Abstract
Tractional force exerted by tissue cells in 3D collagen matrices can be utilized for matrix remodeling or cell migration. The interrelationship between these motile processes is not well understood. The current studies were carried out to test the consequences of oncogenic Ras (H-RasV12) transformation on human fibroblast contraction and migration in 3D collagen matrices. Beginning with hTERT-immortalized cells, we prepared fibroblasts stably transformed with E6/E7 and with the combination E6/E7 and H-RasV12. Oncogenic Ras-transformed cells lost contact inhibition of cell growth, formed colonies in soft agar and were unable to make adherens junctions. We observed no changes in the extent or growth factor dependence of collagen matrix contraction (floating or stress-relaxation) by oncogenic Ras-transformed cells. On the other hand, transformed cells in nested collagen matrices lost not only growth factor selectivity, but also cell matrix density-dependent inhibition of migration. These findings demonstrate differential regulation of collagen matrix contraction and cell migration in 3D collagen matrices.
Keywords: Fibroblast, Collagen, Contraction, Migration, Mechanoregulation, Oncogenic Ras, Adherens Junctions
INTRODUCTION
Fibrous connective tissue provides mechanical support and frameworks for the other tissues of the body. Type 1 collagen is the major protein component of fibrous connective tissue. Fibroblasts are the cell type primarily responsible for collagen biosynthesis and remodeling. During mechanical remodeling of fibrous connective tissues, collagen and other ECM molecules can stretch, slip, and undergo stable reorganization [1]. Such remodeling has been implicated in diverse aspects of normal physiology and pathology including wound repair [2, 3], fibrosis [4–6], scar formation [7, 8], tumorigenesis [9, 10], and aging [11]. Matrix remodeling also is an important design feature in tissue engineering [12–14].
Unlike conventional 2D surfaces, with which most research on cell-matrix interactions has been carried out, 3D collagen matrices exhibit mechanical properties that resemble connective tissues [15–17]. When fibroblasts interact with 3D collagen matrices, adhesion sites are limited to matrix fibrils. The cells can penetrate into the matrix, and they can reorganize and contract the matrix. Given this diversity, research on fibroblasts and other cells interacting with 3D matrices can be used to replicate and analyze a broad range of cell-matrix interactions under different biomechanical conditions [18, 19].
Fibroblasts can contract collagen matrices by more than one mechanism. Floating collagen matrix contraction leads to contraction of the matrix that depends on cell ruffling [20–22], whereas stress-relaxation matrix contraction depends on cell contraction [23–25]. Tractional force exerted by tissue cells in 3D collagen matrices can be utilized for matrix remodeling or cell migration [26]. However, the interrelationship between these motile processes is not well understood. Recently, we developed a new model of nested collagen matrices to study fibroblast migration in the 3D tissue like environment [27]. Using this model, we found that different sets of physiological growth factors stimulate human fibroblast migration and contraction suggesting that these processes are under separate regulatory control [28].
SV40-transformed human fibroblasts and rat sarcoma cells exhibit reduced contraction of floating collagen matrices [29, 30], and Ras-transformed fibroblasts show increased migration on 2D surfaces [31–34]. Given the foregoing differences, we reasoned that a more systematic comparison of control and transformed human fibroblasts might provide new insights into the regulation of contraction and migration of cells interacting with 3D matrices. Beginning with hTERT-immortalized human fibroblasts, we prepared cell lines stably transformed with E6/E7 and with the combination E6/E7 and H-RasV12. Oncogenic Ras-transformed cells lost contact inhibition of cell growth, formed colonies in soft agar and were unable to make adherens junctions. Compared to control human fibroblasts, no changes in extent or growth factor dependence were observed in contraction of collagen matrices (floating or stress-relaxation) by oncogenic Ras-transformed cells. On the other hand, transformed cells in nested collagen matrices lost not only growth factor selectivity, but also cell matrix density-dependent inhibition of migration. These findings indicate that collagen matrix contraction and cell migration are differentially regulated.
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle medium (DMEM), CO2-independent DMEM, Opti-MEM and 0.25% trypsin/EDTA solution were purchased from Invitrogen, Gaithersburg, MD. Fetal bovine serum (FBS) was purchased from Gemini, West Sacramento, CA. Type I collagen (3 mg/ml, Vitrogen) was purchased from Cohesion Technologies, Palo Alto, CA. Fatty acid-free bovine serum albumin (BSA), lysophosphatidic acid (LPA), sphingosine-1-phosphate (S1P), and epidermal growth factor (EGF) were acquired from Sigma-Aldrich, St. Louis, MO. Platelet-derived growth factor (BB isotype) (PDGF) was obtained from Upstate Biotechnology, Lake Placid, NY. SV589 cells (SV40-transformed human fibroblasts, originally Human Genetic Cell repository GM-00639) -- were provided by Dr. Richard Anderson (UT Southwestern).
Cell culture
Use of human foreskin fibroblasts was approved by the University Institutional Review Board (Exemption #4). BR5 cells are hTERT-immortalized, early passage human foreskin fibroblasts [35]. BR5 and SV589 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum at 37°C in a 5% CO2 humidified incubator. Experimental incubation media was DMEM with 10% FBS or 5 mg/ml BSA and growth factors as indicated in the figure legends.
Anchorage independent cell growth was tested in soft agar [36]. Briefly, cells in DMEM plus 10% FBS in 0.35% (w/v) agar were plated on top of a 0.5% agar layer in a 6-well plate. 1 ml DMEM containing 10% FBS was added weekly to each well and the incubations continued for two weeks.
Transfection of BR5 cells with HPV16 E6/E7 and H-RasV12
Initial experiments to prepare oncogenic Ras-transformed BR5 cells were unsuccessful because the cells became growth-arrested. To overcome growth-arrest, BR5 cells first were treated to stably express human papillomavirus E6 and E7, which causes the degradation of p53 and inactivation of pRb and thus bypasses oncogenic Ras-induced growth-arrest [37–41].
Stable transfection of BR5 cells with HPV16 E6 and E7 and with H-RasV12 was accomplished as has been described [42, 43]. Briefly, proviral plasmid DNA containing the E6 and E7 genes was transfected into Phoenix E cells by calcium phosphate precipitation. Viral supernatants from transfected cells were used to infect PA317 cells to generate stable lines expressing unrearranged retrovirus. Following infection and a 7-day drug selection in 1 mg/ml G-418, the supernatant of antibiotic-selected PA317 was harvested, passed through a 0.45 µm filter and added to a BR5 culture in the presence of 8 µg/ml polybrene. Infected cells were selected for 7 days in DMEM containing 10% FBS and 1 mg/ml G-418. H-RasV12 retroviral supernatants were obtained by transiently transfecting 293T cells with pCLAmpho retrovirus-packaging vector and pBabe-hygro-H-RasV12 using Opti-MEM and FuGENE6 (Roche, Basel, Switzerland) as carriers of vectors. Forty-eight hours after transfection, 293T supernatant was harvested, passed through a 0.45 µm filter and added to BR5-E6/E7 cultures in the presence of 8 µg/ml polybrene. Infected cells were selected for 10 days in DMEM containing 10% FBS and 50 µg/ml hygromycin. Phoenix E and PA317 cell lines, plasmids pLXSN and pLXSN-E6/E7, and cDNA primers for HPV-16 E6 and E7 were provided by the Wright/Shay Laboratory (UT Southwestern). pCLAmpho, pBabe-hygro-H-RasV12 plasmids, and 293T cells were provided by Dr. Michael White (UT Southwestern). Throughout the manuscript, for simplicity, BR5-E6/E7 cells are designated BR5-E cells and BR5-E6/E7-H-RasV12 cells are designated BR5-E-Ras.
RT-PCR and western blots
Reverse-transcriptase polymerase chain reaction (RT-PCR) analysis was used to assess E6 and E7 mRNA expression. Primers were designed for either E6 or E7 specific amplification: Forward E6 (nucleotides 201–220): 5’- GCAAGCAACAGTTACTGCGA-3’, Reverse E6 (nucleotides 503–522): 5’- CAACAAGACATACATCGACC-3’; Forward E7 (nucleotides 652–671): 5’- AGCTCAGAGGAGGAGGATGA-3’, Reverse E7 (nucleotides 835–854): 5’- GGTTTCTGAGAACAGATGGG-3’. PCR was performed with Platinum Pfx DNA Polymerase (Invitrogen, Gaithersburg, MD) using GeneAmp PCR system 9700 (Applied Biosystem, Foster City, CA). RT-PCR with GAPDH primer was carried out as an internal control for RT-PCR. To ensure that the assay was in the linear range, the cycle number and amounts of RNA were varied.
Immunoblotting analysis was carried out as described previously [20, 35]. Primary antibodies used were anti-actin and anti-β tubulin from Sigma and anti-P53 and anti-H-Ras(259) from Santa Cruz Biotechnology, Santa Cruz, CA. Secondary HRP-conjugated antibodies were obtained from ICN Biomedical or MP Biomedical, Solon, OH.
Matrix contraction and cell migration
Data are presented as averages ± standard deviations for duplicate or triplicate samples. All experiments were carried out two or more times. Methods for preparing collagen matrix cultures and measuring floating collagen matrix contraction, stressed-released matrix contraction, and cell migration in nested collagen matrices have been described previously [20, 27, 28, 35]. Briefly, to measure floating matrix contraction, collagen matrices (200 µl, 1.5 mg/ml collagen, 2×105 cells/matrix) were polymerized for 1 hr and then released from the culture surface and incubated 4 hr floating in basal medium (DMEM + 5 mg/ml BSA) and growth factors as shown. To measure stressed-released matrix contraction, collagen matrices as above were cultured overnight in DMEM/10% FBS, rinsed, and then released from the culture surface and incubated 1 hr in basal medium and growth factors as shown. At the end of the incubations, samples were fixed with 3% paraformaldehyde in phosphate buffered saline. Overall appearance of nested collagen matrices was recorded using an Epson 4870 photo scanner, and matrix area measured using MetaVue imaging software (Molecular Devices, Sunnyvale, CA). Extent of matrix contraction was calculated by subtracting final matrix area from the starting area (~113 mm2).
To measure cell migration using nested collagen matrices, floating matrices were precontracted for the times indicated in DMEM/10% FBS after which the cell-containing contracted matrices (dermal equivalents) were re-embedded in 200 µl cell-free outer collagen matrices and then incubated for an additional 16 hr in basal medium and growth factors as shown. At the end of the incubations, samples were fixed and stained with 8 µg/ml propidium iodide (Molecular Probes, Inc., Eugene, OR) in the presence of 20 µg/ml RNAse (DNAse-free) Roche, Basel, Switzerland to detect cell nuclei. Cell migration index was calculated by counting the average number of nuclei of cells that had migrated out of dermal equivalents in four 10X microscopic fields selected arbitrarily. Each field included the border of the dermal equivalent detected by dark field microscopy) and the furthest moving cells (detected by nuclear staining with propidium iodide).
To analyze cell migration by scrape-wounding, cells were incubated overnight on collagen-coated coverslips in DMEM/BSA and 0.1% FBS. The samples were scrape-wounded with a pipette tip, and then incubated an additional 24 hr in basal medium and growth factors as shown. At the end of the incubations, samples were fixed and then stained with propidium iodine and with phalloidin to detect actin.
Immunofluorescence, phase contrast, and time-lapse microscopic methods
Immunofluorescence microscopy was accomplished as described previously [20, 27, 35]. Cells were formaldehyde-fixed and Triton-X-100 permeabilized. Primary antibodies used were against H-Ras(259) and β-catenin (Zymed). For actin staining, we used Alexa Fluor® 488 or Alexa Fluor® 594-conjugated phalloidin (Molecular Probes, Inc). Samples were mounted on glass slides with Fluoromount G (Southern Biotechnology Associates, Birmingham, AL). Microscopic images were collected at 22° with a Nikon Elipse 400 fluorescent microscope using 10×/0.45, 20×/0.75, and 40×/0.75 Nikon Plan Apo infinity corrected objectives, Photometrics SenSys camera and MetaView acquisition software. Subsequent image processing was carried out using Adobe Photoshop.
Time lapse microscopy was carried out as described previously [44]. Nested collagen matrices in 24 well culture dishes were placed in an environmental chamber at 37°C. Microscopic images were collected using a Zeiss Axiovert 200M inverted microscope equipped with an A-PLAN 10×/0.25 PH1 Zeiss objective and a Nikon DXM1200F camera. Z stack images were acquired at 20 µm steps and 5–10 min intervals using Metamorph software. The Z stack range was 800 µm to insure complete coverage of the range of cell movement, which typically occurred within 100–200 µm. Z planes in which cells were most in focus were reconstructed into single 2D videos.
RESULTS
Transformation of BR5 cells with H-RasV12
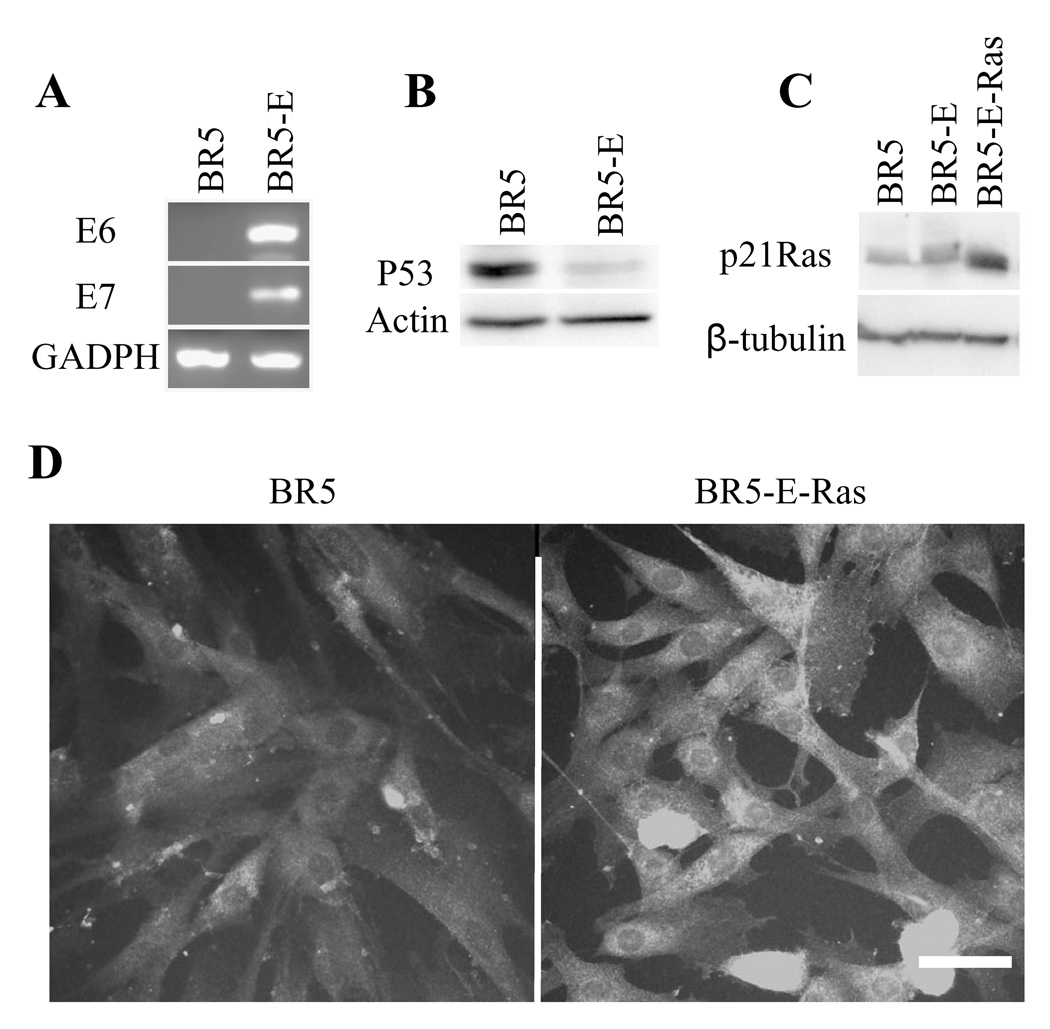
As described in Materials and Methods, preparation of oncogenic Ras-transformed BR5 cells required prior cellular expression of human papillomavirus E6 and E7. Figure 1A shows by RT-PCR that BR5 cells transformed with E6 and E7 (BR5-E) expressed both E6 and E7 messenger RNAs. Figure 1B demonstrates by immunoblotting that levels of P53 were much lower in BR5-E compared to BR5 cells. The results in Figure 1C show that BR5 and BR5-E had lower levels of Ras protein compared to BR5-E-Ras cells, and immunofluorescence analysis (Figure 1D) demonstrated that increased Ras expression occurred in most cells and not just in a subpopulation.
Figure 1. BR5 transfection with E6/E7 and H-RasV12.
(A) RNA extracts of BR5 and BR5-E cells were subjected to RT-PCR to determine E6 and E7 mRNA expression. GAPDH was employed as loading control. (B) Protein extracts of BR5 and BR5-E cells were immunoblotted for p53 protein. Actin was employed as loading control. (C) Protein extracts of BR5, BR5-E and BR5-E-Ras cells were immunoblotted for p21Ras. â-tubulin was employed as loading control. (D) BR5, BR5-E and BR5-E-Ras cells were cultured overnight on glass coverslips, fixed and immunostained for p21Ras. Compared to controls, BR5-E-Ras cells expressed higher levels of p21Ras and most BR5-E-Ras cells appeared to be transformed. Bar = 25µm.
Figure 2A shows that BR5 and BR5-E cells had a similar flattened appearance and tended to remain as a monolayer as cell cultures became confluent. BR5-E-Ras cells were less flattened and more likely to overgrow each other. Loss of normal growth regulation was shown by the ability of BR5-E-Ras cells but not BR5 or BR5-E cells to form colonies when growing on agar (Figure 2B) and to continue cell division beyond confluency (Figure 2C), which for BR5 cells occurred at ~3×106 cells/T75 culture flask.
Figure 2. Growth properties of BR5, BR5-E, and BR5-E-Ras cells.
(A) BR5, BR5-E and BR5-E-Ras cells were cultured to confluence on collagen-coated glass slides and imaged by phase-contrast microscopy. Bar = 50 µm. (B) BR5, BR5-E and BR5- E-Ras cells were incubated in soft-agar-containing 6-well dishes for 2 weeks. At the end of the incubations, samples were fixed and the number of colonies counted in 10 separate fields per dish. Data show the averages ± SD from three separate experiments. (C) BR5, BR5-E and BR5-E-Ras fibroblasts were seeded in T75 flasks at starting concentrations of 1.0, 1.5 or 3.0 ×106 cells and culture for 3 days. At the end of the incubations, cells were recovered by trypsinization and cell numbers counted. Data show the averages ± SD from three separate experiments. Compared to controls, BR5-E-Ras cells appeared less well spread compared to controls, exhibited growth in soft agar and loss of contact inhibition. Bar = 50µm.
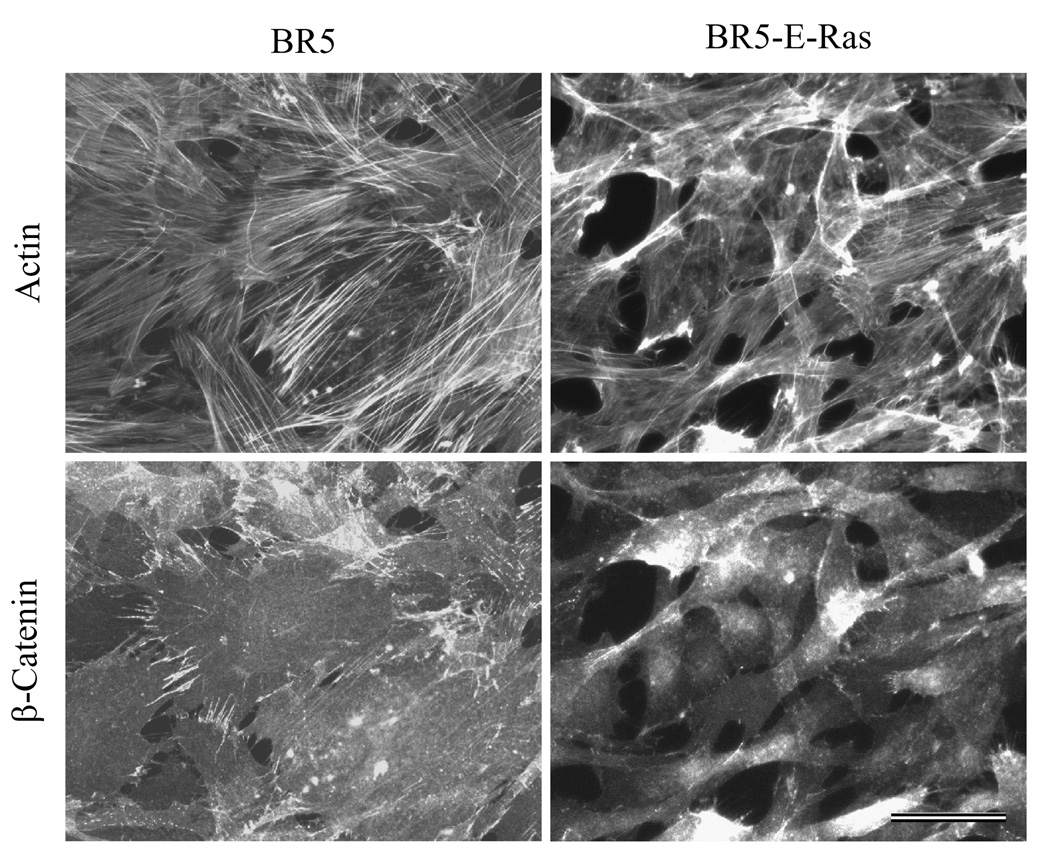
As demonstrated by Figure 3, BR5-E-Ras cells had less well defined actin-staining stress fibers compared to control cells. Fibroblast adherens junctions were readily visualized at sites of cell-cell overlap by immunostaining for β-catenin as has been demonstrated by others [45–47]. These junctions were positive for N-cadherin (not shown). In oncogenic Ras transformed cells, β-catenin distribution was diffuse suggesting that adherens junctions were mostly absent.
Figure 3. Distribution of stress fibers and adherens junctions in BR5 and BR5-E-Ras cells.
BR5 and BR5-E-Ras cells were cultured for 2 days on glass coverslips, fixed and stained to detect actin and β-catenin. Compared to controls, BR5-E-Ras cells displayed less prominent actin stress fibers and exhibited few β-catenin-staining adherens junctions. Bar = 50µm.
Collagen matrix contraction activity of oncogenic Ras-transformed human fibroblasts
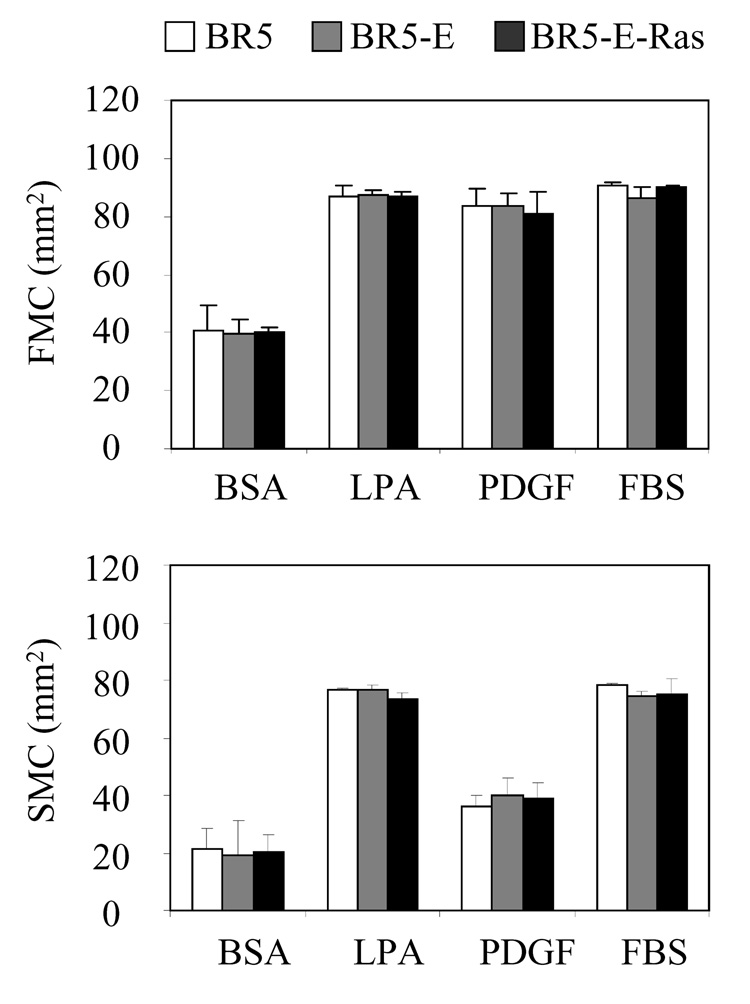
Experiments then were carried out to determine the ability of control and oncogenic Ras-transformed human fibroblasts to contract collagen matrices. Figure 4 compares BR5, BR5-E, and BR5-E-Ras in floating matrix contraction (FMC) and stressed-released matrix contraction (SMC). As described in the Introduction, floating matrix contraction depends on matrix remodeling by cell ruffling, whereas stressed-released matrix contraction depends on matrix remodeling by cell contraction. The results demonstrated that neither the extent nor the growth factor dependence of contraction differed for oncogenic Ras-transformed cells compared to controls.
Figure 4. Contraction of collagen matrices BR5, BR5-E, and BR5-E-Ras cells.
BR5, BR5-E and BR5-E-Ras cells were harvested and polymerized in collagen matrices. To determine floating collagen matrix contraction (FMC), matrices were released for 4 hr in medium containing growth factors and serum as indicated after which the samples were fixed and matrix areas measured. Matrix contraction values (starting – final) shown are the averages ± SD for duplicate samples. To determine stressed-released collagen matrix contraction (SMC), matrices were cultured 24 hr in serum-containing medium, rinsed and released for 1 hr in medium containing growth factors and serum as indicated, and then the samples were fixed and matrix areas measured. Matrix contraction values (starting – final) shown are the averages ± SD for triplicate samples. BR5, BR5-E, and BR5-E-Ras cells exhibited similar collagen matrix contraction activity.
To control for possible differences as a result of cell concentration, we compared BR5 and BR5-E-Ras cells over a 20 fold cell-concentration range. Similar contraction and growth factor dependence profiles were observed (Supplemental Figure 1). As an additional control, we compared BR5 cells with SV40-transformed human fibroblasts (SV589 cells). Consistent with previous work [30], contraction of SV40-transformed cells was decreased compared to control human fibroblasts (Supplemental Figure 2).
Migration of oncogenic Ras-transformed human fibroblasts in nested collagen matrices
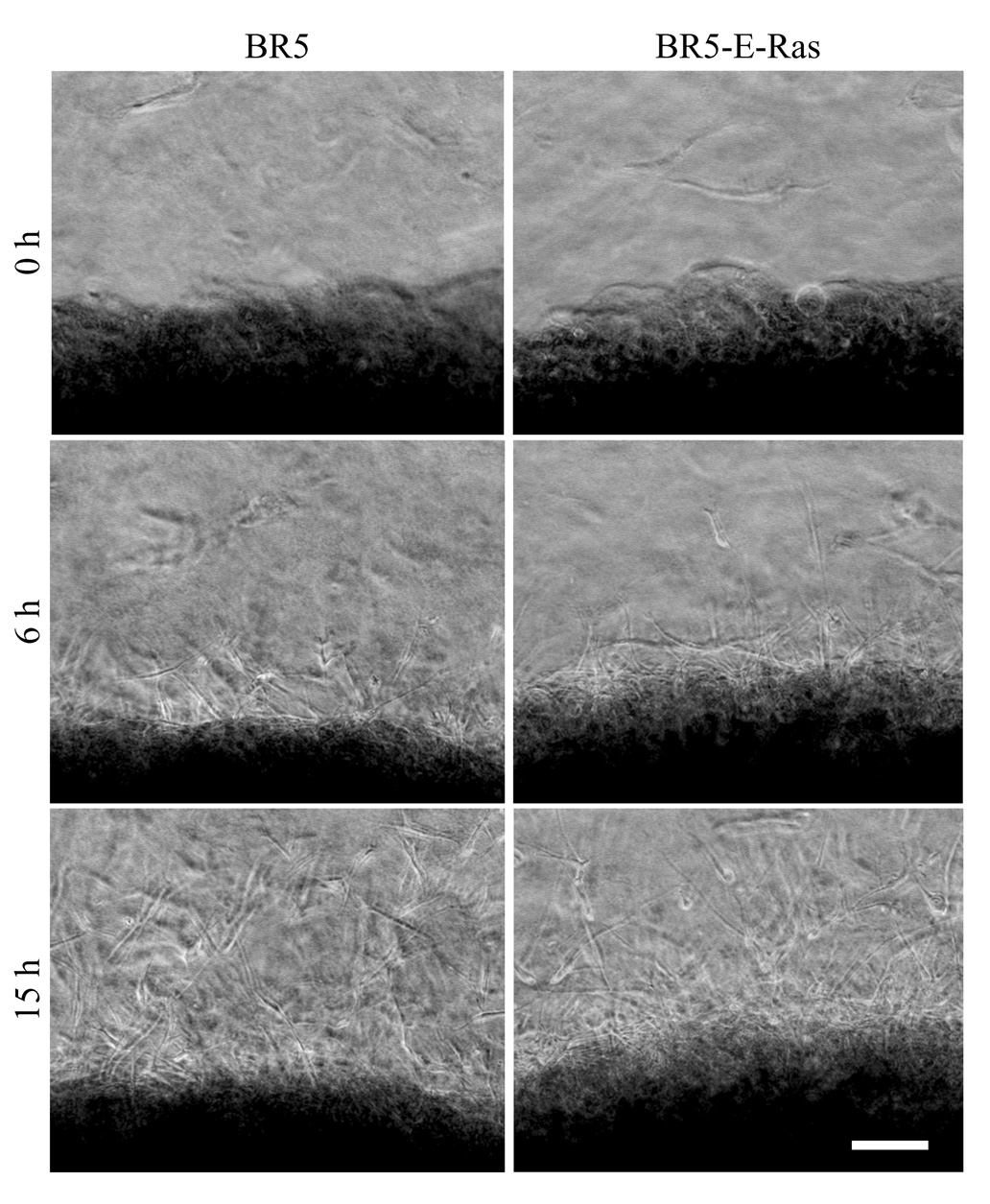
We used nested collagen matrices to analyze migration of oncogenic Ras transformed and control fibroblasts in the 3D matrix environment. To prepare nested matrices, dermal equivalents (fibroblast-contracted floating collagen matrices) were embedded in cell-free, outer collagen matrices. Figure 5 presents representative phase contrast images from time-lapse videos of BR5 and BR5-E-Ras migration in PDGF-containing medium (Supplemental Video 1 and Supplemental Video 2). Viewed from above, the dermal equivalent appears dense (lower portion of the microscopic field) and the surrounding outer matrix more translucent. The overall shape of migrating cells and pattern of migration was similar for BR5 or BR5-E-Ras with a wave of highly elongated cells beginning to migrate out of the dermal equivalent into the outer matrix after a lag phase of several hours.
Figure 5. Migration of BR5 and BR5-E-Ras in nested collagen matrices.
Representative images of the times indicated from time-lapse microscopic videos of BR5 cells (Sup_1.mov) and BR5-E-Ras cells (Sup_2.mov) in nested collagen matrices in medium containing PDGF. Dermal equivalents were precontracted for 6 hr in serum-containing medium. Migration was imaged over 15 hr. Individual cells migrated from the dermal equivalent (dark region at the bottom) into the outer matrix after a lag phase of several hours. BR5 and BR5-E-Ras cells showed the same overall pattern of migration. Bar = 120 µm.
Migration of fibroblasts in nested collagen matrices can be quantified with propidium iodide (PI)-strained samples by counting the number of cell nuclei in a defined region of the outer matrix [27]. Figure 6A shows typical PI stained images of BR5 and BR5-E-Ras migration after 24 hr of migration, and Figure 6B presents quantification of the results. In these experiments, the inner dermal equivalents had been precontracted for different times varying from 6 hr to 48 hr. Previously, we observed that human fibroblasts in nested collagen matrices exhibit cell matrix density-dependent inhibition of migration. That is, at longer times of precontraction, cell matrix density increases and cell migration decreases [44]. Figure 6 shows that BR5-E-Ras cells no longer exhibit cell matrix density-dependent inhibition of migration.
Figure 6. Cell migration in nested matrices prepared with dermal equivalents precontracted for different times.
(A) Nested collagen matrices were prepared with dermal equivalents precontracted in serum-containing medium for the times indicated. Samples were incubated 24 hr in medium containing PDGF and stained to detect cell nuclei. Bar = 120 µm. (B) Same as “A.” Cell migration index values shown are the averages ± SD of duplicate samples. Migration of BR5-E-Ras cells appeared to be relatively unaffected by the prolonged, precontraction period whereas migration of BR5 cells was reduced markedly.
Growth factor dependence of oncogenic Ras-transformed human fibroblast migration
With normal human fibroblasts, platelet-derived growth factor is much more promigratory compared to other physiological agonists; whereas sphingosine-1-phosphate is inhibitory [28]. Figure 7 shows the extent of cell migration after 24 hr in the presence of several different growth factors. Consistent with previous findings, PDGF was much more promigratory for BR5 cells than the other growth factors tested, and S1P inhibited migration. BR5-E-Ras cells not only showed greater migration overall but, in addition, a decreased PDGF growth factor requirement for migration. However, the oncogenic Ras transformed cells remained sensitive to inhibition of migration by S1P. The findings in Figure 7 were based on PI-stained cell preparations as described for Figure 6. Supplemental Video 3 and Supplemental Video 4 show migration of BR5 and BR5-E-Ras cells in serum-containing medium. Consistent with the quantitative measurements, BR5 cells show little migration in serum-containing medium. Migrating BR5-E-Ras cells tended to be less elongated in serum-containing medium compared to PDGF-containing medium (Supplemental Video 1 and Supplemental Video 2).
Figure 7. Growth factor and serum stimulation of BR5 and BR5-E-Ras in nested collagen matrices.
Nested collagen matrices were prepared with dermal equivalents precontracted 10 hr in serum-containing medium. Migration was measured after 24 hr in medium containing growth factors and serum as indicated. Cell migration index values shown are the averages ± SD of duplicate samples from three separate experiments. Unlike control cells for which PDGF selectively stimulated migration, BR5-E-Ras exhibited migration with or without added growth factors except for S1P, which inhibited migration of control and oncogenic Ras-transformed cells.
Oncogenic Ras-transformed fibroblast migration in 2D after scrape wounding
To learn if the differences in Figure 7 were specific to the 3D environment, some experiments also were carried out using scrape-wounded 2D human fibroblast cultures. Figure 8 shows representative images of cells 24 hr after scrape wounding, double-stained for actin (green) and propidium iodide (read). It can be seen from the photomicrographs that compared to controls, BR5-E-Ras cells exhibited a decreased PDGF growth factor requirement for migration.
Figure 8. Migration of BR5, BR5-E, and BR5-E-Ras cells after scrape wounding.
BR5, BR5-E and BR5-E-Ras were cultured overnight, scrape wounded using a pipette tip, and further incubated 24 hr in medium containing growth factors and serum as indicated. At the end of incubation, samples were fixed and stained with to detect actin (green) and cell nuclei (red). Compared to control cells, BR5-E-Ras showed increased migration under all conditions. Bar = 100 µm.
DISCUSSION
The current studies were carried out to test the consequences of human fibroblast transformation with oncogenic Ras on cell-matrix contraction and cell migration in 3D collagen matrices. Beginning with hTERT-immortalized human fibroblasts (BR5), we prepared E6/E7 and E6/E7- H-RasV12 stably transformed cell lines. Compared to control BR5 and BR5-E6/E7 cells, oncogenic Ras expressing fibroblasts lost contact inhibition of cell growth and formed colonies in soft agar. We observed no changes in the extent or growth factor dependence of collagen matrix contraction (floating or stress-relaxation). However, oncogenic Ras-transformed cells in nested collagen matrices lost not only growth factor selectivity, but also cell matrix density-dependent inhibition of migration.
Mechanical force exerted by tissue cells can be used to remodel and contract the extracellular matrix or for cell migration [26]. Some evidence favors a close relationship between cell migration and contraction [48–51], but differences in regulation have been reported as well [52–54]. Complicating interpretation of the foregoing results, mechanisms of cell migration are more diverse in 3D environments compared to 2D surfaces [44, 55–57]. The current findings with oncogenic Ras transformed human fibroblasts along with our recent observations on selectivity of growth factor stimulation [28] support the idea that matrix contraction and cell migration are differentially regulated.
Oncogenic Ras transformation influences diverse downstream targets including cell motile functions [58, 59], and a requirement for Ras activation has been implicated in PDGF-stimulated cell migration [60]. Therefore, one explanation for the loss of growth factor selectivity by oncogenic Ras-transformed cells might have been general downstream activation of PDGF signaling pathways. However, this explanation seems unlikely since BR5-E-Ras cells retained growth factor specificity for collagen matrix contraction, e.g., contraction of floating matrices in basal medium was similar for BR5-E-Ras and control cells.
One intriguing possibility is that loss of growth factor selectivity and cell matrix density-dependent inhibition of migration are related to each other and depend on the loss of adherens junctions in oncogenic-Ras transformed cells. Fibroblast adherens junctions mediated by N-cadherin play diverse roles in fibrous connective tissues [61]. Differentiation of fibroblasts to myofibroblasts during wound repair under the influence of transforming growth factor β has been reported to involve a switch in cadherin types from N to OB, and OB cadherin cell-cell adherens junctions are believed to help stabilize myofibroblasts exerting contractile force [47, 62]. Although often thought of as isolated cells in connective tissue, fibroblasts in 3D collagen matrices form adherens junctions [63] and gap junctions [64]. Disrupting adherens junctions without interfering with cell motile machinery would be expected to increase overall cell migration and to decrease the impact of increased cell matrix density, which occurs when dermal equivalents are precontracted for longer times. Of course, other factors might be equally important such as increased expression of matrix metalloproteinases [65, 66]. Whatever the precise mechanism responsible for Ras activation of migration, S1P retains the ability to exert an inhibitory effect.
Differential regulation of cell migration and matrix contraction helps explain fibroblast/myofibroblast function during wound repair. Regardless whether wound myofibroblasts arise from fibroblasts, fibrocytes or epithelial-mesenchymal transition (EMT) [4, 5, 67, 68], these cells exhibit potentially conflicting migratory and contractile behavior. Strong tractional forces can retard migration, and localized increases in matrix density resulting from contraction can present a steric hindrance to migration. However, differential regulation of cell migration and matrix contraction could permit the two processes to occur independently of each other thereby avoiding potential interference.
Differential regulation of migration and contraction also may be important for epithelial cell invasiveness, since the tumor environment frequently contains both migratory malignant cells and contractile myofibroblasts [10, 69, 70]. Epithelial cell invasiveness depends at least in part on loss of E-cadherin adherens junctions during epithelial-mesenchymal transition [71–73]. Therefore, EMT can give rise to cells that are highly migratory or highly contractile.
Finally, it should be noted that, although not evident with oncogenic Ras transformed fibroblasts, SV40 transformed cells showed decreased collagen matrix contraction as was reported previously [30]. Cell transformation by SV40 also influences multiple downstream cell signaling pathways [74, 75]. Identification of the regulatory mechanisms that account for loss of cell-matrix contraction following SV40 transformation remains an important subject for future studies.
Supplementary Material
Corresponds to text Figure 8. Time of observation was 0–15:00 hr. Frames were collected every 5 min, and display rate is 10 frames/sec.
Corresponds to text Figure 8. Time of observation was 0–15:00 hr. Frames were collected every 5 min, and display rate is 10 frames/sec.
Conditions are similar to text Figure 7. Time of observation was 0–18:00 hr. Frames were collected every 15 min, and display rate is 10 frames/sec.
Conditions are similar to text Figure 7. Time of observation was 0–18:00 hr. Frames were collected every 15 min, and display rate is 10 frames/sec.
Details are the same as Figure 4 except the number of cells/matrix was varied as shown and only BR5 and BR5-E-Ras cells were compared. Each bar represents averages ± SD for duplicate experiments. BR5 and BR5-E-Ras cells displayed similar collagen matrix contraction activity over a wide range of cell concentrations.
BR5 and SV589 cells were harvested and polymerized in collagen matrices. To determine floating collagen matrix contraction, matrices were released for 4 hr in medium containing growth factors and serum as indicated after which the samples were fixed and matrix areas measured. To determine stressed-released collagen matrix contraction, newly polymerized matrices containing cells were cultured 24 hr in serum-containing medium, rinsed and released for 1 hr in medium containing growth factors and serum as indicated, and then the samples were fixed and matrix areas measured. Matrix contraction values (starting – final) shown are the averages ± SD for duplicate samples. SV589 cells showed decreased activity in both floating and stressed-released collagen matrix contraction assays.
ACKNOWLEDGEMENTS
We are grateful to Drs. Michael White, Jerry Shay, and Woodring Wright for their many insights and suggestions regarding this work. This research was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (to G.C.M.) and National Institutes of Health Grant GM31321 (to F.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Research and Technology. 2002;8:1–21. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 2.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 3.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 5.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 8.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 9.Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 2005;15:329–341. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 11.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano- and microstructures by plastic compression. Advanced Functional Materials. 2005;15:1762–1770. [Google Scholar]
- 13.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barocas VH, Moon AG, Tranquillo RT. The fibroblast-populated collagen microsphere assay of cell traction force--Part 2: Measurement of the cell traction parameter. J Biomech Eng. 1995;117:161–170. doi: 10.1115/1.2795998. [DOI] [PubMed] [Google Scholar]
- 16.Wakatsuki T, Kolodney MS, Zahalak GI, Elson EL. Cell mechanics studied by a reconstituted model tissue. Biophys J. 2000;79:2353–2368. doi: 10.1016/S0006-3495(00)76481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlfors JE, Billiar KL. Biomechanical and biochemical characteristics of a human fibroblast-produced and remodeled matrix. Biomaterials. 2007;28:2183–2191. doi: 10.1016/j.biomaterials.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev. 2007;59:1299–1305. doi: 10.1016/j.addr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Rhee S, Grinnell F. P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts. J Cell Biol. 2006;172:423–432. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16:472–479. doi: 10.1111/j.1524-475X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 22.Dahlmann-Noor AH, Martin-Martin B, Eastwood M, Khaw PT, Bailly M. Dynamic protrusive cell behaviour generates force and drives early matrix contraction by fibroblasts. Exp Cell Res. 2007;313:4158–4169. doi: 10.1016/j.yexcr.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanase M, Ikeda H, Matsui A, Maekawa H, Noiri E, Tomiya T, Arai M, Yano T, Shibata M, Ikebe M, Fujiwara K, Rojkind M, Ogata I. Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho-kinase. Biochem Biophys Res Commun. 2000;277:72–78. doi: 10.1006/bbrc.2000.3634. [DOI] [PubMed] [Google Scholar]
- 24.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-Promoted Myofibroblast Contraction: Role of Rho, Myosin Light Chain Kinase, and Myosin Light Chain Phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 25.Mochitate K, Pawelek P, Grinnell F. Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp Cell Res. 1991;193:198–207. doi: 10.1016/0014-4827(91)90556-a. [DOI] [PubMed] [Google Scholar]
- 26.Harris AK. Cell traction in relationship to morphogenesis and malignancy. Dev Biol (N Y 1985) 1986;3:339–357. doi: 10.1007/978-1-4684-5050-7_16. [DOI] [PubMed] [Google Scholar]
- 27.Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Jiang H, Rhee S, Ho C-H, Grinnell F. Distinguishing fibroblast promigratory and procontractile growth factor environments in 3D collagen matrices. Faseb J. 2008 doi: 10.1096/fj.07-097014. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Buttle DJ, Ehrlich HP. Comparative studies of collagen lattice contraction utilizing a normal and a transformed cell line. J Cell Physiol. 1983;116:159–166. doi: 10.1002/jcp.1041160206. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg BM, Smith K, Colozzo M, Pollack R. Establishment and transformation diminish the ability of fibroblasts to contract a native collagen gel. J Cell Biol. 1980;87:304–308. doi: 10.1083/jcb.87.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider L, Klausen TK, Stock C, Mally S, Christensen ST, Pedersen SF, Hoffmann EK, Schwab A. H-ras transformation sensitizes volume-activated anion channels and increases migratory activity of NIH3T3 fibroblasts. Pflugers Arch. 2008;455:1055–1062. doi: 10.1007/s00424-007-0367-3. [DOI] [PubMed] [Google Scholar]
- 32.Varani J, Fligiel SE, Wilson B. Motility of rasH oncogene transformed NIH-3T3 cells. Invasion Metastasis. 1986;6:335–346. [PubMed] [Google Scholar]
- 33.Liu R, Li B, Qiu M. Elevated superoxide production by active H-ras enhances human lung WI-38VA-13 cell proliferation, migration and resistance to TNF-alpha. Oncogene. 2001;20:1486–1496. doi: 10.1038/sj.onc.1204214. [DOI] [PubMed] [Google Scholar]
- 34.Park S, Koch D, Cardenas R, Kas J, Shih CK. Cell motility and local viscoelasticity of fibroblasts. Biophys J. 2005;89:4330–4342. doi: 10.1529/biophysj.104.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee S, Jiang H, Ho CH, Grinnell F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci U S A. 2007;104:5425–5430. doi: 10.1073/pnas.0608030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark GJ, Cox AD, Graham SM, Der CJ. Biological assays for Ras transformation. Methods Enzymol. 1995;255:395–412. doi: 10.1016/s0076-6879(95)55042-9. [DOI] [PubMed] [Google Scholar]
- 37.Fogel S, Riou G. The early HPV16 proteins can regulate mRNA levels of cell cycle genes in human cervical carcinoma cells by p53-independent mechanisms. Virology. 1998;244:97–107. doi: 10.1006/viro.1998.9086. [DOI] [PubMed] [Google Scholar]
- 38.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 39.Deng Q, Li Y, Tedesco D, Liao R, Fuhrmann G, Sun P. The ability of E1A to rescue ras-induced premature senescence and confer transformation relies on inactivation of both p300/CBP and Rb family proteins. Cancer Res. 2005;65:8298–8307. doi: 10.1158/0008-5472.CAN-05-0054. [DOI] [PubMed] [Google Scholar]
- 40.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 41.Hicks GG, Egan SE, Greenberg AH, Mowat M. Mutant p53 tumor suppressor alleles release ras-induced cell cycle growth arrest. Mol Cell Biol. 1991;11:1344–1352. doi: 10.1128/mcb.11.3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halbert CL, Demers GW, Galloway DA. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol. 1992;66:2125–2134. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chien Y, White MA. Characterization of RalB-Sec5-TBK1 Function in Human Oncogenesis. Methods Enzymol. 2008;438:321–329. doi: 10.1016/S0076-6879(07)38022-1. [DOI] [PubMed] [Google Scholar]
- 44.Miron-Mendoza M, Seemann J, Grinnell F. Collagen Fibril Flow and Tissue Translocation Coupled to Fibroblast Migration in 3D Collagen Matrices. Mol Biol Cell. 2008;19:2051–2058. doi: 10.1091/mbc.E07-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gloushankova NA, Krendel MF, Alieva NO, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM. Dynamics of contacts between lamellae of fibroblasts: essential role of the actin cytoskeleton. Proc Natl Acad Sci U S A. 1998;95:4362–4367. doi: 10.1073/pnas.95.8.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ragsdale GK, Phelps J, Luby-Phelps K. Viscoelastic response of fibroblasts to tension transmitted through adherens junctions. Biophys J. 1997;73:2798–2808. doi: 10.1016/S0006-3495(97)78309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell--cell adherens junctions. Mol Biol Cell. 2004;15:4310–4320. doi: 10.1091/mbc.E04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 49.Ehrlich HP, Rajaratnam JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell. 1990;22:407–417. doi: 10.1016/0040-8166(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Moon JJ, Miao H, Jin G, Chen BP, Yuan S, Hu Y, Usami S, Chien S. Signal transduction in matrix contraction and the migration of vascular smooth muscle cells in three-dimensional matrix. J Vasc Res. 2003;40:378–388. doi: 10.1159/000072702. [DOI] [PubMed] [Google Scholar]
- 51.Sawhney RK, Howard J. Molecular dissection of the fibroblast-traction machinery. Cell Motil Cytoskeleton. 2004;58:175–185. doi: 10.1002/cm.20004. [DOI] [PubMed] [Google Scholar]
- 52.Cheresh DA, Leng J, Klemke RL. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J Cell Biol. 1999;146:1107–1116. doi: 10.1083/jcb.146.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shreiber DI, Barocas VH, Tranquillo RT. Temporal variations in cell migration and traction during fibroblast-mediated gel compaction. Biophys J. 2003;84:4102–4114. doi: 10.1016/S0006-3495(03)75135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javelaud D, Laboureau J, Gabison E, Verrecchia F, Mauviel A. Disruption of basal JNK activity differentially affects key fibroblast functions important for wound healing. J Biol Chem. 2003;278:24624–24628. doi: 10.1074/jbc.M301942200. [DOI] [PubMed] [Google Scholar]
- 55.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 58.White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler MH. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 59.Zuber J, Tchernitsa OI, Hinzmann B, Schmitz AC, Grips M, Hellriegel M, Sers C, Rosenthal A, Schafer R. A genome-wide survey of RAS transformation targets. Nat Genet. 2000;24:144–152. doi: 10.1038/72799. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto T, Yokote K, Tamura K, Takemoto M, Ueno H, Saito Y, Mori S. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J Biol Chem. 1999;274:13954–13960. doi: 10.1074/jbc.274.20.13954. [DOI] [PubMed] [Google Scholar]
- 61.El Sayegh TY, Kapus A, McCulloch CA. Beyond the epithelium: cadherin function in fibrous connective tissues. FEBS Lett. 2007;581:167–174. doi: 10.1016/j.febslet.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 62.Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 63.Bellows CG, Melcher AH, Bhargava U, Aubin JE. Fibroblasts contracting three-dimensional collagen gels exhibit ultrastructure consistent with either contraction or protein secretion. J Ultrastruct Res. 1982;78:178–192. doi: 10.1016/s0022-5320(82)80022-1. [DOI] [PubMed] [Google Scholar]
- 64.Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three dimensional collagen matrices. Mol Biol Cell. 2003 doi: 10.1091/mbc.E02-08-0493. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell PM, Der CJ. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol. 2004;14:105–114. doi: 10.1016/j.semcancer.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999;13:781–792. [PubMed] [Google Scholar]
- 67.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- 70.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 71.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal ransition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 74.Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–7755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 75.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Corresponds to text Figure 8. Time of observation was 0–15:00 hr. Frames were collected every 5 min, and display rate is 10 frames/sec.
Corresponds to text Figure 8. Time of observation was 0–15:00 hr. Frames were collected every 5 min, and display rate is 10 frames/sec.
Conditions are similar to text Figure 7. Time of observation was 0–18:00 hr. Frames were collected every 15 min, and display rate is 10 frames/sec.
Conditions are similar to text Figure 7. Time of observation was 0–18:00 hr. Frames were collected every 15 min, and display rate is 10 frames/sec.
Details are the same as Figure 4 except the number of cells/matrix was varied as shown and only BR5 and BR5-E-Ras cells were compared. Each bar represents averages ± SD for duplicate experiments. BR5 and BR5-E-Ras cells displayed similar collagen matrix contraction activity over a wide range of cell concentrations.
BR5 and SV589 cells were harvested and polymerized in collagen matrices. To determine floating collagen matrix contraction, matrices were released for 4 hr in medium containing growth factors and serum as indicated after which the samples were fixed and matrix areas measured. To determine stressed-released collagen matrix contraction, newly polymerized matrices containing cells were cultured 24 hr in serum-containing medium, rinsed and released for 1 hr in medium containing growth factors and serum as indicated, and then the samples were fixed and matrix areas measured. Matrix contraction values (starting – final) shown are the averages ± SD for duplicate samples. SV589 cells showed decreased activity in both floating and stressed-released collagen matrix contraction assays.