Abstract
Astrocytes perform vital maintenance, functional enhancement, and protective roles for their associated neurons; however these same mechanisms may become deleterious for neurons under some conditions. In this review, we highlight two normally protective pathways, the endoplasmic reticulum (ER) stress response and an endogenous antioxidant response, which may become neurotoxic when activated in astrocytes during the inflammation associated with neurodegeneration. Stimulation of these multifaceted pathways affects a panoply of cellular processes. Of particular importance is the effect these pathways have on the homeostasis of the excitatory amino acid neurotransmitter, glutamate. The endogenous antioxidant response increases extracellular glutamate in the pursuit of making the cellular antioxidant, glutathione, by increasing expression of the xCT subunit of the cystine/glutamate antiporter. Meanwhile, inflammatory mediators such as TNFα reduce levels of membrane-bound glutamate scavenging proteins such as the excitatory amino acid transporters. Together, these cellular activities may result in a net increase in extracellular glutamate that could alter neuronal function and lead to excitotoxicity. Here we discuss the role of N-methyl-D-aspartate receptors, which, when excessively stimulated by glutamate, can cause neuronal dysfunction and loss via activation of calpains. While there are other pathways acting in concert or parallel to those we describe here, this review explores a rationale to explain how two protective mechanisms may result in neuronal loss during neurodegeneration.
Keywords: glutamate, endoplasmic reticulum stress, oxidative stress, brain, antioxidant response, Nrf2, PERK, calpain, NMDA receptor, integrated stress response, xCT, excitatory amino acid transporters
Introduction: Insults and Injury in Neurodegeneration
Slow, chronic, progressive damage and loss of neurons underlie the symptoms observed in neurodegenerative diseases including Alzheimer disease (AD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS) and HIV-associated dementia (HAD). Although the precise mechanisms of neuronal loss in these diseases have yet to be elucidated, evidence from human autopsy tissue, animal studies, and in vitro work demonstrate a prominent role for oxidative stress (Lipton et al., 1994; Ehret et al., 1996; Koutsilieri et al., 1997; Kruman et al., 1998; Malorni et al., 1998; Romero-Alvira and Roche, 1998; Mollace et al., 2001; Viviani et al., 2001). Endogenous agents that reduce cellular oxidative damage such as glutathione and catalase are depleted in patients with AD, PD, ALS and HAD (Sofic et al., 1992; Castagna et al., 1995; Sohal and Weindruch, 1996; Lovell et al., 1998; Choi et al., 2000; Sayre et al., 2001). By providing exogenous antioxidants or enzymes that destroy free radicals in animal models or in vitro models of AD, PD, and HAD (Kruman et al., 1998; Malorni et al., 1998; Floyd et al., 1999; Ramassamy et al., 1999; Wang and Xu, 2005) or as therapeutics in human trials (Anonymous, 1998; Muller et al., 2000; Shor-Posner et al., 2002; Turchan et al., 2003; Weinreb et al., 2004), neuronal loss can be attenuated. Though the sufficiency of these treatments in preventing neurodegeneration still remains unclear, these studies suggest that reduction in oxidative stress will mitigate disease progression.
Neuroinflammation can trigger oxidative stress and act as a prominent insult in AD, PD, ALS, and HAD. While there is evidence of microgliosis and astrogliosis in each of these neurodegenerative conditions, neuroinflammation is most closely associated with disease etiology and progression in HAD. Pathologic studies of the brains of patients with HAD suggest an inflammatory mechanism in the progression of this disease (Kaul et al., 2001; Garden et al., 2002; Ghorpade et al., 2003). Neuronal dysfunction and death in HAD is mediated by the release of neurotoxic factors from activated macrophages and microglia including, but not limited to, reactive oxygen species and excitatory amino acids (Pulliam et al., 1991; Yoshioka et al., 1995; Conant et al., 1998; Bagetta et al., 1999; Nath et al., 1999; Kaul et al., 2005). Moreover, these factors stimulate astrocytes and neurons to release additional pro-inflammatory factors, perpetuating the inflammatory state in the CNS, and potentially exacerbating neuronal damage (Gonzalez-Scarano and Martin-Garcia, 2005). Soluble macrophage factors associated with neurologic damage in the CNS of AIDS patients include excitotoxins (i.e. quinolinic acid, glutamate), reactive oxygen species (ROS), cytokines, chemokines and neurotrophic factors (Masliah et al., 1992; Brouwers et al., 1993; Gelbard et al., 1994; Pulliam et al., 1994; Crowe, 1995; Achim and Wiley, 1996; Giulian et al., 1996; Pulliam et al., 1996; Heyes et al., 1998; Sanders et al., 1998). How neurons “interpret” the conflicting signaling environment in HAD or other neurodegenerative diseases ultimately determines viability. Factors such as neurotrophic factor and chemokines have been reported to activate survival pathways in neurons as part of their signaling cascades, while ROS and cytokines can induce death via apoptosis or necrosis (Nicotera et al., 1997; D’Mello, 1998; Pulliam et al., 1998; Bibel and Barde, 2000; Boldyrev, 2000; Holmin and Mathiesen, 2000; Takikita et al., 2001; Annunziato et al., 2003; Barzilai et al., 2003). Understanding the impact of these factors with disparate effects on neuronal viability is essential to unraveling the mechanisms that lead to neurodegeneration.
As important as investigating the mechanisms of neuronal loss in neurodegeneration is the role of neuronal support cells in neuroprotection and function, with particular emphasis on the astrocytes. Astrocytes not only support neuronal survival, but provide significant support of synaptic function by modulating neurotransmitter reuptake, neurotransmitter metabolism, and neurotrophic factor release (reviewed in (Benarroch, 2005)). Astrocytes have been shown to be sufficient to provide neuronal protection from oxidative stress, an insult associated with neurodegeneration (Shih et al., 2003; Shih et al., 2005). The cellular pathway by which astrocytes protect from oxidative stress is part of the integrated stress response (Cullinan et al., 2003; Shih et al., 2003; Shih et al., 2005; Cullinan and Diehl, 2006), a recently described mechanisms made up of at least two pathways important for maintenance of cellular homeostasis. In this review, we will highlight the importance of these protective pathways in the interplay between neurons and astrocytes under neuroinflammatory conditions and propose a model by which protective pathways can be subverted to neurotoxic pathways culminating in neuronal dysfunction and death.
Altered Astrocyte Functions in Response to Neurodegenerative Insults
Integrated Stress Response (ISR)
In the past several years, it has become clear that there is a common mechanism by which cells respond to various stresses. This mechanism has been coined the integrated stress response (ISR), also referred to as the endoplasmic reticulum (ER) stress response and the unfolded protein response (UPR). Although this response has been linked to misfolded or misprocessed proteins (Shen et al., 2004), it is well characterized as part of the cellular response to multiple stresses including oxidative stress, altered lipid metabolism, viral infection, hypoxia, and nutrient deprivation, several of which are triggered in AD, PD, ALS and HAD (Yu et al., 1999; Barbosa-Tessmann et al., 2000; Kudo et al., 2002; Ryu et al., 2002; Ledoux et al., 2003; Katayama et al., 2004; Isler et al., 2005; Mao et al., 2005; Xue et al., 2005; Blais et al., 2006; Fels and Koumenis, 2006; Kikuchi et al., 2006; Wei et al., 2006).
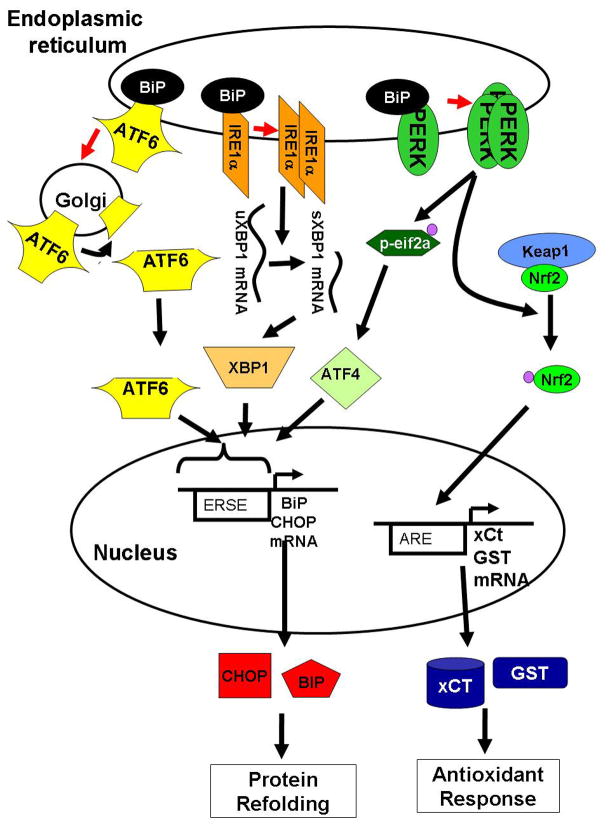
This coordinated response to stress is currently characterized by three pathways, each of which is regulated by its own initiator that is activated upon insult exposure. Specifically, the three ISR initiators are pancreatic ER kinase (PERK), inositol require enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) (Figure 1). In the endoplasmic reticulum, PERK, IRE1α, and ATF6 are kept in inactive forms through complex formation with the chaperone protein BiP (binding protein, also known as GRP78) (Bertolotti et al., 2000; Okamura et al., 2000; Shen et al., 2004). Induction of stress disrupts interaction between BiP and the three ISR regulators (Hurtley and Helenius, 1989) permitting activation of these key regulators. When free of BiP, ATF6 migrates to the transgolgi where the 50 kD N-terminal cytosolic fragment is released by proteolysis, which then translocates to the nucleus and activates expression of genes containing the ER stress response element (ERSE) in their promoter regions (Haze et al., 1999; Li et al., 2000; Ye et al., 2000; Yoshida et al., 2001; Lee et al., 2002; Shen et al., 2004). Disruption of BiP:IRE1α interaction permits dimerization of IRE1α, a serine/threonine kinase with riboendonuclease activity (Nikawa and Yamashita, 1992; Cox et al., 1993). When dimerized, IRE1α autophosphorylates, activating a splicing event that removes an inactivating intron from the unspliced XBP1 (uXBP1) mRNA (Kawahara et al., 1997; Sidrauski and Walter, 1997). Removal of this intron permits efficient translation of the spliced XBP1 (sXBP1) protein, which also activates the ERSE. Finally, disruption of BiP and PERK interactions allows multimerization of PERK, triggering its kinase activity, thereby resulting in autophosphorylation and phosphorylation of eukaryotic initiation factor 2 α (eIF2α) (Shi et al., 1998; Harding et al., 1999; Harding et al., 2000).
Figure 1. The integrated stress response (ISR).
In response to oxidative stress, unfolded proteins, inflammation and other cellular stresses, a stress response is activated to reduce cellular metabolism until cellular homeostasis is restored. In response to stress, the endoplasmic reticulum chaperone protein BiP utilization by unfolded proteins frees the 3 initiators of the ISR: ATF6, IRE1α, and PERK. ATF6 is translocated to the golgi and cleaved releasing ATF6-p50, a transcription factor. Free IRE1α splices uXBP1 to sXBP1 which can now be translated. PERK phosphorylates 2 known substrates, eIF2α and Nrf2. eIF2α depresses general translation initiation, but favors translation of ATF4. ATF6-p53, sXBP1, and ATF4 translocate to the nucleus and activate transcription of chaperone proteins like BiP to re-establish homeostasis. Concomitantly, phosphorylation of Nrf2 by PERK disrupts interaction between Keap1 and Nrf2 leading to translocation of Nrf2 to the nucleus and transactivation of antioxidant genes including GST and xCT. Together, these responses reduce oxidative stress and establish protein refolding.
Of specific relevance for the PERK pathway, phosphorylation of eIF2α leads to inhibition of translation initiation for a majority of cellular proteins (Shi et al., 1998; Harding et al., 1999; Harding et al., 2000) while enhancing translation of select proteins whose functions are to facilitate reestablishment of cellular homeostasis (Fawcett et al., 1999; Harding et al., 2000). eIF2α is a GTP binding protein that delivers the initiator methionine-tRNA to the 40S ribosome (Reviewed in (Brostrom and Brostrom, 1998)). During translation initiation, the eIF2α-bound GTP is hydrolyzed to GDP. Phosphorylation of eIF2α stabilizes the interaction between eIF2α/GDP/eIF2 preventing exchange of the GDP for GTP. Thus, when eIF2α is phosphorylated, translation re-initiation is favored over new initiation, increasing translation of mRNAs with multiple micro open reading frames (called μORF) in their 5′ coding regions. Among the mRNAs translated is the activating transcription factor 4 (ATF4) transcript whose gene product also transactivates expression of the ERSE (Fawcett et al., 1999; Harding et al., 2000). The ERSE is found in the promoter of chaperone genes including BiP and an apoptotic regulator, CHOP (CCAAT enhancer binding protein homologous protein; reviewed in (Kaufman, 1999)). Activation of ERSE genes, including BiP and CHOP, are blunted in PERK−/− cells suggesting that PERK activity contributes significantly to ISR activation (Harding et al., 2000; Novoa et al., 2001).
In addition to inhibition of translation and activation of the ERSE, PERK can modify gene expression through phosphorylation of the Nrf2 transcription factor (Nuclear factor E2-related factor 2), a key regulator of redox homeostasis (Cullinan et al., 2003). In astrocytes, Nrf2 has been shown to upregulate expression of antioxidant genes in response to toxins and is required for protection against oxidative stress (Johnson et al., 2002; Lee et al., 2003; Kraft et al., 2004; Qiang et al., 2004). Nrf2 is normally sequestered in the cytoplasm, but in response to oxidative stress and/or phosphorylation by PERK, Nrf2 translocates to the nucleus and activates expression of detoxifying enzymes and antioxidants (Itoh et al., 1999). Thus, PERK contributes to the activation of the antioxidant pathway (Cullinan and Diehl, 2004).
Antioxidant Pathway of the ISR: Nrf2
When triggered, the antioxidant response can neutralize free radicals, preventing the deleterious effects these radicals have on cells such as direct oxidation of bases within DNA, reaction with unsaturated lipids leading to cellular membrane breakdown, or oxidative modification of amino acids. The enzymes responsible for the endogenous antioxidant response are regulated predominantly by transcriptional activation via Nrf2 (Itoh et al., 1997; Kwak et al., 2001; McMahon et al., 2001). The Nrf2 transcription factor is normally bound in the cytoplasm by the Kelch-like ECH-associated protein 1(Keap1) (Itoh et al., 1999). In response to oxidative stress, sulfhydryl groups on Keap1 become oxidized releasing Nrf2 permitting its translocation into the nucleus (Dinkova-Kostova et al., 2002). Release of Nrf2 from Keap1 is regulated, at least in part, by protein kinase C phosphorylation (Huang et al., 2000, 2002; Bloom and Jaiswal, 2003; Numazawa et al., 2003). These findings implicate Keap1 as a “sensor” of cellular stress that can be activated by oxidative stress and/or activation of second messenger cascades.
Upon translocation to the nucleus, Nrf2 increases transcription of genes containing the cis-acting regulatory antioxidant response element (ARE; also referred to as the electrophilic response element, EpRE) in the promoter region with core sequence 5′-GTGACnnnGC-3′ (Johnson et al., 2002; van Muiswinkel and Kuiperij, 2005). Such ARE-containing genes include antioxidant response enzymes and phase II detoxifying genes which play an essential role in protecting cells against oxidative stress by facilitating the inactivation and elimination of the original insult (Johnson et al., 2002). Among the gene products regulated at the transcriptional level by Nrf2 are the enzymes for glutathione synthesis, an essential antioxidant in the brain. Among the enzymes contributing to glutathione synthesis is the xCT subunit of the glutamate/cystine antiporter, which also contributes to glutamate homeostasis.
Studies have suggested Nrf2 regulation has importance in various neurodegenerative conditions. In pharmacologically induced Parkinsonian mice, induction of Nrf2 can alleviate symptoms (Burton et al., 2006). These findings correspond with work that has demonstrated that downregulation of DJ-1, a PD-associated protein, leads to decreased Nrf2 by affecting its stabilization with Keap1 (Clements et al., 2006). In AD, brains from patients with the disease or with the Lewy body variant exhibit decreased nuclear Nrf2 (Ramsey et al., 2007), implicating an inability of these cells to increase expression of Nrf2 target genes; similar findings have been found in ALS (Kirby et al., 2005). As Nrf2 regulates the expression of a host of genes, these findings encourage further exploration into the regulation of Nrf2 and role of genes under its control.
Maintenance of Glutamate Homeostasis by Astrocytes
Glutamate acts as a major excitatory neurotransmitter in the central nervous system (CNS) and must therefore be carefully regulated at the synaptic cleft. One regulatory mechanism is the glutamate-glutamine cycle. In this process, astrocytes rapidly uptake glutamate from the extracellular space and convert it into glutamine by glutamine synthetase (Norenberg and Martinez-Hernandez, 1979). Glutamine is released from astrocytes and transferred to nerve terminals where it is taken up into neurons and converted into glutamate by glutaminase. Interestingly, this glutamate can be decarboxylated by glutamic acid decarboxylase (GAD) which will generate GABA, an inhibitory transmitter (Battaglioli and Martin, 1991; Wu et al., 2007). Alternatively, glutamate released into the synaptic cleft can diffuse to post-synaptic elements where it can act on a variety of glutamatergic receptors. The two main classes of these receptors are the ionotropic receptors (NMDA and non-NMDA subtypes) and the metabotropic glutamatergic receptors (mGluR). Ionotropic receptors are predominately involved in rapid signaling, whereas mGluRs are involved in more sustained responses. As the glutamate network relies on fast signaling and most receptors are prone to rapid desensitization, excess extracellular glutamate can have deleterious effects including induction of NMDA-receptor-mediated excitotoxicity which can cause cell death. Though some agents, such as glutamate pyruvate transaminase (GPT), can effectively degrade glutamate (Matthews et al., 2000), studies have indicated that astrocytes act as the primary endogenous regulators of extracellular glutamate levels in the CNS (Rothstein et al., 1996; Peghini et al., 1997; Tanaka et al., 1997). Astrocytes scavenge excess glutamate to prevent neuronal excitotoxic death predominantly through two separate transport systems, the XAG- system comprised of the excitatory amino acid transporters (EAATs1-5) and system xc-, consisting of the cystine/glutamate antiporter (xCT antiporter).
System XAG-
EAAT1-5, along with the neutral amino acid transporters ASCT1 and 2, are considered members of the soluble carrier gene (SLC) series within the SLC1 family as assigned by the Human Genome Organization Nomenclature Committee. EAAT-1 and EAAT-2 mediate glutamate transport in mature astrocytes, whereas EAAT-3 and EAAT-4 are found predominantly in mature neurons (Palacin et al., 1998; McBean, 2002). Recent work has suggested that there may be levels of EAAT-2 expression in macrophages (Rimaniol et al., 2000). EAAT-5 is primarily located in retina (Arriza et al., 1994; Palacin et al., 1998). Expression of these transporters is developmentally regulated as EAAT-1 and –2 are expressed in immature neurons (Meaney et al., 1998; Yamada et al., 1998) and EAAT-3 and −4 are found in fetal astrocytes (Hu et al., 2003; Maragakis et al., 2003). There is strong evidence to support regional variation with EAAT-1 expression predominately in the cerebellum (Arriza et al., 1994) and high EAAT-2 mRNA expression in astrocytes of the cerebral cortex (Su et al., 2003). EAAT-1 and −3 mRNA is also expressed in non-nervous tissue. EAAT-1 mRNA has high levels of expression in the heart and skeletal muscle, as well as expression in the placenta and lung; EAAT-3 mRNA is in abundance in the kidney, has lower levels in the brain, placenta, lung, and has very low levels in muscle, liver, and heart (Arriza et al., 1994). These expression patterns in human tissue differ from those in rat tissue even though the rat transporters have high homology to their human counterparts (Velaz-Faircloth et al., 1996).
EAATs 1–3 are sodium cotransporters and potassium countertransporters that have a high affinity for L-glutamate (Palacin et al., 1998). Based on mammalian cell assays, EAATs have an affinity for L-glutamate varying from 48 to 97μM (Arriza et al., 1994), though in HeLa cells, oocytes, or rat brain, EAAT-2 has demonstrated higher affinity for glutamate with Km values between 2 and 18μM (Pines et al., 1992; Arriza et al., 1994; Palacin et al., 1998). These affinity values differ from the millimolar values more typical of amino acid transporters of the epithelium. During transport, one glutamate molecule is coupled with the cotransport of three Na+ and one H+ molecules and countertransport of one K+ molecule with the net result of an inward coupled charged movement of +2 (Zerangue and Kavanaugh, 1996; Levy et al., 1998). These transporters are trimeric proteins consisting of three identical subunits. Though still controversial, crystallographic structures of a glutamate transporter from Pyrococcus horikoshii, which has 37% amino acid identity to human EAAT-2, indicate that each individual subunit has a glutamate binding site (Yernool et al., 2004), supporting the hypothesis of an individual anion pore for each subunit. Recently, sodium-driven glutamate uptake currents have been shown to be independently mediated by each subunit, signifying an active anion conductance even at low glutamate concentrations (Koch et al., 2007).
Expression and localization of EAATs are independently regulated. Epidermal growth factor (EGF), transforming growth factor α (TGF-α), dibutyryl cAMP (dBcAMP), and bromo-cAMP all increased EAAT-2 transcription in human fetal astrocytes (HFA) whereas tumor necrosis factor α (TNFα) decreased expression in HFA (Fine et al., 1996; Su et al., 2003). Recent evidence further suggests that EAAT-2 is highly regulated at the translational level by showing that some EAAT-2 mRNAs have long 5′-untranslated regions (UTR) that, in primary astrocyte cell lines, are translationally regulated by extracellular factors such as corticosterone and retinol (Tian et al., 2007). EAAT-3 localization is highly regulated by neuronal activity (Swanson et al., 1997) and intracellular signaling pathways involving α protein kinase C (α PKC) (Guillet et al., 2005) and phosphatidylinositol-3-kinase (PI3K) (Krizman-Genda et al., 2005) as shown by studies on the rat homolog. Astrocyte-secreted factors have also been shown to affect localization of EAAT-3 (Canolle et al., 2004).
Glutamate-induced neuronal death has been implicated as the cause of neurodegeneration in a host of diseases, with evidence that altered EAAT regulation contributes to the pathology. Neuronal death characterized in HIV-associated dementia (HAD), an infliction that affects 10–20% of HIV-1 infected individuals (Gonzalez-Scarano and Martin-Garcia, 2005), has been linked to glutamate-induced excitotoxicity (Nishida et al., 1996; O’Donnell et al., 2006). When exposed to HIV-related factors in vitro, astrocytes exhibit decreased EAAT-2 expression and activity with EAAT-2 mRNA levels decreasing by approximately 66% (Su et al., 2003) and glutamate uptake decreasing by an average of 76% after 6 hours of HIV-1 exposure(Wang et al., 2003). Similar effects on EAAT expression and activity were found in astrocytes exposed to gp120, the HIV-1 envelope glycoprotein, and TNFα, a cytokine released by HIV-infected macrophages and microglia in the CNS (Pitt et al., 2003; Wang et al., 2003). These data suggest that changes in transcriptional regulation of EAAT-2 may contribute to the increases of extracellular glutamate levels seen in HAD. This mechanism is further corroborated by evidence that interruption of proper glutamate regulation following treatment with gp120 causes memory impairments (Fernandes et al., 2006), a common clinical symptom in HAD. As the neurodegeneration characterized in HAD is an indirect effect of HIV-infected macrophages and microglia that have an activated inflammatory response, it is possible that the changes in glutamate receptors associated with HAD will be translatable to other neuroinflammatory conditions.
Aberrant EAAT expression has been implicated in other neurodegenerative diseases. Knocking out the mouse homolog of EAAT-2 leads to lethal spontaneous seizures, neurodegeneration in the hippocampus, and increased susceptibility to acute cortical injury (Tanaka et al., 1997). Specifically, Tanaka et al (1997) found that knockout mice had spontaneous seizures that resembled NMDA-induced seizures and premature death. In concordance with these findings, the sporadic form of ALS, characterized by loss of motor neurons (Wisman et al., 2003) and by increased glutamate levels in cerebrospinal fluid (CSF) (Spreux-Varoquaux et al., 2002), has up to 95% reduction in EAAT-2 expression in motor cortex and spinal cord (Howland et al., 2002). One hypothesis is that this EAAT reduction is due to RNA processing (Lin et al., 1998), but more recent evidence implicates translation attenuation (Tian et al., 2007), a state that can be induced by ISR as well. In AD, the hippocampus and gyrus frontalis medialis have decreased EAAT-2 expression, especially around the amyloid plaques, whereas the cerebellum has upregulated EAAT expression (Jacob et al., 2007). However, the transgenic APP/Ld/2 mouse, which overexpresses a 695 amino acid form of human amyloid precursor protein (APP), exhibits a decrease in glial-specific glutamate transporter activity that corresponds with decreased protein expression but not mRNA levels (Masliah et al., 2000).
Neuronal EAAT-3 has been similarly studied in seizures as well as in transient ischemia. In patients with temporal lobe epilepsy with hippocampal sclerosis, though there were fewer total EAAT-3 positive cells, EAAT-3 expression was increased in individual neurons in CA2 and in the granule cell layer of the dentate gyrus compared with patients without hippocampal sclerosis (Mathern et al., 1999; Proper et al., 2002). Studies have also looked at EAAT-3 regulation after short ischemic events that act as a form of preconditioning, resulting in resistance to damage after subsequent severe ischemic injury. After induction of a short cerebral ischemic event in rats, there is neuronal upregulation of EAAT-3 with particular intensity at the plasma membrane; this upregulation appears to be regulated by TNFα and the TNFα receptor subfamily member 1 (TNFR1) (Pradillo et al., 2006). Though disease or injury to the CNS seems to affect EAAT-2 and EAAT-3 expression similarly, in contrast to EAAT-2, EAAT-3 does not demonstrate changes in the frontal cortex of AD patients by immunoreactivity or at the mRNA level (Li et al., 1997). As damage to the frontal cortex underlies the symptomatology of AD, in regards to glutamate transport, it is the affects of EAAT-2 regulation that might be important in the neuropathogenesis of this disease.
As the structure and kinetics of EAATs continue to be elucidated and their role in disease explored, there has been a great push to discover pharmacological agents that alter function of these glutamate transporters with a goal of developing therapeutics (reviewed in (Bridges and Esslinger, 2005)). Most inhibitors to date that show selectivity are limited to EAAT-2 inhibitors (Vandenberg et al., 1997; Eliasof et al., 2001; Shimamoto et al., 2004; Moussa et al., 2007). However, as the structural differences between the individual EAATs are further elucidated, it may be easier to exploit these differences to increase selectivity. More importantly, understanding the structure of these receptors may aid in the development of functional enhancers which may be needed to compensate for the lost EAAT activity observed in neurodegenerative diseases.
System xc
The other major glutamate transport system in astrocytes, the xc- system, also referred to as the cystine/glutamate transporter and as the xCT antiporter, mediates over 50% of glutamate transport in glioma cell lines (Ye et al., 1999). It acts as an antiporter by taking up cystine in exchange for glutamate export. By blocking glutamate release from this antiporter, the rat striatum has a 60% decrease in extrasynaptic glutamate levels, implicating that the origin of this glutamate is from non-vesicular release, and that system xc- is a major source (Baker et al., 2002). Studies on cocaine withdrawal in rats have also implicated system xc- as an important regulator of extracellular glutamate (Baker et al., 2003). System xc- also has a vital role in cellular protection against oxidative stress (Lewerenz et al., 2006; Shih et al., 2006), as the import of cystine is vital to the synthesis of glutathione. As the antiporter plays an essential for glutathione production in exchange for increasing extracellular glutamate, its regulation must be carefully controlled. We believe that aberrant expression and/or activity of system xc- may contribute to excitotoxicity during neurodegeneration
The xc- system is a highly regulated electroneutral system that exchanges cystine for glutamate in a Na+-independent 1:1 ratio (Palacin et al., 1998; Sato et al., 1999). The antiporter is composed of two subunits: a heavy chain comprised of a cell surface antigen 4F2hc that is necessary for antiporter translocation to the plasma membrane (Bassi et al., 2001), and a light chain known as xCT that has two variants formed by alternative splicing (McBean, 2002), either of which can confer substrate specificity. In humans, the xCT light chain has been termed SLC7A11 by the Human Genome Organization Nomenclature Committee. In mice, xCT is a 502 amino acid protein with 12 assumed transmembrane domains (Sato et al., 1999; Gasol et al., 2004). Though the predicted size of the xCT protein is approximately 55kDa (Sato et al., 1999), research in mice has found that an xCT antiserum recognizes 40kDa and 80kDa bands that correspond to the xCT protein (Burdo et al., 2006; La Bella et al., 2007). Northern blot analysis has revealed xCT mRNA in mouse brain, rat cortical cells, retinal ganglion cells, and human glioma cells (Sato et al., 2002; Burdo et al., 2006; Dun et al., 2006), but not in mouse heart, kidney, liver, or lung (Sato et al., 1999). Immunocytochemical studies in rat brain have found strong xCT expression in all cell types examined, with increasing expression throughout development (La Bella et al., 2007). In the adult mouse brain, xCT mRNA has robust expression in areas facing the cerebral ventricles, medial habenuclei, and in the meninges, scattered expression in the hypothalamic regions, and low level expression in the cortex and cerebellum (Sato et al., 2002).
Within the 5′-flanking region of the xCT gene are several sequences resembling the cis-acting electrophilic response element (EpRE), also known as the antioxidant response element (ARE), that is responsible for the induction of the adjacent genes. As such, xCT is transcriptionally regulated by electrophilic agents such as diethylmaleate, and hydroquione by activation of the endogenous antioxidant response via the Nrf2 transcription factor (Sasaki et al., 2002). During oxidative stress or other events that cause the activation of the cellular integrated stress response (Cullinan et al., 2003), Nrf2 translocates to the nucleus where, in the presence of small Maf proteins, it binds the ARE and up-regulates transcription of these ARE-containing genes. Nrf2 has been found to regulate the xCT antiporter in astrocytes (Qiang et al., 2004), with overexpression of Nrf2 in rat cortical cultures leading to increased xCT mRNA expression, xCT activity, and intracellular glutathione levels (Shih et al., 2003). Though Nrf2 has been found to control the glutathione pathway (Sun et al., 2005), Nrf2 is not the only regulator of xCT, though other methods of regulation have yet to be determined (Pacchioni et al., 2007). One recently suggested mechanism is through the ISR-regulated transcription factor, ATF4, which seems to be important in controlling xCT expression during amino acid deprivation (Sato et al., 2004). As neurodegenerative conditions can induce oxidative stress, upregulation of the antioxidant response, including the xCT antiporter, can act as a protective mechanism by preventing damage from oxidative stress. In HIV-associated dementia (HAD), studies have found the exposure of retinal pigment epithelial to the transactivator protein, TAT, induces xCT expression (Bridges et al., 2004). As the most abundant source of oxidative stress in the brain is from the respiratory burst of activated microglia (Colton and Gilbert, 1987; Colton et al., 2000), a major pathological hallmark in HAD, it is not surprising to see changes in the antioxidant pathway and, therefore, in xCT in this disease.
Though important in glutathione synthesis, upregulation of the xCT antiporter can have potentially damaging effects, as it is a mechanism for glutamate export and critical in the regulation of extracellular glutamate (Augustin et al., 2007). The pathology behind a variety of neurological insults takes advantage of the xCT antiporter to propagate cell damage. In glial-derived tumors, or gliomas, high levels of glutamate are released, causing excitotoxic neuronal death, mediated by increased xCT expression (Chung et al., 2005). When pharmacological inhibitors are used to block system xc- activity, the progression of tumor growth is disrupted (Chung et al., 2005). Kaposi’s sarcoma-associated herpesvirus (KSHV), a virus linked to lymphoproliferative syndromes that frequently affects immunocompromised individuals, also uses the xCT antiporter, as KSHV cell fusion and virion entry correlates closely with expression of the xCT light chain of the antiporter (Kaleeba and Berger, 2006). In AD, inhibition of system xc- may also prove advantageous for neurons. In neuronal cultures exposed to microglia, exposure to amyloid β-peptides (Aβ) increases microglial xCT mRNA expression and glutamate release which can trigger neuronal death; if system xc- is inhibited, microglia can increase release of apolipoprotein E rescuing neurons from Aβ toxicity (Qin et al., 2006), indicating a potential deleterious role of system xc-. Under these situations, attenuating xCT antiporter activity would be expected to have beneficial outcomes. Thus, regulation of system xc- requires a delicate balance, ensuring adequate cystine for glutathione synthesis, but minimizing glutamate export.
Regulation of Glutathione by Astrocytes
Oxidative stress can lead to endoplasmic reticulum (ER) stress as the ER attempts to handle the accumulation of oxidized proteins. Cystine is one of the required components in the synthesis of glutathione (GSH), an important antioxidant of the CNS. In exchange for glutamate, cystine is transported into the cell through the xCT antiporter. Once in the cell, cystine is reduced to cysteine. A reaction between cysteine and glutamic acid results in γ-glutamyl-cysteine, which in turns reacts with glycine to form GSH (Peuchen et al., 1997). As cysteine acts as the rate limiting element in GSH synthesis, the xCT antiporter is crucial for maintaining GSH homeostasis (Dun et al., 2006), and is critically important in protecting cells from free radicals, peroxides, and other oxidizing agents (O’Connor et al., 1995).
Glutathione is heterogeneously distributed throughout the nervous system, potentially affecting region-and cell-specific capacities to respond to oxidative stress (Philbert et al., 1991). In the central nervous system, GSH is compartmentalized between glia and neurons, with glial cells exhibiting higher levels of GSH (Knollema et al., 1996), especially microglia (Chatterjee et al., 1999). GSH released from astrocytes provides the precursors for neuronal GSH synthesis. GSH acts as an antioxidant by either catalyzing the nucleophilic addition of the sulfur atom in glutathione to an electrophilic group, or by covalently binding with reactive metabolites (Ahlgren-Beckendorf et al., 1999), upon which it is oxidized to glutathione disulfide (GSSG). Therefore, not only is GSH an essential antioxidant, it also participates in a variety of cell processes such as cell proliferation and regulation of apoptosis, detoxification of xenobiotics, thiol redox state, and storage and transport of cysteine, among others (reviewed in ((Cotgreave and Gerdes, 1998; Arrigo, 1999; Dringen, 2000)).
Altered glutathione levels are characterized in a variety of conditions from PD (Sofic et al., 1992) to schizophrenia (Yao et al., 2006) as well as in normal aging (Sohal and Weindruch, 1996). In HIV patients, glutathione levels are altered by viral proteins (Price et al., 2005) and act as a key determinant of patient survival, with decreased GSH associated with a markedly decreased survival rate (Herzenberg et al., 1997); administration of the GSH pro-drug N-acetylcysteine (NAC) replenishes GSH, identifying itself as an important potential therapeutic (De Rosa et al., 2000; Muller et al., 2000). Targeting the consequences of glutathione depletion has proved an effective method to attenuate neuronal loss in models of CNS disorders (Malorni et al., 1998; Anderson et al., 2004; Hart et al., 2004).
As more glutathione is needed, logic stands that xCT activity and/or expression would need to be augmented in order to increase glutathione synthesis. However, as upregulation of this antiporter mediates the regulation of extracellular glutamate (Augustin et al., 2007), careful regulation of system xc- is needed in order to maintain a balance between protective glutathione synthesis and toxic increases in extracellular glutamate.
Neuronal Responses to Altered Astrocyte Function
NMDA Receptor Stimulation
Increased release of glutamate from astrocytes mediates their neurotoxic activities through the NMDA receptors. Functional NMDA receptors are heteromeric assemblies of four subunits: two NMDA-R1 subunits and two NMDA-R2. Together, the four subunits form a channel in the plasma membrane permitting influx of Ca2+ when stimulated. The NMDA-R1 subunits (NR1) are derived from a single gene that can exist as eight different variants based on alternative splicing (Goebel et al., 2005), while four separate genes encode the NMDA-R2 subunits (NR2A, NR2B, NR2C and NR2D) (Lynch and Guttmann, 2001; Waxman and Lynch, 2005). The NR1 subunit binds glycine, while the NR2 subunit binds glutamate and quinolinic acid. Although all four NR2 subunits bind glutamate with equal affinity, NR2A and NR2B trigger greater excitotoxicity than NR2C and NR2D (reviewed in (Lynch and Guttmann, 2001; Waxman and Lynch, 2005). Quinolinic acid activates NR2A- and NR2B-containing receptors, but not those containing NR2C or NR2D subunits (Carvalho et al., 1996). NR2B-containing NMDA receptors are often located extrasynaptically (Charton et al., 1999; Ali and Salter, 2001; Riccio and Ginty, 2002). Interestingly, brain regions enriched in NMDA receptors composed of NR2A and NR2B subunits, which include the hippocampus, striatum, forebrain, are commonly injured in excitotoxic insults such as ischemia, epilepsy and also in HIV-1 infection (Conti et al., 1999; Everall et al., 1999; Heyes et al., 2001; Lynch and Guttmann, 2002; Archibald et al., 2004; Sa et al., 2004; Waxman and Lynch, 2005). Furthermore, regions such as the cerebellum that express relatively high levels of NR2C and low levels of NR2B are commonly spared in excitotoxic insults (Lynch and Guttmann, 2002).
There are two inter-related levels of regulation of NMDA receptor expression and function that ultimately influence their roles in neuronal survival and death: NR subunit expression by developmental stage and phosphorylation state. A major mechanism of regulation of the NMDA receptor is the phosphorylation/de-phosphorylation by neuronal kinases and phosphatases. The phosphorylation state of NR1 and NR2 NMDA receptor subunits regulates their function and can also influence their localization to synaptic and extra-synaptic sites by regulating associations with cellular proteins. NR1 subunits are typically phosphorylated on serine and threonine residues (ser896, ser897) while NR2A and NR2B are typically phosphorylated on tyrosine residues (tyr842, tyr1387, tyr1472), as well as at other residues (Kennedy and Manzerra, 2001; Li et al., 2001; Chung et al., 2004; Besshoh et al., 2005). NMDA receptor function is modulated by tyrosine kinases and phosphatases, as well as serine/threonine kinases, although how this modulation is achieved is not clear (Kennedy and Manzerra, 2001; Li et al., 2001; Goebel et al., 2005). In NR1, phosphorylation of ser896 and ser897 is required for PKC-mediated release of NR1-containing NMDA receptors from the ER and may play a role in NMDA receptor membrane trafficking (Scott et al., 2001). Brain-derived neurotrophic factor (BDNF) causes a rapid phosphorylation of NR1 that is associated with potentiation of synaptic transmission (Suen et al., 1997).
In NR2 subunits, the C-terminal tails of both NR2A and NR2B contain three tyrosines located within YXXØ motifs (NR2A: tyr 842, tyr1325, tyr 1454, and NR2B tyr843, tyr1336, and tyr1472). Phosphorylation of tyr1472 in NR2B located within the YXXØ motif correlates with amyloid-β-induced internalization of the NMDA receptor in cortical neurons (Snyder et al., 2005), while inhibition of tyr842 phosphorylation on NR2A is associated with decreased membrane expression (Vissel et al., 2001). Notably, ischemic insult to neurons is associated with increased NR1 (ser896), NR2A (tyr1387) and NR2B phosphorylation, as well as with enhanced association of these receptors within the postsynaptic density (Cheung et al., 2003; Besshoh et al., 2005). The net effect is the increased synaptic expression of NR2A– and NR2B-containing NMDA receptors. However, loss of tyr1472 phosphorylation in NR2B has been associated with stabilization of NR2B on the cell surface, as well as with increased internalization through endocytosis via clathrin coated pits (Roche et al., 2001; Snyder et al., 2005). It thus remains to be determined how tyr1472 phosphorylation affects hippocampal neuronal sensitivity to excitotoxicity. Recently, cdk5-dependent phosphorylation of NR2A ser1232 in vitro and in vivo has been shown to regulate NMDA-induced long-term potentiation (LTP) in CA1 hippocampal neurons (Kennedy and Manzerra, 2001; Li et al., 2001). Furthermore, enhanced phosphorylation of NR2B tyr1336 might be associated with activation of neuronal survival pathways (Wu et al. J. Biol. Chem. 13: 20075–20087).
Another important consideration regarding the role of NR2 subunits in excitotoxic death is their site of localization. Generally, extrasynaptic NMDA receptors primarily containing NR2B subunits are thought to mediate excitotoxicity, whereas synaptically-associated NMDA receptors mainly containing NR2A subunits at ages when both subunits are expressed, are associated with neuroprotective functions, synaptic plasticity, and long-term potentiation (Rumbaugh and Vicini, 1999; Tovar and Westbrook, 1999; Sattler and Tymianski, 2001; Hardingham and Bading, 2003). Synaptic and extrasynaptic localization of NMDA receptors are differentially regulated by their phosphorylation state (Li et al., 2002), and by their association with post-synaptic density proteins (PSD-95, SAP 102) (Dong et al., 2004). Thus, specific phosphorylation events within the C-terminal tails of NR subunits provide a major level of regulation of neuronal entry into both survival and death pathways.
A second major level of regulation of NMDA receptor function is the developmentally-linked expression of NR subunits (Liu et al. J. Neuroscience 27: 2846–2857). NR2A and NR2B-containing receptors demonstrate varying temporal expression in the rat hippocampus and the expression coincides with hippocampal neuronal susceptibility to age-dependent excitotoxicity in maturing cultures (O’Donnell et al., 2006); mouse cortical cultures demonstrate a similar age-dependent effect in regards to glutamate toxicity (Fogal et al., 2005). These observations suggest a common pathway of neuronal death mediated by NR2A and/or NR2B subunits in classic excitotoxic brain injury, including HIV-1 infection. Accordingly, the development of NR2A- (Siao and Tsirka, 2002) and NR2B-selective antagonists (Lynch and Guttmann, 2001) has been driven by a widespread interest in producing non-toxic, selective NMDA receptor antagonists as neuroprotectants against such brain injury (Lipton, 2004). Most NMDA receptor-mediated toxicity is regulated through calpain activation, hence, selective NR subunit inhibitors and calpain antagonists can provide neuroprotection.
Activation of Calpains in Neurons
Excitotoxicity due to overstimulation of NMDA receptors results in increased cytosolic calcium concentrations ([Ca2+]). A family of calcium-activated cysteine proteases, the calpains, has been implicated in modulating cellular mechanisms in response to changes in [Ca2+] (Guroff, 1964; Wang et al., 1992). Calpains are upregulated in a variety of neurodegenerative conditions, both acute, such as traumatic brain injury (McCracken et al., 1999) and brain ischemia (Yamashima, 2000; Blomgren et al., 2001; Shioda et al., 2006), and chronic, such as multiple sclerosis (Shields et al., 1999), AD (Grynspan et al., 1997; Tsuji et al., 1998; Raynaud and Marcilhac, 2006), Huntington’s disease (Lee et al., 2006), and HAD (Vanderklish and Bahr, 2000; Ray and Banik, 2003; Wang et al., 2007). Calpains are thought mainly to function intracellularly and have been found in virtually all vertebrate cells, although calpains or calpain-like genes have been identified in Drosophila and other invertebrates (Goll et al., 2003). There are 14 identified human genes in the mammalian calpain family encoding the 80kDa cysteine protease domains and two encoding the small 30kDa regulatory subunit domain (Suzuki et al., 2004). The mammalian calpain genes share over 90% homology and there is little evidence of alternative splicing events (Suzuki, 1990). Though calpain substrate selectivity is not completely understood and may depend on the target peptide’s tertiary structure, some substrates can be distinguished by a sequence rich in proline, glutamate, and serine/threonine residues (Rechsteiner and Rogers, 1996; Tompa et al., 2004).
Two calpain isoforms predominate, μ–calpain and m-calpain (Croall and DeMartino, 1991). Both isoforms share substrate specificities (Molinari and Carafoli, 1997) and are heterodimers composed of a regulatory 28 kDa subunit that is identical between the two isoforms and an 80-kDa subunit that shares 55–65% sequence homology (Goll et al., 2003). Although μ–calpains have been described more prevalently in neurons and m-calpains in glia (Hamakubo et al., 1986), they are classically distinguished by the in vitro [Ca2+] requirement for their half maximal activation: μ–calpain (3–50 μM) and m-calpain (400–800 μM) (Suzuki, 1991; Ray et al., 1999; Goll et al., 2003). Since physiologic [Ca2+] generally range from 50–300nM, and [Ca2+] necessary to activate m-calpain would result in immediate cell death regardless of calpain activation, calpain must utilize mechanisms that reduce their required [Ca2+]. In the presence of Ca2+, μ–calpains and m-calpains both undergo autoproteolysis that reduces their [Ca2+] activation requirement by over half (Saido et al., 1994; Goll et al., 2003). In addition, phospholipids, such as phosphatidylinositol 4,5-bisphosphate (PIP2), can also further reduce the [Ca2+] activation requirement (Saido et al., 1992). Phosphorylation of the calpains may also play a role in determining their activation (Goll et al., 2003). ERK phosphorylation of Ser50 of m-calpain can reduce its activation threshold to under 1μM Ca2+ (Glading et al., 2002). These steps may be necessary for calpain activation under physiologic conditions.
Once activated, calpains are known to regulate multiple cellular mechanisms through cleavage of their substrates. The limited number of calpain cleavage sites on a given peptide often results in large, catalytically active fragments and suggests that the role of calpains is often regulatory, as opposed to the degradational roles of proteases in lysomes or proteasomes (Goll et al., 2003; Franco and Huttenlocher, 2005). Along these lines, calpains have been implicated in long-term potentiation in the hippocampus via direct or indirect modulation of kinases, glutamate receptors, or receptor interacting proteins (Tomimatsu et al., 2002). Further alteration of cellular activity may occur due to proteolytic modulation of signal transduction (Kishimoto et al., 1989; Pariat et al., 1997; Pfaff et al., 1999; Pariat et al., 2000), gene expression and transcription factors, cell cycle machinery (Kubbutat and Vousden, 1997; Zhang et al., 1997), and apoptosis (Squier et al., 1999; Chua et al., 2000; Lu et al., 2002).
Both apoptotic- and necrotic-like death have been described following excitotoxic stimulation depending on the stress severity (Lieberthal et al., 1998). Calpains have a variety of roles in regulating apoptotic mechanisms in cells, including prevention of apoptosome formation. This action prevents the induction of the intrinsic mitochondrial apoptotic pathway despite potential mitochondrial cytochrome c release and, therefore, may promote a more necrotic-like mechanism of death (Lankiewicz et al., 2000). Activation of μ-calpains has also been shown to lead to lysosomal membrane degradation and, thus, release of cathespin B and L (Yamashima, 2000). Cathepsin could contribute to breakdown of cellular components and this has been demonstrated in hippocampal neurons (Kitao et al., 2001). However, calpains can also have pro-apoptotic effects due to their cleavage of Bcl-2 family proteins (Gil-Parrado et al., 2002), apoptosis-inducing factor (Polster et al., 2005), Bax (Wood et al., 1998; Gao and Dou, 2000; Hwang et al., 2003), and Bid (Chen et al., 2001; Chen et al., 2002; Mandic et al., 2002; Takano et al., 2005; White et al., 2007). Furthermore, calpains can both directly activate and deactivate caspases through cleavage (Chua et al., 2000; Nakagawa and Yuan, 2000).
Calpains play an important role in disassembling adhesion and cytoskeletal components at the plasma membrane, which are essential for the mechanisms of cell motility (Glading et al., 2002; Perrin and Huttenlocher, 2002). Because of this, calpains may play important roles in neurite outgrowth or remodeling in neurons (Howard et al., 1999). Along these lines, some of the major calpain substrates are adhesion molecules and cytoskeletal proteins, including vimentin, spectrin, tau, actin, tubulin, glial fibrilliary acidic protein, neurofilaments, and microtubule-associated proteins. Activation of calpains during synaptic activity is also crucial for neurotransmitter release, synaptic plasticity, vesicular trafficking, and structural stabilization (Wu and Lynch, 2006). Activation of calpains due to excitotoxic insult can result in synaptic dysfunction or inappropriate disassembly of cellular structures and may contribute to the classic morphologic impact seen following excitotoxic stress (Buki et al., 2003; O’Hanlon et al., 2003). As such, calpains have been implicated in Wallerian degeneration (Zhai et al., 2003), acute axonal degeneration (Kerschensteiner et al., 2005), and in the loss of soma, neurites, and synapses in aged primary neuronal cultures (Kim et al., 2007).
Calpains may further exacerbate excitotoxic conditions by cleaving proteins critical to cytosolic [Ca2+] regulations, such as the plasmalemmal Ca2+ ATPase (Guerini et al., 2003). μ-calpains are thought to be closely linked to NMDA receptor function (Hewitt et al., 1998). In fact, its ability to cleave the NR2 subunits (N-terminal of NR2A, NR2B, and NR2C and C-terminal of NR2A) of the NMDA receptor may lead to modulation of excitotoxic conditions (Bi et al., 1998; Guttmann et al., 2001; Simpkins et al., 2003). Electrophysiological properties of the NMDA receptors are not altered by these cleavages, but these cleavages may regulate localization, turnover, or signaling. Along these lines, the C-terminus of NR2 subunits in the cytoplasmic side of the cellular membrane interact with various kinases and synaptic proteins, such as PSD-95, which is involved in vesicle trafficking. Guttmann et al (2001) have speculated, and transgenic studies have suggested (Sprengel et al., 1998), that cleavage of the NR2 subunit results in NMDA receptors that are prevented from interacting with cytosolic signaling pathways. In addition, it has been suggested that calpain-cleaved NR2B-containing NMDA receptors may linger on the surface of neurons and thus increase neuronal excitotoxic susceptibility (O’Donnell et al., 2006).
Altered cell cycle regulation is another means by which excitoxic calpain activation could induce neuronal dysfunction. Aberrant activation of cell cycle machinery has been linked to a variety of neurodegenerative diseases (reviewed in (Herrup and Yang, 2007)). As suggested by Carragher et al, calpain activity may modulate progression through the G1 phase of the cell cycle, and increased expression of the endogenous calpain inhibitor calpastatin correlates with decreased cyclin A, D, and cdk2, as well as decreased levels of retinoblastoma protein phosphorylation (Carragher et al., 2002). Calpains have also been implicated in the regulation of the E2F1 transcription factor, which is known to regulate proliferation and neuronal death (Strachan et al., 2005).
Other calpain substrates whose cleavage could alter neuronal function include ion channels and transporters, growth factor receptors, phospholipases, kinases and phosphatases (Goll et al., 2003). Since calpains themselves can be phosphorylated, their activation may have autoregulatory roles. Additionally, calpains may alter neuronal function by modifying kinase and signal transduction pathways in neurons through cyclin dependent kinase 5 (CDK5) activation. Calpain cleavage of p35 to its more stable isoform, p25, increases the level, activity, and subcellular distribution of the p25:CDK5 complex (Musa et al., 1998; O’Hare et al., 2005). This complex has been associated with CDK5 neurotoxic function (Patrick et al., 1999), and pharmacologic inhibition of calpains and CDK5 has shown to be protective in some excitotoxicity models (Wang et al., 2007).
The most stringently specific calpain inhibitor is the endogenously expressed calpastatin, which is a major mediator of calpains and is, itself, dependent on Ca2+ for its inhibition of calpains (Tullio et al., 1999; Goll et al., 2003). Synthetic calpain inhibitors, on the other hand, lack the exact calpain specificity of calpastatin, but do successfully protect neurons from excitotoxic stress. Calpain inhibitors PD 150606 and MDL-28170 can reduce degeneration in cultured neurons following excitotoxic or other stress stimuli (Wang et al., 2007). MDL-28170 is neuroprotective in both in vitro and in vivo models of traumatic brain injury (Wang and Yuen, 1997; Laurer and McIntosh, 2001) and several calpain inhibitors have shown significant neuroprotection in animal models of CNS injury and disease (Ray and Banik, 2003). However, clinical trials involving calpain inhibitors have thus far shown little promise (Wang et al., 2006).
Because calpains have seemingly contradictory roles in preventing and initiating apoptotic processes, as well as in regulating critical cellular functions, it is hard to define their role as simply neurodegenerative or neuroprotective. Indeed, the system that may be responsible for the blebbing and disintegration of neuronal processes might also serve a critical role in remodeling cytoskeletal proteins important for the neurite regrowth that is seen following sublethal excitotoxic insults. Most likely, the role of calpains is dependent on both the type and severity of the stress (Moore et al., 2002). Likewise, the function of calpain activation could be site-specific within a cell, as non-homogeneous distribution of [Ca2+] can locally activate calpains (Valeyev et al., 2006). Alternatively, the effect of calpains could be dependent on the isoform composition of the local calpain pool. The resultant magnitude, duration, and localization of cytosolic [Ca2+] following an excitotoxic insult could all be critical determinants of the role calpains will play in excitotoxic responses.
Summary and Model
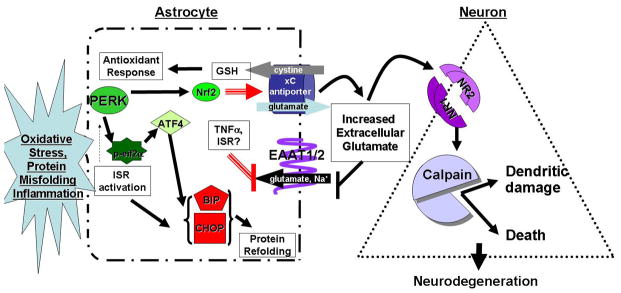
Taken together, the pathways described in this review have known physiologic roles in CNS function and response to stress. Certainly, astrocytes need to activate Nrf2 to produce glutathione and reduce oxidative stress. However, we propose that overstimulation of protective pathways, such as the antioxidant response and the integrated stress response, in astrocytes during chronic, progressive exposure to neurodegenerative stimuli will result in neuronal damage and death by altering the balance of glutamate transport. In our model (Figure 2), activation of the ISR in astrocytes will increase glutamate export by the cystine/glutamate antiporter to increase import of cystine for glutathione production. At the same time, inflammatory mediators such as TNFα or the ISR via altered ER and golgi processing will repress EAAT-1 and EAAT-2 expression and activity removal of excess glutamate from the extracellular milieu. Such changes will alter astrocytic glutamate homeostasis leading to net extracellular accumulation of glutamate. Failure of the astrocytes to regulate glutamate will result in stimulation of neuronal NMDA receptors, perhaps extra-synaptically, leading to Ca+2 release and activation of calpains. Initially, activation of calpains may have limited local effects on dendritic morphology, but overtime, its activation may lead to neuronal death. We propose that the initial local changes in dendritic morphology would cause neuronal dysfunction, which would manifest clinically, but would be reversible if treated. However, chronic, progressive activation of calpains would ultimately culminate in neuronal loss making the clinical deficits fixed. These studies suggest several therapeutic targets for patients with neurodegenerative diseases, but also highlight the complexity of treating astrocytic dysfunction, as reducing effects of glutamate toxicity may reduce antioxidant activity, thus trading one mechanism of cellular toxicity for another. As we expand our understanding of the regulatory mechanisms governing astrocytic and neuronal responses to stress, comprehensive treatment programs for patients with neurodegenerative diseases will emerge.
Figure 2. Model for ISR activation in altering astrocyte neuroprotection.
In neurodegeneration, ISR activation occurs due to chronic exposure to reactive oxygen species, unfolded proteins and/or inflammatory mediators. Chronic activation of ISR leads to constant elevation of the glutamate/cystine antiporter (xCT) via transcriptional activation by Nrf2. Increased xCT exports glutamate out of the astrocyte in order to import cystine for glutathione generation to reduce reactive oxygen species. Inflammatory mediators and, potentially, ISR activation results in decreased EAAT1/2 activity blocking glutamate uptake from the extracellular milieu, leading to an increase in extracellular glutamate. Increased extracellular glutamate is free to stimulate NMDA receptors on neurons causing influx of Ca2+ ions. Increased Ca2+ levels activate calpain proteases causing damage to dendritic spines and dendrites. Such damage to neurons will lead to neuronal dysfunction. Finally, chronic or extreme activation of calpain will lead to cell death.
References
- Achim CL, Wiley CA. Inflammation in AIDS and the role of the macrophage in brain pathology. Current Opinion in Neurology. 1996;9:221–225. doi: 10.1097/00019052-199606000-00013. [DOI] [PubMed] [Google Scholar]
- Ahlgren-Beckendorf JA, Reising AM, Schander MA, Herdler JA, Johnson JA. Coordination regulation of NAD(P)H:Quinone Oxidoreductase and Glutathione-S-Transferases in primary cultures of rat neurons and glia: Role of the antioxidant/electrophilic response element. GLIA. 1999;25:121–142. [PubMed] [Google Scholar]
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Anderson MF, Nilsson M, Eriksson PS, Sims NR. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neuroscience Letters. 2004;354:163–165. doi: 10.1016/j.neulet.2003.09.067. [DOI] [PubMed] [Google Scholar]
- Annunziato L, Amoroso S, Pannaccione A, Cataldi M, Pignataro G, D’Alessio A, Sirabella R, Secondo A, Sibaud L, Di Renzo GF. Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicology Letters. 2003;139:125–133. doi: 10.1016/s0378-4274(02)00427-7. [DOI] [PubMed] [Google Scholar]
- Anonymous. A randomized, double-blind, placebo-controlled trial of deprenyl and thioctic acid in human immunodeficiency virus-associated cognitive impairment. Dana Consortium on the Therapy of HIV Dementia and Related Cognitive Disorders.[see comment] Neurology. 1998;50:645–651. doi: 10.1212/wnl.50.3.645. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. ArchNeurol. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Gene expression and the thiol redox state. Free Radical Biology & Medicine. 1999;27:936–944. doi: 10.1016/s0891-5849(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. Nonvesicular Release of Glutamate by Glial xCT Transporters Suppresses Glutamate Receptor Clustering In Vivo. Journal of Neuroscience. 2007;27:111–123. doi: 10.1523/JNEUROSCI.4770-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Berliocchi L, Nistico R, Giammarioli AM, Malorni W, Aloe L, Finazzi-Agro A. Involvement of interleukin-1[beta] in the mechanism of human immunodeficiency virus type 1 (HIV-1) recombinant protein gp120-induced apoptosis in the neocortex of rat. Neuroscience. 1999;89:1051–1066. doi: 10.1016/s0306-4522(98)00363-7. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW. The Origin and Neuronal Function of In Vivo Nonsynaptic Glutamate. Journal of Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang X-C, Toda S, Kalivas PW. Neuroadaptions in cystine-glutamate exchange underlie cocaine relapse. Nature Neuroscience. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Barbosa-Tessmann IP, Chen C, Zhong C, Siu F, Schuster SM, Nick HS, Kilberg MS. Activation of the Human Asparagine Synthetase Gene by the Amino Acid Response and the Endoplasmic Reticulum Stress Response Pathways Occurs by Common Genomic Elements. J Biol Chem. 2000;275:26976–26985. doi: 10.1074/jbc.M000004200. [DOI] [PubMed] [Google Scholar]
- Barzilai A, Daily D, Zilkha-Falb R, Ziv I, Offen D, Melamed E, Shirvan A. The molecular mechanisms of dopamine toxicity. Advances in Neurology. 2003;91:73–82. [PubMed] [Google Scholar]
- Bassi M, Gasol E, Manzoni M, Pineda M, Riboni M, Martin R, Zorzano A, Borsani G, Palacin M. Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc- Pflugers Archiv European Journal of Physiology. 2001;442:286. doi: 10.1007/s004240100537. [DOI] [PubMed] [Google Scholar]
- Battaglioli G, Martin D. GABA synthesis in brain slices is dependent on glutamine produced in astrocytes. Neurochemical Research. 1991;16:151–156. doi: 10.1007/BF00965703. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clinic Proceedings. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biology. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- Bi X, Rong Y, Chen J, Dang S, Wang Z, Baudry M. Calpain-mediated regulation of NMDA receptor structure and function. Brain Research. 1998;790:245–253. doi: 10.1016/s0006-8993(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes & Development. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Blais JD, Addison C, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, Bell JC. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Molecular and Cellular Biology. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? Journal of Biological Chemistry. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2S40 by PKC in response to antioxidants leads to the release of Nrf2 from INrf2 but not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of ARE-mediated NQ01 Gene expression. Journal of Biological Chemistry. 2003 doi: 10.1074/jbc.M307633200. In Press. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA. Discrimination between apoptosis and necrosis of neurons under oxidative stress. Biochemistry (Moscow) 2000;65:834–842. [PubMed] [Google Scholar]
- Bridges CC, Hu H, Miyauchi S, Siddaramappa UN, Ganapathy ME, Ignatowicz L, Maddox DM, Smith SB, Ganapathy V. Induction of Cystine-Glutamate Transporter xc- by Human Immunodeficiency Virus Type 1 Transactivator Protein Tat in Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci. 2004;45:2906–2914. doi: 10.1167/iovs.03-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS. The excitatory amino acid transporters: Pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacology & Therapeutics. 2005;107:271–285. doi: 10.1016/j.pharmthera.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Brostrom CO, Brostrom MA. Regulation of translational initiation during cellular responses to stress. Progress in Nucleic Acid Research & Molecular Biology. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- Brouwers P, Heyes MP, Moss HA, Wolters PL, Poplack DG, Markey SP, Pizzo PA. Quinolinic acid in the cerebrospinal fluid of children with symptomatic human immunodeficiency virus type 1 disease: relationships to clinical status and therapeutic response. Journal of Infectious Diseases. 1993;168:1380–1386. doi: 10.1093/infdis/168.6.1380. [DOI] [PubMed] [Google Scholar]
- Buki A, Farkas O, Doczi T, Povlishock JT. Preinjury administration of the calpain inhibitor MDL-28170 attenuates traumatically induced axonal injury. Journal of Neurotrauma. 2003;20:261–268. doi: 10.1089/089771503321532842. [DOI] [PubMed] [Google Scholar]
- Burdo J, Dargusch R, Schubert D. Distribution of the Cystine/Glutamate Antiporter System xc- in the Brain, Kidney, and Duodenum. J Histochem Cytochem. 2006;54:549–557. doi: 10.1369/jhc.5A6840.2006. [DOI] [PubMed] [Google Scholar]
- Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. NeuroToxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Canolle B, Masmejean F, Melon C, Nieuollon A, Pisano P, Lortet S. Glial soluble factors regulate the activity and expression of the neuronal glutamate transporter EAAC1: implication of cholesterol. Journal of Neurochemistry. 2004;88:1521–1532. doi: 10.1046/j.1471-4159.2003.02301.x. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Westhoff MA, Riley D, Potter DA, Dutt P, Elce JS, Greer PA, Frame MC. v-Src-induced modulation of the calpain-calpastatin proteolytic system regulates transformation. Molecular & Cellular Biology. 2002;22:257–269. doi: 10.1128/MCB.22.1.257-269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho DP, Dupuy C, Gorin Y, Legue O, Pommier J, Haye B, Virion A. The Ca2+- and reduced nicotinamide adenine dinucleotide phosphate-dependent hydrogen peroxide generating system is induced by thyrotropin in porcine thyroid cells. Endocrinology. 1996;137:1007–1012. doi: 10.1210/endo.137.3.8603567. [DOI] [PubMed] [Google Scholar]
- Castagna A, Grazie CL, Accordini A, Giulidori P, Cavalli G, Bottiglieri T, Lazzarin A. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: Effect of parenteral treatment with SAMe. Neurology. 1995;45:1678–1683. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- Charton JP, Herkert M, Becker CM, Schroder H. Cellular and subcellular localization of the 2B-subunit of the NMDA receptor in the adult rat telencephalon. Brain Res. 1999;816:609–617. doi: 10.1016/s0006-8993(98)01243-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Noack H, Possel H, Keilhoff G, Wolf G. Glutathione levels in primary glial cultures: monochlorobimane provides evidence of cell type-specific distribution. GLIA. 1999;27:152–161. [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. Journal of Biological Chemistry. 2001;276:30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- Chen W, Sulcove J, Frank I, Jaffer S, Ozdener H, Kolson DL. Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary Isolates and evidence for involvement of the Bcl2/Bcl-xL-sensitive intrinsic apoptosis pathway. Journal of Virology. 2002;76:9407–9419. doi: 10.1128/JVI.76.18.9407-9419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HH, Teves L, Wallace MC, Gurd JW. Inhibition of protein kinase C reduces ischemia-induced tyrosine phosphorylation of the N-methyl-d-aspartate receptor. J Neurochem. 2003;86:1441–1449. doi: 10.1046/j.1471-4159.2003.01951.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Liu R-M, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ. Molecular Mechanism of Decreased Glutathione Content in Human Immunodeficiency Virus Type 1 Tat-transgenic Mice. Journal of Biological Chemistry. 2000;275:3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- Chua BT, Guo K, Li P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. Journal of Biological Chemistry. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of Cystine Uptake Disrupts the Growth of Primary Brain Tumors. Journal of Neuroscience. 2005;25:7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JPY. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. PNAS. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Letters. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- Colton CA, Chernyshev ON, Gilbert DL, Vitek MP. Microglial Contribution to Oxidative Stress in Alzheimer’s Disease. Ann NY Acad Sci. 2000;899:292–307. doi: 10.1111/j.1749-6632.2000.tb06195.x. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. PNAS. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, Barbaresi P, Melone M, Ducati A. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. CerebCortex. 1999;9:110–120. doi: 10.1093/cercor/9.2.110. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry--glutathione-protein interactions: a molecular link between oxidative stress and cell proliferation? Biochemical & Biophysical Research Communications. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiological Reviews. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Crowe SM. Role of macrophages in the pathogenesis of human immunodeficiency virus (HIV) infection. Australian & New Zealand Journal of Medicine. 1995;25:777–783. doi: 10.1111/j.1445-5994.1995.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. Journal of Biological Chemistry. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. The International Journal of Biochemistry & Cell Biology. 2006;38:317. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 Is a Direct PERK Substrate and Effector of PERK-Dependent Cell Survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello SR. Molecular regulation of neuronal apoptosis. Current Topics in Developmental Biology. 1998;39:187–213. doi: 10.1016/s0070-2153(08)60456-1. [DOI] [PubMed] [Google Scholar]
- De Rosa SC, Zaretsky MD, Dubs JG, Roederer M, Anderson M, Green A, Mitra D, Watanabe N, Nakamura H, Tjioe I, Deresinski SC, Moore WA, Ela SW, Parks D, Herzenberg LA, Herzenberg LA. N-acetylcysteine replenishes glutathione in HIV infection. [see comment] European Journal of Clinical Investigation. 2000;30:915–929. doi: 10.1046/j.1365-2362.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YN, Waxman EA, Lynch DR. Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J Neurosci. 2004;24:11035–11045. doi: 10.1523/JNEUROSCI.3722-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Progress in Neurobiology. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith S. Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell and Tissue Research. 2006;324:189. doi: 10.1007/s00441-005-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret A, Westendorp MO, Herr I, Debatin KM, Heeney JL, Frank R, Krammer PH. Resistance of chimpanzee T cells to human immunodeficiency virus type 1 Tat-enhanced oxidative stress and apoptosis. Journal of Virology. 1996;70:6502–6507. doi: 10.1128/jvi.70.9.6502-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasof S, McIlvain HB, Petroski RE, Foster AC, Dunlop J. Pharmacological characterization of threo-3-methylglutamic acid with excitatory amino acid transporters in native and recombinant systems. Journal of Neurochemistry. 2001;77:550–557. doi: 10.1046/j.1471-4159.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochemical Journal. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biology and therapy. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- Fernandes SP, Edwards TM, Ng KT, Robinson SR. HIV-1 protein gp120 rapidly impairs memory in chicks by interrupting the glutamate-glutamine cycle. Neurobiology of Learning and Memory. 2006 doi: 10.1016/j.nlm.2006.03.006. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, Gelbard HA. Tumor Necrosis Factor alpha Inhibits Glutamate Uptake by Primary Human Astrocytes. Journal of Biological Chemistry. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K, Jaffery F, Maidt L, Robinson K, Pye Q, Stewart C. Increased oxidative stress brought on by pro-inflammatory cytokines in neurodegenerative processes and the protective role of nitrone-based free radical traps. Life Sciences. 1999;65:1893–1899. doi: 10.1016/s0024-3205(99)00443-9. [DOI] [PubMed] [Google Scholar]
- Fogal B, Trettel J, Uliasz TF, Levine ES, Hewett SJ. Changes in secondary glutamate release underlie the developmental regulation of excitotoxic neuronal cell death. Neuroscience. 2005;132:929–942. doi: 10.1016/j.neuroscience.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. Journal of Cell Science. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- Gao G, Dou QP. N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. Journal of Cellular Biochemistry. 2000;80:53–72. doi: 10.1002/1097-4644(20010101)80:1<53::aid-jcb60>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase Cascades in Human Immunodeficiency Virus-Associated Neurodegeneration. Journal of Neuroscience. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane Topology of System Xc-Light Subunit Reveals a Re-entrant Loop with Substrate-restricted Accessibility. Journal of Biological Chemistry. 2004;279:31228–31236. doi: 10.1074/jbc.M402428200. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Nottet HSLM, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi Y-B, Zhang D, Lipton SA, Tourtellotte WW, Epstein LG, Gendelman HE. Platelet activating factor: a candidate HIV-1-induced neurotoxin. J Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorpade A, Holter S, Borgmann K, Persidsky R, Wu L. HIV-1 and IL-1 beta regulate Fas ligand expression in human astrocytes through the NF-kappa B pathway. Journal of Neuroimmunology. 2003;141:141–149. doi: 10.1016/s0165-5728(03)00222-4. [DOI] [PubMed] [Google Scholar]
- Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, Knoch TA, Auerswald EA, Welsh K, Reed JC, Fritz H, Fuentes-Prior P, Spiess E, Salvesen GS, Machleidt W. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. Journal of Biological Chemistry. 2002;277:27217–27226. doi: 10.1074/jbc.M202945200. [DOI] [PubMed] [Google Scholar]
- Giulian D, Yu J, Li X, Tom D, Li J, Wendt E, Lin S-N, Schwarcz R, Noonan C. Study of Receptor-Mediated Neurotoxins Released by HIV-1-Infected Mononuclear Phagocytes Found in Human Brain. Journal of Neuroscience. 1996;16:3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends in Cell Biology. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- Goebel SM, Alvestad RM, Coultrap SJ, Browning MD. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor is enhanced in synaptic membrane fractions of the adult rat hippocampus. Brain Res Mol Brain Res. 2005;142:65–79. doi: 10.1016/j.molbrainres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiological Reviews. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The Neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Grynspan F, Griffin WR, Cataldo A, Katayama S, Nixon RA. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer’s disease. Brain Research. 1997;763:145–158. doi: 10.1016/s0006-8993(97)00384-3. [DOI] [PubMed] [Google Scholar]
- Guerini D, Pan B, Carafoli E. Expression, purification, and characterization of isoform 1 of the plasma membrane Ca2+ pump: focus on calpain sensitivity. Journal of Biological Chemistry. 2003;278:38141–38148. doi: 10.1074/jbc.M302400200. [DOI] [PubMed] [Google Scholar]
- Guillet BA, Velly LJ, Canolle B, Masmejean FM, Nieoullon AL, Pisano P. Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochemistry International. 2005;46:337–346. doi: 10.1016/j.neuint.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Guroff G. A neutral, calcium-activated proteinase from the soluble fraction of rat brain. Journal of Biological Chemistry. 1964;239:149–155. [PubMed] [Google Scholar]
- Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. Journal of Neurochemistry. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- Hamakubo T, Kannagi R, Murachi T, Matus A. Distribution of calpains I and II in rat brain. Journal of Neuroscience. 1986;6:3103–3111. doi: 10.1523/JNEUROSCI.06-11-03103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase.[see comment][erratum appears in Nature 1999 Mar 4;398(6722):90] Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Molecular Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hart AM, Terenghi G, Kellerth JO, Wiberg M. Sensory neuroprotection, mitochondrial preservation, and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscience. 2004;125:91–101. doi: 10.1016/j.neuroscience.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Molecular Biology of the Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nature Reviews Neuroscience. 2007;8:368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC, Herzenberg LA. Glutathione deficiency is associated with impaired survival in HIV†disease. PNAS. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt KE, Lesiuk HJ, Tauskela JS, Morley P, Durkin JP. Selective coupling of mu-calpain activation with the NMDA receptor is independent of translocation and autolysis in primary cortical neurons. Journal of Neuroscience Research. 1998;54:223–232. doi: 10.1002/(SICI)1097-4547(19981015)54:2<223::AID-JNR10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Lackner A, Wiley CA, Achim CL, Markey SP. Sources of the neurotoxin quinolinic acid in the brain of HIV-1 infected patients and retrovirus-infected macaques. FASEB J. 1998;12:881–896. doi: 10.1096/fasebj.12.10.881. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Ellis RJ, Ryan L, Childers ME, Grant I, Wolfson T, Archibald S, Jernigan TL, Center HGHNR. Elevated cerebrospinal fluid quinolinic acid levels are associated with region-specific cerebral volume loss in HIV infection. Brain. 2001;124:1033–1042. doi: 10.1093/brain/124.5.1033. [DOI] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T. Intracerebral administration of interleukin-1beta and induction of inflammation, apoptosis, and vasogenic edema. Journal of Neurosurgery. 2000;92:108–120. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- Howard MJ, David G, Barrett JN. Resealing of transected myelinated mammalian axons in vivo: evidence for involvement of calpain. Neuroscience. 1999;93:807–815. doi: 10.1016/s0306-4522(99)00195-5. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) PNAS. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Walters W, Xia X, Karmally S, Bethea J. Neuronal glutamate transporter EAAT4 is expressed in astrocytes. GLIA. 2003;44:13–25. doi: 10.1002/glia.10268. [DOI] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. [erratum appears in Proc Natl Acad Sci U S A 2001 Jan 2;98(1):379] Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. Journal of Biological Chemistry. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annual Review of Cell Biology. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Paik S, Park SH, Kim HS, Lee IS, Kim SP, Baek WK, Suh MH, Kwon TK, Park JW, Park JB, Lee JJ, Suh SI. N-phenethyl-2-phenylacetamide isolated from Xenorhabdus nematophilus induces apoptosis through caspase activation and calpain-mediated Bax cleavage in U937 cells. International Journal of Oncology. 2003;22:151–157. [PubMed] [Google Scholar]
- Isler JA, Skalet AH, Alwine JC. Human Cytomegalovirus infection activates and regulates the unfolded protein response. Journal of Virology. 2005;79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & Development. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical & Biophysical Research Communications. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Gr√∘nblatt E. Alterations in Expression of Glutamatergic Transporters and Receptors in Sporadic Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. Journal of Neurochemistry. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- Kaleeba JAR, Berger EA. Kaposi’s Sarcoma-Associated Herpesvirus Fusion-Entry Receptor: Cystine Transporter xCT. Science. 2006;311:1921–1924. doi: 10.1126/science.1120878. [DOI] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer’s disease. Journal of Chemical Neuroanatomy. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes & Development. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death and Differentiation. 2005;12:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kawahara T, Yanagi H, Yura T, Mori K. Endoplasmic Reticulum Stress-induced mRNA Splicing Permits Synthesis of Transcription Factor Hac1p/Ern4p That Activates the Unfolded Protein Response. Mol Biol Cell. 1997;8:1845–1862. doi: 10.1091/mbc.8.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB, Manzerra P. Telling tails. Proc Natl Acad Sci U S A. 2001;98:12323–12324. doi: 10.1073/pnas.231486398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Medicine. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Almer G, Yamashita S, Guegan C, Nagai M, Xu Z, Sosunov AA, McKhann GM, 2nd, Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Oh SJ, Park SH, Kang HJ, Won MH, Kang TC, Park JB, Kim JI, Kim J, Lee JY. Neuronal loss in primary long-term cortical culture involves neurodegeneration-like cell death via calpain and p35 processing, but not developmental apoptosis or aging. Experimental & Molecular Medicine. 2007;39:14–26. doi: 10.1038/emm.2007.3. [DOI] [PubMed] [Google Scholar]
- Kirby J, Halligan E, Baptista MJ, Allen S, Heath PR, Holden H, Barber SC, Loynes CA, Wood-Allum CA, Lunec J, Shaw PJ. Mutant SOD1 alters the motor neuronal transcriptome: implications for familial ALS. Brain. 2005;128:1686–1706. doi: 10.1093/brain/awh503. [DOI] [PubMed] [Google Scholar]
- Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) Journal of Biological Chemistry. 1989;264:4088–4092. [PubMed] [Google Scholar]
- Kitao Y, Ozawa K, Miyazaki M, Tamatani M, Kobayashi T, Yanagi H, Okabe M, Ikawa M, Yamashima T, Stern DM, Hori O, Ogawa S. Expression of the endoplasmic reticulum molecular chaperone (ORP150) rescues hippocampal neurons from glutamate toxicity. Journal of Clinical Investigation. 2001;108:1439–1450. doi: 10.1172/JCI12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollema S, Hom HW, Schirmer H, Korf J, Ter Horst GJ. Immunolocalization of glutathione reductase in the murine brain. Journal of Comparative Neurology. 1996;373:157–172. doi: 10.1002/(SICI)1096-9861(19960916)373:2<157::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Koch HP, Lane Brown R, Larsson HP. The Glutamate-Activated Anion Conductance in Excitatory Amino Acid Transporters Is Gated Independently by the Individual Subunits. Journal of Neuroscience. 2007;27:2943–2947. doi: 10.1523/JNEUROSCI.0118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsilieri E, Gotz ME, Sopper S, Sauer U, Demuth M, ter Meulen V, Riederer P. Regulation of glutathione and cell toxicity following exposure to neurotropic substances and human immunodeficiency virus-1 in vitro. Journal of Neurovirology. 1997;3:342–349. doi: 10.3109/13550289709030748. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear Factor E2-Related Factor 2-Dependent Antioxidant Response Element Activation by tert-Butylhydroquinone and Sulforaphane Occurring Preferentially in Astrocytes Conditions Neurons against Oxidative Insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizman-Genda E, Gonzalez M, Zelenaia O, Robinson MB. Evidence that Akt mediates platelet-derived growth factor-dependent increases in activity and surface expression of the neuronal glutamate transporter, EAAC1. Neuropharmacology. 2005;49:872–882. doi: 10.1016/j.neuropharm.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Experimental Neurology. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Molecular & Cellular Biology. 1997;17:460–468. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Katayama T, Imaizumi K, Yasuda Y, Yatera M, Okochi M, Tohyama M, Takeda M. The Unfolded Protein Response Is Involved in the Pathology of Alzheimer’s Disease. Ann NY Acad Sci. 2002;977:349–355. doi: 10.1111/j.1749-6632.2002.tb04837.x. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Molecular Medicine. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- La Bella V, Valentino F, Piccoli T, Piccoli F. Expression and developmental regulation of the cystine/glutamate exchanger (xc-) in the rat. Neurochemical Research. 2007;32:1081–1090. doi: 10.1007/s11064-006-9277-6. [DOI] [PubMed] [Google Scholar]
- Lankiewicz S, Marc Luetjens C, Truc Bui N, Krohn AJ, Poppe M, Cole GM, Saido TC, Prehn JH. Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. Journal of Biological Chemistry. 2000;275:17064–17071. doi: 10.1074/jbc.275.22.17064. [DOI] [PubMed] [Google Scholar]
- Laurer HL, McIntosh TK. Pharmacologic therapy in traumatic brain injury: update on experimental treatment strategies. Current Pharmaceutical Design. 2001;7:1505–1516. doi: 10.2174/1381612013397285. [DOI] [PubMed] [Google Scholar]
- Ledoux S, Yang R, Friedlander G, Laouari D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Research. 2003;63:7284–7290. [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. Journal of Biological Chemistry. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes & Development. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Park JE, Kang L, Ko SY, Jung KH, Kim M. Memantine reduces striatal cell death with decreasing calpain level in 3-nitropropionic model of Huntington’s disease. Brain Research. 2006;1118:199–207. doi: 10.1016/j.brainres.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the Glial Glutamate Transporter GLT-1 Expressed Inducibly in a Chinese Hamster Ovary Cell Line Selected for Low Endogenous Na+-Dependent Glutamate Uptake. Journal of Neuroscience. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Klein M, Methner A. Cooperative action of glutamate transporters and cystine/glutamate antiporter system Xc protects from oxidative glutamate toxicity. Journal of Neurochemistry. 2006;1:1–10. doi: 10.1111/j.1471-4159.2006.03921.x. [DOI] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci U S A. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Baumeister P, Roy B, Phan T, Foti D, Luo S, Lee AS. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Molecular & Cellular Biology. 2000;20:5096–5106. doi: 10.1128/mcb.20.14.5096-5106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mallory ME, Alford M, Tanaka S, Masliah E. Glutamate Transporter Alterations in Alzheimer Disease are Possibly Associated with Abnormal APP Expression. Journal of Neuropathology and Experimental Neurology. 1997;56:910–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. American Journal of Physiology. 1998;274:F315–327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- Lin C-LG, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA Processing in a Neurodegenerative Disease: the Cause for Absent EAAT2, a Glutamate Transporter, in Amyotrophic Lateral Sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer’s disease and other neurologic disorders. Journal of Alzheimers Disease. 2004;6:S61–S74. doi: 10.3233/jad-2004-6s610. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Yeh M, Dreyer EB. Update on current models of HIV-related neuronal injury: platelet-activating factor, arachidonic acid and nitric oxide. Advances in Neuroimmunology. 1994;4:181–188. doi: 10.1016/s0960-5428(06)80255-x. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology. 1998;51:1562–1566. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- Lu T, Xu Y, Mericle MT, Mellgren RL. Participation of the conventional calpains in apoptosis. Biochimica et Biophysica Acta. 2002;1590:16–26. doi: 10.1016/s0167-4889(02)00193-3. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Guttmann RP. NMDA receptor pharmacology: perspectives from molecular biology. Curr Drug Targets. 2001;2:215–231. doi: 10.2174/1389450013348434. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Guttmann RP. Excitotoxicity: perspectives based on N-Methyl-D-Aspartate receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 2002;300:717–723. doi: 10.1124/jpet.300.3.717. [DOI] [PubMed] [Google Scholar]
- Malorni W, Rivabene R, Lucia BM, Ferrara R, Mazzone AM, Cauda R, Paganelli R. The role of oxidative imbalance in progression to AIDS: effect of the thiol supplier N-acetylcysteine. AIDS Research & Human Retroviruses. 1998;14:1589–1596. doi: 10.1089/aid.1998.14.1589. [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Molecular & Cellular Biology. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W, Iwai C, Qin F, Liang C-s. Norepinephrine induces endoplasmic reticulum stress and downregulation of norepinephrine transporter density in PC12 cells via oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H2381–2389. doi: 10.1152/ajpheart.00904.2004. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Dietrich J, Wong V, Xue H, Mayer-Proschel M, Rao MS, Rothstein JD. Glutamate transporter expression and function in human glial progenitors. GLIA. 2003;45:133–143. doi: 10.1002/glia.10310. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA. Selective neuronal vulnerability in HIV encephalitis. Journal of Neuropathology and Experimental Neurology. 1992;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Mallory ME, Rockenstein E, Moechars D, Van Leuven F. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Experimental Neurology. 2000;163:381–387. doi: 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, Sakamoto AC, Assirati JA, Fried I, Peacock WJ, Ojemann GA, Adelson PD. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–473. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Matthews CC, Zielke HR, Wollack JB, Fishman PS. Enzymatic Degradation Protects Neurons from Glutamate Excitotoxicity. Journal of Neurochemistry. 2000;75:1045–1052. doi: 10.1046/j.1471-4159.2000.0751045.x. [DOI] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends in Pharmacological Sciences. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- McCracken E, Hunter AJ, Patel S, Graham DI, Dewar D. Calpain activation and cytoskeletal protein breakdown in the corpus callosum of head-injured patients. Journal of Neurotrauma. 1999;16:749–761. doi: 10.1089/neu.1999.16.749. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap‘n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Research. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Meaney J, Balcar V, Rothstein JD, Jeffrey P. Glutamate transport in cultures from developing avian cerebellum: presence of GLT-1 immunoreactivity in Purkinje neurons. Journal of Neuroscience Research. 1998;54:595–603. doi: 10.1002/(SICI)1097-4547(19981201)54:5<595::AID-JNR4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Molinari M, Carafoli E. Calpain: a cytosolic proteinase active at the membranes. Journal of Membrane Biology. 1997;156:1–8. doi: 10.1007/s002329900181. [DOI] [PubMed] [Google Scholar]
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends in Neurosciences. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Moore JD, Rothwell NJ, Gibson RM. Involvement of caspases and calpains in cerebrocortical neuronal cell death is stimulus-dependent. British Journal of Pharmacology. 2002;135:1069–1077. doi: 10.1038/sj.bjp.0704538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa CE-H, Rae C, Bubb WA, Griffin JL, Deters NA, Balcar VJ. Inhibitors of glutamate transport modulate distinct patterns in brain metabolism. Journal of Neuroscience Research. 2007;85:342–350. doi: 10.1002/jnr.21108. [DOI] [PubMed] [Google Scholar]
- Muller F, Svardal AM, Nordoy I, Berge RK, Aukrust P, Froland SS. Virological and immunological effects of antioxidant treatment in patients with HIV infection. European Journal of Clinical Investigation. 2000;30:905–914. doi: 10.1046/j.1365-2362.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- Musa FR, Tokuda M, Kuwata Y, Ogawa T, Tomizawa K, Konishi R, Takenaka I, Hatase O. Expression of cyclin-dependent kinase 5 and associated cyclins in Leydig and Sertoli cells of the testis. Journal of Andrology. 1998;19:657–666. [PubMed] [Google Scholar]
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. Journal of Cell Biology. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient Exposure to HIV-1 Tat Protein Results in Cytokine Production in Macrophages and Astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Ankarcrona M, Bonfoco E, Orrenius S, Lipton SA. Neuronal necrosis and apoptosis: two distinct events induced by exposure to glutamate or oxidative stress. Advances in Neurology. 1997;72:95–101. [PubMed] [Google Scholar]
- Nikawa J, Yamashita S. IRE1 encodes a putative protein kinase containing a membrane-spanning domain and is required for inositol phototrophy in Saccharomyces cerevisiae. Molecular Microbiology. 1992;6:1441–1446. doi: 10.1111/j.1365-2958.1992.tb00864.x. [DOI] [PubMed] [Google Scholar]
- Nishida K, Markey SP, Kustova Y, Morse HC, Skolnick P, Basile AS, Sei Y. Increased Brain Levels of Platelet-Activating Factor in a Murine Acquired Immune Deficiency Syndrome Are NMDA Receptor-Mediated. Journal of Neurochemistry. 1996;66:433–435. doi: 10.1046/j.1471-4159.1996.66010433.x. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Research. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. Journal of Cell Biology. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. American Journal of Physiology -Cell Physiology. 2003;285:C334–342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- O’Connor E, Devesa A, Garcia C, Puertes IR, Pellin A, Vina JR. Biosynthesis and maintenance of GSH in primary astrocyte cultures: role of L-cystine and ascorbate. Brain Research. 1995;680:157–163. doi: 10.1016/0006-8993(95)00257-q. [DOI] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: Roles for the NMDA receptor subtypes. J Neurosci. 2006;26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon GM, Humphreys PD, Goldman RS, Halstead SK, Bullens RW, Plomp JJ, Ushkaryov Y, Willison HJ. Calpain inhibitors protect against axonal degeneration in a model of anti-ganglioside antibody-mediated motor nerve terminal injury. Brain. 2003;126:2497–2509. doi: 10.1093/brain/awg254. [DOI] [PubMed] [Google Scholar]
- O’Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. Journal of Neuroscience. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Kimata Y, Higashio H, Tsuru A, Kohno K. Dissociation of Kar2p/BiP from an ER Sensory Molecule, Ire1p, Triggers the Unfolded Protein Response in Yeast. Biochemical and Biophysical Research Communications. 2000;279:445–450. doi: 10.1006/bbrc.2000.3987. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Vallone J, Melendez RI, Shih AY, Murphy TH, Kaliva PW. Nrf2 gene deletion fails to alter psychostimulant-induced behavior or neurotoxicity. Brain Research. 2007;1127:26–35. doi: 10.1016/j.brainres.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertan J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiological Reviews. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Pariat M, Carillo S, Molinari M, Salvat C, Debussche L, Bracco L, Milner J, Piechaczyk M. Proteolysis by calpains: a possible contribution to degradation of p53. Molecular & Cellular Biology. 1997;17:2806–2815. doi: 10.1128/mcb.17.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariat M, Salvat C, Bebien M, Brockly F, Altieri E, Carillo S, Jariel-Encontre I, Piechaczyk M. The sensitivity of c-Jun and c-Fos proteins to calpains depends on conformational determinants of the monomers and not on formation of dimers. Biochemical Journal 345 Pt. 2000;1:129–138. [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration.[see comment] Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. The EMBO Journal. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Huttenlocher A. Calpain. International Journal of Biochemistry & Cell Biology. 2002;34:722–725. doi: 10.1016/s1357-2725(02)00009-2. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Bolanos JP, Heales SJR, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Progress in Neurobiology. 1997;32:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Pfaff M, Du X, Ginsberg MH. Calpain cleavage of integrin beta cytoplasmic domains. FEBS Letters. 1999;460:17–22. doi: 10.1016/s0014-5793(99)01250-8. [DOI] [PubMed] [Google Scholar]
- Philbert MA, Beiswanger CM, Waters DK, Reuhl KR, Lowndes HE. Cellular and regional distribution of reduced glutathione in the nervous system of the rat: Histochemical localization by mercury orange and o-phthaldialdehyde-induced histofluorescence. Toxicology and Applied Pharmacology. 1991;107:215–227. doi: 10.1016/0041-008x(91)90204-r. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. Journal of Biological Chemistry. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Hurtado O, Romera C, Cardenas A, Fernandez-Tome P, Alonso-Escolano D, Lorenzo P, Moro MA, Lizasoain I. TNFR1 mediates increased neuronal membrane EAAT3 expression after in vivo cerebral ischemic preconditioning. Neuroscience. 2006;138:1171–1178. doi: 10.1016/j.neuroscience.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Research. 2005;1045:57. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MGA, Schrama LH, van Veelen CWM, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PNE. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125:32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Herndier BG, Tang NM, McGrath MS. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alteration in cultures human brains. J Clin Invest. 1991;87:503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Clarke JA, McGuire D, McGrath MS. Investigation of HIV-infected macrophage neurotoxin production from patients with AIDS dementia. Advances in Neuroimmunology. 1994;4:195–198. doi: 10.1016/s0960-5428(06)80257-3. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Zhou M, Stubblebine M, Bitler CM. Differential modulation of cell death proteins in human brain cells by tumor necrosis factor alpha and platelet activating factor. Journal of Neuroscience Research. 1998;54:530–538. doi: 10.1002/(SICI)1097-4547(19981115)54:4<530::AID-JNR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Clarke JA, McGrath MS, Moore D, McGuire D. Monokine products as predictors of AIDS dementia. AIDS. 1996;10:1495–1500. doi: 10.1097/00002030-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol. 2004;78:11926–11938. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Colin C, Hinners I, Gervais A, Cheret C, Mallat M. System Xc- and Apolipoprotein E Expressed by Microglia Have Opposite Effects on the Neurotoxicity of Amyloid-beta Peptide 1–40. J Neurosci. 2006;26:3345–3356. doi: 10.1523/JNEUROSCI.5186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramassamy C, Averill D, Beffert U, Bastianetto S, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Davignon J, Quirion R, Poirier J. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer’s disease is related to the apolipoprotein E genotype. Free Radical Biology and Medicine. 1999;27:544–553. doi: 10.1016/s0891-5849(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL. Expression of Nrf2 in Neurodegenerative Diseases. Journal of Neuropathology and Experimental Neurology. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Current Drug Targets -CNS & Neurological Disorders. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Ray SK, Shields DC, Saido TC, Matzelle DC, Wilford GG, Hogan EL, Banik NL. Calpain activity and translational expression increased in spinal cord injury. Brain Research. 1999;816:375–380. doi: 10.1016/s0006-8993(98)01128-7. [DOI] [PubMed] [Google Scholar]
- Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer’s disease. FEBS Journal. 2006;273:3437–3443. doi: 10.1111/j.1742-4658.2006.05352.x. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends in Biochemical Sciences. 1996;21:267–271. [PubMed] [Google Scholar]
- Riccio A, Ginty DD. What a privilege to reside at the synapse: NMDA receptor signaling to CREB. Nat Neurosci. 2002;5:389–390. doi: 10.1038/nn0502-389. [DOI] [PubMed] [Google Scholar]
- Rimaniol A-C, Haik S, Martin M, Le Grand R, Boussin FD, Dereuddre-Bosquet N, Gras G, Dormont D. Na+-Dependent High Affinity Glutamate Transport in Macrophages. Journal of Immunology. 2000;164:5430–5438. doi: 10.4049/jimmunol.164.10.5430. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Romero-Alvira D, Roche E. The keys of oxidative stress in acquired immune deficiency syndrome apoptosis. Medical Hypotheses. 1998;51:169–173. doi: 10.1016/s0306-9877(98)90113-x. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. Journal of Neuroscience. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol(Berl) 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Saido TC, Shibata M, Takenawa T, Murofushi H, Suzuki K. Positive regulation of mu-calpain action by polyphosphoinositides. Journal of Biological Chemistry. 1992;267:24585–24590. [PubMed] [Google Scholar]
- Saido TC, Nagao S, Shiramine M, Tsukaguchi M, Yoshizawa T, Sorimachi H, Ito H, Tsuchiya T, Kawashima S, Suzuki K. Distinct kinetics of subunit autolysis in mammalian m-calpain activation. FEBS Letters. 1994;346:263–267. doi: 10.1016/0014-5793(94)00487-0. [DOI] [PubMed] [Google Scholar]
- Sanders V, Pittman C, White M, Wang G, Wiley C, Achim C. Chemkines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. Journal of Biological Chemistry. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and Expression of a Plasma Membrane Cystine/Glutamate Exchange Transporter Composed of Two Distinct Proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochemical and Biophysical Research Communications. 2004;325:109. doi: 10.1016/j.bbrc.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of Cystine/Glutamate Exchange Transporter, System Xc-, in the Mouse Brain. J Neurosci. 2002;22:8028–8033. doi: 10.1523/JNEUROSCI.22-18-08028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Current Medicinal Chemistry. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Zhang K, Kaufman RJ. The Unfolded Protein Response - a Stress Signaling Pathway of the Endoplasmic Reticulum. Journal of Chemical Neuroanatomy. 2004;28:79–92. doi: 10.1016/j.jchemneu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Molecular & Cellular Biology. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. Journal of Neuroscience. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/Glutamate Exchange Modulates Glutathione Supply for Neuroprotection from Oxidative Stress and Cell Proliferation. J Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate Regulation of Glutathione Biosynthesis and Release by Nrf2-Expressing Glia Potently Protects Neurons from Oxidative Stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto K, Sakai R, Takaoka K, Yumoto N, Nakajima T, Amara SG, Shigeri Y. Characterization of Novel L-threo-beta-Benzyloxyaspartate Derivatives, Potent Blockers of the Glutamate Transporters. Molecular Pharmacology. 2004;65:1008–1015. doi: 10.1124/mol.65.4.1008. [DOI] [PubMed] [Google Scholar]
- Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K. Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. Journal of Neurochemistry. 2006;98:310–320. doi: 10.1111/j.1471-4159.2006.03874.x. [DOI] [PubMed] [Google Scholar]
- Shor-Posner G, Lecusay R, Morales G, Campa A, Miguez-Burbano M-J. Neuroprotection in HIV-positive drug users: Implications for antioxidant therapy. JAIDS. 2002;31:S84–S88. doi: 10.1097/00126334-200210012-00009. [DOI] [PubMed] [Google Scholar]
- Siao C-J, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. JNeurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Simpkins KL, Guttmann RP, Dong Y, Chen Z, Sokol S, Neumar RW, Lynch DR. Selective activation induced cleavage of the NR2B subunit by calpain. Journal of Neuroscience. 2003;23:11322–11331. doi: 10.1523/JNEUROSCI.23-36-11322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neuroscience Letters. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative Stress, Caloric Restriction, and Aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Spreux-Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Le Forestier N, Marouan A, Dib M, Meininger V. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. Journal of the Neurological Sciences. 2002;193:73–78. doi: 10.1016/s0022-510x(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Squier MK, Sehnert AJ, Sellins KS, Malkinson AM, Takano E, Cohen JJ. Calpain and calpastatin regulate neutrophil apoptosis. Journal of Cellular Physiology. 1999;178:311–319. doi: 10.1002/(SICI)1097-4652(199903)178:3<311::AID-JCP5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Strachan GD, Koike MA, Siman R, Hall DJ, Jordan-Sciutto KL. E2F1 induces cell death, calpain activation, and MDMX degradation in a transcription independent manner implicating a novel role for E2F1 in neuronal loss in SIV encephalitis. Journal of Cellular Biochemistry. 2005;96:728–740. doi: 10.1002/jcb.20574. [DOI] [PubMed] [Google Scholar]
- Su Z-z, Leszczyniecka M, Kang D-c, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: Cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) PNAS. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci U S A. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Erb H, Murphy TH. Coordinate regulation of glutathione metabolism in astrocytes by Nrf2. Biochemical and Biophysical Research Communications. 2005;326:371–377. doi: 10.1016/j.bbrc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Suzuki K. The structure of the calpains and the calpain gene. In: Mellgren RL, Murachi T, editors. Intracellular Calcium-Dependent Proteolysis. Boca Raton, FL: CRC; 1990. pp. 25–35. [Google Scholar]
- Suzuki K. Nomenclature of calcium dependent proteinase. Biomedica Biochimica Acta. 1991;50:483–484. [PubMed] [Google Scholar]
- Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl 1):S12–18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell KA, Stein B, Longuemare MC. Neuronal Regulation of Glutamate Transporter Subtype Expression in Astrocytes. J Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Tomioka M, Tsubuki S, Higuchi M, Iwata N, Itohara S, Maki M, Saido TC. Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways in adult brains: evidence from calpastatin mutant mice. Journal of Biological Chemistry. 2005;280:16175–16184. doi: 10.1074/jbc.M414552200. [DOI] [PubMed] [Google Scholar]
- Takikita S, Takano T, Narita T, Takikita M, Ohno M, Shimada M. Neuronal apoptosis mediated by IL-1 beta expression in viral encephalitis caused by a neuroadapted strain of the mumps virus (Kilham Strain) in hamsters. Experimental Neurology. 2001;172:47–59. doi: 10.1006/exnr.2001.7773. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and Exacerbation of Brain Injury in Mice Lacking the Glutamate Transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tian G, Lai L, Guo H, Lin Y, Butchbach MER, Chang Y, Lin C-lG. Translational Control of Glial Glutamate Transporter EAAT2 Expression. J Biol Chem. 2007;282:1727–1737. doi: 10.1074/jbc.M609822200. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y, Idemoto S, Moriguchi S, Watanabe S, Nakanishi H. Proteases involved in long-term potentiation. Life Sciences. 2002;72:355–361. doi: 10.1016/s0024-3205(02)02285-3. [DOI] [PubMed] [Google Scholar]
- Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilagyi A, Banoczi Z, Hudecz F, Friedrich P. On the sequential determinants of calpain cleavage. Journal of Biological Chemistry. 2004;279:20775–20785. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer’s disease brains. Neuroscience Letters. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- Tullio RD, Passalacqua M, Averna M, Salamino F, Melloni E, Pontremoli S. Changes in intracellular localization of calpastatin during calpain activation. Biochemical Journal 343 Pt. 1999;2:467–472. [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger J, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- Valeyev NV, Downing AK, Skorinkin AI, Campbell ID, Kotov NV. A calcium dependent de-adhesion mechanism regulates the direction and rate of cell migration: a mathematical model. In Silico Biology. 2006;6:545–572. [PubMed] [Google Scholar]
- van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling Pathway: Promising Drug Target to Combat Oxidative Stress in Neurodegenerative Disorders. Current Drug Targets - CNS & Neurological Disorders. 2005;4:267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Mitrovic AD, Chebib M, Balcar VJ, Johnston GAR. Contrasting Modes of Action of Methylglutamate Derivatives on the Excitatory Amino Acid Transporters, EAAT1 and EAAT2. Molecular Pharmacology. 1997;51:809–815. doi: 10.1124/mol.51.5.809. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Bahr BA. The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states. International Journal of Experimental Pathology. 2000;81:323–339. doi: 10.1111/j.1365-2613.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaz-Faircloth M, McGraw TS, alandro MS, Fremeau RT, Jr, Kilberg MS, Anderson KJ. Characterization and distribution of the neuronal glutamate transporter EAAC1 in rat brain. Am J Physiol Cell Physiol. 1996;270:C67–75. doi: 10.1152/ajpcell.1996.270.1.C67. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- Viviani B, Corsini E, Binaglia M, Galli CL, Marinovich M. Reactive oxygen species generated by glia are responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience. 2001;107:51–58. doi: 10.1016/s0306-4522(01)00332-3. [DOI] [PubMed] [Google Scholar]
- Wang KK, Yuen PW. Development and therapeutic potential of calpain inhibitors. Advances in Pharmacology. 1997;37:117–152. doi: 10.1016/s1054-3589(08)60949-7. [DOI] [PubMed] [Google Scholar]
- Wang KK, Larner SF, Robinson G, Hayes RL. Neuroprotection targets after traumatic brain injury. Current Opinion in Neurology. 2006;19:514–519. doi: 10.1097/WCO.0b013e3280102b10. [DOI] [PubMed] [Google Scholar]
- Wang S, Lees GJ, Rosengren LE, Karlsson JE, Hamberger A, Haglid KG. Proteolysis of filament proteins in glial and neuronal cells after in vivo stimulation of hippocampal NMDA receptors. Neurochemical Research. 1992;17:1005–1009. doi: 10.1007/BF00966828. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Xu JX. Salvianic acid A protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Neuroscience Research. 2005;51:129–138. doi: 10.1016/j.neures.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, White MG, Akay C, Chodroff R, Robinson J, Lindl KA, Dichter MA, Qian Y, Mao Z, Kolson D, Jordan-Sciutto K. Activation of cyclin-dependent kinase 5 by calpain contributes to human immunodeficiency virus-induced neurotoxicity. Journal of Neurochemistry. 2007 doi: 10.1111/j.1471-4159.2007.04746.x. In Press. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American Journal of Physiology -Endocrinology & Metabolism. 2006;291:E275–281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Mandel S, Amit T, Youdim MBH. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. The Journal of Nutritional Biochemistry. 2004;15:506. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- White MG, Luca LE, Nonner D, Saleh O, Hu B, Barrett EF, Barrett JN. Cellular mechanisms of neuronal damage from hyperthermia. Progress In Brain Research. 2007:162. doi: 10.1016/S0079-6123(06)62017-7. [DOI] [PubMed] [Google Scholar]
- Wisman LAB, van Muiswinkel FL, de Graan PNE, Hol EM, Bar PR. Cells over-expressing EAAT2 protect motoneurons from excitotoxic death in vitro. NeuroReport. 2003;14:1967–1970. doi: 10.1097/00001756-200310270-00017. [DOI] [PubMed] [Google Scholar]
- Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- Wu H, Jin Y, Buddhala C, Osterhaus G, Cohen E, Jin H, Wei J, Davis K, Obata K, Wu J-Y. Role of glutamate decarboxylase (GAD) isoform, GAD65, in GABA synthesis and transport into synaptic vesicles--Evidence from GAD65-knockout mice studies. Brain Research. 2007;1154:80. doi: 10.1016/j.brainres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Wu HY, Lynch DR. Calpain and synaptic function. Molecular Neurobiology. 2006;33:215–236. doi: 10.1385/MN:33:3:215. [DOI] [PubMed] [Google Scholar]
- Xue X, Piao J-H, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNF-alpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR conteracts ROS accumulation by TNF-alpha. Journal of Biological Chemistry. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- Yamada K, Watanabe M, Shibata T, Nagashima M, Tanaka K, Inoue Y. Glutamate Transporter GLT-1 Is Transiently Localized on Growing Axons of the Mouse Spinal Cord before Establishing Astrocytic Expression. Journal of Neuroscience. 1998;18:5706–5713. doi: 10.1523/JNEUROSCI.18-15-05706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashima T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Progress in Neurobiology. 2000;62:273–295. doi: 10.1016/s0301-0082(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Disease Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Molecular Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Ye Z-C, Rothstein JD, Sontheimer H. Compromised Glutamate Transport in Human Glioma Cells: Reduction-Mislocalization of Sodium-Dependent Glutamate Transporters and Enhanced Activity of Cystine-Glutamate Exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Bradley WG, Shapshak P, Nagano I, Stewart RV, Xin KQ, Srivastava AK, Nakamura S. Role of immune activation and cytokine expression in HIV-1-associated neurologic diseases. Advances in Neuroimmunology. 1995;5:335–358. doi: 10.1016/0960-5428(95)00012-q. [DOI] [PubMed] [Google Scholar]
- Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Experimental Neurology. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Lu Q, Xie ZJ, Mellgren RL. Inhibition of the growth of WI-38 fibroblasts by benzyloxycarbonyl-Leu-Leu-Tyr diazomethyl ketone: evidence that cleavage of p53 by a calpain-like protease is necessary for G1 to S-phase transition. Oncogene. 1997;14:255–263. doi: 10.1038/sj.onc.1200841. [DOI] [PubMed] [Google Scholar]