Abstract
Over the past decade, it has become clear that T cell immune responses in both murine and human neonates are very heterogeneous, running the gamut from poor or deviant responsiveness to mature, adult-like inflammatory function. How this variability arises is not well understood but there is now a great deal of information suggesting that differences in the T cell compartments in neonates vs adults play important roles. A number of cell types or processes are qualitatively or quantitatively different in the neonate. These include (a) alternate epigenetic programs at the Th2 cytokine locus, (b) enhanced homeostatic proliferation, (c) a relative abundance of fetal-origin cells, (d) a greater representation of recent thymic emigrants, (e) high proportions of potentially self-reactive cells, (f) a developmental delay in the production of regulatory T cells, and (g) cells bearing TCR with limited N region diversity. Different conditions of antigen exposure may lead to different environmental signals that promote the selective responsiveness of one or more of these populations. Therefore, the variability of neonatal responses may be a function of the heterogeneous nature of the responding T cell population. In this review, we will describe these various subpopulations in detail and speculate as to the manner in which they could contribute to the heterogeneity of neonatal immune responses.
Keywords: Ontogeny, animals, newborn, Th1/Th2 cells, immune plasticity, cytokines, comparative immunology, vaccination
INTRODUCTION
Neonates are particularly susceptible to infections and diseases which only mildly affect adults. While the absence of specific immune memory almost certainly contributes, immaturity in the neonatal CD4+ cell compartment may play a central role in this sensitivity. Neonatal responses in both humans and mice are often deficient in the proinflammatory Th1 arm of immunity and are of poor protective value. Typically in mice (reviewed in [1–3]) and sometimes in humans [4–7], this is also characterized by the predominance of Th2 anti-inflammatory responses. In spite of this default deficiency in Th1 function, it has become clear that neonates are competent to develop mature Th1 inflammatory immune responses under the right conditions of antigenic exposure (reviewed in [8]). The picture that has emerged is that neonatal Th responses are heterogeneous, ranging from highly Th2 skewed to balanced Th1/Th2 function. This variability of responsiveness could be of evolutionary value since newborns must combat many newly encountered pathogens while simultaneously developing tolerance for the first time in life to a variety of peripheral self and common environmental antigens. However, how this flexibility of responsiveness arises is not well understood. The challenge lies in identifying the basis for this phenomenon – i.e., why do poor responses occur most of the time and what are the mechanisms underlying the production of mature protective responses? There is a great deal of emerging information on the importance of the APC compartment in modulating T cell responses in neonates. An excellent review of this field has recently been published [9]. Here, we will focus on the characteristics of neonatal T cells which may contribute to the variability of the response.
In the past several decades, we have witnessed a huge expansion in our understanding of the cellular properties of neonatal CD4+ cells. One of the unique characteristics of the newborn peripheral T cell compartment is its extreme heterogeneity, compared with adults. Both older and newly emerging data indicate that there are quantitative and qualitative differences in the compositions of the CD4+ T cell compartments in neonates and adults. In many cases, these differences could be predicted to have important functional consequences. Thus, we propose that the variability of newborn immune responses derives, at least in part, from this heterogeneity. Under different conditions of antigen exposure, the dominant responding subpopulation may shift, producing the wide range of observed responses. In this review, we will summarize the currently described, major differences in the compositions of the neonatal and adult T cell compartments and discuss the potential impact of these subsets on neonatal immunity.
EPIGENETIC MODIFICATIONS AS A POTENTIAL STRATEGY FOR REGULATING T HELPER CELL RESPONSES DURING ONTOGENY
The Th2 cytokine locus contains the tandomly arranged Il5, Il13, and Il4 genes. In naïve adult CD4+ cells, this locus exists in a transcriptionally silent state characterized by condensed chromatin and locus-wide methylation of CpG residues. Activation under Th2-polarizing conditions leads to extensive epigenetic modifications, including the appearance of DNase I hypersensitivity sites [10–13], histone modifications [14, 15], and extensive DNA demethylation [11, 12, 16, 17]. These modifications, together with the expression of specific transcription factors, are thought to be essential for the high level transcription of Th2 cytokine genes [18–21].
Total murine neonatal CD4+ lymph node cells rapidly produce copious amounts of the Th2 cytokines IL-4 and IL-13 [22–24], something that only occurs in adults following extensive epigenetic modifications at the Th2 locus. Thus, we reasoned that the Th2 cytokine locus in neonates may have already undergone at least some of those modifications favorable for high level expression of Th2 cytokine genes. To test this, we examined CpG methylation in several important areas of the Th2 cytokine locus, including the conserved non-coding sequence 1 (CNS-1), an enhancer and coordinate regulator of Th2 cytokine production [25]. Indeed, total neonatal CD4+ cells showed extensive hypomethylation in this key regulatory region [24]. Since the total neonatal lymph node population contains naïve cells as well as some cells which have undergone homeostatic proliferation (see below), it was possible that this hypomethylation arose during division of the latter cells. However, this state did not require prior cell division since a high frequency (36%) of the CpG dinucleotides were unmethylated in the CNS-1 region of naïve (CD44lo) neonatal CD4+ lymph node cells, compared to 3% for naïve adult cells. Importantly, low DNA methylation at CNS-1 in adults has been linked with high IL-4 expression [16]. By analogy, in an environment favorable for the expression of the appropriate transcription factors, relative hypomethylation at CNS-1 may predispose murine neonates to the early and high level production of Th2 cytokines. Indeed, the epigenetic state of the naïve cells may allow them to acquire Th2 function more rapidly than adult cells. In response to polyclonal stimulation, naïve neonatal cells developed robust IL-4 production within 48 hours of stimulation whereas none was produced by adult cells at this time point (Fig. 1) [26]. Interestingly, this phenomenon could be traced back to the neonatal thymus since CD4 single positive thymocytes also showed relative hypomethylation at CNS-1 [24]. Thus, the propensity of murine neonates for Th2 cytokine production could be largely due to developmental-specific epigenetic patterns that are established during thymic maturation.
Fig. 1. Neonatal CD4+ cells that have undergone homeostatic proliferation rapidly produce both Th1 and Th2 cytokines.
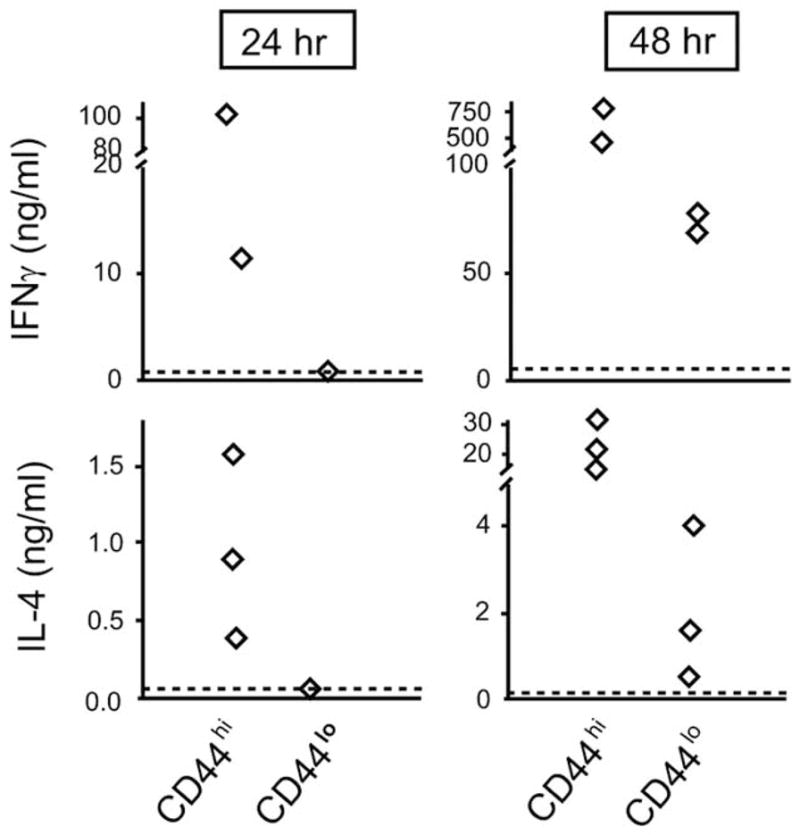
Lymph node cells from 7 day old BALB/c mice were stained with anti-CD4 and anti-CD44 and sorted into CD4+CD44hi and CD4+CD44lo populations. The sorted cells were stimulated with anti-CD3 and anti-CD28 for the indicated times, supernatants were collected, and the amounts of IFNγ and IL-4 were measured by specific ELISA. Each symbol represents an independent experiment.
Recent work from Vercelli and colleagues [5] suggests that there are also developmental specific epigenetic patterns in human neonatal cells that may favor the Th2 anti-inflammatory pathway. In cord blood CD4+ cells, the Th2 locus is remodeled even when cells are polarized to the Th1 lineage. The remodeling includes both DNase I hypersensitivity and CpG demethylation. Perhaps not surprisingly, cord blood Th1 cells retained the ability to produce the Th2 cytokine IL-13, albeit at reduced levels. Thus, cord blood CD4+ cells have a relatively permissive chromatin configuration at the Th2 locus, even in Th1 polarized cells, and this “openness” is associated with the ongoing capacity to produce Th2 cytokines at low levels.
While epigenetic events clearly favorable for Th2 gene expression occur in neonates, the opposite may apply to the Th1 cytokine gene γIFN. Holt and colleagues [27] described hypermethylation of the γIFN promoter in cord blood naïve CD4+ cells. While the precise mechanisms of regulation of γIFN gene expression remain to be elucidated, these findings imply that the γIFN gene may be relatively inaccessible in neonatal life.
Together, these observations indicate that both human and murine neonates may use epigenetic strategies to favor the development and maintenance of Th2 function while simultaneously dampening Th1 responses in early life. Therefore, selective chromatin modifications may be at least one of the causes underlying the tendency toward a Th2 bias in neonatal responses.
HOMEOSTATIC PROLIFERATION IN EARLY LIFE
In the mouse, T cell numbers are reduced several logs in neonates compared with adult mice. As a result, neonates are considered to be lymphopenic. It is well established that T cells introduced into a lymphopenic adult environment undergo spontaneous or homeostatic proliferation (reviewed in [28, 29]. Therefore, it might be expected that neonates would support the proliferation of adoptively transferred T cells. Indeed, this has been shown to be the case for adult CD4+ cells [30] as well as CD8+ cells [31] – i.e., both populations proliferate, in the absence of exogenous antigen, upon transfer to normal neonatal mice. Importantly, a portion of the endogenous neonatal T cells also undergo this proliferation in situ, as shown by in vivo BrDU labeling experiments [32]. Short-term labeling revealed at least 10x more cells in both the murine neonatal CD4+ and CD8+ populations to be in cycle, compared with their adult counterparts [32]. These results were corroborated by Min et al. [30] using TREC analyses. In humans, this has been examined by staining for the expression of a nuclear antigen, Ki67, indicative of cell cycling. Both CD4 and CD8 cells directly isolated from cord blood showed substantially greater expression of Ki67 than the comparable cell populations from adult PBMC [33].
The spontaneous proliferation in both human and murine neonates occurs in the absence of overt immunization. Therefore, the question that arises is, “what drives this proliferation?”. In human newborns, cytokines may be a primary stimulus eliciting proliferation. Common γ-chain cytokines, notably IL-7 for CD4+ and IL-15 for CD8+ cells, drive the division of cord blood naïve cells, in the absence of any other stimulus [33–40]. For IL-7, this is associated with the higher levels of expression of IL-7Rα on cord blood naïve phenotype (CD4+CD45RA+) cells relative to that on naïve adult CD4+ cells [37, 41]. It is not known whether endogenous neonatal cells in the mouse undergo similar γ-chain cytokine-induced proliferation. However, for adult cells adoptively transferred to neonates, CD4+ cell proliferation may be IL-7 independent [30] whereas CD8+ cells appear to be dependent on IL-7 signals [31]. For endogenous murine neonatal CD4+ cells, one intriguing idea is that the intestinal flora may provide the stimulus for antigen-driven proliferation. This is, in part, supported by the observation that germ-free mice remained “locked into” a Th2 bias into adulthood [42] – i.e., perhaps antigenic stimulation from commensal bacteria is contributing to homeostatic proliferation and maturation of the peripheral T cell pool. Thus far, however, attempts in our laboratory to directly demonstrate Th responses to the endogenous commensal flora have been unsuccessful. Altogether, it appears that cytokines as well as unidentified antigenic-specific signals may be important in promoting homeostatic proliferation in neonates.
The next question that arises is, “are there any functional consequences of this proliferation?”. In human newborns, cells cultured in common γ-chain cytokines change functionally and the extent of the change may be dependent on the period of exposure to IL-7. Hassan and Reen [35] reported that cord CD4+CD45RA+ cells (naïve phenotype) cultured in IL-7 for < 1 week maintained their naïve CD45RA+ phenotype but acquired the ability to proliferate as well as adult CD45RA+ cells. They did not, however, acquire Th1/Th2 effector function. On the other hand, Fukui et al. [36] described that naïve cord blood CD4+CD45RA+ cells cultured for 1–2 weeks in IL-7 acquired the capacity to secrete both Th1 and Th2 cytokines. In mice, it appears that cells acquire effector function in vivo during the process of homeostatic proliferation. Using the marker CD44, we separated neonatal CD4+ cells into those which had undergone proliferation (CD44bright) from those considered naïve (CD44low). The CD44bright subset of CD4+ neonatal cells rapidly produced both γ-IFN and IL-4 (Fig. 1). Therefore, homeostatic proliferation in murine neonates leads to the acquisition of rapid Th1/Th2 effector function.
It is important to point out that most of the cells in the neonate are not undergoing division. The process is restricted to ≤ 10% of the cells in either the CD4+ or CD8+ population [32, 33]. Nonetheless, this process provides a means for rapidly expanding the pool of T cells present in early life. In addition, in the absence of specific memory, these naturally arising primed T cells may provide rapid effector responses of mixed Th1/Th2 type. The antigenic specificities of the homeostatically expanded cells have not been identified. Nonetheless, it is possible that protective Th1/Th2 responses may arise when cross-reacting exogenous antigens are recognized by this population. Thus, part of the flexibility of neonatal immunity may rely on the fine antigen specificities of the homeostatically expanded population.
THE NEONATAL T CELL COMPARTMENT: THE FETAL CONNECTION
During murine ontogeny, the fetal thymus is first seeded by a wave of hematopoietic precursors, most likely from the fetal liver, between embryonic days 12–14 [43–45]. These precursors proliferate and differentiate throughout the rest of fetal life. Near birth, ~ 19–21 days of gestation in inbred mice, there is a second major colonization by hematopoietic cells [43, 46]. These two precursor entries are referred to as the fetal and adult waves, respectively. Following their entry, the adult precursors also proliferate and differentiate and are thought to eventually completely replace cells from the fetal wave.
There is now a wealth of data that the process of thymopoiesis is not identical in fetal and adult life. One stunning difference is the period of time over which T cell maturation occurs. In the postnatal thymus, it is estimated to take about fourteen days to differentiate into double positive (DP) cells [47]. In contrast, the first fetal precursors arrive in the thymus at 12–13 gestational days (E12–13) and the first DP thymocytes emerge by E16, a total span of only 3–4 days [48]. In addition to more rapid maturation, there are some differentiation programs that appear to be restricted to the fetal thymus. Perhaps the best known example of such fetal specific programs is the production of Vγ3-bearing T cells. In an elegant series of experiments, Havran and Allison [49, 50] showed that these cells arise early in the fetal thymus (day 14 of gestation) but are no longer detectable at birth. They further showed that Vγ3+ cells in the adult arise from the fetal thymus and that even the newborn thymus can no longer make Vδ3+ cells. More recently, genetic manipulated mice have revealed differential requirements for selected gene products in fetal vs adult life. One of these mutant strains showed a selective impairment in fetal thymopoiesis. In mice deficient in the transcription factor Ikaros, the thymus was devoid of thymocytes throughout gestation and for the first days after birth. Thymocytes were first detected in the postnatal thymus between 3–5 days post birth and expanded to reach nearly normal numbers in adults [51]. However, in all of the other strains described thus far, the major impairment is in adult thymopoiesis. The targeted genes include IL-7R [52], a T cell specific DNA-binding nuclear protein named Tcf-1 [53], signal transducer and activator of transcription (Stat) 3 [54], and α4 integrin [55]. Mice deficient in any of these genes showed normal or nearly normal fetal thymopoiesis but, in adulthood, the thymuses became severely atrophic. Collectively, these results indicate that (a) the factors driving thymopoiesis in fetal and adult life are not identical and (b) fetal thymopoiesis may be relatively independent of a number of factors that are required in later life.
The different fetal and adult thymic programs lead to the idea that the mature progeny produced by the fetal and adult waves of precursors may exhibit distinct functional properties once they leave the thymus and seed peripheral lymphoid organs. Indeed, there is evidence that cells of fetal origin may contribute to the variability in neonatal immune responses in both mice and humans. We [56] compared the antigen-specific responses of fetal-derived and adult-derived peripheral lymphocytes in chimeric animals in vivo. CD4+ cells of fetal origin produced copious amounts of both the Th1 cytokine γ-IFN and the Th2 cytokine IL-4 in response to immunization. Interestingly, the relative Th1 and Th2 responses of the fetal-derived cells fluctuated, depending on the antigen concentration. At low antigen concentrations, responses were Th2-skewed whereas, at high antigen concentrations, the responses showed a mature, adult-like Th1: Th2 ratio. In humans, it is clear that Th responses can be generated during fetal development and the nature of these responses is also quite variable. This is well illustrated with malaria, in which infection during pregnancy can lead to the transplacental passage of antigens that induce immune responses in utero. Malarial specific responses of cord blood cells can be Th1-biased, Th2-biased, or mixed Th1/Th2 [57, 58]. Therefore, in both mice and humans, it seems likely that at least some of the variability of the neonatal response pattern harkens from the fetal origin of many of the cells.
RECENT THYMIC EMIGRANTS
In the mouse, the rate of emigration from the thymus during newborn life is similar to that in adult life [59]. However, the relative proportions of recent thymic emigrants (RTE) would be expected to be higher due to the small number of resident peripheral T cells in neonates. This has recently been shown directly in mice transgenic for GFP driven by the RAG-2 promoter (RAG2p-GFP) [60]. In this model system, RTE retain high levels of GFP for approximately 1 week after their exit from the thymus [61]. In transgenic newborns, > 50% of splenic CD4+ cells are GFPhi out to 1 week post birth, demonstrating the large accumulation of RTE in the periphery in the first week of life [61]. This contrasts with the ≤ 25% of CD4+ cells in the adult spleen that are GFPhi. However, the murine neonatal spleen contains few T cells [62]; the major T cell populations are seen in the lymph nodes which contain mature proportions in 7 day old neonates [63]. Therefore, we compared the levels of GFPhi cells in the lymph nodes of neonates and adults from this strain of mouse. These studies revealed that RTE were also very abundant in neonatal lymph nodes; approximately 1/3 of neonatal lymph node cells were CD4+GFPhi, compared with only 10% of adult lymph node cells (Fig. 2). The relative abundance of RTE in neonates has also been demonstrated in a second experimental setting, using the more “traditional” approach of examining RTE. In this method, the thymus is injected with FITC and ~ 1 day later, RTE appear in peripheral tissues as FITC+ cells [59]. This approach differs from the transgenic system in that only RTE that have exited very recently, within the previous 24 hours, are analyzed. Using this technique, we have found that RTE are nearly 25 fold more abundant in the lymph nodes of 7 day old, relative to adult, mice (Fig. 2). The disparities in the fold differences between neonates and adults in the two systems may be related to the time of analysis. Over a one week period in the RAG-GFP model, dilution of some of the FITC signal may have occurred specifically in neonates because of their high rate of homeostatic proliferation [32]. In contrast, in the FITC-injection model, the time period is too short for significant dilution by proliferation and the relative percentages appear much higher in neonates than in adults. While we know they are more prevalent in the neonatal mouse, there is no information at present on the functional properties of murine neonatal RTE.
Fig. 2. Comparison of RTE in neonatal and adult lymph nodes using two different experimental systems.
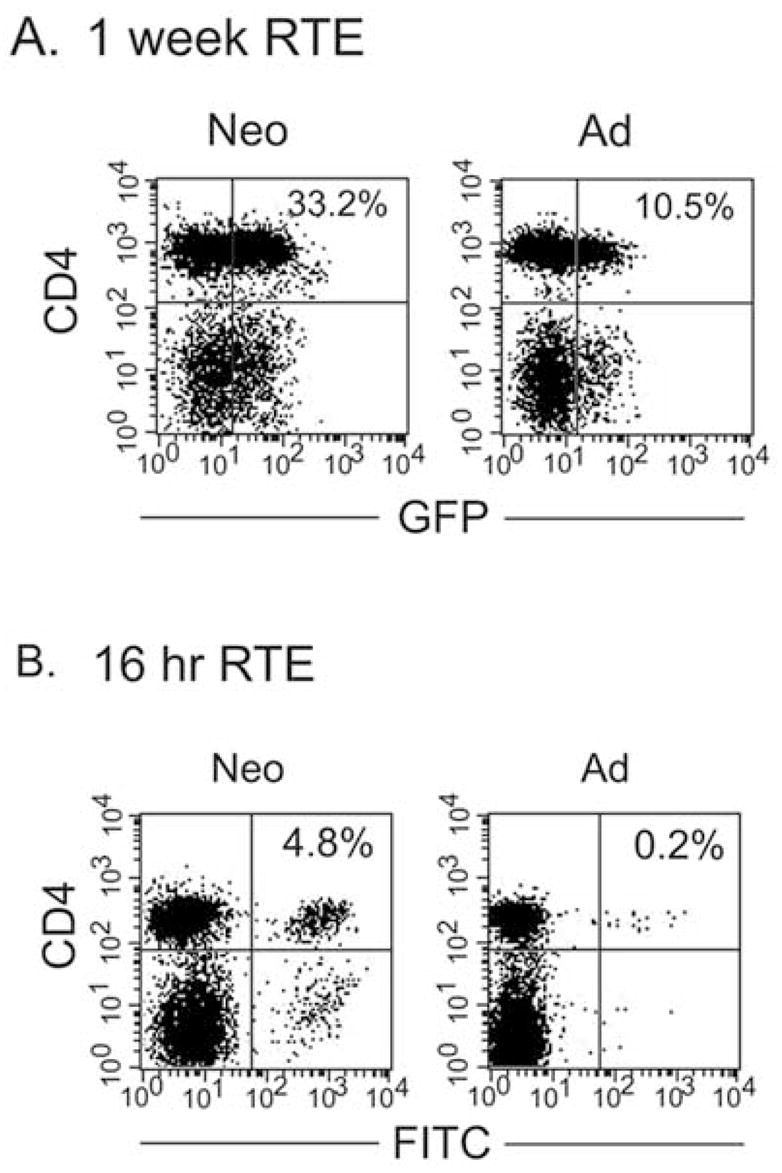
(A) Lymph node cells were prepared from 7 day old and adult RAG2p-GFP mice and stained with anti-CD4. (B) Six day old and adult C57BL/6 mice were injected intrathymically with 20 μg (in 20 μl) of fluorescein isothiocyanate per thymic lobe. Sixteen hr later, lymph node cells were prepared and stained with anti-CD4.
Analyses of the functional properties of RTE in adult mice may give important insights into the potential consequences of such a large RTE population in newborns. Unfortunately, there is not good agreement among reports about the capacities of RTE in adult mice. Fink and colleagues reported [61] that proliferation and IL-2 production were both diminished in RTE, relative to resident cells, in adult RAG-GFP transgenic mice. Reduced IL-2 production by RTE was also observed when genetically marked thymocytes were injected intrathymically [64]. In this system, RTE in the adult acquired the capacity to secrete mature levels of IL-2 over a 3–8 day period following exit from the thymus, consistent with a period of post-thymic maturation. In contrast, using the FITC injection method to examine emigrants ≤ 24 hr after thymus exit, Bendelac et al. [65] found that RTE in adult animals produced mature levels of IL-2 but up to 20 fold more IL-4 than did resident CD4+ cells. IL-4-production declined over the next six days, again consistent with a period of post-thymic maturation in the periphery [61, 64]. Swain and colleagues [66] also used the FITC injection method but waited 10 days before examing FITC+ cells in the periphery. They reported that their adult CD4+ RTE secreted mature levels of IL-2 and proliferated as well as resident naïve cells. At present, it is difficult to reconcile these seemingly disparate results into a cohesive picture of the functional capacities of adult RTE.
In humans, RTE are detected by the presence of T cell receptor excision circles (TREC). Cord blood cells contain nearly 10 fold higher levels of TREC, relative to adult cells [33, 34] and the TREC are found almost exclusively in the abundant CD45RA+, naïve phenotype population [34]. CD45RA+ cells in cord blood have been extensively described as having reduced proliferative and cytokine secreting capacities relative to adult resident CD45RA+CD4+ cells.
The available data in humans would suggest that neonatal RTE are functionally compromised. However, it is important to point out that it is not clear how neonatal RTE would compare with adult RTE. I.e., in humans, the “deficient” responses of neonatal RTE have been compared to total, resident naïve CD4+ cells from adults. A direct comparison between neonatal and adult RTE is possible only in experimental animal models at present. However, because of the discordance in results on adult RTE from different laboratories, it is difficult to predict how neonatal and adult RTE will compare in the different experimental settings outlined above. We also do not know whether the processes of post-thymic maturation are similar or different – this could potentially be affected by the gross differences in homeostatic proliferation at different stages in ontogeny. A true understanding of the impact of RTE on early life immune function will only be achieved when we understand how RTE themselves and post-thymic maturation compare in neonates and adults.
DEVELOPMENTAL DELAY IN CENTRAL DELETION
It was initially shown nearly 20 years ago that certain Vβ-bearing cells deleted in adult mice were readily detected both in the thymus and spleen of neonatal mice [67, 68]. Cells bearing forbidden Vβ began to decline in the thymus on day 4 of life and were no longer detectable by day 10 [67]. Therefore, the process of central deletion apparently requires several days post birth to become fully mature. The basis for this developmental delay is uncertain but is unlikely to be due to limiting levels of Aire since abundant expression of this autoimmune regulator gene was detected in the fetal thymus as early as E16 [69]. While a delay in deletion has been clearly shown in mice, leakage of self-reactive T cells to the periphery during fetal life in humans has also been postulated [70].
What are the consequences of such potentially autoreactive cells? Under normal (i.e., non-diseased or otherwise manipulated) circumstances, they may have little effect since self-tolerance seems to be preserved through peripheral clonal anergy [71]. However, under certain conditions, these cells can apparently become activated. Three experimental settings support this idea. First, Kojima and Prehn [72] originally showed that the incidence of autoimmunity is increased following neonatal thymectomy. In that subset of mice that did develop autoimmunity, the T cell compartment was enriched in cells bearing forbidden Vβ [68] – i.e., these cells are produced in the neonatal period but are then maintained and later naturally expanded in adult life. Second, it was found that treatment of neonatal thymectomized mice with high doses of IL-2 abrogated the in vitro anergy of the forbidden Vβ-bearing cells. This reversal of anergy in vitro was associated with the development of autoimmune manifestations in vivo [73]. Lastly and more recently, using an elegant double transgenic system, Gallegos and Bevan [74] showed that cells expressing the forbidden Vβ were detectable in peripheral tissues for weeks post birth. These cells were rapidly expanded following immunization, leading to the rapid development of autoimmune disease in the majority of animals. Therefore, cells bearing forbidden Vβ are probably quiescent most of the time. However, specific immunization, exogenous IL-2, and other unknown agents can elicit functionally robust responses from these cells.
The reason(s) for the developmental delay in central tolerance induction are not well understood. However, it could be speculated that, early in life, it may be dangerous to delete any TCR specificities. I.e., neonates encounter many new antigens for which they have no specific memory. When these new “antigens” are pathogenic infectious agents, the lack of memory may become life-threatening. Therefore, to mount efficient primary responses to newly encountered microbes, it may be important to have as many specificities as possible in peripheral tissues. The normal non-responsiveness of the cells could potentially be reversed in the presence of high level “danger signals” generated by, e.g., microbial compounds. The recruitment of forbidden Vβ-bearing cells to these responses, together with the Th1-promoting properties of microbial products, may contribute to the times when neonatal Th1 responses appear to be mature and fully protective.
NATURAL REGULATORY T CELLS (TREG) IN EARLY LIFE
Thymectomy of 3 day old neonatal mice leads to the increased incidence of autoimmune disease with target organs such as the ovaries, thyroid gland, and intestine [72]. These autoimmune syndromes are prevented by the early transfer into neonates of adult natural regulatory cells, or CD4+25+ cells [75]. One straightforward interpretation of these results is that murine neonates have not yet developed sufficient numbers of functional Treg to suppress the development of autoimmune function. Indeed, early reports [75] suggested that CD4+25+ cells were only significantly exported to the periphery after the third day of postnatal life. Several groups subsequently analyzed neonates for the expression of the protein scurfin (encoded by the Foxp3 gene), a specific marker for regulatory activity [76]. They reported a delay during ontogeny in the appearance of FoxP3-expressing CD4 single positive thymocytes. Mature frequencies of these cells did not become apparent by flow cytometric analyses until ≥ 6 days post birth [77, 78]. However, Bandeira and colleagues [79] reported that CD4+25+ cells from the thymus or spleen of newborns contained readily detectable FoxP3 mRNA, albeit at a level 2–3 times lower than that expressed by adults. Moreover, the FoxP3-expressing cells appeared to be capable of mature function since CD4+25+ cells from adult mice that had been thymectomized as neonates were able to suppress the development of autoimmune gastritis in vivo. These latter results suggest that there may be at least some functionally mature Treg already present in the periphery of 3 day old animals. This idea is supported by the observation that only a minority of neonatally thymectomized mice develop autoimmune disease [72] – i.e., the Treg present on day 3 of life may have acted to suppress autoimmunity in the unaffected mice. A synthesis of these data leads to the idea that fully functional natural Treg are present in the periphery of neonatal mice but their numbers are not yet fully mature. The appearance of functional Treg during early ontogeny appears to be shared by mice and humans. There is substantial evidence that CD4+CD25+FoxP3-expressing cells appear in the early second trimester of human pregnancy and these cells have potent suppressive activity in vitro [80–82].
The presence of functional Treg in neonates suggests that this cellular compartment could have major impact on the variability of neonatal T cell responses. For example, conditions favoring Treg suppression of Th1 responses could lead to the default Th2 bias. Such a state may occur in neonatal transplantation tolerance. Field and colleagues [83] reported that Treg activity capable of suppressing alloreactive Th1-associated CTL responses developed in animals neonatally tolerized to alloantigens. On the other hand, conditions favoring Treg downregulation of Th2 responses may promote mature, adult-level Th1 responses. In support of these ideas, endogenous neonatal CD25+ Treg appear to be able to downregulate both neonatal Th1 and Th2 responses in the mouse (Fig. 3).
Fig. 3. Endogenous natural Treg in neonates are competent to downregulate both Th1 and Th2 responses by neonatal CD4+ cells.
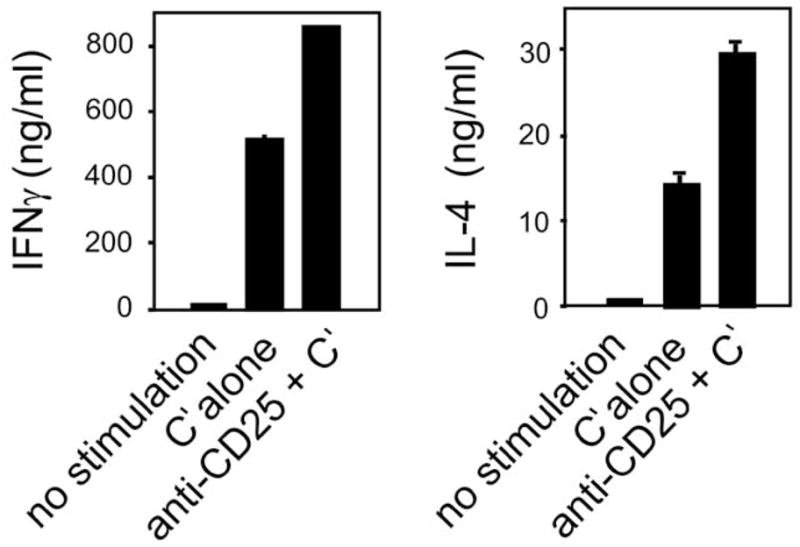
CD4+ lymph nodes from 7 day neonatal BALB/c mice were treated with anti-CD25 plus complement or with complement alone (control cells). Both the control and the specifically treated cells were then incubated with anti-CD3 plus anti-CD28 for 48 hr and the cytokine content of the supernatants was tested in specific ELISA.
A DEVELOPMENTAL DELAY IN TDT EXPRESSION
In the mouse, TdT is poorly detected in fetal hematopoietic organs and is not fully expressed in developing thymocytes until 3–5 days after birth [84–86]. As a consequence, TCR chains formed during fetal and early postnatal life lack significant N region addition in the CDR3 region [87, 88]. In humans, N region addition is also limited in early gestation (out to 16 weeks) [89, 90] but may be at mature levels by birth [91, 92].
The conservation of limited N region addition during ontogeny in both mice and humans suggests this phenomenon has important biological consequences. In mice, the potential importance of N region diversity has been extensively examined in genetically manipulated adult animals lacking TdT. Although Th1/Th2 activity was not analyzed, an impressive array of functional assays were compared in TdT-deficient and wild-type adult mice by Mathis and colleagues [93]. They found that responses to immunization or infection appeared to be normal in TdT-deficient mice - i.e., the absence of N regions did not compromise either the quality or the quantity of the immune response. Gavin and Bevan [94] looked at the fine specificity of the TdT-deficient CD8+ TCR repertoire by examining clones from individual mice immunized with a single H-2Db-restricted peptide. Although affinity did not seem to be grossly affected, each individual clone showed peptide promiscuity – i.e., the TdT-deficient clones cross-reacted with many more different peptides than wild-type clones. Together, these results suggest that the promiscuous nature of TCR reactivity in normal neonates may allow the small number of peripheral T cells to more effectively mount responses following immunization and infection. This is particularly important in early life because of the lack of specific memory in the newborn. However, this interpretation would seem at odds with more recent reports describing a reduced incidence of autoimmune disease in several strains of autoimmune prone mice bred to the TdT-deficient background [95–97]. The authors speculate that the neonatal repertoire may be deficient in autoreactive cells with high enough affinities to induce disease. Thus, the responsiveness of N region-deficient neonatal T cells may depend on the context in which antigen is encountered. In the case of a self-antigen, the TdT-deficient repertoire may be limited in autoreactive specificities or may have insufficient affinity to become pathogenic, in the absence of some adjuvant effect. However, in the presence of strong proinflammatory signals, as with immunization or infection, the affinity and promiscuous reactivity of the neonatal TCR may be sufficiently high to mount mature-like responses. Overall, the lack of N region diversity in the neonatal repertoire could be viewed as both protective against the development of autoimmune disease as well as protective against foreign invasion.
CONCLUDING REMARKS
Subsets of cells or physiological processes are either differently represented or uniquely present in the neonate. As a result, different combinations of cell types may be activated under different conditions, leading to the wide range of responses observed (Table 1). Conservation of T cell heterogeneity between mouse and man adds strength to the idea that this heterogeneity is critical in defining neonatal T cell responses. As development ensues, this heterogeneity becomes more restricted and, by young adulthood, the T cell compartment is much more homogeneous. Thus, as with development of the entire organism, the T lymphoid lineage appears to have extreme flexibility in early life and then gradually acquire a more limited and uniform potential.
Table 1.
Potential Impact of Populations/Processes Either Differentially Represented in or Unique to the Neonate
| Cell Subset/Process | Potential Consequences |
|---|---|
| Developmental specific epigenetic patterns at the Th2 and Th1 locus | Rapid development of robust Th2 function (mouse); persistence of low level Th2 function in Th1 cells (human); typically reduced Th1 responses (human) |
| ↑ homeostatically proliferating cells | Natural memory population; both Th1 and Th2 rapid effector activity; may provide mature levels of protection against cross-reactive exogenous antigens (human, mouse) |
| ↑ fetal origin cells | Robust cytokine production; variable Th1/Th2 ratios, depending on antigen concentration (mouse) or unknown conditions (human) |
| ↑ RTE | Variable IL-2, IL-4 production and proliferation |
| ↑ cells with forbidden TCR | May contribute to early life autoimmunity but also to responses against cross-reacting infectious agents |
| ↓ Treg activity/numbers | May modulate the development of either Th1 or Th2 function |
| ↑ TCR with no N region | Promiscuous reactivity, beneficial in infections; Low affinity or reduced reactivity to self-antigens, protective against autoimmunity |
References
- 1.Siegrist CA. Vaccination in the neonatal period and early infancy. Int Rev Immunol. 2000;19:195–219. doi: 10.3109/08830180009088505. [DOI] [PubMed] [Google Scholar]
- 2.Fadel S, Sarzotti M. Cellular immune responses in neonates. Int Rev Immunol. 2000;19:173–93. doi: 10.3109/08830180009088504. [DOI] [PubMed] [Google Scholar]
- 3.Adkins B. Development of neonatal Th1/Th2 function. Int Rev Immunol. 2000;19:157–171. doi: 10.3109/08830180009088503. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro-do-Couto LM, Boeije LC, Kroon JS, et al. High IL-13 production by human neonatal T cells: neonate immune system regulator? Eur J Immunol. 2001;31:3394–402. doi: 10.1002/1521-4141(200111)31:11<3394::aid-immu3394>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Webster RB, Rodriguez Y, Klimecki WT, Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. J Biol Chem. 2007;282:700–9. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- 6.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–12. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott SL, Macaubas C, Holt BJ, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–7. [PubMed] [Google Scholar]
- 8.Adkins B, Marshall-Clarke S, Leclerc C. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 9.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–75. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 11.Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. 2004;21:865–76. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Lee DU, Rao A. Molecular analysis of a locus control region in the T helper 2 cytokine gene cluster: a target for STAT6 but not GATA3. Proc Natl Acad Sci U S A. 2004;101:16010–5. doi: 10.1073/pnas.0407031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takemoto N, Koyano-Nakagawa N, Yokota T, Arai N, Miyatake S, Arai K. Th2-specific DNase I-hypersensitive sites in the murine IL-13 and IL-4 intergenic region. Int Immunol. 1998;10:1981–5. doi: 10.1093/intimm/10.12.1981. [DOI] [PubMed] [Google Scholar]
- 14.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–51. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 15.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–50. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 16.Guo L, Hu-Li J, Zhu J, et al. In TH2 cells the Il4 gene has a series of accessibility states associated with distinctive probabilities of IL-4 production. Proc Natl Acad Sci U S A. 2002;99:10623–8. doi: 10.1073/pnas.162360199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16:649–60. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson CB, Merkenschlager M. Chromatin structure and gene regulation in T cell development and function. Curr Opin Immunol. 2006;18:143–51. doi: 10.1016/j.coi.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005;17:105–19. doi: 10.1016/j.smim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–79. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–56. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 22.Adkins B, Ghanei A, Hamilton K. Developmental regulation of IL-4, IL-2, and IFN-gamma production by murine peripheral T lymphocytes. J Immunol. 1993;151:6117–6126. [PubMed] [Google Scholar]
- 23.Adkins B, Hamilton K, Ghanei A. Upregulation of murine neonatal T helper cell function by accessory cell factors. J Immunol. 1994;153:3378–3385. [PubMed] [Google Scholar]
- 24.Rose S, Lichtenheld M, Foote M, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J Immunol. 2007;178:2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loots GG, Locksley RM, Blankespoor CM, et al. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science. 2000;288:136–40. doi: 10.1126/science.288.5463.136. [DOI] [PubMed] [Google Scholar]
- 26.Adkins B, Guevara P, Rose S. Thymic and extrathymic contributions to T helper cell function in murine neonates. Heamatologica reports. 2006;2:9–13. [PMC free article] [PubMed] [Google Scholar]
- 27.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168:2820–7. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 28.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–91. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Min B, Paul WE. Endogenous proliferation: burst-like CD4 T cell proliferation in lymphopenic settings. Semin Immunol. 2005;17:201–7. doi: 10.1016/j.smim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–40. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 31.Schuler T, Hammerling GJ, Arnold B. Cutting Edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells. J Immunol. 2004;172:15–9. doi: 10.4049/jimmunol.172.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Le Campion A, Bourgeois C, Lambolez F, et al. Naive T cells proliferate strongly in neonatal mice in response to self- peptide/self-MHC complexes. Proc Natl Acad Sci USA. 2002;99:4538–43. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonland SO, Zimmer JK, Lopez-Benitez CM, et al. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–34. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- 34.Hassan J, Reen DJ. Human recent thymic emigrants--identification, expansion, and survival characteristics. J Immunol. 2001;167:1970–6. doi: 10.4049/jimmunol.167.4.1970. [DOI] [PubMed] [Google Scholar]
- 35.Hassan J, Reen DJ. IL-7 promotes the survival and maturation but not differentiation of human post-thymic CD4+ T cells. Eur J Immunol. 1998;28:3057–65. doi: 10.1002/(SICI)1521-4141(199810)28:10<3057::AID-IMMU3057>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 36.Fukui T, Katamura K, Abe N, et al. IL-7 induces proliferation, variable cytokine-producing ability and IL-2 responsiveness in naive CD4+ T-cells from human cord blood. Immunol Lett. 1997;59:21–8. doi: 10.1016/s0165-2478(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 37.Dardalhon V, Jaleco S, Kinet S, et al. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc Natl Acad Sci USA. 2001;98:9277–82. doi: 10.1073/pnas.161272698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109:1034–42. doi: 10.1182/blood-2006-06-027912. [DOI] [PubMed] [Google Scholar]
- 39.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol. 2003;171:61–8. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–17. [PubMed] [Google Scholar]
- 41.Hassan J, Reen DJ. Human Recent Thymic Emigrants-Identification, Expansion, And Survival Characteristics. J Immunol. 2001;167:1970–1976. doi: 10.4049/jimmunol.167.4.1970. [DOI] [PubMed] [Google Scholar]
- 42.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Jotereau F, Heuze F, Salomon-Vie V, Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol. 1987;138:1026–30. [PubMed] [Google Scholar]
- 44.Owen JJ, Ritter MA. Tissue interaction in the development of thymus lymphocytes. J Exp Med. 1969;129:431–42. doi: 10.1084/jem.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douagi II, Andre II, Ferraz JC, Cumano A. Characterization of T cell precursor activity in the murine fetal thymus: evidence for an input of T cell precursors between days 12 and 14 of gestation. Eur J Immunol. 2000;30:2201–2210. doi: 10.1002/1521-4141(2000)30:8<2201::AID-IMMU2201>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–74. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–62. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David-Fung ES, Yui MA, Morales M, et al. Progression of regulatory gene expression states in fetal and adult pro-T-cell development. Immunol Rev. 2006;209:212–36. doi: 10.1111/j.0105-2896.2006.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 50.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–5. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 51.Wang JH, Nichogiannopoulou A, Wu L, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–49. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 52.Crompton T, Outram SV, Buckland J, Owen MJ. Distinct roles of the interleukin-7 receptor alpha chain in fetal and adult thymocyte development revealed by analysis of interleukin-7 receptor alpha-deficient mice. Eur J Immunol. 1998;28:1859–66. doi: 10.1002/(SICI)1521-4141(199806)28:06<1859::AID-IMMU1859>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 53.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–91. [PubMed] [Google Scholar]
- 54.Sano S, Takahama Y, Sugawara T, et al. Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity. 2001;15:261–73. doi: 10.1016/s1074-7613(01)00180-7. [DOI] [PubMed] [Google Scholar]
- 55.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 56.Adkins B. Peripheral CD4(+) Lymphocytes Derived from Fetal versus Adult Thymic Precursors Differ Phenotypically and Functionally. J Immunol. 2003;171:5157–64. doi: 10.4049/jimmunol.171.10.5157. [DOI] [PubMed] [Google Scholar]
- 57.Malhotra I, Mungai P, Muchiri E, et al. Distinct Th1- and Th2-Type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect Immun. 2005;73:3462–70. doi: 10.1128/IAI.73.6.3462-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metenou S, Suguitan AL, Jr, Long C, Leke RG, Taylor DW. Fetal Immune Responses to Plasmodium falciparum Antigens in a Malaria-Endemic Region of Cameroon. J Immunol. 2007;178:2770–7. doi: 10.4049/jimmunol.178.5.2770. [DOI] [PubMed] [Google Scholar]
- 59.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 60.Yu W, Nagaoka H, Jankovic M, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–7. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 61.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–25. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 62.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: Turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 63.Adkins B, Hamilton K. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J Immunol. 1992;149:3448–3455. [PubMed] [Google Scholar]
- 64.Chang J-F, Thomas CA, III, Kung JT. Induction of high level IL-2 production in CD4+8− T helper lymphocytes requires post-thymic development. J Immunol. 1991;147:851. [PubMed] [Google Scholar]
- 65.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J Exp Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and Intrinsic Factors Lead to Antigen Unresponsiveness in CD4+ Recent Thymic Emigrants from Aged Mice. J Immunol. 2007;178:1321–31. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- 67.Schneider R, Lees RK, Pedrazzini T, Zinkernagel RM, Hengartner H, MacDonald HR. Postnatal disappearance of self-reactive (Vγ6+) cells from the thymus of Mlsa mice. J Exp Med. 1989;169:2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith H, Chen I-M, Kubo R, Tung KSK. Neonatal thymectomy results in a repertoire enriched in T cells deleted in the adult thymus. Science. 1989;245:749. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- 69.Zuklys S, Balciunaite G, Agarwal A, Fasler-Kan E, Palmer E, Hollander GA. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APE-CED) J Immunol. 2000;165:1976–83. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- 70.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–97. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 71.Jones LA, Chin LT, Merriam GR, Nelson LM, Kruisbeck AM. Failure of clonal deletion in neonatally thymectomized mice: Tolerance is preserved through clonal anergy. J Exp Med. 1990;172:1277. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 73.Andreu-Sanchez JL, Moreno de Alboran IM, Marcos MA, Sanchez-Movilla A, Martinez AC, Kroemer G. Interleukin 2 abrogates the nonresponsive state of T cells expressing a forbidden T cell receptor repertoire and induces autoimmune disease in neonatally thymectomized mice. J Exp Med. 1991;173:1323. doi: 10.1084/jem.173.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–6. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 75.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 77.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–6. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang Q, Su H, Knudsen G, Helms W, Su L. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dujardin HC, Burlen-Defranoux O, Boucontet L, Vieira P, Cumano A, Bandeira A. Regulatory potential and control of Foxp3 expression in newborn CD4+ T cells. Proc Natl Acad Sci USA. 2004;101:14473–8. doi: 10.1073/pnas.0403303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–8. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 81.Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. Eur J Immunol. 2005;35:383–90. doi: 10.1002/eji.200425763. [DOI] [PubMed] [Google Scholar]
- 82.Darrasse-Jeze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–21. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- 83.Field EH, Matesic D, Rigby S, Fehr T, Rouse T, Gao Q. CD4+CD25+ regulatory cells in acquired MHC tolerance. Immunol Rev. 2001;182:99–112. doi: 10.1034/j.1600-065x.2001.1820108.x. [DOI] [PubMed] [Google Scholar]
- 84.Gregoire KE, Goldschneider I, Barton RW, Bollum FJ. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979;123:1347. [PubMed] [Google Scholar]
- 85.Bogue M, Gilfillan S, Benoist C, Mathis D. Regulation of N-region diversity in antigen receptors through thymocyte differentiation and thymus ontogeny. Proc Natl Acad Sci USA. 1992;89:11011–5. doi: 10.1073/pnas.89.22.11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rothenberg E, Triglia D. Clonal proliferation unlinked to terminal deoxynucleotidyl transferase synthesis in thymocytes of young mice. J Immunol. 1983;130:1627–33. [PubMed] [Google Scholar]
- 87.Bogue M, Candeias S, Benoist C, Mathis D. A special repertoire of alpha: beta T cells in neonatal mice. EMBO J. 1991;10:3647–54. doi: 10.1002/j.1460-2075.1991.tb04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feeney AJ. Junctional sequences of fetal T cell receptor beta chains have few N regions. J Exp Med. 1991;174:115–24. doi: 10.1084/jem.174.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raaphorst FM, Kaijzel EL, van Tol MJ, Vossen JM, van den Elsen PJ. Non-random employment of V beta 6 and J beta gene elements and conserved amino acid usage profiles in CDR3 regions of human fetal and adult TCR beta chain rearrangements. Int Immunol. 1994;6:1–9. doi: 10.1093/intimm/6.1.1. [DOI] [PubMed] [Google Scholar]
- 90.George JF, Jr, Schroeder HW., Jr Developmental regulation of D beta reading frame and junctional diversity in T cell receptor-beta transcripts from human thymus. J Immunol. 1992;148:1230–9. [PubMed] [Google Scholar]
- 91.Hall MA, Reid JL, Lanchbury JS. The distribution of human TCR junctional region lengths shifts with age in both CD4 and CD8 T cells. Int Immunol. 1998;10:1407–19. doi: 10.1093/intimm/10.10.1407. [DOI] [PubMed] [Google Scholar]
- 92.Schelonka RL, Raaphorst FM, Infante D, Kraig E, Teale JM, Infante AJ. T cell receptor repertoire diversity and clonal expansion in human neonates. Pediatr Res. 1998;43:396–402. doi: 10.1203/00006450-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 93.Gilfillan S, Bachmann M, Trembleau S, et al. Efficient immune responses in mice lacking N-region diversity. Eur J Immunol. 1995;25:3115–3122. doi: 10.1002/eji.1830251119. [DOI] [PubMed] [Google Scholar]
- 94.Gavin MA, Bevan MJ. Increased peptide promiscuity provides a rationale for the lack of N regions in the neonatal T cell repertoire. Immunity. 1995;3:793–800. doi: 10.1016/1074-7613(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 95.Conde C, Weller S, Gilfillan S, Marcellin L, Martin T, Pasquali JL. Terminal deoxynucleotidyl transferase deficiency reduces the incidence of autoimmune nephritis in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 1998;161:7023–30. [PubMed] [Google Scholar]
- 96.Feeney AJ, Lawson BR, Kono DH, Theofilopoulos AN. Terminal deoxynucleotidyl transferase deficiency decreases autoimmune disease in MRL-Fas(lpr) mice. J Immunol. 2001;167:3486–93. doi: 10.4049/jimmunol.167.6.3486. [DOI] [PubMed] [Google Scholar]
- 97.Robey IF, Peterson M, Horwitz MS, et al. Terminal deoxynucleotidyltransferase deficiency decreases autoimmune disease in diabetes-prone nonobese diabetic mice and lupus-prone MRL-Fas(lpr) mice. J Immunol. 2004;172:4624–9. doi: 10.4049/jimmunol.172.7.4624. [DOI] [PubMed] [Google Scholar]


