Abstract
GABAA receptors have an age-adapted function in the brain. During early development, they mediate depolarizing effects, which result in activation of calcium-sensitive signaling processes that are important for the differentiation of the brain. In more mature stages of development and in adults, GABAA receptors acquire their classical hyperpolarizing signaling. The switch from depolarizing to hyperpolarizing GABAA-ergic signaling is triggered through the developmental shift in the balance of chloride cotransporters that either increase (ie NKCC1) or decrease (ie KCC2) intracellular chloride. The maturation of GABAA signaling follows sex-specific patterns, which correlate with the developmental expression profiles of chloride cotransporters. This has first been demonstrated in the substantia nigra, where the switch occurs earlier in females than in males. As a result, there are sensitive periods during development when drugs or conditions that activate GABAA receptors mediate different transcriptional effects in males and females. Furthermore, neurons with depolarizing or hyperpolarizing GABAA-ergic signaling respond differently to neurotrophic factors like estrogens. Consequently, during sensitive developmental periods, GABAA receptors may act as broadcasters of sexually differentiating signals, promoting gender-appropriate brain development. This has particular implications in epilepsy, where both the pathophysiology and treatment of epileptic seizures involve GABAA receptor activation. It is important therefore to study separately the effects of these factors not only on the course of epilepsy but also design new treatments that may not necessarily disturb the gender-appropriate brain development.
Keywords: GABA, substantia nigra, hippocampus, sex, autism, epilepsy
1.1 INTRODUCTION
Over the last few decades, many studies have highlighted the differences between the male and female brain and showed that they are not just limited to structures involved primarily in sexual behavior. These include morphological, functional and ontogenetic differences in a variety of neurotransmitter signaling systems and cell types that influence the way the male and female brains operate under normal and pathological conditions. Such differences are also applicable during the very early stages of life, even before gonadal function fully matures. In epilepsy, epidemiological and clinical studies have suggested that males and females may have different susceptibility to specific seizure types and epileptic syndromes, especially those first manifesting during the early postnatal life. In this review, I will focus on the GABAA receptor signaling system, known both as an important neurotrophic factor during development but also as the major inhibitory neurotransmitter signaling system in the mature brain. I will introduce why sex differences in the physiology of GABAAergic system are important for normal, sex-appropriate brain development and how they influence the consequences of seizures and antiepileptic drugs.
1.2 GABAA receptors and chloride cotransporters in normal brain development
1.2.1 Molecular biology of developmental changes in GABAA receptor physiology
GABAA receptors are pentameric ligand-gated ion channels that are permeable to chloride ions and to a lesser extent bicarbonate [reviewed in (Farrant and Kaila 2007; Galanopoulou 2008b)]. The subunit composition of each receptor complex determines its subcellular localization, pharmacological, and kinetic properties. Albeit GABAA receptors are largely known as the principal inhibitory receptors in the mature brain, early in life they depolarize immature neurons (Ben-Ari et al. 1989; Cherubini et al. 1990). These paradoxical depolarizing effects of GABA are promoted by the relative accumulation of chloride inside the immature neurons, leading to chloride efflux once GABAA receptor channels open.
Chloride regulation and the control of GABAA receptor signaling are effected through chloride cotransporters and channels (Table I) (Payne et al. 1996; Delpire 2000; Farrant and Kaila 2007; Galanopoulou 2008b). The main proteins that increase intracellular chloride are the sodium-potassium-chloride cotransporters (NKCCs) and sodium-chloride cotransporters (NCCs), which import chloride along with the respective cations (sodium with or without potassium) in an electroneutral manner. To counteract their effects, the potassium chloride cotransporters (KCCs) export these ions decreasing intracellular chloride (Figure I). The best-studied representatives of these proteins are the neuronal-specific KCC2 isoform (KCC class) and the almost ubiquitous NKCC1 (NKCC class). During development, there is a gradual shift in the balance of these cotransporters from a NKCC1-dominant to a KCC2-dominant state, that ultimately favors the maintenance of reduced chloride concentration in more mature neurons and appearance of hyperpolarizing GABAA responses (Plotkin et al. 1997; Rivera et al. 1999; Mikawa et al. 2002; Wang et al. 2002; Galanopoulou et al. 2003). In most studied rodent neuronal cells, this shift occurs during the first four weeks of life, a time that coincides with the switch of GABAA receptor signaling from depolarizing to hyperpolarizing (herein referred as GABAA switch) (Rivera et al. 1999; Galanopoulou et al. 2003; Khazipov et al. 2004; Banke and McBain 2006; Kyrozis et al. 2006; Galanopoulou 2008a). The GABAA switch follows region and cell type specific tempos that can be gender-specific. It may also differ across the somatodendritic space (Romo-Parra et al. 2008).
Table I.
Selected proteins involved in Cl- homeostasis with known effects on GABAA signaling.
| Protein | Function | Disease linkage | References | |
|---|---|---|---|---|
| Favoring hyperpolarizing GABAA responses | ||||
| Potassium Chloride co-transporters (KCCs) | Efflux of K+ and Cl− | Humans: Agenesis of corpus callosum with peripheral neuropathy and variants with bipolar disease | (Payne et al. 1996; Delpire and Mount 2002; Howard et al. 2002; Meyer et al. 2005; Salin-Cantegrel et al. 2007) | |
| Knockout mice epilepsy | ||||
| Chloride channel 2 | Efflux of Cl− | Humans: Idiopathic generalized epilepsy; Lesion-related epilepsy. | (Haug et al. 2003; D'Agostino et al. 2004; Niemeyer et al. 2004; Bertelli et al. 2007; Blanz et al. 2007; Everett et al. 2007) | |
| Knockout mice: Vacuolar leukoencephalopathy, bindness, decreased conduction velocity in central auditory paths, normal neuronal morphology. | ||||
| Na+ dependent anion exchanger | Influx of Na+ , HCO3− ; Efflux of H+ , Cl−. | N/A | (Kintner et al. 2007) | |
| Favoring depolarizing GABAA responses | ||||
| Sodium potassium chloride cotransporters (NKCCs) | Influx of Na+, K+and 2Cl− | Humans: Bartter’s syndrome type 1 | (Delpire and Mount 2002) | |
| Sodium chloride cotransporters (NCCs) | Influx of Na+, and Cl− | Humans: Gitelman’s syndrome | (Nicolet-Barousse et al. 2005) | |
| Na+ independent anion exchanger | Influx of Cl− ; Efflux of HCO3−. | Humans: small susceptibility to Idiopathic generalized epilepsy (AE3). | (Sander et al. 2002; Hentschke et al. 2006) | |
| Knockout mice: Reduced seizure threshold | ||||
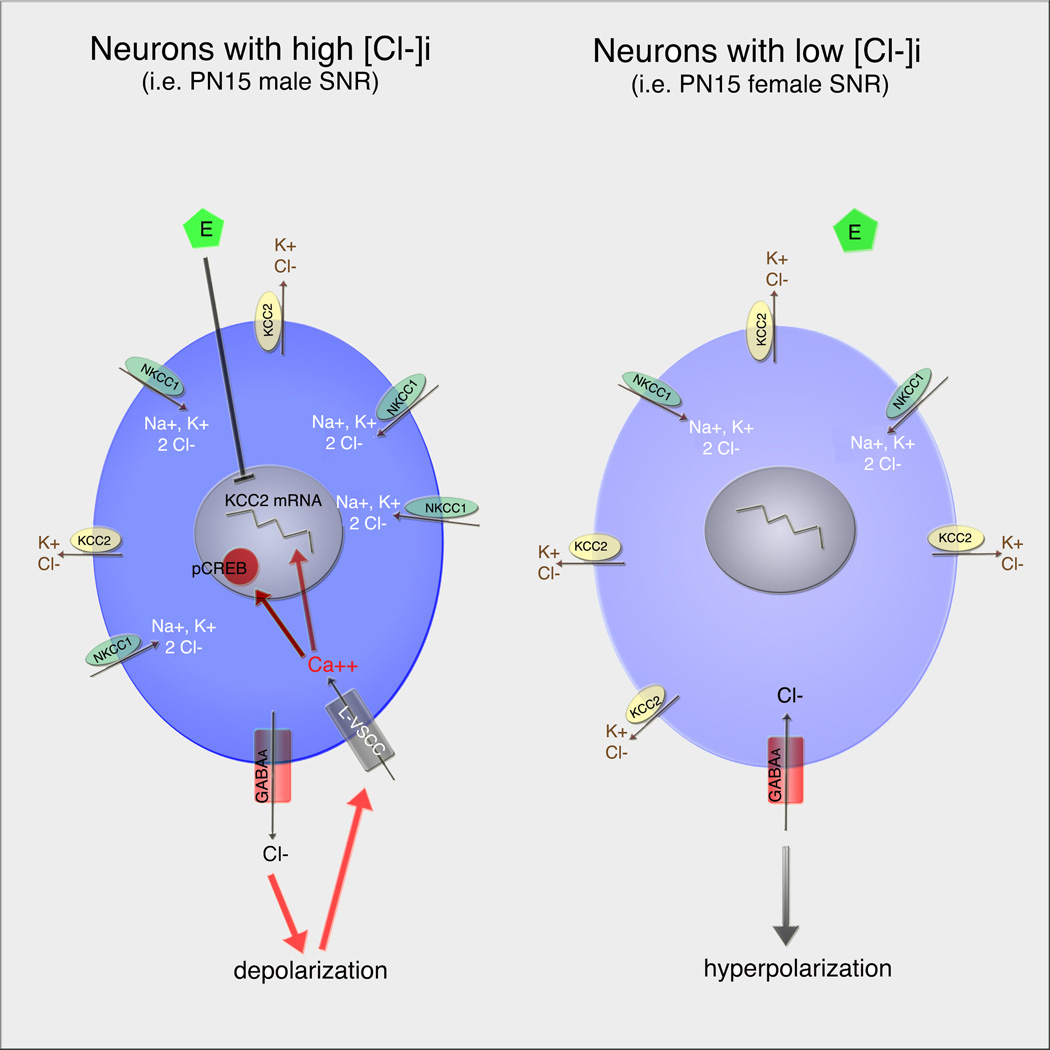
Figure I. Sex-specific patterns in GABAA signaling and ensuing control of KCC2 expression in PN15 rat GABAergic SNR neurons.
In PN15 male SNR (left panel), there is relatively low expression of KCC2 compared to NKCC1, leading to high intracellular Cl- ([Cl-]i). As a result, activation of GABAA receptors depolarizes them and activates L-VSCCs, increasing intracellular calcium. This leads to increased expression of pCREB-ir and KCC2 mRNA expression. In these neurons, estradiol (E) downregulates KCC2 mRNA, through pathways interacting with GABAA-mediated activation of L-VSCCs.
In PN15 female SNR (right panel), there is relative abundance of KCC2 expression, decreasing [Cl-]i. GABAA receptor activation results in hyperpolarization, without altering intracellular calcium or calcium-sensitive processes. Estradiol also cannot influence KCC2 expression in these neurons.
1.2.2 Implications for normal brain development
GABAA-mediated depolarizations are essential for normal brain development. The physiological processes affected by them have been detailed previously [reviewed in (Galanopoulou 2005; Akerman and Cline 2007; Ben-Ari et al. 2007; Farrant and Kaila 2007)]. In brief, GABAA receptor mediated depolarizations activate voltage-sensitive calcium and sodium channels and relieve the blockade from N-methyl-D-aspartate (NMDA) receptors (Ben-Ari et al. 1997). Such effects influence the neuronal activity but also trigger cascades of calcium-sensitive signaling processes that ultimately control DNA synthesis, proliferation and migration, neuronal differentiation and communication (Reichling et al. 1994; Owens et al. 1996; Ben-Ari et al.1997; Ganguly et al. 2001; Ben-Ari 2002; Kandler et al. 2002; Galanopoulou et al. 2003;Cancedda et al. 2007).
It is becoming increasingly more evident that disruption of the normal developmental patterns of depolarizing or hyperpolarizing GABAA signaling can result in developmental abnormalities. Precocious termination of depolarizing GABAA signaling in maturing neurons may have severe consequences for brain development. This is supported by a number of in vitro and in vivo studies that emphasize the importance of correct timing of the GABAA switch. Precocious termination of depolarizing GABAA receptor signaling via selective overexpression of KCC2 in utero in rat embryonic (E17–18) ventricular progenitors impairs their morphological differentiation and dendritic arborization, although it does not affect cortical migration (Cancedda et al. 2007). Similarly, global overexpression of KCC2 in zebrafish embryos decreased the number of spinal motoneurons and interneurons, decreased the axonal tracts and the size of the brain and spinal cord (Reynolds et al. 2008). Premature switch of GABAA responses to hyperpolarizing by overexpression of KCC2 may shift the balance of glutamatergic versus GABAAergic postsynaptic responses in favor of the GABAA-mediated inhibitory input (Chudotvorova et al. 2005; Akerman and Cline 2006). NKCC1 knockout mice exhibit a variety of deficits including impaired locomotion and pain perception, deafness, problems with fertility, salivation, and intestinal function (Delpire et al. 1999; Sung et al. 2000; Delpire and Mount 2002).
In contrast, prolongation of the developmental periods with depolarizing GABAA receptor signaling, in KCC2 knockout mice, is lethal soon after birth due to motor and respiratory impairments (Hubner et al. 2001). Partial deficit in KCC2 increases the susceptibility to seizures and seizure-related hippocampal injury (Delpire and Mount 2002). To further corroborate the experimental data, mutations in chloride cotransporters and channels can be pathogenic in humans. Mutations in another KCC isoform, KCC3, have been linked with Agenesis of Corpus Callosum and Peripheral Neuropathy (ACCPN), a syndrome also associated with bipolar disease, and at least in transgenic mice lowers the threshold for seizures (Howard et al. 2002; Meyer et al. 2005) (Table I).
The importance of the age-related changes in the direction of GABAA receptor signaling in normal development has long been known. In the current review, I will rather present more recent data implicating chloride cotransporters and GABAA receptor signaling in the sexual differentiation of the brain and will discuss how these sex-specific developmental processes modify the effects of seizures and other early life stressors.
1.3 GABAA receptors as mediators of sex-specific differentiating signals
The GABAergic system has been a favorite research target in studies of sexual dimorphism in the brain. Several sex differences in GABAergic indices have been documented in both developing animals (Table II) and adults (Table III). These refer to the numbers of GABAergic neurons, expression of proteins involved in GABA synthesis or metabolism or GABAA receptors, as well as the responsiveness to GABA-acting drugs. The sex-specific phenotypic and functional differences in the GABAergic system may play key roles in the way the male and female mature brains operate. In adulthood, however, many of these differences are under the control of sex hormones and can often be reversed by gonadectomy or hormonal manipulations (Segovia and Guillamon 1993; Becu-Villalobos et al. 1997; Cooke et al. 1998; Knickmeyer and Baron-Cohen 2006a). To better understand how the sexual differentiation of the GABAAergic system evolves, it is important to trace it back to the early developmental stages. Sexual differentiation of the brain starts very early, driven by the genomic differences, such as those dictated by the sex-determining region of the Y chromosome (sry) (Sinclair et al. 1990). The perinatal and early postnatal periods are viewed as critical or sensitive periods for defining the sexual differentiation of several brain regions. Such effects are usually linked to the perinatal testosterone surge and the influence of its estrogenic or androgenic metabolites. Among the signaling pathways involved in the sexual differentiation of the brain is the GABAAergic. In the following paragraphs, I will present recent findings from the substantia nigra (SN) and hippocampus detailing how differences in the timing of GABAA switch contribute to this process.
Table II.
Sex differences in the developing brain, as they relate to the GABAergic system.
| Parameter | Species / Age / brain region | Male vs female | Reference |
|---|---|---|---|
| GAD mRNA | Rat Sprague Dawley, dorsomedial nucleus, arcuate nucleus, CA1. | PN1 : M>F; | (Davis et al. 1996) |
| PN15: M=F | |||
| GABAA receptor .1 subunit mRNA | Rat Sprague-Dawley SNR (PN15 and PN30) | M<F | (Ravizza et al. 2003) |
| Number of GABA-ir neurons | Rat Sprague-Dawley striatum (E16 – E21); SNR (PN15, PN30); | M<F | (Ovtscharoff et al. 1992; Galanopoulou et al. 2001; Ravizza et al. 2003) |
| GABAA switch to hyperpolarizing | Sprague-Dawley rats: SNR, SNC, CA1 pyramidal neurons of the hippocampus | Earlier in females | (Galanopoulou et al. 2003; Galanopoulou 2006; Kyrozis et al. 2006; Galanopoulou 2008a) |
| KCC2 expression | Sprague-Dawley rats: PN15–30 SNR, PN10 CA1 pyramidal neurons of the hippocampus | M<F | (Galanopoulou 2008a) |
| Sprague-Dawley rats: Mediobasal hypothalamic nucleus (neonatal) | M<F | (Perrot-Sinal et al. 2007). | |
| NKCC1 expression or activity | Sprague-Dawley rats: SNR, CA1 pyramidal neurons of the hippocampus | bumetanide-sensitive NKCC1-like activity: M>F | (Galanopoulou 2008a) |
| Sprague-Dawley rats: Mediobasal hypothalamic nucleus (neonatal) | mRNA: M>F active protein: M=F | (Perrot-Sinal et al. 2007) | |
| Muscimol effects | Neonatal Rat hypothalamus, CA1 pyramidal neurons | M: increase in pCREB | (Auger et al. 2001) |
| F: decrease in pCREB | |||
| Sprague-Dawley rat SN, (PN15) | M: increase in pCREB-ir, KCC2 mRNA. | (Galanopoulou et al. 2003; Galanopoulou 2006) | |
| F: no effect | |||
| Effects of infusions of GABAA-acting drugs in the SNR | Sprague-Dawley rat SN, (PN15–30) | Sex specific changes in the function of SNR-mediated seizure control | (Veliskova and Moshé 2001) |
| Effects of pre- or perinatal exposure to GABAAergic drugs | Rats, mice | Sex and region specific effects on neurosteroid system, behavior, BDNF levels, accessory olfactory bulb, locus coeruleus, susceptibility to PTZ seizures | (Kellogg et al. 2006) (Sobrian and Nandedkar 1986; Pankaj and Brain 1991; Rodriguez-Zafra et al. 1993; Perez-Laso et al. 1994; Kellogg et al. 2000; Cannizzaro et al. 2001; Christensen et al. 2003; Nicosia et al. 2003) |
| Bicuculline effects on somatostatin. | Rats (periventricular median eminence) | M: stimulates somatostatin release (PN5-PN17, PN75) except for transient inhibitory effects at PN40). | (Murray et al. 1999) |
| F: decreases somatostatin, starting at PN25. | |||
M: males; F: females. PTZ: pentyleneterazole. BDNF: brain derived neurotrophic factor.
Table III.
Sex differences in the adult brain, as they relate to the GABAergic system.
| Parameter | Species / Age / brain region | Male vs female | Reference |
|---|---|---|---|
| Rodents | |||
| GAD activity | Rat hypothalamus, substantia nigra | M=F | (Pericic et al. 1986; Manev and Pericic 1987) |
| GAD67 mRNA | Adult Sprague-Dawley rats: Medial amygdaloid nucleus | M>F. | (Searles et al. 2000) |
| GAD65 mRNA | Diagonal Band of Broca: Dorsomedial nucleus: | M>F; M<F. | |
| GABA content | Adult Sprague-Dawley rats: Nucleus accumbens, Medial preoptic area, Ventromedial hypothalamus. | M>F (diestrous) | (Frankfurt et al. 1984) |
| Diagonal Band of Broca, | M>F (diestrous) | ||
| GABA turnover | Adult Sprague Dawley rat hypothalamus | M>F | (Searles et al. 2000) |
| Number of GABA-ir neurons | Adult rat Sprague-Dawley: Bed Nucleus Stria Terminalis, amygdala. | M>F | (Stefanova 1998) |
| GABA transaminase | Rat brain | M<F | (Sherif et al. 1991) |
| Muscimol binding | Adult wood mice: Caudate-putamen, Anterior Hypothalamic Nucleus. | M>F | (Canonaco et al. 1996) |
| Bed Nucleus of the Stria Terminalis, Ventromedial hypothalamic nucleus, Ventral lateral Thalamic nucleus, Pontine Central Gray, SNR. | M<F | ||
| Adult Sprague Dawley rats: Cortex | M>F | (Kokka et al. 1992) | |
| Benzodiazepine (BZ) binding (flunitrazepam) | Adult Wistar rats: Frontal Cortex | Affinity: M>F BZ receptors: M<F | (Farabollini et al. 1996) |
| Picrotoxin or bicuculline-induced seizures or EEG changes | Adult Sprague-Dawley and Wistar rats, cats, CBA/H and swiss mice | Sex and species-specific effects on latencies to seizures, ED50, mortality, EEG findings | (Pericic et al. 1986) (Bujas et al. 1997; Pericic and Bujas 1997; Matejovska et al. 1998; Tan and Tan 2001) |
| Bicuculline induced inhibition of post-gonadectomy rise in LH | Adult rats | M: no effect | (Hood and Schwartz 2000) |
| F: present | |||
| Bicuculline induced inhibition of post-gonadectomy rise in LH | Adult rats | M: no effect | (Hood and Schwartz 2000) |
| F: present | |||
| Pentylenetetrazole-induced seizures | Adult Sprague Dawley rats, Adult Swiss mice | Threshold to seizures: M≤F | (Kokka et al. 1992; Medina et al. 2001) |
| Humans | |||
| GABA levels (Magnetic resonance spectroscopy) | Humans, occipital cortex | M<F | (Sanacora et al. 1999) |
| sDiazepam | Humans | Sex differences in EEG power and coherent activity | (Romano-Torres et al. 2002) |
| Time to recovery from anesthesia | Humans | M>F | (Bajaj et al. 2007) |
| Zolpidem, triazolam effects | Humans | No significant sex difference on sedation, poor recall, impaired psychomotor performance, beta EEG | (Greenblatt et al. 2000) |
M: males; F: females. GAD: glutamic acid decarboxylase.
1.3.1 Sex-specific patterns of GABAAergic signaling in infantile rat SN
Although the SN is not among the classical nuclei involved in reproduction, it has a central role in networks involved in movement and information processing (Deniau et al. 2007; Field and Pellis 2007), functions known to be distinctly different in males and females. The SN is also involved in the pathophysiology of diseases that exhibit age and sex-specific patterns of vulnerability, like Parkinson’s, Tourette syndrome, and epilepsy (Peterson et al. 1992; Moshé 1997; Kotsopoulos et al. 2005; Shulman 2007; Baym et al. 2008). It consists of two main compartments. The SN pars compacta (SNC) contains almost exclusively dopaminergic neurons, which are affected in Parkinson’s disease and also participate in the networks implicated in Tourette’s syndrome. The SN pars reticulata (SNR) consists of predominantly GABAergic neurons and a minority of dopaminergic neurons located in its posterior region (Gonzalez-Hernandez and Rodriguez 2000). It is now widely accepted that the GABAergic SN neurons form an important subcortical center controlling seizure propagation, as demonstrated in a variety of animal models of seizures (Iadarola and Gale 1982; Gonzalez and Hettinger 1984; McNamara et al. 1984; Moshé and Albala 1984) (De Sarro et al. 1984; Turski et al. 1990). Experiments in which intra-SNR infusions of GABAAergic drugs were done, revealed that the function of the SNR in seizure control changes with age (Moshé and Albala 1984; Sperber et al. 1987; Sperber et al. 1989) but is also different between sexes (Veliskova and Moshé 2001). A number of studies have outlined the age- and sex-specific features of SNR neurons, which might relate to its changing role in seizure control. These differences start very early during the embryonic stage, when neurogenesis appears to occur later in male than in female SNR (Galanopoulou et al. 2001) and continue postnatally. The developmental patterns in the expression of gonadal hormone receptors, at least as early as the day of birth in the rat, are sex-specific, suggesting different levels of responsiveness to sex hormones (Ravizza et al. 2002). During early postnatal development, the responsiveness of the SN neurons to gonadal hormones and GABA appears to be different in male and female pups, based on the documented differences in the expression of their receptors (Ravizza et al. 2002; Ravizza et al. 2003).
Given the abundant GABAAergic afferent input on the SN and the important role of depolarizing actions of GABA in neuronal differentiation, our first experiments explored whether the phenotypic differences between the male and female SNR might relate to differences in GABAA receptor signaling. In situ hybridization experiments confirmed that KCC2 mRNA expression increased through development in both sexes, between PN15 (rat infantile stage) and PN30 (rat prepubertal stage) (Galanopoulou et al. 2003), similar to what had previously been reported for other structures (Rivera et al. 1999). However, at any given age, it was always higher in the female than in the male SNR (Galanopoulou et al. 2003). The earlier developmental rise in KCC2 expression in females correlated indeed with important physiological differences. In male PN15 rat SNR neurons, the GABAA receptor agonist muscimol triggered depolarizing currents, increased calcium intracellularly, as well as the expression of the phosphorylated and active form of CREB (pCREB-ir; cAMP responsive element binding protein-immunoreactivity), and the expression of calcium-regulated mRNAs, like KCC2 (Galanopoulou et al. 2003; Galanopoulou 2006) (Figure I). In contrast, muscimol caused hyperpolarizing currents in female PN15 SNR neurons and failed to activate calcium signaling, as assessed by the lack in activation of pCREB or KCC2 transcription (Galanopoulou et al. 2003; Galanopoulou 2006). Muscimol, however, decreased KCC2 mRNA in female PN15 SNR neurons, probably through inhibition of a yet unknown regulatory pathway (Galanopoulou et al. 2003). These sex differences are not just pharmacological effects but can also be triggered by physiologic stimuli, such as synaptic stimulation of GABAAergic synapses. Using synaptic stimulation of GABAergic SNR neurons in the presence of glutamatergic inhibitors, which leave only the GABAAergic synapses operative, the timing of GABAA receptor switch from depolarizing to hyperpolarizing was found to occur earlier in female (PN10) than in male (PN17) SNR (Kyrozis et al. 2006). In terms of ontogenesis, and considering the faster development and shorter life span of rats, this 7-day difference may have enormous repercussions for brain development. Definitely, this is not the beginning of sexual differentiation. However, physiological activities, drugs or experiences that lead to activation of GABAAergic synapses in the infantile SNR may have different translational effects in males and females, influencing calcium-sensitive differentiation.
1.3.2 GABAA receptor signaling as sex-specific modifier of estradiol effects
To further understand the mechanisms underlying the higher expression of KCC2 in the female SNR, we examined the in vivo regulation of KCC2 mRNA by gonadal hormones. As previously stated, the perinatal surge of testosterone in male rats is required for the masculinization of most studied sexually brain structures. Unlike humans, in rats, this is usually through the estrogenic derivatives of testosterone, produced through aromatization, and less often through the androgenic metabolites, like dihydrotestosterone (DHT) (Cooke et al. 1998). To determine whether KCC2 is regulated by gonadal hormones, the effects of systemic administration of testosterone, 17β-estradiol or DHT on KCC2 mRNA expression in PN15 SNR were studied (Galanopoulou and Moshé 2003). Testosterone and DHT increased KCC2 mRNA expression in both male and female PN15 SNR neurons. In contrast, 17β-estradiol decreased KCC2 mRNA in males but not in females. These effects were seen both after short (4 hours) or long periods (52 hours) of exposure to the hormones. However, they occurred only in neurons in which active GABAA-mediated depolarizations were operative (naïve male PN15 SNR neurons). Estradiol failed to downregulate KCC2 in neurons in which GABAA receptors or L-type voltage sensitive calcium channels (L-VSCCs) were blocked (bicuculline or nifedipine pretreated PN15 male rat SNR), and in those that had already hyperpolarizing GABAA signaling (female PN15 SNR neurons). This indicated that 17β-estradiol-mediated downregulation of certain calcium-regulated genes, like KCC2, shows a requirement for active GABAA-mediated activation of L-VSCCs (Galanopoulou and Moshé 2003). In agreement with this model, in vivo administration of 17β-estradiol decreased pCREB-ir in male but not in female PN15 SNR neurons (Galanopoulou 2006). The idea that the effects of estradiol on chloride cotransporters or GABAA signaling may depend upon the direction of GABAA responses is also reverberated in other publications. In hippocampal pyramidal neurons of adult ovariectomized female rats, where GABAA signaling is thought to be hyperpolarizing, 17β-estradiol had no effect on KCC2 expression (Nakamura et al. 2004). In contrast, in cultured neonatal hypothalamic neurons that still respond with muscimol-triggered calcium rises, thought to be due to the depolarizing effects of GABAA receptors, 17β-estradiol delays the period with excitatory GABAA signaling (Perrot-Sinal et al. 2001). However, a direct involvement of KCC2 in this process has not been demonstrated yet. Such findings indicate that GABAA signaling can not only augment the existing sex differences through pathways directly regulated by its own receptors, but can also interact indirectly and modify the effects of important neurotrophic and morphogenetic factors, like estradiol, at least in some neuronal types (Galanopoulou 2005; Galanopoulou 2006). It is possible that perinatal exposure to higher levels of the estrogenic metabolites produced by the testosterone surge in male pups could be one factor that maintains KCC2 expression lower in males. In agreement, daily administration of 17β-estradiol in neonatal female rat pups, during the first 5 days of life, reduces KCC2 mRNA at postnatal day 15. This does not occur if 17β-estradiol is given only during the first 3 days of postnatal life (personal unpublished data).
1.3.3 Applicability to other sexually dimorphic regions
Such signaling differences are not unique to the rat substantia nigra. In the CA1 pyramidal region of the male hippocampus, most prior studies done on male rats or rats of unspecified sex had placed the switch of GABAA signaling at PN14 (Khazipov et al. 2004; Banke and McBain 2006; Tyzio et al. 2007). In our recent work on CA1 pyramidal neurons of the hippocampus of Sprague-Dawley rats, we found that GABAA signaling acquired its hyperpolarizing mode of signaling at PN14 in males only. Female CA1 pyramidal neurons exhibit, however, earlier onset of hyperpolarizing GABAA responses to physiological synaptic stimulation, which are already present by PN4 (Galanopoulou 2008a). This correlates with the higher expression of KCC2 and decreased activity of NKCC1-like, bumetanide-sensitive chloride cotransporters in females (Galanopoulou 2008a). The finding that GABAA inhibition matures earlier in the female than in the male CA1 pyramidal region is also in agreement with earlier reports that in vivo muscimol administration increases pCREB-ir in male but decreases it in female rat hippocampus on the day of their birth (Auger 2003). On the other hand, Tyzio et al reported that at the time of birth, oxytocin triggers the transient appearance of hyperpolarizing GABAA signaling in CA3 pyramidal neurons from rats of unspecified sex, by decreasing the activity of NKCC1 (Tyzio et al. 2006). Direct electrophysiological experiments are therefore warranted for that time period to determine whether sex or regional differences may explain these observations.
In the bed nucleus stria terminalis of PN5 Wistar rats, in vivo diazepam administration increased pCREB-ir and neuronal nitric oxide synthase (nNOS) only in male but not in female pups (Mantelas et al. 2007). This was blocked by L-VSCC inhibitors, implicating depolarizing GABAA signaling in males (Mantelas et al. 2007). No such sex differences in response to diazepam were observed in the anterior or posterior rat PN5 hypothalamus (Mantelas et al. 2007). In a different study, high doses of muscimol, injected in Sprague-Dawley rats on the day of their birth, increased pCREB-ir in the median preoptic and mediobasal hypothalamic nuclei in males but decreased it in females (Auger et al. 2001). The same group of investigators identified differences in KCC2 mRNA (higher in females) and NKCC1 mRNA and protein (higher in males) that could potentially be linked with these pharmacological effects (Perrot-Sinal et al. 2007). However, similar levels of activated NKCC1 (phosphorylated form) was found (Perrot-Sinal et al. 2007).
In support of the sexually differentiating effects of GABAA signaling in the developing brain are a number of studies that confirm that prenatal or early postnatal exposure of the developing brain to GABAAergic drugs may alter the behavior of the offspring in adulthood (Table IV). Further studies are however needed to directly test whether such effects are mediated by sex-specific patterns of GABAA responses in the pertinent brain regions.
Table IV.
Sex-specific effects of perinatal exposure to GABAergic drugs on brain development and behavior.
| Treatment | Endpoint | Male vs female | Reference |
|---|---|---|---|
| Diazepam (DZ), 1.5mg/kg/day SC, E14–20, Wistar rats | Open field activity (OFA) and acoustic startle reflex in adulthood. | M-DZ: reduced total distance traveled, higher rearing frequency. | (Cannizzaro et al. 2001) |
| F-DZ: less time spent in central squares. | |||
| M-DZ and F-DZ: higher acoustic startle amplitude | |||
| DZ 2.5 – 10 mg/kg/day, E14–20, rats. | Dopamine-β-hydroxylase (DBH)-ir and corticotropin releasing factor (CRF) neurons in adult males. | M-DZ: Decreased DBH-ir and CRF neurons in paraventricular nucleus of the hypothalamus. | (Inglefield et al. 1993) |
| 1–2.5 mg/kg/day, E14–20, rats | Elevated plus maze DZ | M-DZ: more time spent in open arms; | (Kellogg et al. 1991) |
| F-DZ: no difference. | |||
| Social interaction in adult rats. | M-DZ: increased in unfamiliar but decreased in familiar environment. | ||
| F-DZ: decreased in unfamiliar environment. | |||
| DZ 10 mg/kg/day prenatally, rats | Acquisition or retention of a simultaneous choice discrimination task in adults. | DZ in 2nd gestational week: No effect. | (Frieder et al. 1984) |
| DZ in 3rd gestational week: impaired in males. | |||
| DZ given E3–18: impaired in both sexes, worse in F. | |||
| DZ 2.5 mg/kg/day prenatally or postnatally. | OFA, continuously reinforced lever-pressing responses in adults. | DZ in 3rd gestational week: no sex differences in adulthood. | (Guillamon et al. 1990) |
| DZ at PN0–16: no sex differences in OFA; impaired level-pressing behavior in M only. | |||
| DZ 1–2.5 mg/kg/day, postnatally, Wistar rats | Maternal behavior. | DZ at PN0–16: Positive effects in both F and M. | (Del Cerro et al. 1995; Segovia et al. 1996) |
| DZ 2.5 mg/kg/day E14–21, Wistar rats | Neonatal reflexes (cliff aversion, forelimb pacing and grasping, bar holding). | Offspring-DZ: delayed appearance of neonatal reflexes. | (Nicosia et al. 2003) |
| DZ prenatal (E14–21) or postnatal (PN0–21) Wistar rats | PTZ seizures (PN50). | Offspring-DZ: M>F | (Nicosia et al. 2003) |
| Prenatal DZ. | Sensitivity of GABAA receptors to Cl-, sensitivity to bicuculline. | Offspring-DZ: Altered sensitivity in adults; in M environmental stressors further alter these effects. | Reviewed in (Kellogg 1999) |
| Prenatal (E15–20) exposure to antiepileptics (AEDs: Phenobarbital 2mg/kg/day, clonazepam 0.15mg/kg/day, valproic acid 20 mg/kg/day), Sprague-Dawley rats. | Spontaneous alternation (PN43–45). | Controls:M: persevered, F: random alternations. | (Sobrian and Nandedkar 1986) |
| AED in utero: M: random alternations, F: persevered. | |||
| Spontaneous activity (PN43–45) | M-clonazepam: hypoactive. | (Sobrian and Nandedkar 1986) | |
| F-phenobarbital/valproic acid: hyperactive. | |||
| Prenatal benzodiazepine(BZ) antagonists: Ro15-1788 (10–20 mg/kg/day) or CGS8216, 2nd half of pregnancy (Swiss Webster albino mice). | Righting reflex (PN1–14); | Righting reflex: prenatal treatments resulted in sporadic days with significant changes. | (Pankaj and Brain 1991) |
| Resident-intruder test | M-Ro: immobility, more attacks. | ||
| (PN21). | F-Ro: more digging, immobility. | ||
| M-CGSP: less grooming. | |||
| F-CGS: less defensive. | |||
| Alprazolam (AL) 0.32 mg/kg/day E18, C57BL/6 mice. | Social play, sleep/wake patterns, aggression at prejuvenile (PN17), juvenile (PN25), adults. | Offspring-AL (PN17): Less desire to escape, isolated behavior, shorter awake periods. | (Christensen et al. 2003) |
| M-AL (juvenile, adult): more aggression on food restriction and cage changing. | |||
M: male; F: female; AED: antiepileptic; AL: alprazolam; BZ: benzodiazepine; CRF: corticotropin releasing factor; DBH: dopamine-β-hydroxylase; DZ: diazepam; E: embryonic day; OFA: open field activity; PN: postnatal day; PTZ: pentyleneterazole; M-DZ or F-DZ: male or female offspring exposed to DZ; M-AL or F-AL: male or female offspring exposed to AL; M-Ro or F-Ro: male or female offspring exposed to Ro15-1788; M-CGS or F-CGS: male or female offspring exposed to CGS8216.
1.4 GABAA receptors as sex-specific modifiers of the consequences of seizures
Depolarizing GABAA signaling is thought to contribute to the enhanced excitability of the immature brain. In certain in vitro models of neonatal seizures, inhibitors of GABAA receptors or of NKCC1 also inhibit or decrease the ictal epileptiform discharges (Khalilov et al. 2003; Khazipov et al. 2004; Dzhala et al. 2005), although model-specific differences have been suggested (Kilb et al. 2007). These raise the possibility that the longer maintenance of depolarizing GABAA signaling in certain brain regions of the developing male brain that participate in seizure networks may be one of the biological factors that render males more susceptible to seizures (Hauser et al. 1993; Kotsopoulos et al. 2005). However, direct proof of this hypothesis has not yet been offered by the available in vivo models of seizure induction, which test the behaviors of seizure networks rather than of individual neuronal structures. This can partly be attributed to the fact that, until recently, males and females were used indifferently in studies involving developing rats. When focusing on individual brain regions, like the SNR, sex differences in their function in seizure control start to become evident. Pharmacological activation of GABAAergic SNR receptors has proconvulsant effects in male but not female PN15 rats (Moshé and Albala 1984; Sperber et al. 1987; Veliskova and Moshé 2001). Multiple factors appear to contribute to these sex specific effects, including the type and level of GABAA receptor expression (Ravizza et al. 2003), hormonal influences (Veliskova and Moshé 2001), and possibly GABAA-driven sexual differentiation (Galanopoulou et al. 2003; Galanopoulou and Moshé 2003; Galanopoulou 2005).
Of special importance is the need to clarify the mechanisms underlying the adverse outcomes of neonatal seizures that include cognitive deficits and epilepsy. In agreement with the human studies, late cognitive and developmental deficits have been demonstrated in a number of animal models of early life seizures (Holmes 1991; de Rogalski Landrot et al. 2001; Lee et al. 2001; Hoffmann et al. 2004; Sayin et al. 2004) (Wasterlain 1977; Hesdorffer et al. 1998; Pisani et al. 2007). Could such adverse effects be at least partially mediated by the disruption of GABAA-sensitive brain development from the neonatal SE? The existence of sex-specific patterns of GABAA signaling in the neonatal hippocampus (Galanopoulou 2008a) presented the opportunity to determine, in neurons with the same chronological age, whether the direction of GABAA signaling influences the effects of neonatal SE. Three episodes of KA-SE, each induced at PN4-6 were utilized to simulate severe seizures. In the absence of neuronal injury in the hippocampus, 3KA-SE reversed the direction of GABAA signaling in both sexes (Galanopoulou 2007; Galanopoulou 2008a). In male pups, 3KA-SE at PN4-6 led to a precocious switch of EGABA to hyperpolarizing values. This was attributed to increased KCC2 expression and decrease in bumetanide-sensitive NKCC1-like activity. On the other hand, in female pups, 3KA-SE led to a transient re-appearance of depolarizing GABAA signaling between PN8–14, associated with an increase in NKCC1-like activity (Galanopoulou 2008a). Considering the rapid rate of brain development, the disruption of GABAA-driven neuronal differentiation in 3KA-SE pups during the second postnatal week may have potentially severe neurodevelopmental consequences. However, there is no evidence yet that 3KA-SE pups develop epilepsy, as no spontaneous seizures were seen in young adulthood (Galanopoulou 2008a). Unlike the findings with neonatal 3KA-SE, repetitive but brief flurothyl-induced seizures (5 per day, PN1-PN5) did not alter the timing of GABAA switch in the CA3 region of the hippocampus of rats of unspecified sex (Isaeva et al. 2006). Although model or sex-specific differences may be involved, these findings corroborate the epidemiological data that indicate that prolonged seizures may have graver outcomes than recurrent brief seizures do (Hesdorffer et al. 1998; Pisani et al. 2007).
Interestingly, in our study, prolonged maternal separation also influenced the timing of GABAA receptor switch. Both male and female pups subjected to prolonged sessions of maternal separation (6 hours daily, between PN4–6) manifested earlier switch of GABAA signaling to hyperpolarizing, in the CA1 pyramidal region (Galanopoulou 2008a). Decreased bumetanide-sensitive NKCC1-like activity was noted in stressed pups, of both sexes, whereas stressed males also showed an increase in KCC2-ir (Galanopoulou 2008a). Duration of maternal separation appears to be important, as short daily periods of separation did not influence GABAA switch (Isaeva et al. 2006). Although the effects of prolonged maternal separation were different than the effects of 3KA-SE, both were mediated by GABAA receptors, as they were not observed in pups pretreated with the GABAA receptor antagonist bicuculline.
1.5 Sex-specific GABAA signaling: clinical implications
How relevant are these findings in the clinical field? Are there certain situations where sex-specific patterns in GABAA signaling may influence the function of the human brain under normal or pathological conditions? The limitations of experimentation in humans have been an obstacle in obtaining direct evidence for or against any such associations. I will discuss therefore various situations where possible links may be worth exploring, as technology advances, as well as the challenges in doing so.
First, which is the developmental stage in humans that may be considered as equivalent to the time when GABAA signaling may still exhibit its immature patterns? The only indirect evidence so far, suggesting that the switch probably occurs around the perinatal period, has been derived from post-mortem analysis of chloride cotransporter expression in the parietal cortices of patients dying from non-neurological causes (Dzhala et al. 2005). This study demonstrated a shift from a NKCC1-dominant state to a KCC2-dominant state around the time of birth (Dzhala et al. 2005).
It is tempting to associate such findings with occasional reports of ictogenic effects of GABAA-acting drugs, like midazolam in low-birth-weight premature neonates (Montenegro et al. 2001; Ng et al. 2002). These are only sporadic reports and, at present, GABAA-acting drugs, including benzodiazepines and barbiturates still remain the first line treatment for neonatal seizures. Even in cases where the reversal potential of GABAA responses is depolarizing, it can still shunt excess excitation, as it occurs during seizures (Staley and Mody 1992). However, GABAA-mediated shunting inhibition is less efficient than GABAA inhibition occurring in more mature neurons with hyperpolarizing GABAA signaling. If indeed depolarizing GABAA signaling also occurs for longer developmental periods in the brain of male neonates, it could contribute to their higher susceptibility to early life seizures (Hauser et al. 1993). Specialized in vivo functional tests of GABAA signaling would be however needed to test these associations.
Are disease syndromes with sex-specific predominance attributable to the reported differences in GABAA signaling? For certain diseases, like Tourette’s syndrome or autism, where clear distinction exists in their prevalence in males versus females, such associations appear meaningful. Autism, for instance, has been proposed as a paradigm of “extreme male brain”, based on psychometric assessments that suggest that the male-type of thinking and functioning, i.e. “systemizing”, are overrepresented over the female-type attributes, i.e. “empathizing” (Baron-Cohen 2002). Perinatal exposure to high levels of testosterone has been proposed to contribute to the exaggeration of the male-type features in autism (Knickmeyer and Baron-Cohen 2006b). Polymorphisms in GABAA receptor subunits have been proposed as susceptibility traits for autism, suggesting that factors that control GABAA signaling may be important in the pathogenesis of the disease (Maddox et al. 1999; Maestrini et al. 1999; Bass et al. 2000; Martin et al. 2000; Menold et al. 2001; Nurmi et al. 2001; Buxbaum et al. 2002; Hellings et al. 2002; McCauley et al. 2004; Muhle et al. 2004; Ma et al. 2005; Collins et al. 2006; Vincent et al. 2006).
Among the epileptic or seizure syndromes linked with mutations of GABAA receptor subunits of chloride cotransporters, we can identify a few with mild male predominance. These include the autosomal dominant epilepsy with febrile seizure plus, febrile seizures, rare cases with severe myoclonic epilepsy of infancy, and epilepsy with grand mal upon awakening (Cossette et al. 2002; Harkin et al. 2002; Kananura et al. 2002; Wallace 2002; Haug et al. 2003; Marini et al. 2003; Dibbens et al. 2004; Macdonald et al. 2004; Audenaert et al. 2006). However, the same mutations have also been linked with syndromes without clear preference towards males or females. The contrast between the easiness in identifying biological sex differences in the experimental literature and the often underwhelming findings of the clinical studies may be because the observational studies are strongly influenced by a combination of factors, including genetic, biological, epigenetic that cannot be as easily isolated as in experimental animals. Awareness though about the diversity of these processes between sexes will allow us to design studies and functional tests sensitive in identifying different sex-specific patterns in the expression, natural course, semiology, and response to treatments of these syndromes.
A direct implication of the finding that GABAAergic signaling is involved in the sexual differentiation of the brain is that GABAA-acting drugs may have sex-specific effects in brain development. In the experimental literature, this has been demonstrated with multiple studies (Table II, Table IV). In humans, this is relatively unexplored and it is therefore important to further evaluate not only the acute but also the late and sex-specific effects of exposure to GABAA-acting drugs that are commonly used for episodic or chronic disorders of early life or pregnancy, to better evaluate their consequences. If the primary goal in our practice and research is to cure epilepsy without side effects, as has been emphasized in the Epilepsy Research Benchmarks set during the “Curing Epilepsy 2007: Translating Discoveries into Therapies” NINDS-funded conference (Bethesda, MD), it will be important to design and implement therapies that take into account not only the developmental stage of the patient but also respect the specific needs of the male and female brain.
ACKNOWLEDGEMENTS
I would like to acknowledge the grant support from NIH NINDS (grant 45243) and the Rett Syndrome Research Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26(19):5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30(8):382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Audenaert D, Schwartz E, Claeys KG, Claes L, Deprez L, Suls A, Van Dyck T, Lagae L, Van Broeckhoven C, Macdonald RL, De Jonghe P. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67(4):687–690. doi: 10.1212/01.wnl.0000230145.73496.a2. [DOI] [PubMed] [Google Scholar]
- Auger AP. Sex differences in the developing brain: crossroads in the phosphorylation of cAMP response element binding protein. J Neuroendocrinol. 2003;15(6):622–627. doi: 10.1046/j.1365-2826.2003.01041.x. [DOI] [PubMed] [Google Scholar]
- Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci U S A. 2001;98(14):8059–8064. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj P, Raiger LK, Jain SD, Kumar S. Women emerge from general anesthesia faster than men. Middle East J Anesthesiol. 2007;19(1):173–183. [PubMed] [Google Scholar]
- Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci. 2006;26(45):11720–11725. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Bass MP, Menold MM, Wolpert CM, Donnelly SL, Ravan SA, Hauser ER, Maddox LO, Vance JM, Abramson RK, Wright HH, Gilbert JR, Cuccaro ML, DeLong GR, Pericak-Vance MA. Genetic studies in autistic disorder and chromosome 15. Neurogenetics. 2000;2(4):219–226. doi: 10.1007/s100489900081. [DOI] [PubMed] [Google Scholar]
- Baym CL, Corbett BA, Wright SB, Bunge SA. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain. 2008;131(Pt 1):165–179. doi: 10.1093/brain/awm278. [DOI] [PubMed] [Google Scholar]
- Becu-Villalobos D, Gonzalez Iglesias A, Diaz-Torga G, Hockl P, Libertun C. Brain sexual differentiation and gonadotropins secretion in the rat. Cell Mol Neurobiol. 1997;17(6):699–715. doi: 10.1023/A:1022542221535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87(4):1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated 'menage a trois'. Trends Neurosci. 1997;20(11):523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Bertelli M, Cecchin S, Lapucci C, de Gemmis P, Danieli D, d'Amore ES, Buttolo L, Giunta F, Mortini P, Pandolfo M. Quantification of chloride channel 2 (CLCN2) gene isoforms in normal versus lesion- and epilepsy-associated brain tissue. Biochim Biophys Acta. 2007;1772(1):15–20. doi: 10.1016/j.bbadis.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hubner CA, Jentsch TJ. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci. 2007;27(24):6581–6589. doi: 10.1523/JNEUROSCI.0338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujas M, Pericic D, Jazvinscak M. Influence of gender and gonadectomy on bicuculline-induced convulsions and on GABAA receptors. Brain Res Bull. 1997;43(4):411–416. doi: 10.1016/s0361-9230(97)00027-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr, Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7(3):311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27(19):5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro C, Martire M, Cannizzaro E, Provenzano G, Gagliano M, Carollo A, Mineo A, Steardo L. Long-lasting handling affects behavioural reactivity in adult rats of both sexes prenatally exposed to diazepam. Brain Res. 2001;904(2):225–233. doi: 10.1016/s0006-8993(01)02462-3. [DOI] [PubMed] [Google Scholar]
- Canonaco M, Tavolaro R, Facciolo RM, Carelli A, Cagnin M, Cristaldi M. Sexual dimorphism of GABAA receptor levels in subcortical brain regions of a woodland rodent (Apodemus sylvaticus) Brain Res Bull. 1996;40(3):187–194. doi: 10.1016/0361-9230(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Rovira C, Gaiarsa JL, Corradetti R, Ben Ari Y. GABA mediated excitation in immature rat CA3 hippocampal neurons. Int J Dev Neurosci. 1990;8(4):481–490. doi: 10.1016/0736-5748(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Christensen HD, Gonzalez CL, Rayburn WF. Effects from prenatal exposure to alprazolam on the social behavior of mice offspring. Am J Obstet Gynecol. 2003;189(5):1452–1457. doi: 10.1067/s0002-9378(03)00756-7. [DOI] [PubMed] [Google Scholar]
- Chudotvorova I, Ivanov A, Rama S, Hubner CA, Pellegrino C, Ben-Ari Y, Medina I. Early expression of KCC2 in rat hippocampal cultures augments expression of functional GABA synapses. J Physiol. 2005;566(Pt 3):671–679. doi: 10.1113/jphysiol.2005.089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7(3):167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19(4):323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31(2):184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- D'Agostino D, Bertelli M, Gallo S, Cecchin S, Albiero E, Garofalo PG, Gambardella A, St Hilaire JM, Kwiecinski H, Andermann E, Pandolfo M. Mutations and polymorphisms of the CLCN2 gene in idiopathic epilepsy. Neurology. 2004;63(8):1500–1502. doi: 10.1212/01.wnl.0000142093.94998.1a. [DOI] [PubMed] [Google Scholar]
- Davis AM, Grattan DR, Selmanoff M, McCarthy MM. Sex differences in glutamic acid decarboxylase mRNA in neonatal rat brain: implications for sexual differentiation. Horm Behav. 1996;30(4):538–552. doi: 10.1006/hbeh.1996.0057. [DOI] [PubMed] [Google Scholar]
- de Rogalski Landrot I, Minokoshi M, Silveira DC, Cha BH, Holmes GL. Recurrent neonatal seizures: relationship of pathology to the electroencephalogram and cognition. Brain Res Dev Brain Res. 2001;129(1):27–38. doi: 10.1016/s0165-3806(01)00177-8. [DOI] [PubMed] [Google Scholar]
- De Sarro G, Meldrum BS, Reavill C. Anticonvulsant action of 2-amino-7-phosphonoheptanoic acid in the substantia nigra. Eur J Pharmacol. 1984;106(1):175–179. doi: 10.1016/0014-2999(84)90692-7. [DOI] [PubMed] [Google Scholar]
- Del Cerro MC, Izquierdo MA, Perez-Laso C, Rodriguez-Zafra M, Guillamon A, Segovia S. Early postnatal diazepam exposure facilitates maternal behavior in virgin female rats. Brain Res Bull. 1995;38(2):143–148. doi: 10.1016/0361-9230(95)00080-x. [DOI] [PubMed] [Google Scholar]
- Delpire E. Cation-Chloride Cotransporters in Neuronal Communication. News Physiol Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 1999;22(2):192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu Rev Physiol. 2002;64:803–843. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: a window to basal ganglia output. Prog Brain Res. 2007;160:151–172. doi: 10.1016/S0079-6123(06)60009-5. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13(13):1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11(11):1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Everett K, Chioza B, Aicardi J, Aschauer H, Brouwer O, Callenbach P, Covanis A, Dooley J, Dulac O, Durner M, Eeg-Olofsson O, Feucht M, Friis M, Guerrini R, Heils A, Kjeldsen M, Nabbout R, Sander T, Wirrell E, McKeigue P, Robinson R, Taske N, Gardiner M. Linkage and mutational analysis of CLCN2 in childhood absence epilepsy. Epilepsy Res. 2007;75(2–3):145–153. doi: 10.1016/j.eplepsyres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Farabollini F, Fluck E, Albonetti ME, File SE. Sex differences in benzodiazepine binding in the frontal cortex and amygdala of the rat 24 hours after restraint stress. Neurosci Lett. 1996;218(3):177–180. doi: 10.1016/s0304-3940(96)13158-x. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Field EF, Pellis SM. The Brain as the Engine of Sex Differences in the Organization of Movement in Rats. Arch Sex Behav. 2007 doi: 10.1007/s10508-007-9270-4. pp. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Fuchs E, Wuttke W. Sex differences in gamma-aminobutyric acid and glutamate concentrations in discrete rat brain nuclei. Neurosci Lett. 1984;50(1–3):245–250. doi: 10.1016/0304-3940(84)90493-2. [DOI] [PubMed] [Google Scholar]
- Frieder B, Epstein S, Grimm VE. The effects of exposure to diazepam during various stages of gestation or during lactation on the development and behavior of rat pups. Psychopharmacology (Berl) 1984;83(1):51–55. doi: 10.1007/BF00427422. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. GABA receptors as broadcasters of sexually differentiating signals in the brain. Epilepsia. 2005;46 Suppl 5:107–112. doi: 10.1111/j.1528-1167.2005.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sex- and cell-type-specific patterns of GABAA receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur J Neurosci. 2006;23(9):2423–2430. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Developmental patterns in the regulation of chloride homeostasis and GABA(A) receptor signaling by seizures. Epilepsia. 2007;48 Suppl 5:14–18. doi: 10.1111/j.1528-1167.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008a;28(7):1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. GABAA receptors in normal development and seizures: friends or foes. Current Neuropharmacology. 2008b;6(1):1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp Neurol. 2003;183(2):628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Liptakova S, Veliskova J, Moshé SL. Sex and regional differences in the time and patterns of neurogenesis of the rat substantia nigra. Epilepsia. 2001;42 Suppl. 7:109. [Google Scholar]
- Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184(2):1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez LP, Hettinger MK. Intranigral muscimol suppresses ethanol withdrawal seizures. Brain Res. 1984;298(1):163–166. doi: 10.1016/0006-8993(84)91162-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Rodriguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol. 2000;421(1):107–135. doi: 10.1002/(sici)1096-9861(20000522)421:1<107::aid-cne7>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Harmatz JS, von Moltke LL, Wright CE, Durol AL, Harrel-Joseph LM, Shader RI. Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: evaluation of sex-dependent differences. J Pharmacol Exp Ther. 2000;293(2):435–443. [PubMed] [Google Scholar]
- Guillamon A, Cales JM, Rodriguez-Zafra M, Perez-Laso C, Caminero A, Izquierdo MA, Segovia S. Effects of perinatal diazepam administration on two sexually dimorphic nonreproductive behaviors. Brain Res Bull. 1990;25(6):913–916. doi: 10.1016/0361-9230(90)90187-5. [DOI] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70(2):530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug K, Warnstedt M, Alekov AK, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H, Heils A. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet. 2003;33(4):527–532. doi: 10.1038/ng1121. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Hellings JA, Hossain S, Martin JK, Baratang RR. Psychopathology, GABA, and the Rubinstein-Taybi syndrome: a review and case study. Am J Med Genet. 2002;114(2):190–195. doi: 10.1002/ajmg.10156. [DOI] [PubMed] [Google Scholar]
- Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl-/HCO3- exchanger AE3 display a reduced seizure threshold. Mol Cell Biol. 2006;26(1):182–191. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol. 1998;44(6):908–912. doi: 10.1002/ana.410440609. [DOI] [PubMed] [Google Scholar]
- Hoffmann AF, Zhao Q, Holmes GL. Cognitive impairment following status epilepticus and recurrent seizures during early development: support for the "two-hit hypothesis". Epilepsy Behav. 2004;5(6):873–877. doi: 10.1016/j.yebeh.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Holmes GL. The long-term effects of seizures on the developing brain: clinical and laboratory issues. Brain Dev. 1991;13(6):393–409. doi: 10.1016/s0387-7604(12)80037-4. [DOI] [PubMed] [Google Scholar]
- Hood SC, Schwartz NB. Sex difference in serum luteinizing hormone postgonadectomy in the rat: role of gamma-aminobutyric acid-ergic inhibition. Endocrine. 2000;12(1):35–40. doi: 10.1385/ENDO:12:1:35. [DOI] [PubMed] [Google Scholar]
- Howard HC, Mount DB, Rochefort D, Byun N, Dupre N, Lu J, Fan X, Song L, Riviere JB, Prevost C, Horst J, Simonati A, Lemcke B, Welch R, England R, Zhan FQ, Mercado A, Siesser WB, George AL, Jr, McDonald MP, Bouchard JP, Mathieu J, Delpire E, Rouleau GA. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet. 2002;32(3):384–392. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30(2):515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218(4578):1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Inglefield JR, Bitran D, Olschowka JA, Kellogg CK. Selective effects on CRF neurons and catecholamine terminals in two stress-responsive regions of adult rat brain after prenatal exposure to diazepam. Brain Res Bull. 1993;31(3–4):353–359. doi: 10.1016/0361-9230(93)90227-3. [DOI] [PubMed] [Google Scholar]
- Isaeva E, Isaev D, Khazipov R, Holmes GL. Selective impairment of GABAergic synaptic transmission in the flurothyl model of neonatal seizures. Eur J Neurosci. 2006;23(6):1559–1566. doi: 10.1111/j.1460-9568.2006.04693.x. [DOI] [PubMed] [Google Scholar]
- Kananura C, Haug K, Sander T, Runge U, Gu W, Hallmann K, Rebstock J, Heils A, Steinlein OK. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch Neurol. 2002;59(7):1137–1141. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- Kandler K, Kullmann PH, Ene FA, Kim G. Excitatory action of an immature glycinergic/GABAergic sound localization pathway. Physiol Behav. 2002;77(4–5):583–587. doi: 10.1016/s0031-9384(02)00905-8. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Kenjarski TP, Pleger GL, Frye CA. Region-, age-, and sex-specific effects of fetal diazepam exposure on the postnatal development of neurosteroids. Brain Res. 2006;1067(1):115–125. doi: 10.1016/j.brainres.2005.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg CK, Yao J, Pleger GL. Sex-specific effects of in utero manipulation of GABA(A) receptors on pre- and postnatal expression of BDNF in rats. Brain Res Dev Brain Res. 2000;121(2):157–167. doi: 10.1016/s0165-3806(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Kellogg CK. Sex differences in long-term consequences of prenatal diazepam exposure: possible underlying mechanisms. Pharmacol Biochem Behav. 1999;64(4):673–680. doi: 10.1016/s0091-3057(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Primus RJ, Bitran D. Sexually dimorphic influence of prenatal exposure to diazepam on behavioral responses to environmental challenge and on gamma-aminobutyric acid (GABA)-stimulated chloride uptake in the brain. J Pharmacol Exp Ther. 1991;256(1):259–265. [PubMed] [Google Scholar]
- Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6(10):1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19(3):590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Kilb W, Sinning A, Luhmann HJ. Model-specific effects of bumetanide on epileptiform activity in the in-vitro intact hippocampus of the newborn mouse. Neuropharmacology. 2007;53(4):524–533. doi: 10.1016/j.neuropharm.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Wang Y, Sun D. Role of membrane ion transport proteins in cerebral ischemic damage. Front Biosci. 2007;12:762–770. doi: 10.2741/2099. [DOI] [PubMed] [Google Scholar]
- Knickmeyer CR, Baron-Cohen S. Fetal testosterone and sex differences. Early Hum Dev. 2006a;82(12):755–760. doi: 10.1016/j.earlhumdev.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol. 2006b;21(10):825–845. doi: 10.1177/08830738060210101601. [DOI] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Witte U, Olsen RW. Sex differences in sensitivity to pentylenetetrazol but not in GABAA receptor binding. Pharmacol Biochem Behav. 1992;43(2):441–447. doi: 10.1016/0091-3057(92)90174-e. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos I, de Krom M, Kessels F, Lodder J, Troost J, Twellaar M, van Merode T, Knottnerus A. Incidence of epilepsy and predictive factors of epileptic and non-epileptic seizures. Seizure. 2005;14(3):175–182. doi: 10.1016/j.seizure.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Chudomel O, Moshé SL, Galanopoulou AS. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci Lett. 2006;398(1–2):1–5. doi: 10.1016/j.neulet.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Spatial learning deficits without hippocampal neuronal loss in a model of early-onset epilepsy. Neuroscience. 2001;107(1):71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77(3):377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Gallagher MJ, Feng HJ, Kang J. GABA(A) receptor epilepsy mutations. Biochem Pharmacol. 2004;68(8):1497–1506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Maddox LO, Menold MM, Bass MP, Rogala AR, Pericak-Vance MA, Vance JM, Gilbert JR. Autistic disorder and chromosome 15q11–q13: construction and analysis of a BAC/PAC contig. Genomics. 1999;62(3):325–331. doi: 10.1006/geno.1999.6017. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Lai C, Marlow A, Matthews N, Wallace S, Bailey A, Cook EH, Weeks DE, Monaco AP. Serotonin transporter (5-HTT) and gamma-aminobutyric acid receptor subunit beta3 (GABRB3) gene polymorphisms are not associated with autism in the IMGSA families. The International Molecular Genetic Study of Autism Consortium. Am J Med Genet. 1999;88(5):492–496. doi: 10.1002/(sici)1096-8628(19991015)88:5<492::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Manev H, Pericic D. Sex difference in the turnover of GABA in the rat substantia nigra. J Neural Transm. 1987;70(3–4):321–328. doi: 10.1007/BF01253606. [DOI] [PubMed] [Google Scholar]
- Mantelas A, Stamatakis A, Fameli M, Stylianopoulou F. Sex differences in the control of neuronal nitric oxide synthase by GABA-A receptors in the developing rat diencephalon. Brain Res. 2007;1149:38–49. doi: 10.1016/j.brainres.2007.02.075. [DOI] [PubMed] [Google Scholar]
- Marini C, Harkin LA, Wallace RH, Mulley JC, Scheffer IE, Berkovic SF. Childhood absence epilepsy and febrile seizures: a family with a GABA(A) receptor mutation. Brain. 2003;126(Pt 1):230–240. doi: 10.1093/brain/awg018. [DOI] [PubMed] [Google Scholar]
- Martin ER, Menold MM, Wolpert CM, Bass MP, Donnelly SL, Ravan SA, Zimmerman A, Gilbert JR, Vance JM, Maddox LO, Wright HH, Abramson RK, DeLong GR, Cuccaro ML, Pericak-Vance MA. Analysis of linkage disequilibrium in gamma-aminobutyric acid receptor subunit genes in autistic disorder. Am J Med Genet. 2000;96(1):43–48. doi: 10.1002/(sici)1096-8628(20000207)96:1<43::aid-ajmg9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Matejovska I, Veliskova J, Velisek L. Bicuculline-induced rhythmic EEG episodes: gender differences and the effects of ethosuximide and baclofen treatment. Epilepsia. 1998;39(12):1243–1252. doi: 10.1111/j.1528-1157.1998.tb01321.x. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, Jacobs MM, Folstein SE, Haines JL, Sutcliffe JS. A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. Am J Med Genet B Neuropsychiatr Genet. 2004;131(1):51–59. doi: 10.1002/ajmg.b.30038. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Galloway MT, Rigsbee LC, Shin C. Evidence implicating substantia nigra in regulation of kindled seizure threshold. J Neurosci. 1984;4(9):2410–2417. doi: 10.1523/JNEUROSCI.04-09-02410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Manhaes AC, Schmidt SL. Sex differences in sensitivity to seizures elicited by pentylenetetrazol in mice. Pharmacol Biochem Behav. 2001;68(3):591–596. doi: 10.1016/s0091-3057(01)00466-x. [DOI] [PubMed] [Google Scholar]
- Menold MM, Shao Y, Wolpert CM, Donnelly SL, Raiford KL, Martin ER, Ravan SA, Abramson RK, Wright HH, Delong GR, Cuccaro ML, Pericak-Vance MA, Gilbert JR. Association analysis of chromosome 15 gabaa receptor subunit genes in autistic disorder. J Neurogenet. 2001;15(3–4):245–259. doi: 10.3109/01677060109167380. [DOI] [PubMed] [Google Scholar]
- Meyer J, Johannssen K, Freitag CM, Schraut K, Teuber I, Hahner A, Mainhardt C, Mossner R, Volz HP, Wienker TF, McKeane D, Stephan DA, Rouleau G, Reif A, Lesch KP. Rare variants of the gene encoding the potassium chloride co-transporter 3 are associated with bipolar disorder. Int J Neuropsychopharmacol. 2005;8(4):495–504. doi: 10.1017/S1461145705005821. [DOI] [PubMed] [Google Scholar]
- Mikawa S, Wang C, Shu F, Wang T, Fukuda A, Sato K. Developmental changes in KCC1, KCC2 and NKCC1 mRNAs in the rat cerebellum. Brain Res Dev Brain Res. 2002;136(2):93–100. doi: 10.1016/s0165-3806(02)00345-0. [DOI] [PubMed] [Google Scholar]
- Montenegro MA, Guerreiro MM, Caldas JP, Moura-Ribeiro MV, Guerreiro CA. Epileptic manifestations induced by midazolam in the neonatal period. Arq Neuropsiquiatr. 2001;59(2A):242–243. doi: 10.1590/s0004-282x2001000200018. [DOI] [PubMed] [Google Scholar]
- Moshé SL. Sex and the substantia nigra: administration, teaching, patient care, and research. J Clin Neurophysiol. 1997;14(6):484–494. doi: 10.1097/00004691-199711000-00004. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Albala BJ. Nigral muscimol infusions facilitate the development of seizures in immature rats. Brain Res. 1984;315(2):305–308. doi: 10.1016/0165-3806(84)90165-2. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Murray HE, Rantle CM, Simonian SX, DonCarlos LL, Herbison AE, Gillies GE. Sexually dimorphic ontogeny of GABAergic influences on periventricular somatostatin neurons. Neuroendocrinology. 1999;70(6):384–391. doi: 10.1159/000054500. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, Rosell DR, Akama KT, McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80(5):308–323. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- Ng E, Klinger G, Shah V, Taddio A. Safety of benzodiazepines in newborns. Ann Pharmacother. 2002;36(7–8):1150–1155. doi: 10.1345/aph.1A328. [DOI] [PubMed] [Google Scholar]
- Nicolet-Barousse L, Blanchard A, Roux C, Pietri L, Bloch-Faure M, Kolta S, Chappard C, Geoffroy V, Morieux C, Jeunemaitre X, Shull GE, Meneton P, Paillard M, Houillier P, De Vernejoul MC. Inactivation of the Na-Cl co-transporter (NCC) gene is associated with high BMD through both renal and bone mechanisms: analysis of patients with Gitelman syndrome and Ncc null mice. J Bone Miner Res. 2005;20(5):799–808. doi: 10.1359/JBMR.041238. [DOI] [PubMed] [Google Scholar]
- Nicosia A, Giardina L, Di Leo F, Medico M, Mazzola C, Genazzani AA, Drago F. Long-lasting behavioral changes induced by pre- or neonatal exposure to diazepam in rats. Eur J Pharmacol. 2003;469(1–3):103–109. doi: 10.1016/s0014-2999(03)01729-1. [DOI] [PubMed] [Google Scholar]
- Niemeyer MI, Yusef YR, Cornejo I, Flores CA, Sepulveda FV, Cid LP. Functional evaluation of human ClC-2 chloride channel mutations associated with idiopathic generalized epilepsies. Physiol Genomics. 2004;19(1):74–83. doi: 10.1152/physiolgenomics.00070.2004. [DOI] [PubMed] [Google Scholar]
- Nurmi EL, Bradford Y, Chen Y, Hall J, Arnone B, Gardiner MB, Hutcheson HB, Gilbert JR, Pericak-Vance MA, Copeland-Yates SA, Michaelis RC, Wassink TH, Santangelo SL, Sheffield VC, Piven J, Folstein SE, Haines JL, Sutcliffe JS. Linkage disequilibrium at the Angelman syndrome gene UBE3A in autism families. Genomics. 2001;77(1–2):105–113. doi: 10.1006/geno.2001.6617. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Eusterschulte B, Zienecker R, Reisert I, Pilgrim C. Sex differences in densities of dopaminergic fibers and GABAergic neurons in the prenatal rat striatum. J Comp Neurol. 1992;323(2):299–304. doi: 10.1002/cne.903230212. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16(20):6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankaj V, Brain PF. Effects of prenatal exposure to benzodiazepine-related drugs on early development and adult social behaviour in Swiss mice--II. Antagonists. Gen Pharmacol. 1991;22(1):43–51. doi: 10.1016/0306-3623(91)90307-r. [DOI] [PubMed] [Google Scholar]
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem. 1996;271(27):16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- Perez-Laso C, Valencia A, Rodriguez-Zafra M, Cales JM, Guillamon A, Segovia S. Perinatal administration of diazepam alters sexual dimorphism in the rat accessory olfactory bulb. Brain Res. 1994;634(1):1–6. doi: 10.1016/0006-8993(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Pericic D, Bujas M. Sex differences in the response to GABA antagonists depend on the route of drug administration. Exp Brain Res. 1997;115(1):187–190. doi: 10.1007/pl00005681. [DOI] [PubMed] [Google Scholar]
- Pericic D, Manev H, Geber J. Sex related differences in the response of mice, rats and cats to administration of picrotoxin. Life Sci. 1986;38(10):905–913. doi: 10.1016/0024-3205(86)90258-4. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JP, McCarthy MM. Estradiol enhances excitatory gamma-aminobutyric [corrected] acid-mediated calcium signaling in neonatal hypothalamic neurons. Endocrinology. 2001;142(6):2238–2243. doi: 10.1210/endo.142.6.8180. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM. Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol. 2007;19(4):302–308. doi: 10.1111/j.1365-2826.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Scahill L, Naftolin F, Keefe D, Charest NJ, Cohen DJ. Steroid hormones and CNS sexual dimorphisms modulate symptom expression in Tourette's syndrome. Psychoneuroendocrinology. 1992;17(6):553–563. doi: 10.1016/0306-4530(92)90015-y. [DOI] [PubMed] [Google Scholar]
- Pisani F, Cerminara C, Fusco C, Sisti L. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology. 2007;69(23):2177–2185. doi: 10.1212/01.wnl.0000295674.34193.9e. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33(6):781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Friedman LK, Moshé SL, Veliskova J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 2003;21(5):245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Galanopoulou AS, Veliskova J, Moshé SL. Sex differences in androgen and estrogen receptor expression in rat substantia nigra during development: an immunohistochemical study. Neuroscience. 2002;115(3):685–696. doi: 10.1016/s0306-4522(02)00491-8. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Kyrozis A, Wang J, MacDermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994;476(3):411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Brustein E, Liao M, Mercado A, Babilonia E, Mount DB, Drapeau P. Neurogenic role of the depolarizing chloride gradient revealed by global overexpression of KCC2 from the onset of development. J Neurosci. 2008;28(7):1588–1597. doi: 10.1523/JNEUROSCI.3791-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Zafra M, De Blas MR, Perez-Laso C, Cales JM, Guillamon A, Segovia S. Effects of perinatal diazepam exposure on the sexually dimorphic rat locus coeruleus. Neurotoxicol Teratol. 1993;15(2):139–144. doi: 10.1016/0892-0362(93)90072-v. [DOI] [PubMed] [Google Scholar]
- Romano-Torres M, Borja-Lascurain E, Chao-Rebolledo C, del-Rio-Portilla Y, Corsi-Cabrera M. Effect of diazepam on EEG power and coherent activity: sex differences. Psychoneuroendocrinology. 2002;27(7):821–833. doi: 10.1016/s0306-4530(01)00082-8. [DOI] [PubMed] [Google Scholar]
- Romo-Parra H, Trevino M, Heinemann U, Gutierrez R. GABA actions in hippocampal area CA3 during postnatal development: differential shift from depolarizing to hyperpolarizing in somatic and dendritic compartments. J Neurophysiol. 2008;99(3):1523–1534. doi: 10.1152/jn.01074.2007. [DOI] [PubMed] [Google Scholar]
- Salin-Cantegrel A, Riviere JB, Dupre N, Charron FM, Shekarabi M, Karemera L, Gaspar C, Horst J, Tekin M, Deda G, Krause A, Lippert MM, Willemsen MA, Jarrar R, Lapointe JY, Rouleau GA. Distal truncation of KCC3 in non-French Canadian HMSN/ACC families. Neurology. 2007;69(13):1350–1355. doi: 10.1212/01.wnl.0000291779.35643.15. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56(11):1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sander T, Toliat MR, Heils A, Leschik G, Becker C, Ruschendorf F, Rohde K, Mundlos S, Nurnberg P. Association of the 867Asp variant of the human anion exchanger 3 gene with common subtypes of idiopathic generalized epilepsy. Epilepsy Res. 2002;51(3):249–255. doi: 10.1016/s0920-1211(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45(12):1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- Searles RV, Yoo MJ, He JR, Shen WB, Selmanoff M. Sex differences in GABA turnover and glutamic acid decarboxylase (GAD(65) and GAD(67)) mRNA in the rat hypothalamus. Brain Res. 2000;878(1–2):11–19. doi: 10.1016/s0006-8993(00)02648-2. [DOI] [PubMed] [Google Scholar]
- Segovia S, del Cerro MC, Ortega E, Perez-Laso C, Rodriguez-Zafra C, Izquierdo MA, Guillamon A. Role of GABAA receptors in the organization of brain and behavioural sex differences. Neuroreport. 1996;7(15–17):2553–2557. doi: 10.1097/00001756-199611040-00030. [DOI] [PubMed] [Google Scholar]
- Segovia S, Guillamon A. Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors. Brain Res Brain Res Rev. 1993;18(1):51–74. doi: 10.1016/0165-0173(93)90007-m. [DOI] [PubMed] [Google Scholar]
- Sherif F, Eriksson L, Oreland L. GABA-transaminase activity in rat and human brain: regional, age and sex-related differences. J Neural Transm Gen Sect. 1991;84(1–2):95–102. doi: 10.1007/BF01249113. [DOI] [PubMed] [Google Scholar]
- Shulman LM. Gender differences in Parkinson's disease. Gend Med. 2007;4(1):8–18. doi: 10.1016/s1550-8579(07)80003-9. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Nandedkar AK. Prenatal antiepileptic drug exposure alters seizure susceptibility in rats. Pharmacol Biochem Behav. 1986;24(5):1383–1391. doi: 10.1016/0091-3057(86)90199-1. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Wong BY, Wurpel JN, Moshé SL. Nigral infusions of muscimol or bicuculline facilitate seizures in developing rats. Brain Res. 1987;465(1–2):243–250. doi: 10.1016/0165-3806(87)90245-8. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Wurpel JN, Zhao DY, Moshé SL. Evidence for the involvement of nigral GABAA receptors in seizures of adult rats. Brain Res. 1989;480(1–2):378–382. doi: 10.1016/0006-8993(89)90211-4. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68(1):197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Stefanova N. Gamma-aminobutyric acid-immunoreactive neurons in the amygdala of the rat - sex differences and effect of early postnatal castration. Neurosci Lett. 1998;255(3):175–177. doi: 10.1016/s0304-3940(98)00735-6. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20(20):7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Tan U. Sex difference in susceptibility to epileptic seizures in rats: importance of estrous cycle. Int J Neurosci. 2001;108(3–4):175–191. doi: 10.3109/00207450108986513. [DOI] [PubMed] [Google Scholar]
- Turski L, Andrews JS, Loschmann PA, Bressler K, Bortolotto ZA, Calderazzo-Filho LS, Cavalheiro EA. Substantia nigra regulates action of antiepileptic drugs. Brain Res. 1990;520(1–2):232–239. doi: 10.1016/0006-8993(90)91710-x. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314(5806):1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. 2007;48 Suppl 5:96–105. doi: 10.1111/j.1528-1167.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Moshé SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50(5):596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Vincent JB, Horike SI, Choufani S, Paterson AD, Roberts W, Szatmari P, Weksberg R, Fernandez B, Scherer SW. An inversion inv(4)(p12–p15.3) in autistic siblings implicates the 4p GABA receptor gene cluster. J Med Genet. 2006;43(5):429–434. doi: 10.1136/jmg.2005.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. Mutations in GABA-receptor genes cause human epilepsy. Lancet Neurol. 2002;1(4):212. doi: 10.1016/s1474-4422(02)00098-4. [DOI] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139(1):59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG. Effects of neonatal seizures on ontogeny of reflexes and behavior. An experimental study in the rat. Eur Neurol. 1977;15(1):9–19. doi: 10.1159/000114783. [DOI] [PubMed] [Google Scholar]