Abstract
The physiology of microvessels limits the growth and development of tumours. Tumours gain nutrients and excrete waste through growth-associated microvessels. New anticancer therapies target this microvasculature by inhibiting vascular endothelial growth factor A (VEGF-A) splice isoforms that promote microvessel growth. However, certain VEGF-A splice isoforms in normal tissues inhibit growth of microvessels. Thus, it is the VEGF-A isoform balance, which is controlled by mRNA splicing, that orchestrates angiogenesis. Here, we highlight the functional differences between the pro-angiogenic and the anti-angiogenic VEGF-A isoform families and the potential to harness the synthetic capacity of cancer cells to produce factors that inhibit, rather than aid, cancer growth.
The growth and progression of tumours, in line with that of all expanding cellular structures such as the placenta and the developing embryo, depends on a proliferating vasculature ensuring adequate supply of nutrients and efficient removal of waste products. The advent of anti-angiogenic therapies such as sorafenib1, sunitinib2 and bevacizumab3,4 stems from a huge leap in our mechanistic understanding of the initiation, development, refinement and maintenance of new vessels and microvessels. This in turn originates from the discovery in the 1980s by Ferrara5, Senger6 and Keck7 of the principal player in angiogenesis, vascular endothelial growth factor A (VEGF-A, also referred to as VEGF). VEGF-A exists in multiple isoforms of variable exon content and strikingly contrasting properties and expression patterns. This range of products from the 8-exon VEGF-A gene on chromosome 6 renders VEGF-A biology complex (FIG. 1), and alterations in isoform expression in cancer may be instructive for other genes involved in malignant change in general8 and in the pro-angiogenic cascade in particular. Indeed, the products of VEGF-A, rather than just being targets for inhibition, may hold the key to impeding tumour growth and act as a model for controlling the qualitative expression of other malignancy-associated genes.
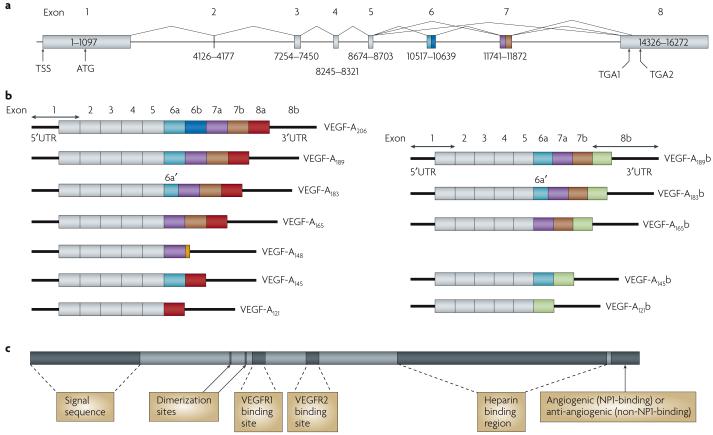
Figure 1. Protein and mRNA products of human vascular endothelial growth factor A (VEGF-A).
a | Gene structure of human VEGF-A. VEGF-A spans 16,272 bp of chromosome 6p12 and consists of eight exons. Alternate 5′ and 3′ splice site selection in exons 6, 7 and 8 generate multiple isoforms. Exons 6 and 7 encode heparin binding domains. The transcriptional start site (TSS) and translational start site (ATG) in exon 1 are indicated. Alternative stop codons within exon 8 are also indicated (TGA1 and TGA2). b | Alternative splicing can occur either at the 5′ donor splice site (for example, VEGF-A189 versus VEGF-A206) or the 3′ acceptor splice site (for example, VEGF-A189 versus VEGF-A165). Two mRNA isoform families are generated. The pro-angiogenic isoforms (VEGF-Axxx, left) are generated by proximal splice site (PSS) selection in exon 8 and the anti-angiogenic family (VEGF-Axxxb, right) from exon 8 distal splice site (DSS) choice. Thus, VEGF-A165, formed by PSS selection in exon 8, has VEGF-A165b as its DSS sister isoform11, the DSS-selected mRNA encoding a protein of exactly the same length. Exon 6a’ occurs in VEGF-A183 as a result of a conserved alternative splicing donor site in exon 6a and is 18 bp shorter than full-length exon 6a. VEGF-A148 is a truncated isoform splicing from exon 7a into exon 8a out of frame and resulting in a premature stop codon71. VEGF-A206b has not yet been identified. c | Protein structure of VEGF-A containing the dimerization sites and binding sites for heparin, VEGF-A receptor 1 (VEGFR1; encoded by exon 3) and VEGFR2 (encoded by exon 4), which are present in all isoforms. The six amino acids at the extreme carboxyl terminus of the protein can be either pro-angiogenic (CDKPRR, encoded by exon 8a) or anti-angiogenic (SLTRKD, encoded by exon 8b). The epitopes recognized by most commercial antibodies are in the region of the VEGF-A receptor-binding domains, present in VEGF-A isoforms of both families. UTR, untranslated region.
In tumours, and most other angiogenic situations, new vessel development is primarily dependent on this 46 kDa glycoprotein acting on its endothelial cell receptors VEGF receptor 1 (VEGFR1), VEGFR2 and the co-receptor neuropilin 1. This view is supported by the finding that even heterozygous Vegfa knockouts are embryonically lethal9. The first VEGF-A isoform described, VEGF-A165 (REF. 5), has been extensively investigated for its function, signalling, expression and roles in cancer10. Other isoforms including VEGF-A121, VEGF-A145, VEGF-A148, VEGF-A183, VEGF-A189 and VEGF-A206, identified between 1989 and 2003, are generated by alternative splicing of exons 6 and 7, which code for motifs that bind to the highly negatively charged glycosaminoglycan carbohydrate heparin and similar molecules. In 2002, an additional isoform was identified11: VEGF-A165b, which is generated by exon 8 distal splice site (DSS) selection. This DSS choice can also occur in conjunction with exon 6 and 7 inclusion or exclusion. It therefore became apparent that VEGF-A mRNA splicing generates two families of proteins that differ by their C’ terminal six amino acids (FIG. 1), and these are termed VEGF-Axxx (pro-angiogenic) and VEGF-Axxxb (anti-angiogenic)12, xxx denoting the amino acid number of the mature protein.
Details of the molecular control of C’ terminal splice site choice (and the pro-angiogenic-anti-angiogenic balance) are emerging13 (FIG. 2). Upstream factors governing VEGF-A expression include hypoxia, cytokines, sex hormones, chemokines and growth factors (reviewed in REFS 10,14), although most studies have assessed VEGF-A expression using agents that would not distinguish between the two VEGF-A families. Subsequent downstream VEGF-A signalling of the conventional pro-angiogenic VEGF-Axxx isoforms has been identified (reviewed in REFS 15,16) (FIG. 3a). Alterations in these pathways have not been identified in as much detail for the VEGF-Axxxb family (FIG. 3b).
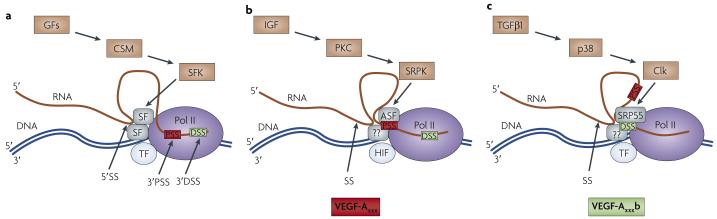
Figure 2. Vascular endothelial growth factor A (VEGF-A) C’ terminal splicing regulation.
a | The C’ terminal domain of RNA polymerase II (Pol II) interacts with both transcription factors (TFs) and splicing factors (SFs). SFs are recruited to the transcriptional machinery owing to their interaction with Pol II72,73. These SFs recognize cis-acting RNA splicing sequences in the pre-mRNA and both splicing sites (SS) — 5′ donor (5′SS) or 3′ acceptor sites — can be recognized. Both 3′ proximal SS (3′PSS) and distal SS (3′DSS) are indicated. The particular splicing factors recruited are dependent on the sequence. These SFs can be regulated by SF kinases (SFKs), which are regulated by cell signalling molecules (CSMs) downstream of growth factors (GFs). b | Regulation of VEGF-A C’ terminal PSS selection by insulin-like growth factor (IGF). IGF activates protein kinase C (PKC), which results in phosphorylation of SR protein kinases (SRPKs). These can activate the ASF-SF2 splicing factor, which favours PSS selection. This process may be dependent on the presence of hypoxia-inducible factor (HIF), a transcription factor involved in VEGF-Axxx upregulation13. Other SFs and kinases may also be involved in PSS and DSS selection, denoted by ‘??’. c | Factors affecting VEGF-A C’ terminal DSS selection. Transforming growth factor β1 (TGFβ1) results in p38 mitogen-activated protein kinase activation and subsequent activation of the kinases CLK1 and CLK4. CLK1 and CLK4 phosphorylate the splicing factor SRP55, resulting in DSS selection and production of VEGF-Axxxb13. It is also possible that ASF-SF2 is inactivated by CLK1 and CLK4, or that phosphorylation of the SFs could change their location, degradation or binding affinity. This scheme summarizes the limited data available.
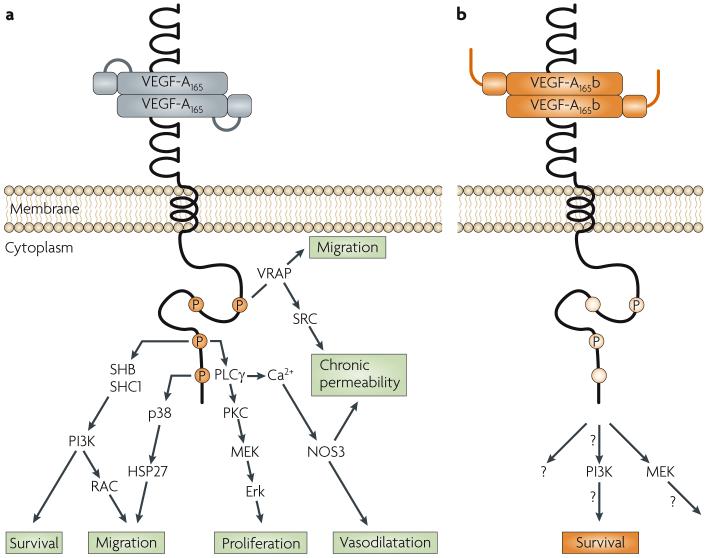
Figure 3. Signalling pathways downstream of vascular endothelial growth factor (VEGF-A)xxx and VEGF-Axxxb.
a | The VEGF-Axxx-mediated angiogenic response acts primarily through VEGF receptor 2 (VEGFR2) to initiate multiple downstream pathways15,16. b | VEGF-A165b results in transient, weak phosphorylation and the downstream signalling (denoted ‘?’) from such qualitatively different phosphorylation is largely unknown (see REF. 25 for details). Erk, extracellular signal-regulated kinase; HSP27, heat shock protein 27; NOS3, endothelial nitric oxide synthase; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; RAC, Ras-related C3 botulinum toxin substrate; PLCγ, phospholipase Cγ; SHB, SH2 domain-containing adaptor protein B; SHC1, SH2 domain-containing transforming protein 1; VRAP, VEGF receptor-associated protein.
In this article we consider the significant functional differences between the isoform families and the progress made in determining the mechanistic differences between them.
Expression of VEGF-Axxxb isoforms
Increased expression of VEGF-A appears to be a characteristic in several pathologies, including cancer, arthritis and cardiovascular disease, but it is upregulated from a basal level in normal tissues. The development of antibodies and probes that specifically detect VEGF-Axxxb isoforms by enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, western blotting and quantitative PCR has revealed that basal expression is dominated by VEGF-Axxxb isoforms in many tissues17. In human vitreous fluid, circulating plasma, urine, renal cortex, colonic epithelium, bladder smooth muscle, lung and pancreatic islets, VEGF-Axxxb isoforms constitute more than or close to half of the total VEGF-A expressed12,17,18. To date, the placenta, in which angiogenesis is known to occur, is the only normal tissue identified to have VEGF-Axxxb constituting significantly less than half its total VEGF-A17.
In primary cultured cells, such as differentiated visceral glomerular epithelial cells (podocytes), retinal pigmented epithelial cells and colonic epithelial cells, VEGF-Axxxb isoforms predominate13,19,20. However, in melanoma, colorectal carcinoma and bladder cancer cells as well as proliferating dedifferentiated podocytes, VEGF-Axxx isoforms comprise the majority of VEGF-A19-21.
VEGF-Axxxb structure and properties
Receptor binding, downstream signalling and pharmacology
VEGF-A165b differs from VEGF-A165 only in the carboxy-terminal six amino acids, a change from CDKPRR to SLTRKD11. The unique C’-terminal six amino acids encoded by exon 8b endow VEGF-A165b (and other VEGF-Axxxb isoforms) with radically different properties to those of VEGF-A165. The key residue alterations are the loss of the cysteine and the replacement of the highly positively charged arginines present in VEGF-A165 with neutral lysine-aspartic acid in VEGF-A165b22. These differences have profound implications for structure (FIG. 4), receptor interaction (FIG. 5) and function (as discussed below). VEGF-A165 binding to VEGFR2 and neuropilin 1 induces a conformational change in VEGFR2 (REF. 23), which is thought to be similar to that of the ERBB2 receptor24, resulting in internal rotation of the intracellular domain. VEGF-A165 binding, after resulting in dimerization of the receptor, leads to re-positioning of the kinase domain by rotation to the inside of the dimer, and hence induces tyrosine autophosphorylation. By contrast, VEGF-A165b is predicted not to have this full rotational effect and so autophosphorylation is not efficient (FIG. 5). Recent experiments from L. Claessen-Welsh’s group in Uppsala, Sweden support this concept, showing that VEGFR2 is actively phosphorylated by VEGF-A165b binding but on different tyrosine residues, suggesting it may not simply be an inactive competitive inhibitor25. Phospho-peptide mapping and site-specific phospho-antibody experiments show that VEGF-A165b only partially activates VEGFR2 suggesting a partial intracellular rotation, such that the kinase domain is activated but tyrosine 1054, which is in the kinase regulatory site, is not phosphorylated, presumably due to insufficient torsional rotation. This results in rapid closure of the ATP binding site of the kinase and rapid inactivation25, leading to a poorly activated kinase and weak, transient phosphorylation of extracellular-signal-regulated kinase 1 (ERK1) and ERK2 (REF. 26) (FIG. 5). VEGF-A165 also stimulates robust phosphorylation of tyrosine 1175, resulting in activation of phospholipase Cγ, phosphoinositide 3-kinase and diacylglycerol production, and activation of the Raf-MEK-Erk pathway in a protein kinase C-dependent27, Ras-independent manner28 (FIG. 3). These events are crucial for the activation of pro-angiogenic gene expression in endothelial cells, particularly as they lead to the production of the matrix metalloproteinases that are required for invasion through the basement membrane and the initiation of endothelial cell migration and angiogenesis29. Notably, VEGF-A165b does not bind neuropilin 1, as the basic carboxy-terminal amino acids essential for neuropilin 1 binding are absent22. The functional difference between VEGF-A165 and VEGF-A165b might be determined by absence of neuropilin 1 co-signalling, or it might be due to unique downstream signalling resulting from the different tertiary structures of the neuropilin 1-VEGFR2-VEGF-A165 triple dimer complex and the VEGFR2-VEGF-A165b double dimer. This question remains to be clarified.
Figure 4. The structure of vascular endothelial growth factor A (VEGF-A).
a | Crystal structure of amino acids 4-108 of VEGF-A, which are present in all isoforms. The crystal structure of the full-length VEGF-A protein is not known as a hinge region after amino acid 108 prevents crystallization. Modified, with permission, from REF. 74 © 1999 Elsevier B.V. b | Amino acids 4-108 of VEGF-A are shown along with the crystal structure of the final 55 residues. Crystallization of the final 55 residues of VEGF-A165 indicates two cysteine (C)-bonded double anti-parallel β sheet structures (brown arrows) separated by an α helix (blue cylinder). This structure is highly mobile and rotates around the hinge, and could pass through the VEGF receptor 2 (VEGFR2) binding region but not the VEGFR1 region (yellow circles)75,76. c | Proposed structure of amino acids 108-165 of VEGF-A165. The C’ terminal six residues include a cysteine with two positively charged arginines (RR) that are proposed to interact with the VEGFR binding domain22 to activate intracellular torsional rotation of VEGFR2. The RR motif therefore acts as a molecular switch by inducing a conformational change in VEGFR2. A disulphide bond77 (shown in orange) between cysteines 146 and 160 is required for VEGF-A165 activity78 and ensures that the C terminus is maintained at close proximity to the neuropilin 1 binding domain (NPBD). d | Proposed structure of amino acids 108-165 of VEGF-A165b. The C’ terminal cysteine and the positively charged RR motif present in VEGF-A165 are replaced by a serine (S) and a neutral DK motif on VEGF-A165b respectively. Although the VEGFR binding domain is present, the cysteine disulphide bond is absent. Thus, the molecular interaction with VEGFR is likely to be significantly different.
Figure 5. Vascular endothelial growth factor A (VEGF-A)165b and VEGF-A165 interaction with VEGF receptor 2 (VEGFR2).
a | The VEGFR2 binding site of VEGF-A165 interacts with the VEGFR2 extracellular domain. VEGF-A165 functions as a dimer and promotes the formation of VEGFR2 dimers (only one receptor is shown here for clarity) resulting in activation of the split kinase domains (green lines) and the phosphorylation of tyrosine residues 951, 1152 and 1214 (orange) and 1054 (purple). The charged residues at the carboxy-terminal end of the VEGF-A165 molecule (omitted for clarity) are required for VEGFR activation and, in receptor tyrosine kinases, this is thought to occur through torsional rotation of the intracellular domain bringing together the split kinase domains. Tyrosine 1054 is located at the mouth of the ATP binding pocket of the tyrosine kinase and, once phosphorylated, prevents the binding pocket from closing, thus resulting in a stable open structure. This results in formation of a persistently functional kinase from the split kinase domains, resulting in sustained cis- and trans- phosphorylation of the tyrosine residues on the intracellular tail, even in the presence of phosphatases. Robust tyrosine phosphorylation also results in the activation of angiogenic signalling pathways (FIG. 3). b | VEGF-A165b binds the VEGFR2 binding site with equal affinity to VEGF-A165 but does not bind neuropilin 1 (NRP1). The C’ terminus of VEGF-A165b is neutral and there is insufficient torsional rotation for tyrosine 1054 to be phosphorylated, although weak phosphorylation of the other tyrosines can occur. Thus, the ATP binding pocket closes and the phosphorylated tyrosines can be rapidly dephosphorylated by phosphatases and trafficked much more quickly. As a result, angiogenic signalling pathways are not activated25.
VEGF-A functions as a dimer. The theoretical formation of heterodimers — either of paired isoforms (for example, VEGF-A165-VEGF-A165b) or non-paired isoforms (for example, VEGF-A121-VEGF-A189b) — adds yet more layers of potential complexity to the subject. The existence of heterodimers in vivo is unproven and their subsequent signalling and function is speculative.
Effect of VEGF-Axxxb on non-endothelial cells
VEGF-A has long been regarded as a family of pro-angiogenic, pro-permeability vasodilator peptides. Two key discoveries have emerged in recent years that have resulted in a radical re-evaluation of VEGF-A biology. One was the identification of the anti-angiogenic VEGF-Axxxb family. Although, 6 years after its description, the number of papers on this anti-angiogenic family equate to 40% of those published in the 6 years after the first discovery of VEGF, this group of isoforms has yet to attract the attention of the majority of VEGF investigators. The reasons for this are unclear but may simply be because it has been overlooked in the vast VEGF-A literature (approximately 200 publications per month) or because the identification of this group has unpalatable implications for all of us. Academically it suggests additional layers of complexity and, in terms of resource allocation, the existence of VEGF-Axxxb suggests that many of the thousands of published manuscripts on VEGF-A may, at best, need re-interpretation or, at worst, require repeating with reagents that differentiate between isoform families. As tools for investigating VEGF-Axxxb isoforms (for example, antibodies, probes and ELISA kits) have now become available, our understanding of the role of VEGF-Axxxb should become clearer.
The second revolution in VEGF-A biology has been that, despite its nomenclature, VEGF-A is not specific to endothelial cells and can also be vital in the function and maintenance of non-endothelial cells. Mutations in the hypoxia response element of the Vegfa promoter can, for example, result in a form of motor neuron disease in mice30, which is not associated with angiogenesis. VEGF-A165 was subsequently shown to be neuroprotective30. Inhibition of VEGF-A also results in retinal neurotoxicity both in vitro and in vivo31 and proteinuria in humans32 and in rodents33. This latter effect could be due to podocyte cytotoxicity, for which both VEGF-A165 (REF. 34) and VEGF-A165b18 are in vitro survival factors, perhaps through VEGF-A-dependent phosphorylation of nephrin35. VEGF-A165b also acts as a paracrine or autocrine survival factor. Treatment of podocytes, retinal pigmented epithelial cells or colonic adenoma cells in vitro with a neutralizing antibody to VEGF-A165b that does not bind VEGF-A165, even when present in 50-fold excess18,20,36, results in increased cytotoxicity13,18,20. Conversely, treatment of these cell types with VEGF-A165b reduces cytotoxicity when induced by multiple agents13,18,20. The receptor-mediated mechanism of action of VEGF-A165b-dependent cytoprotection in epithelial cells has not been well defined and indeed may be cell-type-dependent and VEGFR-phenotype-dependent, but these results support the concept that VEGF-A165b may elicit distinct signalling pathways.
Effect of VEGF-A165b on angiogenesis and tumour growth
The properties of VEGF-A165b have been published by nine laboratories worldwide using VEGF-A165b-transfected cells20,36,37, VEGF-A165b-encoding adenoviral constructs36 and recombinant human VEGF-A165b38,39.
VEGF-A165 stimulates endothelial cell migration and proliferation in vitro, vasodilatation40, increased endothelial monolayer permeability in vitro18, chronically increased vascular permeability in vivo41, in vivo angiogenesis42 and pathological retinal neovascularization in vivo43. By stark contrast, VEGF-A165b does not stimulate these responses, and inhibits several VEGF-A165-mediated processes: endothelial cell migration in vitro11, proliferation in vitro11 and vasodilatation ex vivo11. VEGF-A165b does not increase chronic microvascular permeability in vivo44 and reduces conditionally immortalized human glomerular endothelial cell monolayer permeability in vitro18. VEGF-A165b also inhibits in vivo angiogenesis in the rat mesentery when VEGF-A165 overexpression is driven by an adenoviral vector36. In addition, VEGF-A165b inhibits pathological angiogenesis in murine tumour models20,37,39, physiological angiogenesis in mammary tissue in transgenic mice45, VEGF-A165-mediated angiogenesis in the chick chorioallantoic membrane assay26 and VEGF-A165-mediated angiogenesis in the rabbit corneal eye pocket model36. Finally, recombinant human VEGF-A165b inhibits hypoxia-mediated retinal angiogenesis in vivo in murine models of retinopathy of prematurity38 and human tumour growth in mice39.
Both VEGF-A165 and VEGF-A165b bind VEGFR2 with equal affinity20,36 but VEGF-A165b fails to stimulate angiogenesis in vivo26,36,45. These initial observations lent credence to the view that VEGF-A165b was likely to demonstrate classical competitive ‘key-in-the-lock’ inhibition, even if the molecule was completely inert. However, further work by Ballmer-Hofer’s group26 has shown that a truncated isoform of VEGF-A165, VEGF-A159, which lacks the amino acids encoded by exons 8a and 8b, does not inhibit VEGF-A165-mediated angiogenesis, despite binding to VEGFR2 and lacking angiogenic activity itself, which suggests that the presence of exon 8b in VEGF-A165b may have a specific inhibitory contribution. Therefore, it is as yet unclear whether the profound difference in cellular behaviour induced by VEGF-A165b relative to VEGF-A165 is due to a qualitative alteration in signalling (that is, differing signalling molecules are used) or a quantitative alteration in signalling (that is, the downstream signalling is insufficient), or whether both mechanisms are functional. There is data supporting the quantitative hypothesis25 but the qualitative hypothesis has only circumstantial evidence to date.
In tumours, overexpression of transfected VEGF-A165b delays the growth of melanoma36, kidney37, colon20, prostate37 and Ewing sarcoma37 tumours. Furthermore, recombinant human VEGF-A165b inhibits developing and established solid tumour growth in nude mice when given subcutaneously or by intra-peritoneal injection39. Tumours treated with VEGF-A165b are paler, less haemorrhagic and visibly less vascularized, with reduced microvascular density and increased necrosis39. Dose-response studies show complete inhibition of established tumour growth by 100 μg biweekly injection of recombinant VEGF-A165b39. Furthermore, parenteral treatment with recombinant human VEGF-A165b can reduce the growth of disseminated metastatic melanoma tumours46. All these data are consistent with a cancer-associated switch from anti- to pro-angiogenic VEGF-A isoform expression by alteration of splicing.
Heterogeneity of VEGF-A mRNA proximal splice site (PSS) selection in disease
Many cancers are associated with a switch from a VEGF-Axxxb-dominated milieu in normal tissue to a proliferative phenotype in which VEGF-Axxx isoforms dominate. In their study, Varey et al. showed by quantitative PCR and ELISA that the switch from VEGF-Axxxb, which makes up 90% of the VEGF-A expressed by normal colonic tissue17, to VEGF-Axxx (in other words, a switch from DSS to PSS selection) is variable in patients with colorectal cancer20. Approximately 30% of patients still have a modest excess of VEGF-Axxxb over VEGF-Axxx, about half an excess of up to threefold VEGF-Axxx, and the remainder a much greater VEGF-Axxx excess, up to 60-fold20. This switch to pro-angiogenic VEGF-A isoforms has also been shown at the mRNA level in prostate37, renal11 and bladder cancer21, and at the protein level in bladder cancer21 and metastatic but not non-metastatic melanoma47. The study in melanoma demonstrated that primary melanomas from patients that later developed distant metastases expressed less VEGF-A165b than those from patients that were disease-free 8 years later47. Furthermore, a low level of VEGF-A165b expression is a potential biomarker for poor prognosis in colonic carcinoma48.
Therapeutic implications
Interaction with established anti-VEGF-A agents
VEGF-A165b contains binding domains for the vast majority of anti-VEGF-A antibodies, including therapeutic antibodies such as bevacizumab and most of the commercially available antibodies for laboratory use. Western blotting and Biacore experiments show that VEGF-A165b binds bevacizumab with the same affinity as VEGF-A165 (REF. 20). However, a preliminary report suggests that pegaptinib, the VEGF-A aptamer (an oligonucleotide ligand that displays high-affinity binding to a molecular target), does not bind VEGF-A165b49, although similar data are not yet available for most other anti-VEGF-A agents, including VEGF-TRAP (aflibercept — a unique fusion protein that has a high affinity for all isoforms of VEGF-A as well as for placental growth factor), and VEGFR tyrosine kinase inhibitors (TKIs), such as sunitinib and sorafenib. However, it is possible that the combined effect of recombinant VEGF-A165b and TKIs that target VEGFR2 may have increased efficacy over treatment with a VEGFR TKI alone, as VEGF-A165b is not simply a non-specific inhibitor of VEGFR2 but can actively antagonize VEGFR2 angiogenic signalling25 and possibly also VEGFR1 (REF. 44).
VEGF-A165b expression has a profound effect on the efficacy of bevacizumab. In mice injected with VEGF-A165b-expressing colonic cancer cells, the tumours grow more slowly than in those bearing VEGF-A165-expressing cancer cells. However, the dose of bevacizumab required to prevent tumour growth in VEGF-A165-expressing tumours had absolutely no effect on VEGF-A165b-expressing tumours20. This startling finding suggests that treatment of patients with tumours expressing significant levels of VEGF-Axxxb with bevaciumab may not be effective, because VEGF-A165b will inhibit the effect of this anti-VEGF-A antibody. Conversely, this model would predict that bevacizumab treatment would be most effective in patients whose tumours produce an excess of VEGF-Axxx isoforms.
Recombinant human VEGF-A165b
It has yet to be established whether the inhibition of bevacizumab by VEGF-A165b expression can be predicted by assessing the VEGF-A165:VEGF-A165b ratio in patients. If this is indeed the case, current assays for VEGF-Axxxb will need to be developed for clinical use or standardized immunohistochemical procedures will be required. However, an alternative approach is that VEGF-A165b (the most widely studied VEGF-Axxxb isoform) or other VEGF-Axxxb isoforms may be therapeutic themselves. In principle, VEGF-A165b would have potential advantages over a number of existing anti-angiogenic therapies. These include its endogenous nature and the lack of side effects such as hypertension and proteinuria that are associated with the inhibition of VEGF-Axxx39. Anti-VEGF-A therapy has been shown to cause normal capillary loss50, and endothelial cell-specific knockout of all VEGF-A isoforms (including VEGF-A165b) results in adult mortality in mice due to endothelial cell apoptosis and subsequent haemorrhage51. There are therefore sound reasons to suspect that VEGF-A165b-based therapy will be less problematic than agents that target all VEGF-A isoforms. Thus, the identification of VEGF-Axxx-specific antagonists (for example, anti-exon 8a C’-terminal antibodies) may benefit from precise targeting. The next generation of anti-VEGF-A therapies might derive from such a design.
Tumour splicing hypothesis
The control of divergent physiological properties from one gene resides with mRNA splicing, stability and translation. The transcripts of the majority (70%) of human genes splice52, in that they code for multiple isoforms, many of which have strikingly different properties. Splicing is co-transcriptional, and the consensus sequences at the 5′ and 3′ sites are recognized by the splicing apparatus early in the splicing process — that is, the splicing choice occurs early in the birth of an RNA molecule. Splicing mechanisms in mammals are being elucidated using models such as fibronectin53 and β-globin54 and there is now considerable evidence that regulation of splicing is a key event in cancer progression8,55. The process is mediated by splicing proteins, which form the spliceosome56, and is regulated by splicing regulatory factors. Progress made in defining the mechanisms of VEGF-A exon 8 splice site choice13 are summarized in FIG. 2. Alternate 5′ transcriptional start sites have been demonstrated for VEGF-A57, and these may result in alternative splicing through the recruitment of different splice factors. Other mechanisms, such as polypyrimidine tract binding protein-mediated repression of alternative exon splicing58, are also possible regulators of VEGF-A splicing59.
Thus, the cellular machinery underlying control of splice site choice, and hence which isoforms are expressed, are potential therapeutic targets, particularly as agents that inhibit the actions of specific splicing regulatory factors are now emerging60. Regulation of alternative splicing is unlikely to be restricted to VEGF-A during angiogenesis. Many proteins in the angiogenic cascade have alternative splice variants with antagonistic properties. In active angiogenesis they all splice such that the pro-angiogenic isoforms predominate. VEGFR1 (REF. 61), VEGFR2 (REF. 62), VEGFR3, platelet-derived growth factor receptor-β (PDGFRβ), fibroblast growth factor receptor 1 (FGFR1), FGFR2, FGFR4 (REF. 63) and neuropilin 1 (REF. 64) all have soluble splice variants that can, or are predicted to, act as natural inhibitors. Moreover, anti-angiogenic forms of collagen XVII (endostatin)65 and collagen IV (tumstatin)66 have also been characterized. Common splicing mechanisms allowing anti-angiogenesis to be switched to angiogenesis67 in disease or remodelling have been proposed67, and these may extend to many non-angiogenesis-related proteins that also exist as multiple isoforms and drive tumour progression.
Indeed there are many non-angiogenic cancer-related genes that have splice isoforms with antagonistic properties and it is becoming increasingly apparent that similar factors can orchestrate the splicing of angiogenic and non-angiogenic malignancy-associated genes8. For example, exclusion of exon 3 of the FGFR1 in gliomas produces the tumour-promoting isoform FGFR1β. This exon exclusion results from the loss of the splicing factor SRP55 (REF. 68), which also reduces VEGF-A C’-terminal DSS selection, shifting the VEGF-Axxx versus VEGF-Axxxb balance towards angiogenesis13.
Therefore, the dual phenomena of a molecular switch to stimulate unregulated malignant cell proliferation and the angiogenic switch as described by the late J. Folkman69 may have different cellular mechanisms, but alternate splicing may well provide a mechanism explaining the connection between malignancy and angiogenesis, as Folkman hypothesized.
In summary, VEGF-Axxxb isoforms are key regulators of angiogenesis in health and disease. Exogenously (intravenously) administered recombinant VEGF-A165b appears to accumulate in tumours (presumably because it targets VEGFR2-bearing tumour microvessels) and therefore has increased tumour bio-availability39. Thus, administration of recombinant VEGF-Axxxb isoforms could be a novel therapeutic approach in the short term. However, the most effective but also the most challenging approach in the long term may be to allow cancer VEGF-A transcription to proceed unhindered but to control splicing such that the spliceosome opts for exon 8 DSS selection in place of exon 8 PSS selection. This would effectively cause the cancer to switch off its own nutrient supply. Indeed, given that the VEGF-A promoter contains a hypoxia response element70, the more hypoxic the tumour became, the more effective this switch might be.
DATABASES
National Cancer Institute: http://www.cancer.gov/
bladder cancer | colorectal carcinoma | Ewing sarcoma | kidney cancer | melanoma | prostate cancer
National Cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary/
aflibercept | bevacizumab | sorafenib | sunitinib
UniProtKB: http://www.uniprot.org
β-globin | collagen IV | collagen XVII | FGFR1 | FGFR2 | FGFR4 | fibronectin | nephrin | neuropilin 1 | PDGFRβ | placental growth factor | SRP55 | VEGFA | VEGFR1 | VEGFR2 | VEGFR3
FURTHER INFORMATION
S. J. Harper’s homepage: http://www.mvrl.org
Footnotes
Competing interests statement
The authors declare competing financial interests: see web version for details.
References
- 1.Mendel DB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 2.Motzer RJ, et al. Sunitinib versus interferon α in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin. Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Comm. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 7.Keck PJ, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 8.Venables JPE. Alternative Splicing in Cancer. Transworld Research Network; Kerala: 2006. [Google Scholar]
- 9.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 11.Bates DO, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 12.Perrin RM, et al. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 13.Nowak DG, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by known splicing and growth factors. J. Cell Sci. doi: 10.1242/jcs.016410. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 15.Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling-in control of vascular function. Nature Rev. Mol. Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 16.Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bevan HS, Harper SJ, Bates DO. In: Angiogenesis: Basic Science and Clinical Applications. Maragoudakis ME, Papadimitriou E, editors. Transworld Research Network; Kerala: 2007. pp. 1–26. [Google Scholar]
- 18.Bevan HS, et al. The alternatively spliced anti-angiogenic family of VEGF isoforms, VEGFxxxb, in human kidney development. Nephron Physiol. doi: 10.1159/000177614. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui TG, et al. Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am. J. Physiol. Renal Physiol. 2004;286:F767–F773. doi: 10.1152/ajprenal.00337.2003. [DOI] [PubMed] [Google Scholar]
- 20.Varey AH, et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br. J. Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopi SS, Zadeh MH, Harper SJ, Bates DO, Gillatt GA. Expression of anti-angiogenic isoform, VEGF165b in transitional cell carcinoma of bladder. BJU Int. 2008;101:29–30. [Google Scholar]
- 22.Cebe-Suarez S, et al. Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J. doi: 10.1096/fj.08-107219. in the press. [DOI] [PubMed] [Google Scholar]
- 23.Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nature Struct. Mol. Biol. 2007;14:249–250. doi: 10.1038/nsmb1202. [DOI] [PubMed] [Google Scholar]
- 24.Burgess AW, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. VEGF-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of co-receptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 26.Cebe Suarez S, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell. Mol. Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia P, et al. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J. Clin. Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 29.Lamoreaux WJ, Fitzgerald M, Reiner A, Hasty KA, Charles ST. Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc. Res. 1998;55:29–42. doi: 10.1006/mvre.1997.2056. [DOI] [PubMed] [Google Scholar]
- 30.Lambrechts D, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nature Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 31.Nishijima K, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eremina V, et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimoto H, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 34.Foster RR, et al. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am. J. Physiol. Renal Physiol. 2003;284:F1263–F1273. doi: 10.1152/ajprenal.00276.2002. [DOI] [PubMed] [Google Scholar]
- 35.Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ. Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am. J. Physiol. Renal Physiol. 2005;288:F48–F57. doi: 10.1152/ajprenal.00146.2004. [DOI] [PubMed] [Google Scholar]
- 36.Woolard J, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 37.Rennel ES, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br. J. Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularisation in mice. Mol. Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- 39.Rennel ES, et al. Recombinant human vascular endothelial growth factor (VEGF165b) protein is an effective anti-cancer agent in mice. Eur. J. Cancer. 2008;44:1883–1894. doi: 10.1016/j.ejca.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 1993;265:H586–H592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 41.Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am. J. Physiol. Heart Circ. Physiol. 1996;271:H2520–H2528. doi: 10.1152/ajpheart.1996.271.6.H2520. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J. Cell Biochem. 1991;47:211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell CA, et al. Unique vascular phenotypes following over-expression of individual VEGF-A isoforms from the developing lens. Angiogenesis. 2006;9:209–224. doi: 10.1007/s10456-006-9056-7. [DOI] [PubMed] [Google Scholar]
- 44.Glass CA, Harper SJ, Bates DO. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J. Physiol. 2006;572:243–257. doi: 10.1113/jphysiol.2005.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu Y, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- 46.Budge JR, Fryer JD, Bates DO. Intraperitoneal administration of recombinant human VEGF165b inhibits dissemination of metatatic melanoma cells in vivo. Microcirculation. 2008;17:18–19. [Google Scholar]
- 47.Pritchard-Jones RO, et al. Expression of VEGFxxxb, the inhibitory isoforms of VEGF, in malignant melanoma. Br. J. Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz R, et al. p73 isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int. J. Cancer. 2008;123:1060–1067. doi: 10.1002/ijc.23619. [DOI] [PubMed] [Google Scholar]
- 49.Magnussen A, et al. VEGF165b is more potent at inhibiting endothelial cell migration than Pegabtanib and is cytoprotective for retinal pigmented epithelial cells. FASEB J. 2008;22:746.14. [Google Scholar]
- 50.Baffert F, L. T, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghigna C, et al. In: Alternative Splicing in Cancer. Venables JP, editor. Transworld Research Network; Kerala: 2006. pp. 197–208. [Google Scholar]
- 53.Muro AF, Iaconcig A, Baralle FE. Regulation of the fibronectin EDA exon alternative splicing. Cooperative role of the exonic enhancer element and the 5′ splicing site. FEBS Lett. 1998;437:137–141. doi: 10.1016/s0014-5793(98)01201-0. [DOI] [PubMed] [Google Scholar]
- 54.Schaal TD, Maniatis T. Multiple distinct splicing enhancers in the protein-coding sequences of a constitutively spliced pre-mRNA. Mol. Cell Biol. 1999;19:261–273. doi: 10.1128/mcb.19.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venables JP. Aberrant and alternative splicing in cancer. Cancer Res. 2004;64:7647–7654. doi: 10.1158/0008-5472.CAN-04-1910. [DOI] [PubMed] [Google Scholar]
- 56.Caceres JF, Kornblihtt AR. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 2002;18:186–193. doi: 10.1016/s0168-9525(01)02626-9. [DOI] [PubMed] [Google Scholar]
- 57.Neufeld G, et al. Similarities and differences between the vascular endothelial growth factor (VEGF) splice variants. Cancer Metastasis Rev. 1996;15:153–158. doi: 10.1007/BF00437467. [DOI] [PubMed] [Google Scholar]
- 58.Amir-Ahmady B, Boutz PL, Markovtsov V, Phillips ML, Black DL. Exon repression by polypyrimidine tract binding protein. RNA. 2005;11:699–716. doi: 10.1261/rna.2250405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coles LS, et al. A multi-protein complex containing cold shock domain (Y-box) and polypyrimidine tract binding proteins forms on the vascular endothelial growth factor mRNA. Potential role in mRNA stabilization. Eur. J. Biochem. 2004;271:648–660. doi: 10.1111/j.1432-1033.2003.03968.x. [DOI] [PubMed] [Google Scholar]
- 60.Bakkour N, et al. Small-molecule inhibition of HIV pre-mRNA splicing as a novel antiretroviral therapy to overcome drug resistance. PLoS Pathog. 2007;3:1530–1539. doi: 10.1371/journal.ppat.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kou B, et al. In vivo inhibition of tumor angiogenesis by a soluble VEGFR-2 fragment. Exp. Mol. Pathol. 2004;76:129–137. doi: 10.1016/j.yexmp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Jin P, et al. Novel splice variants derived from the receptor tyrosine kinase superfamily are potential therapeutics for rheumatoid arthritis. Arthritis Res. Ther. 2008;10:R73. doi: 10.1186/ar2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gagnon ML, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc. Natl Acad. Sci. USA. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saarela J, Ylikarppa R, Rehn M, Purmonen S, Pihlajaniemi T. Complete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol. 1998;16:319–328. doi: 10.1016/s0945-053x(98)90003-8. [DOI] [PubMed] [Google Scholar]
- 66.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc. Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bates DO, Harper SJ. Therapeutic potential of inhibitory VEGF splice variants. Fut. Oncol. 2005;1:467–473. doi: 10.2217/14796694.1.4.467. [DOI] [PubMed] [Google Scholar]
- 68.Jin W, Cote GJ. Enhancer-dependent splicing of FGFR1α-exon is repressed by RNA interference-mediated down-regulation of SRp55. Cancer Res. 2004;64:8901–8905. doi: 10.1158/0008-5472.CAN-04-0716. [DOI] [PubMed] [Google Scholar]
- 69.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann. Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nature Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 71.Whittle C, Gillespie K, Harrison R, Mathieson PW, Harper SJ. Heterogeneous vascular endothelial growth factor (VEGF) isoform mRNA and receptor mRNA expression in human glomeruli, and the identification of VEGF148 mRNA, a novel truncated splice variant. Clin. Sci. (Lond.) 1999;97:303–312. [PubMed] [Google Scholar]
- 72.Hirose Y, Tacke R, Manley JL. Phosphorylated RNA polymerase II stimulates pre-mRNA splicing. Genes Dev. 1999;13:1234–1239. doi: 10.1101/gad.13.10.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koenigsberger C, Chicca JJ, 2nd, Amoureux MC, Edelman GM, Jones FS. Differential regulation by multiple promoters of the gene encoding the neuron-restrictive silencer factor. Proc. Natl Acad. Sci. USA. 2000;97:2291–2296. doi: 10.1073/pnas.050578797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Starovasnik MA, et al. Solution structure of the VEGF-binding domain of Flt-1: comparison of its free and bound states. J. Mol. Biol. 1999;293:531–544. doi: 10.1006/jmbi.1999.3134. [DOI] [PubMed] [Google Scholar]
- 75.Muller YA, Christinger HW, Keyt BA, de Vos AM. The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 Å resolution: multiple copy flexibility and receptor binding. Structure. 1997;5:1325–1338. doi: 10.1016/s0969-2126(97)00284-0. [DOI] [PubMed] [Google Scholar]
- 76.Fairbrother WJ, Champe MA, Christinger HW, Keyt BA, Starovasnik MA. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure. 1998;6:637–648. doi: 10.1016/s0969-2126(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 77.Keck RG, Berleau L, Harris R, Keyt BA. Disulfide structure of the heparin binding domain in vascular endothelial growth factor: characterization of posttranslational modifications in VEGF. Arch. Biochem. Biophys. 1997;344:103–113. doi: 10.1006/abbi.1997.0145. [DOI] [PubMed] [Google Scholar]
- 78.Claffey KP, Senger DR, Spiegelman BM. Structural requirements for dimerization, glycosylation, secretion, and biological function of VPF/VEGF. Biochim. Biophys. Acta Prot. Struct. Mol. Enzymol. 1995;1246:1–9. doi: 10.1016/0167-4838(94)00144-6. [DOI] [PubMed] [Google Scholar]