortical fibers (Fig. 2A). The amplitude of evoked EPSPs ranged from 5.7 to 35.5 mV and, when more depolarizing than the action potential threshold, APs were evoked in both preadolescent and adult rats. The mean maximal EPSP amplitude was not different between young (14.6 ± 6.6 mV) and adult (14.3 ± 6.9 mV) rats. EPSP duration was calculated by measuring the time to decay to half-amplitude. EPSP half-decay was longer in preadolescent rats than in adult rats (20.4 ± 7.9 ms, n = 44, in preadolescent rats; 16.4 ± 3.9 ms, n = 29, in adult rats; P = 0.013). Bath application of the AMPA antagonist CNQX (5 μM) nearly abolished EPSPs in both preadolescent and adult rats (P < 0.001, repeated-measures ANOVA, n = 4 preadolescent and n = 6 adult; Fig. 2B and C). These observations show that cortical inputs to NA neurons evoked an AMPA response at negative membrane potentials in this preparation in both preadolescent and adult rats. For the rest of the study, stimuli were delivered at an intensity that evoked EPSPs with half of the maximal amplitude, allowing us to observe either increases or decreases in synaptic responses by DA treatment.
Fig. 2.
EPSPs evoked by electrical stimulation of the white matter adjacent to the NA and carrying cortical fibers in preadolescent and adult rats. (A) Tyrosine hydroxylase-stained slice illustrating the position of recording and stimulating electrodes. Inset shows an IR-DIC image of a MSN in the NA (arrow). (B) Representative corticoaccumbens EPSP in a slice from a preadolescent rat showing an overlay of EPSP evoked before (baseline) and after CNQX (10 μM). Traces are average of five repetitions. (C) Population data of EPSP amplitude normalized to baseline levels. Values are averages per minute. (D) Overlay of representative corticoaccumbens EPSPs in a slice from an adult rat showing blockade with CNQX. (E) Time course of normalized EPSP amplitudes for all the neurons tested. Calibration bar, 25 μm.
D2 modulation of synaptic responses in preadolescent and adult rats
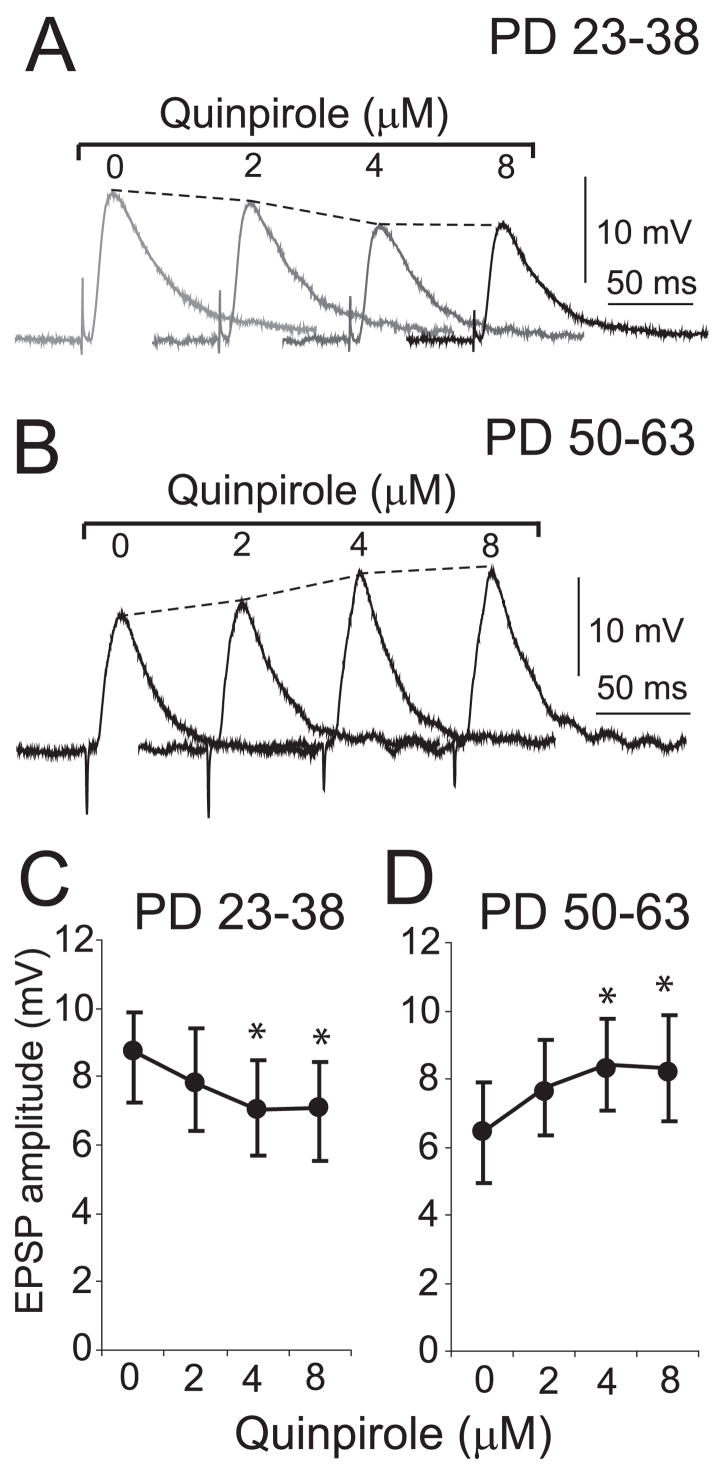
The effect of D2 receptor activation on corticoaccumbens EPSPs was determined by measuring EPSP amplitude before and after bath application of quinpirole at 2, 4 and 8 μM in both preadolescent and adult rats. Quinpirole had a significant effect on EPSP amplitude at the two higher concentrations in both groups, with a decrease observed in slices from preadolescent rats (Fig. 3A and C) and an increase in slices from adult rats (Fig. 3B and D). For the rest of the study quinpirole was delivered at 5 μM. In preadolescent rats, 5 μM quinpirole significantly decreased EPSP amplitude from 9.8 ± 1.3 to 8.4 ± 0.9 mV (P = 0.004, n = 5; Fig. 4A). This decrease was observed in most of the neurons recorded and did not affect the resting membrane potential or input resistance. Switching the bath to ACSF solution did not affect corticoaccumbens EPSPs (Fig. 4A). In adult rats, however, the D2 agonist increased EPSP amplitude from 6.3 ± 1.2 to 7.9 ± 1.8 mV (P = 0.005, n = 7; Fig. 4B) without changing input resistance or resting membrane potential, and this effect was blocked by the selective D2 antagonist eticlopride (20 μM; Fig. 4B). Thus, D2 receptors attenuated corticoaccumbens synaptic responses in slices from young rats, as described previously (O’Donnell & Grace, 1994), but in adult rats this modulation changed to an enhancement of EPSP amplitude.
Fig. 3.
Dose-dependent effects of a D2 agonist on corticoaccumbens responses are different in young and adult slices. (A) Examples of corticoaccumbens EPSPs following exposure to different concentrations of quinpirole at 10-min intervals in a representative MSN from a preadolescent rat. Each trace is an average of five repetitions. (B) Representative EPSP with increasing quinpirole concentrations in an MSN from an adult rat. (C) Plot indicating average EPSP amplitude for each concentration of quinpirole in preadolescent rats (mean ± SD). (D) Plot of EPSP amplitude after quinpirole in adult rats. *P < 0.02.
Fig. 4.
D2 modulation of EPSPs. (A) Representative traces of cortico-NA EPSPs before (baseline) and after changing the bath to ACSF (top) or quinpirole (bottom) in slices from preadolescent rats. Each trace is the average of 10 repetitions. The graph to the right plots normalized EPSP amplitude after changing the bath to ACSF (open circles), quinpirole (black circles) or a cocktail of the selective D2 antagonist eticlopride and quinpirole (grey circles). Values are averages per minute. (B) Representative traces of cortico-NA EPSPs and normalized EPSP amplitude plot in slices from adult rats.
D2 modulation of adult NA MSNs required GABA
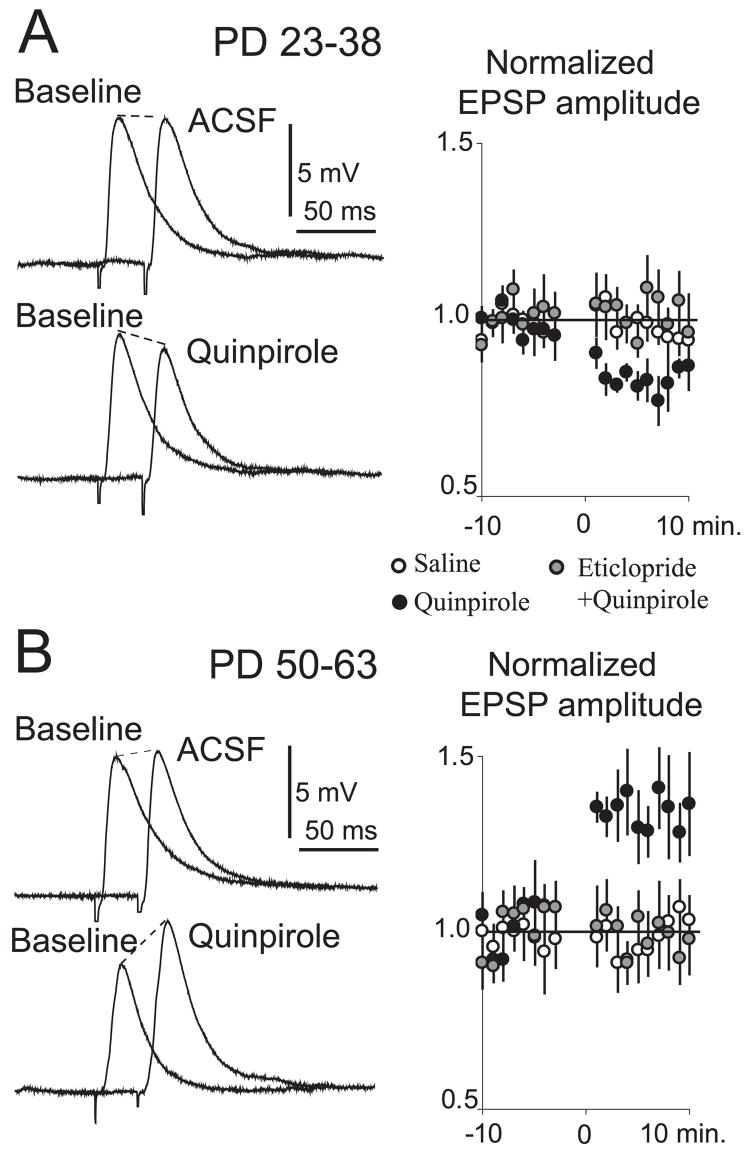
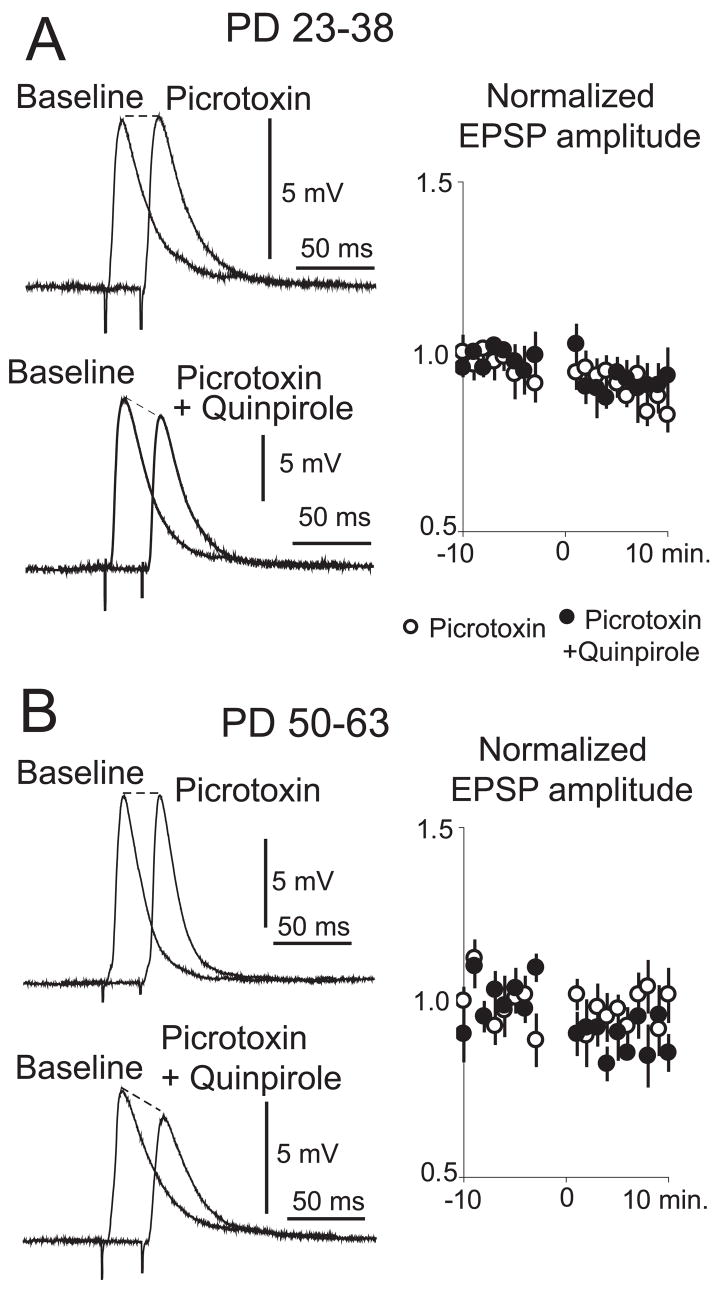
The larger EPSP amplitude observed with D2 activation in slices from adult compared to preadolescent rats could be due to a change in D2 effects on NA MSNs or to the recruitment of an additional depolarizing component. Cortical afferents can activate striatal fast-spiking inter-neurons (Mallet et al., 2005), and a similar interneuron population in the PFC acquires a modulation by D2 receptors during adolescence (Tseng & O’Donnell, 2006). Thus, it is possible that a late maturation of D2 actions exists in NA interneurons, yielding a potential contribution of GABA (which at the negative membrane potential recorded in slices would be depolarizing) to the D2 modulation of corticoaccumbens responses. To investigate the possible role of GABA transmission in the D2 effects on adult NA MSNs, we performed recordings before and after blocking GABA-A receptors with picrotoxin. EPSP amplitude did not change after bath application of picrotoxin (50 μM) in adult or preadolescent rats (Fig. 5A and B). The GABA-A antagonist did not affect the D2 attenuation of EPSPs in slices from preadolescent rats (Fig. 5A). In slices from adult rats in which GABA-A receptors were blocked, quinpirole (5 μM) decreased EPSP amplitude from 7.4 ± 2.9 to 6.5 ± 2.8 mV (P = 0.002, n = 7; Fig. 5B), instead of the increase observed without GABA blockade (see Fig. 4B). This decrease was smaller than that elicited by quinpirole in preadolescent rats and was observed in most neurons recorded without changes in resting membrane potential or input resistance. These results suggest that D2 receptor activation can attenuate corticoaccumbens transmission in both adult and preadolescent rats, but in the adult rat this effect is over-compensated by a D2 facilitation of a cortically evoked GABA component, which is depolarizing at the negative resting membrane potential of MSNs.
Fig. 5.
GABA-A component in the D2 modulation of corticoaccumbens EPSPs. (A) Representative traces of corticoaccumbens EPSPs before and after changing the bath to picrotoxin (top) or picrotoxin + quinpirole (bottom) in slices from preadolescent rats. Each trace is an average of 10 repetitions. The graph to the right shows normalized EPSP amplitude for each treatment. Values are averages per minute. (B) Similar display of EPSP amplitude and normalized plots obtained with slices from adult rats.
D2 modulation of spontaneous synaptic events in preadolescent and adult rats
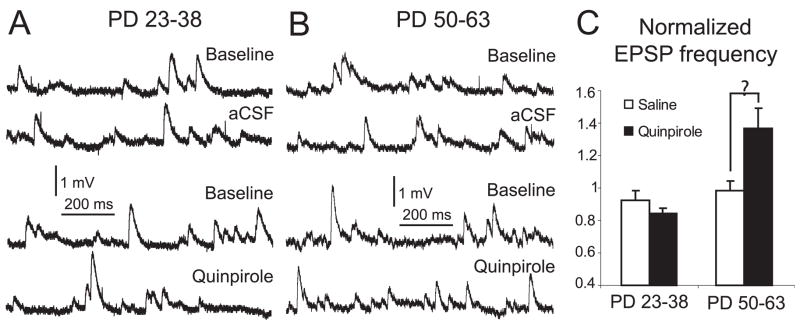
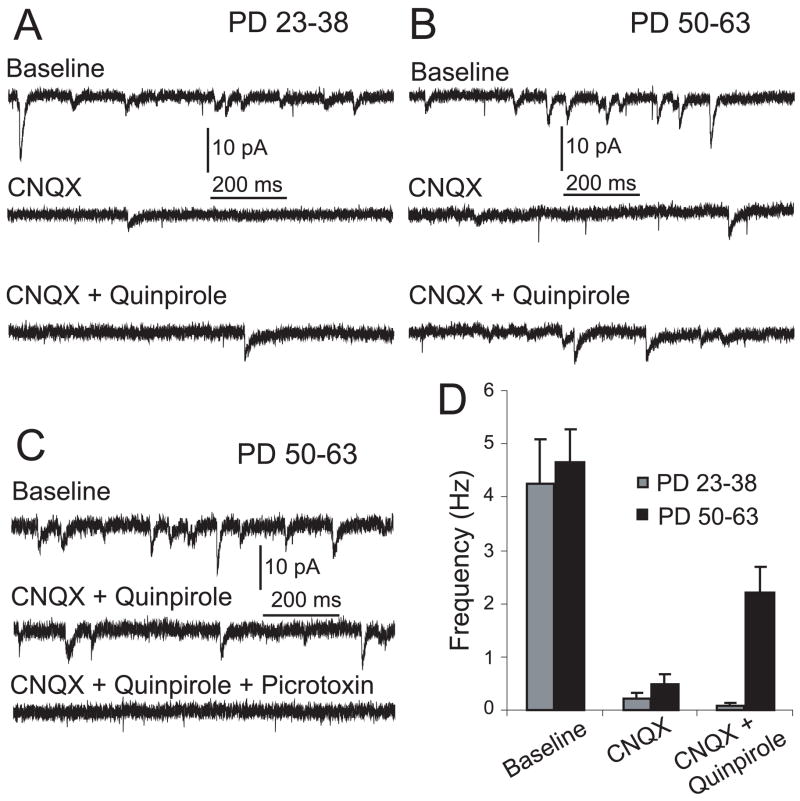
If the D2 enhancement of corticoaccumbens EPSPs in slices from adult rats involves a GABA-A component, D2 agonists should enhance GABA synaptic responses in MSNs. We tested this possibility by assessing the effect of quinpirole on spontaneous synaptic events using current-clamp and voltage-clamp recordings. First, the frequency of spontaneous depolarizing postsynaptic potentials (dPSPs) was analysed before and after quinpirole bath application in brain slices from both age groups. Spontaneous depolarizing events were recorded at membrane potential values similar to those observed with the drug treatments by using current clamp, to obtain insight into changes in spontaneous synaptic events that could be compared to the modulation of evoked responses. Baseline frequency of dPSP was similar in adult and preadolescent rats, 7.8 ± 3.5 and 9.1 ± 3.2 Hz, respectively. In slices from adult rats, quinpirole significantly increased dPSP frequency from 7.3 ± 1.5 to 9.8 ± 2.2 Hz (P = 0.005, n = 6; Fig. 6). Second, we assessed spontaneous synaptic currents recorded while holding the neuron at −70 mV in voltage-clamp conditions. The frequency of spontaneous inward current events was not different between adult and preadolescent rats (4.7 ± 1.4 and 3.8 ± 1.8 Hz, respectively; Fig. 7A and B). The AMPA antagonist CNQX (10 μM) decreased the frequency of inward synaptic currents from 3.8 ± 1.8 to 0.3 ± 0.2 Hz in preadolescent rats and from 4.7 ± 1.4 to 0.5 ± 0.4 Hz in adult rats (Fig. 7C). After blockade of AMPA currents, bath application of a cocktail of CNQX and quinpirole had no effect in preadolescent slices (Fig. 7A and B), but increased the frequency of spontaneous synaptic currents in slices from adult rats (from 0.5 ± 0.4 to 2.2 ± 1.1 Hz; P = 0.02, n = 5; Fig. 7B and C). These CNQX-resistant (i.e. nonglutamatergic) and quinpirole-enhanced synaptic currents were abolished by the GABA-A receptor antagonist picrotoxin (P = 0.03, n = 5; Fig. 7D). Thus, at negative membrane potentials and with AMPA receptors blocked, a D2 agonist was able to increase the occurrence of GABA-A synaptic currents in MSNs, indicating that D2 receptors may have recruited local inhibitory synapses in adult rats.
Fig. 6.
Spontaneous depolarizing potentials in slices from adult and preadolescent rats. (A) Representative traces of spontaneous dPSPs before and after changing the bath to ACSF (top) or quinpirole (bottom) in slices from preadolescent rats. (B) Similar display of representative traces obtained in slices from adult rats. (C) Bar graph showing normalized frequency of spontaneous dPSP in adult and preadolescent rats after ACSF or quinpirole treatment.
Fig. 7.
Effect of D2 activation on spontaneous inward currents recorded in voltage-clamp at −70 mV. (A) Representative traces of spontaneous inward current events before and after changing the bath to ACSF (top), CNQX (middle) and CNQX + quinpirole (bottom) in slices from preadolescent rats. (B) Representative traces of spontaneous inward currents with similar treatments in slices from adult rats. (C) Representative traces of spontaneous inward currents after changing the bath to ACSF (top), CNQX + quinpirole (middle) and CNQX + quinpirole + picrotoxin (bottom) in slices from adult rats. (D) Bar graph depicting the frequency of spontaneous inward current events in slices from preadolescent and adult rats for each treatment.
Discussion
We examined basic membrane properties and D2 responses of NA MSNs in slices obtained from preadolescent and adult rats. Although MSNs recorded before and after adolescence presented similar membrane properties and synaptic responses, there were differences in the effects of a D2 agonist. Quinpirole attenuated synaptic responses in slices from preadolescent rats but enhanced evoked synaptic responses to cortical stimulation in MSNs from adult rats. Moreover, D2 activation attenuated corticoaccumbens responses in adult rats in the presence of a GABA antagonist. Glutamate-independent spontaneous inward currents recorded at −70 mV were more frequently observed in the presence of the D2 agonist, and were blocked by a GABA-A antagonist, but only in slices from adult rats. These results suggest that D2 receptors modulate MSNs differently in young and adult rats, due to the recruitment of a depolarizing GABA component after puberty.
Basal NA electrophysiological properties
Passive and active membrane properties recorded from NA MSNs were similar across the age groups tested here. MSNs had a resting membrane potential between −70 and −80 mV, displayed a depolarizing ramp preceding action potential in response to current injection, and exhibited inward rectification when hyperpolarized from the resting membrane potential, as described previously in young rats (Yim & Mogenson, 1982, 1988; Yang & Mogenson, 1984; Chang & Kitai, 1986; Kawaguchi et al., 1989, 1995; Uchimura et al., 1989; Kawaguchi, 1992, 1993; Pennartz et al., 1992; O’Donnell & Grace, 1993). The similarity we observed between preadolescent and adult rats regarding MSN membrane properties is consistent with previous studies showing that those properties are acquired during the first three postnatal weeks (Tepper et al., 1998; Belleau & Warren, 2000).
Electrical stimulation in the white matter located between the rostral PFC and NA activated corticoaccumbens fibers, evoking EPSPs that were blocked by the AMPA antagonist CNQX. The recording sites were located ~1 mm away from the stimulating site, making it unlikely that current spread directly activated the recorded neuron or other cells in the vicinity. Instead, the small angle of the parasagittal slice allowed preservation of corticoaccumbens fibers. Stimulating and recording electrodes could therefore be separated by long distances and still evoke large-amplitude EPSPs, which exhibited the same amplitude in both groups of animals but faster decay in slices from adult rats. As passive membrane properties were not different between neurons from the two age groups, it is likely that other synaptically evoked factors contributed to the difference. Candidates include a different profile of AMPA and/or NMDA receptor activation in these synapses, with a higher presence of long-lasting NMDA responses in juvenile slices, and differences in voltage-gated channels during development. These results suggest that NA MSNs already exhibit mature membrane properties during puberty but there may be differences in corticoaccumbens synaptic responses.
Differential effect of D2 receptor activation before and after puberty
In vivo and in vitro recordings have shown that DA decreases EPSP amplitude in dorsal and ventral striatal MSNs (Cepeda et al., 1993; O’Donnell & Grace, 1994; Harvey & Lacey, 1996; Levine et al., 1996; Nicola & Malenka, 1997). The receptor subtype involved has been a matter of controversy. Although some studies identified a D1-like modulation of corticoaccumbens responses (Harvey & Lacey, 1996; Nicola & Malenka, 1997; Brady & O’Donnell, 2004), others revealed a D2 modulation (O’Donnell & Grace, 1994; West & Grace, 2002; Charara & Grace, 2003; Brady & O’Donnell, 2004). Here, the D2 modulation of synaptic responses was different between preadolescent and adult rats. Consistent with previous in vitro studies conducted in the NA (O’Donnell & Grace, 1994) and striatum (Hsu et al., 1995) from young rats (Sprague–Dawley rats weighing < 300 g are not yet adults), quinpirole decreased the amplitude of cortically evoked EPSPs in preadolescent rats. In slices from adult rats, however, NA synaptic responses to cortical stimulation were enhanced by D2 activation. Thus, the nature of DA–glutamate interactions in the NA changes during adolescence.
D2 effects on corticoaccumbens synaptic responses in adult rats involved a GABA component
The D2 agonist reduced corticoaccumbens EPSPs in slices from young animals but increased EPSP amplitude in slices from adult animals. As basic membrane properties were not affected by quinpirole in any age group, it is possible that this effect is due to a direct modulation of corticoaccumbens responses by D2 receptors. The pre- or postsynaptic nature of the receptors involved cannot be ascertained with the techniques used here, but there is strong evidence of D2 heterosynaptic receptors being present in corticostriatal terminals and attenuating glutamate release (Bamford et al., 2004).
The change in D2 modulation of synaptic responses after adolescence could be attributed to a modification of the corticoaccumbens network. In addition to their glutamatergic afferents, MSNs receive considerable GABA inputs that could also be modulated by DA, contributing to the effects observed in corticoaccumbens EPSPs. We tested this possibility by repeating the experiments in the presence of the GABA-A antagonist picrotoxin. Before adolescence, picrotoxin did not affect the D2 attenuation of corticoaccumbens EPSPs. However, GABA blockade in adult rats rendered the D2 actions similar to those of preadolescent rats, i.e. decreasing EPSP amplitude instead of enhancing it. This suggests that the recruitment of depolarizing GABA responses by D2 receptors emerges during adolescence, changing the manner in which DA modulates cortical information in the NA. This hypothesis is supported by the finding that spontaneous synaptic events recorded while rendering NMDA receptors ineffective by holding the neuron at −70 mV were reduced but not completely eliminated by blocking AMPA receptors. Furthermore, administration of quinpirole increased the frequency of those glutamate-independent depolarizing events. The inward current events that were AMPA antagonist-resistant and enhanced by quinpirole were GABA-A antagonist-sensitive. The frequency of those events was not increased by quinpirole in preadolescent slices, suggesting that intra-accumbens GABA-A currents can be increased by D2 activation, but only after adolescence. In addition, the amplitude of cortically evoked EPSPs decayed faster in slices from adult than in slices from preadolescent rats, further suggesting that components other than glutamate are activated in the adult slice. GABA fibers contacting NA neurons could originate from local collaterals from other MSNs or from local inhibitory interneurons (Meredith, 1999). In the dorsal striatum, MSNs send local collaterals that could provide some degree of feedback inhibition when MSNs are activated (Czubayko & Plenz, 2002; Tepper et al., 2004). It is unlikely that local collaterals of neighbouring MSNs are involved in the effects reported here, as they have minimal impact on somatic membrane potential of other MSNs (Tepper et al., 2004). On the other hand, it has been recently shown that cortical stimulation activates dorsal striatal GABA interneurons with a shorter latency than MSNs (Mallet et al., 2005). This neuronal population may be activated in the NA by our cortical stimulation protocol combined with the D2 agonist, and is likely to more effectively affect somatic membrane potential of MSNs. Thus, D2 receptors may have a dual action in adult NA networks: on the one hand they directly attenuate corticoaccumbens EPSPs, and on the other hand they enhance feed-forward mechanisms by recruiting GABA interneurons that exert a depolarizing effect onto neighbouring MSNs with negative membrane potentials, but may effectively shunt responses to excitatory inputs when depolarized.
In the slice preparation we used, D2 receptors may enhance corticoaccumbens EPSPs after puberty by engaging GABA transmission. However, we have also recently reported that D2 receptors decrease the amplitude of corticostriatal synaptic responses in vivo in adult rats (Brady & O’Donnell, 2004). This discrepancy can be resolved if one takes into consideration the membrane potential at which this modulation is tested. Synaptic responses can be attenuated by endogenous DA in vivo (via D2 receptors) only during the depolarized up-state (Brady & O’Donnell, 2004). In our preparation, EPSPs were tested at resting membrane potential (equivalent to the in vivo down-state), which is below the chloride reversal potential, causing GABA responses to be depolarizing. In vivo, activation of GABA receptors is likely to exert a depolarizing effect during the down-state as well, but this effect would be minimal during up-states that are closer to the chloride reversal potential. Thus, during up-states or under GABA-A blockade in our preparation, the direct D2 receptor reduction in EPSP amplitude will be the dominant component.
Functional implications
The dopaminergic control of corticoaccumbens activity is critical for a variety of functions. DA cells are known to fire bursts of APs in the presence of reward or reward-predicting stimuli (Schultz, 1998) and may encode reward prediction errors (Tobler et al., 2003). Based on in vivo recordings, we had proposed that DA bursting sustains ongoing up-states in NA MSNs (bringing the membrane potential closer to threshold) and yet reduces the impact of weak cortical inputs (O’Donnell, 2003; Brady & O’Donnell, 2004). Indeed, VTA stimulation with a train of pulses resembling DA cell bursting depolarizes MSNs to a value close to their up-state, and such depolarization could be sustained for a few hundred milliseconds via D1 or D2 receptors (Goto & O’Donnell, 2001). During this depolarization, MSNs rarely fire and incoming PFC inputs are attenuated. This was discussed as a mechanism for filtering irrelevant inputs (Brady & O’Donnell, 2004). The results presented here add another dimension to this network of mesocortical projections. The ability of D2 receptors to recruit a GABA response in the adult PFC (Tseng & O’Donnell, 2006) and NA (data presented here) contribute to further reducing the impact of irrelevant information in the integration of corticolimbic inputs. Its acquisition during adolescence implies a refinement in this circuitry, and could be a mechanism by which adolescents become better at selecting appropriate behavioural responses to a given context. Conversely, a failure in the periadolescent maturation of interneurons reported here could contribute to the delayed onset of neuropsychiatric conditions in which a dysfunction in GABA transmission has been implicated, such as schizophrenia.
Acknowledgments
This work was supported by USPHS grants MH60131 and DA14020 (P.O’D.)
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AP
action potential
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- dPSP
depolarizing postsynaptic potential
- DA
dopamine
- EPSP
excitatory postsynaptic potential
- MSN
medium spiny neuron
- NA
nucleus accumbens
- PD
postnatal day
- PFC
prefrontal cortex
- VTA
ventral tegmental area
References
- Akaike A, Ohno Y, Sasa M, Takaori S. Excitatory and inhibitory effects of dopamine on neuronal activity of the caudate nucleus neurons in vitro. Brain Res. 1987;418:262–272. doi: 10.1016/0006-8993(87)90094-1. [DOI] [PubMed] [Google Scholar]
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol. 2000;84:2204–2216. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Brady AM, O’Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the ‘accumbens’ part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT, Kitai ST. Intracellular recordings from rat nucleus accumbens neurons in vitro. Brain Res. 1986;366:392–396. doi: 10.1016/0006-8993(86)91326-0. [DOI] [PubMed] [Google Scholar]
- Charara A, Grace AA. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology. 2003;28:1412–1421. doi: 10.1038/sj.npp.1300220. [DOI] [PubMed] [Google Scholar]
- Czubayko U, Plenz D. Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci USA. 2002;99:15764–15769. doi: 10.1073/pnas.242428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446:151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Quantification of morphological differences in boutons from different afferent populations to the nucleus accumbens. Brain Res. 2004;1007:167–177. doi: 10.1016/j.brainres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Network synchrony in the nucleus accumbens in vivo. J Neurosci. 2001;21:4498–4504. doi: 10.1523/JNEUROSCI.21-12-04498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Becker NEHM, Lohman AHM. Subcortical afferents of the nucleus accumbens septi in the cat, studied with retrograde axonal transport of horseradish peroxidase and bisbenzimid. Neuroscience. 1980;5:1903–1916. doi: 10.1016/0306-4522(80)90038-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. Endogenous and exogenous dopamine depress EPSCs in rat nucleus accumbens in vitro via D1 receptors activation. J Physiol (Lond) 1996;492:143–154. doi: 10.1113/jphysiol.1996.sp021296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Yang CH, Gean PW. Presynaptic D2 dopaminergic receptors mediate inhibition of excitatory synaptic transmission in rat neostriatum. Brain Res. 1995;690:264–268. doi: 10.1016/0006-8993(95)00734-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Levine MS, Li Z, Cepeda C, Cromwell HC, Altemus KL. Neuromodulatory actions of dopamine on synaptically-evoked neostriatal responses in slices. Synapse. 1996;24:65–78. doi: 10.1002/syn.890240102. [DOI] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann NY Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Dopaminergic reduction of excitability in nucleus accumbens neurons recorded in vitro. Neuropsychopharmacology. 1996;15:87–97. doi: 10.1016/0893-133X(95)00177-F. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of cell firing in the nucleus accumbens. Ann NY Acad Sci. 1999;877:157–175. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Campbell A, Yeghiayan SK, Baldessarini RJ. Localization of dopamine receptor subtypes in corpus striatum and nucleus accumbens septi of rat brain: comparison of D1-, D2-, and D4-like receptors. Neuroscience. 1998;83:169–176. doi: 10.1016/s0306-4522(97)00386-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Sharpe NA, Koos TZ, Trent F. Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci. 1998;20:125–145. doi: 10.1159/000017308. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2006;vv:xx–yy. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N, Cherubini E, North RA. Inward rectification in rat nucleus accumbens neurons. J Neurophysiol. 1989;62:1280–1286. doi: 10.1152/jn.1989.62.6.1280. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res. 1984;324:69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Response of nucleus accumbens neurons to amygdala stimulation and its modification by dopamine. Brain Res. 1982;239:401–415. doi: 10.1016/0006-8993(82)90518-2. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Neuromodulatory action of dopamine in the nucleus accumbens: an in vivo intracellular study. Neuroscience. 1988;26:403–415. doi: 10.1016/0306-4522(88)90158-3. [DOI] [PubMed] [Google Scholar]