Abstract
Xenobiotic-DNA adducts are used as biomarkers to assess the genotoxic effects of carcinogens. Rats were dosed with 4-aminobiphenyl (4-ABP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), or 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). DNA was isolated from the colons of vehicle and carcinogen-treated rats and digested using different nucleases and alkaline phosphatase. Deoxyribonucleoside adducts were quantified by capillary liquid chromatography-tandem mass spectrometry (LC-MS/MS) using isotope dilution methods with deuterated internal standards. Major adducts were those bound to the C8 position of deoxyguanosine. 3′- and 5′-Exonucleases were the most efficient nucleases at isolating dG-C8-ABP adducts. However, bulky adducts such as dG-C8-MeIQx and dG-C8-PhIP were better isolated using nuclease P1 rather than a combination of micrococcal nuclease and spleen phosphodiesterase. The use of DNase I enhanced the detection of all three adducts. We describe LC-MS/MS methods for DNA adduct detection and support the testing of different nucleases that increase DNA digestion efficiency and make available more DNA adducts for detection.
Keywords: 4-aminobiphenyl; 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; MeIQx; 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; PhIP
INTRODUCTION
The development of the 32P-postlabeling method over 25 years ago (1) greatly increased the ability to detect chemically modified DNA for the assessment of genotoxic effects of carcinogens. Since that time, several new methods have been developed. These methods include immunoassays, gas or liquid chromatography-mass spectrometry, fluorescence or electrochemical detection, and atomic absorbance. As reviewed previously (2,3), each method has advantages and disadvantages in terms of the amount of DNA required (1–100 μg), sensitivity (1 adduct/107 nucleosides – 1/1010), and specificity for adducted bases.
As novel approaches to DNA adduct analysis have been developed, the challenge has been to maintain the sensitivity afforded by radiolabeling while increasing the information derived from the analysis. The use of on-line liquid chromatography (4) and tandem mass spectrometric (MS/MS) methods has shown great promise in this application (5,6). The hydrolysis of DNA to yield a mixture of natural and adducted nucleosides provides sensitive and specific DNA adduct structural information. Following DNA isolation, enzymatic digestion of DNA by nucleases greatly influences the ability of the detection methods to identify DNA adducts. Detection methods identify either adducted nucleosides or nucleotides produced by the hydrolysis of duplex DNA. Incomplete hydrolysis results in oligonucleotides, which are difficult to identify by mass spectrometry or immunoaffinity (due to antibody specificity) or are incorrectly identified as other adducts by 32P-postlabeling (due to differences in migration distances by thin layer chromatography). Therefore, it is important to determine optimal conditions for DNA digestion for MS/MS sample preparation.
4-Aminobiphenyl (4-ABP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) are each implicated as important arylamine carcinogens for humans (7). These carcinogens undergo N-oxidation by cytochrome P450s to form the corresponding N-hydroxyarylamine metabolites (8,9). The hydroxylated metabolites can then be O-acetylated by N-acetyltransferase to form electrophilic metabolites that react with DNA to produce DNA adducts (9–11). Since rat colon has very high N-acetyltransferase catalytic activity (12), it was used to generate the DNA adducts for our optimization studies.
LC-MS/MS methods for characterizing and quantifying 4-ABP DNA adducts (6,13,14), MeIQx DNA adducts (15) and PhIP DNA adducts (5,16) have been reported previously. This paper describes improved LC-MS/MS methods and compares the efficiency of different nucleases at digesting DNA isolated from the colon of rats treated with 4-ABP, MeIQx, and PhIP.
MATERIALS AND METHODS
Materials
Alkaline phosphatase, 4-ABP, DNase I, micrococcal nuclease, nuclease P1, phenol solution, phenol/chloroform/isoamyl alcohol solution, and spleen phosphodiesterase were obtained from Sigma Aldrich (St. Louis, MO). Proteinase K solution was purchased from U. S. Biological (Swampscott, MA). Standards dG-C8-ABP, d5 dG-C8-ABP, MeIQx, PhIP, dG-C8-PhIP, and d3 dG-C8-PhIP were obtained from Toronto Research Chemicals (Ontario, Canada). Standards dG-C8-MeIQx and d3-dG-C8-MeIQx were generously provided by Dr. Robert Turesky of the Wadsworth Center of the New York State Department of Health.
Animals and Treatments
Female F344/WKY hybrid rats (200–250 g) were housed in groups of 1–3 per cage on a 12 h dark/light cycle with ad libitum access to rodent diet (Lab Diet, Brentwood, MO) and tap water. Each rat received a single oral dose by gavage of 50 mg/kg of 4-ABP, 25 mg/kg of MeIQx, or 50 mg/kg of PhIP dissolved in DMSO. Injection volumes were 1 mL/kg. Twenty-four hours after injection, rats were euthanized by CO2 asphyxiation. Colons were collected, cleaned, snap frozen in liquid nitrogen, and stored at −80°C.
DNA isolation and digestion
Colons were homogenized in two volumes of 20 mM sodium phosphate buffer (pH 7.4). One-tenth volumes each of proteinase K solution (20 mg/mL) and 10% SDS were added to the tissue homogenate, and the mixture was incubated at 37°C for 1 hour. One volume of phenol equilibrated with 10 mM Tris HCl (pH 8.0), was added to the mixture, which was then vortexed and centrifuged at 3600 x g for 15 minutes. The aqueous layer was removed and added to 1 volume of phenol:chloroform:isoamyl alcohol (25:24:1) saturated with 10 mM Tris HCl (pH 8.0), which was vortexed and centrifuged. The aqueous layer was removed and added to 1 volume of cold (−20°C) isopropanol, and the mixture was vortexed and centrifuged. The DNA pellet was washed with 70% ethanol and redissolved in 5 mM Tris (pH 7.4) containing 1 mM CaCl2, 1 mM ZnCl2, and 10 mM MgCl2. The DNA was quantified by A260. DNA quality was monitored by A260/280 and was consistently above 1.9. DNA (85–150 μg) was spiked with 500 pg of deuterated standard and then digested as follows: Test 1: Five units of micrococcal nuclease and 0.01 units of spleen phosphodiesterase were added and incubated for 6 hours at 37°C. Five units of alkaline phosphatase were then added, and the samples were incubated overnight at 37°C. Test 2: Five units of nuclease P1 were added and incubated for 6 hours at 37°C. Five units of alkaline phosphatase were added, and the samples were incubated overnight at 37°C. Test 3: Ten units of DNase I were added and incubated for 1 hour at 37°C. Five units of nuclease P1 were then added and incubated for 6 hours followed by the addition of 5 units of alkaline phosphatase and overnight incubation at 37°C. Test 4: Ten units of DNase I were added and incubated for 1 hour at 37°C. Then 0.01 units of spleen phosphodiesterase and 0.01 units of snake venom phosphodiesterase were added and incubated for 6 hours followed by the addition of 5 units of alkaline phosphatase for overnight incubation at 37°C. Test 5: Ten units of DNase I were added and incubated for 1 hour at 37°C. Five units of micrococcal nuclease, 5 units of nuclease P1, 0.01 units of spleen phosphodiesterase, and 0.01 units of snake venom phosphodiesterase were added and incubated for 6 hours followed by the addition of 5 units of alkaline phosphatase for overnight incubation at 37°C. The final volume of all digestion tests was approximately 100 μL.
Digestions were stopped by the addition of 2 volumes of acetonitrile. Samples were filtered using a 5,000 nominal molecular weight limit centrifugal filter device (Millipore, Bedford, MA). Samples were then evaporated to approximately 50 μL, reconstituted in 5% aqueous acetonitrile to a final volume of 100 μL, and taken for LC-MS/MS analysis.
Capillary high performance liquid chromatography (HPLC)
Samples were loaded onto a Inertsil C18 precolumn (5 mm × 300 μm i.d., 5 μm, LC Packings) using Perkin Elmer ABI 140D syringe pumps and a Hewlett Packard 1100 Series autosampler (solid lines in Figure 1). Binary gradient HPLC separation was adapted from methods described previously (Doerge et al., 1999). Solvent A was water with 0.05% formic acid, and solvent B was acetonitrile with 0.05% formic acid. Loading conditions were 5% solvent B for 15 minutes at a flow rate of 20 μl/min. During sample loading, the capillary column was equilibrated at 5% solvent B. After 15 minutes of on-line sample clean-up, a switching valve was used to back flush the analyte onto the Intersil C18 capillary column (15 cm × 75 μm i.d., 5 μm, LC Packings) (dashed lines in Figure 1). The sample was eluted using Hewlett Packard 1100 Series binary pumps set at a flow rate of 0.4 ml/min equipped with a pre-column flow splitter having a fixed ratio flow split of 2000:1. Gradient conditions were 5% B to 60% B over 5 minutes; 60% B for 10 minutes; 60% B to 90% B over 5 minutes; 90% B for 5 minutes; 90% B to 5% B over 10 minutes; 5% B for 5 minutes. During sample elution onto the analytical column, the precolumn was washed using the following gradient conditions: 5% B to 90% B over 10 minutes; 90% B for 10 minutes; 90% B to 5% B over 10 minutes; 5% B for 10 minutes.
Figure 1.
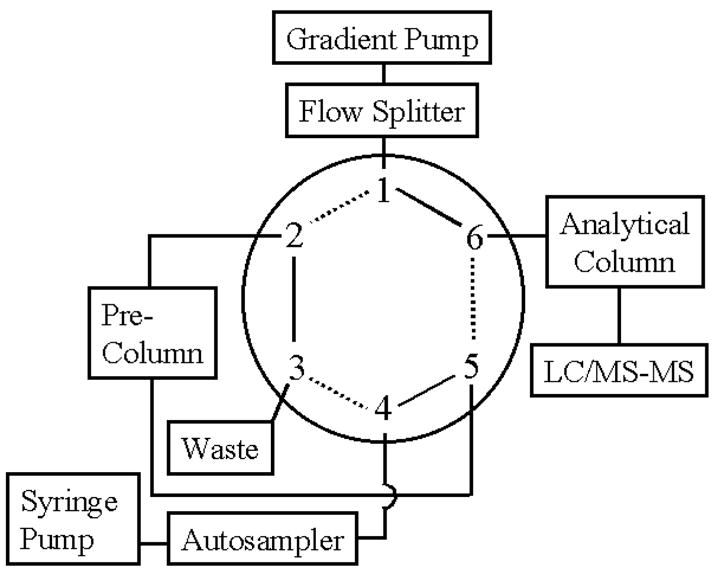
Schematic diagram of the switching valve used for on-line sample preparation. Solid lines represent sample loading, concentration, and clean-up. Dashed lines indicate analyte elution into the mass spectrometer.
Mass spectrometry
Samples were introduced into a Micromass Quattro LC triple quadrupole mass spectrometer using a custom-built nanospray with 20 μm i.d. fused silica tubing inserted through 125 μm i.d. PEEK tubing. The other end of the capillary was inserted through a blunted 20 gauge needle, which was fitted to a tee union connected to nitrogen gas as a sheath gas. Connection to the LC system was made using an internal union. All samples were analyzed using the positive ionization mode. Capillary voltages, cone voltages, collision energies, and collision gas (argon) pressures were optimized by monitoring ion intensities while infusing 10 ng/μL of standards in 5% aqueous acetonitrile, 0.05% formic acid at a flow rate of 0.5 μL/min. Electrospray ionization was set at 2.5 kV for dG-C8-ABP and dG-C8-MeIQx and 3.0 kV for dG-C8-PhIP. Cone voltages were 25 V for dG-C8-ABP and dG-C8-MeIQx and 30 V for dG-C8-PhIP. Argon was used as the collision gas and set at 2.0e−4 mBar. Collision energies were 25 V for dG-C8-ABP and dG-C8-MeIQx and 20 V for dG-C8-PhIP. Multiple reaction monitoring (MRM) (dwell time 0.5 sec; span 0.4 Daltons) was used to measure the [M+H]+ to [(M-116) + H]+ (loss of deoxyribose) mass transition of each adduct (435 to 319 for dG-C8-ABP, 440 to 324 for d5 dG-C8-ABP, 479 to 363 for dG-C8-MeIQx, 482 to 366 for d3 dG-C8-MeIQx, 490 to 374 for dG-C8-PhIP, and 493 to 377 for d3 dG-C8-PhIP).
RESULTS
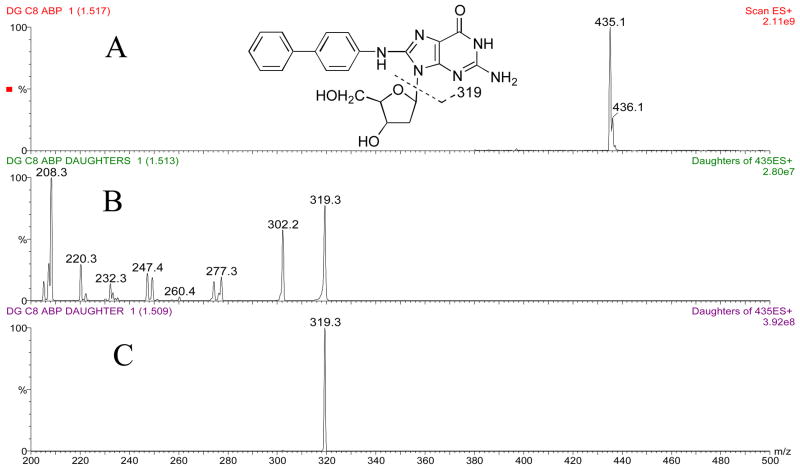
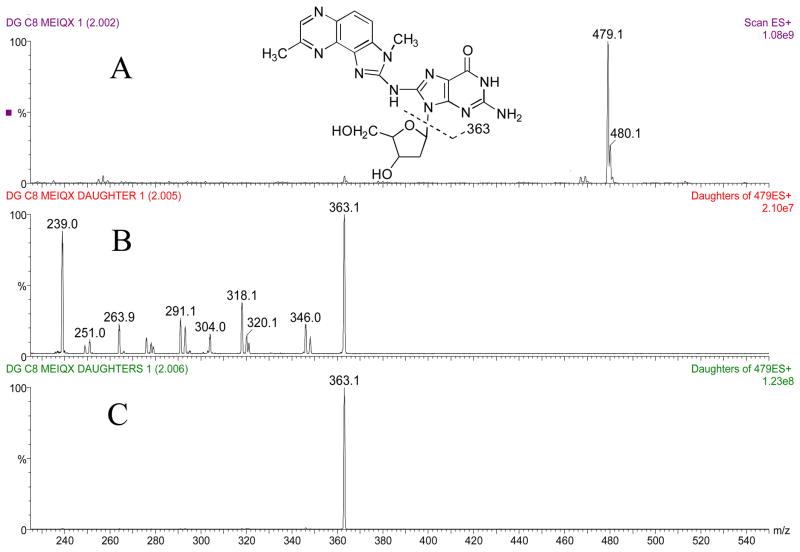
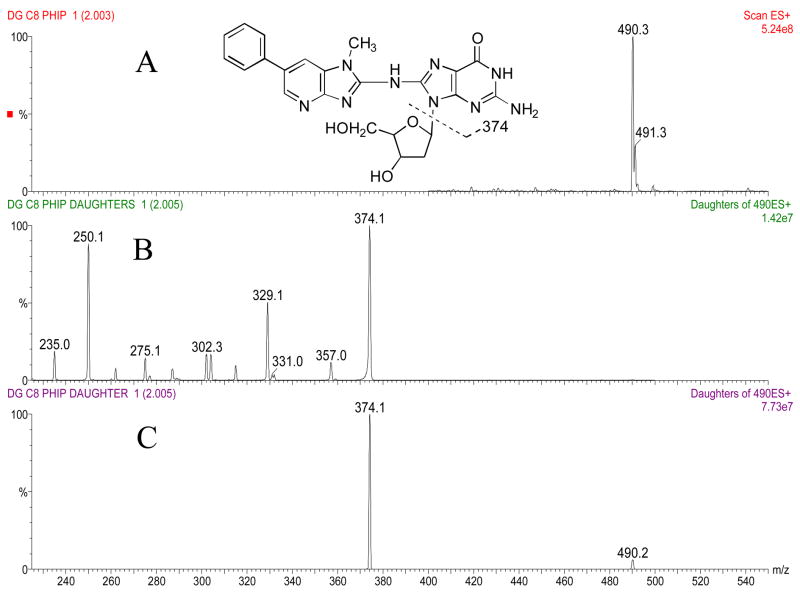
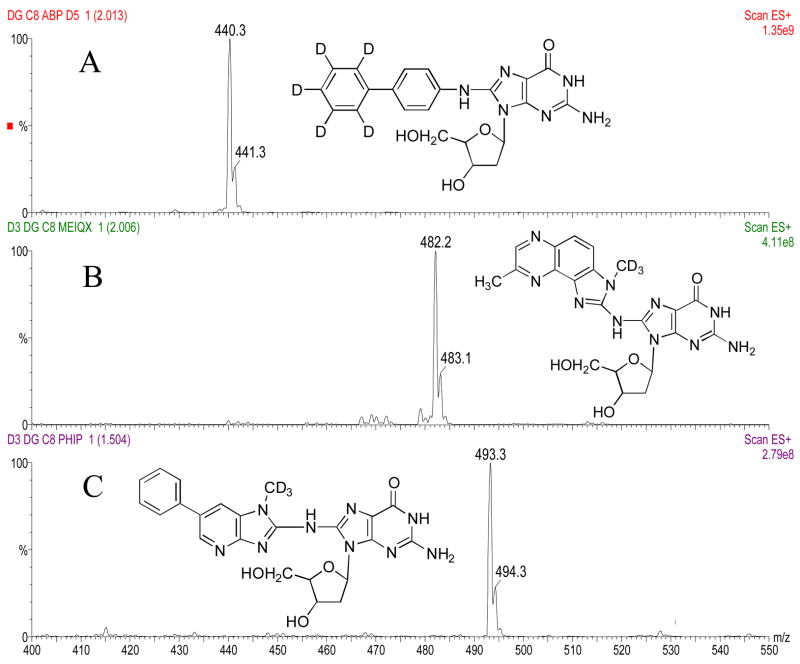
The masses and structures of dG-C8-ABP, dG-C8-MeIQx, and dG-C8-PhIP are shown in Figures 2a, 3a and 4a, respectively. Masses and structures of the deuterated internal standards d5 dG-C8-ABP, d3 dG-C8-MeIQx, and d3 dG-C8-PhIP are shown in Figure 5. High capillary and cone voltages produced in-source fragmentation that reduced sensitivity by reducing the amount of parent compound that passed through the first quadrupole (data not shown). High collision energies also reduced sensitivity by producing multiple fragments (Figures 2b, 3b, and 4b). Cleavage of the glycosidic bond linking the adducted base to the deoxyribose is the most easily broken bond for all three DNA adducts. Therefore, to increase sensitivity, collision energies were set to only cleave the glycolytic bond and produce one major fragment, which was [(M –116) + H]+ (Figures 2c, 3c, 4c).
Figure 2.
Panel A: Structure and ion spectra of dG-C8-ABP are shown. Panel B: Collision induced dissociation fragmentation of dG-C8-ABP at −50 V collision energy. Panel C: Collision induced dissociation fragmentation of dG-C8-ABP at −25 V collision energy. The major fragment produced is the aglycone ion of m/z 319.
Figure 3.
Panel A: Structure and ion spectra of dG-C8-MeIQx are shown. Panel B: Collision induced dissociation fragmentation of dG-C8-MeIQx at −50 V collision energy. Panel C: Collision induced dissociation fragmentation of dG-C8-MeIQx at −25 V collision energy. The major fragment produced is the aglycone ion of m/z 363.
Figure 4.
Panel A: Structure and ion spectra of dG-C8-PhIP are shown. Panel B: Collision induced dissociation fragmentation of dG-C8-PhIP at −50 V collision energy. Panel C: Collision induced dissociation fragmentation of dG-C8-PhIP at −25 V collision energy. The major fragment produced is the aglycone ion of m/z 374.
Figure 5.
Structure and parent ion spectra of d5 dG-C8-ABP (Panel A), d3 dG-C8-MeIQx (Panel B), and d3 dG-C8-PhIP (Panel C) are shown.
No DNA adducts were detected in the colons of vehicle-treated rats. The dG-C8 carcinogen adduct was identified in rat colon following treatment with ABP, MeIQx, or PhIP. However, the DNA adduct levels varied with different nuclease digestion test as shown in Figure 6. Micrococcal nuclease and spleen phosphodiesterase are commonly used nucleases in DNA adduct analyses. Spleen phosphodiesterase together with snake venom phosphodiesterase were the most efficient nucleases at digesting 4-ABP DNA adducts (test 4). The combination of micrococcal nuclease, nuclease P1, spleen phosphodiesterase, and snake venom phosphodiesterase (test 5) did not increase the number of 4-ABP adducts detected in comparison to the use of only micrococcal nuclease and spleen phosphodiesterase (test 1). However, the combination of all four nucleases (test 5) significantly increased the number of both MeIQx and PhIP adducts detected when compared to only using micrococcal nuclease and spleen phosphodiesterase. Although MeIQx and PhIP DNA adducts are detected after the use of micrococcal nuclease and spleen phosphodiesterase, the use of only these two nucleases is the least efficient DNA digestion protocol tested (test 1). As indicated by higher amounts of MeIQx and PhIP adducts being detected, the use of nuclease P1 (test 2) increased digestion efficiency and in combination with DNase I (test 3) further increased digestion efficiency when compared to only using micrococcal nuclease and spleen phosphodiesterase.
Figure 6.
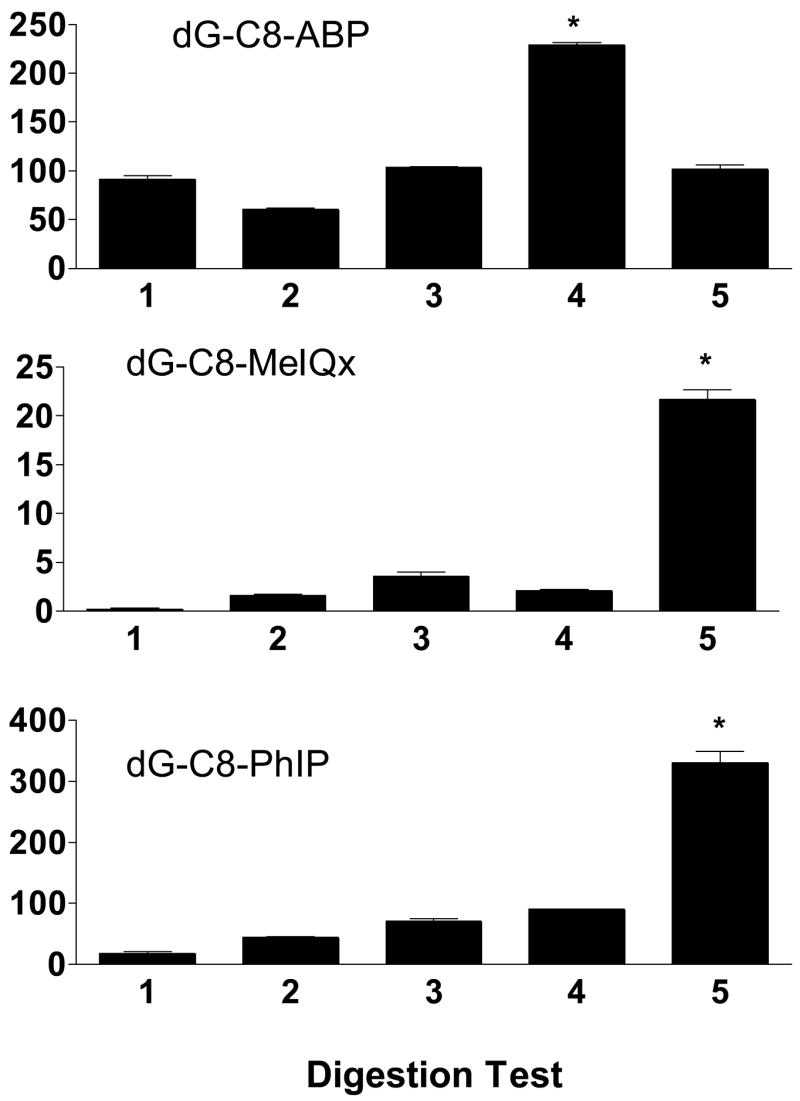
Carcinogen-DNA adduct levels (adducts/108 nucleosides) in carcinogen-treated rat colon following various digestion strategies. Each bar represents mean ± SE for three biological samples. *Significantly (p < 0.001) higher than other digestion tests following ANOVA and Tukey-Kramer Multiple Comparisons Test.
DISCUSSION
LC-MS has been successfully used by others to characterize and quantitate DNA adducts. On-line sample preparation and clean-up using switching valves followed by microbore LC-ESI-MS was used for the characterization and quantitation of 4-ABP adducts (13,14). Capillary LC-μESI-MS/MS was utilized for the detection of 4-ABP adducts in human pancreatic tissue (6). Microbore LC-ESI-MS (16) and capillary LC-μESI-MS/MS (5) were also used for the detection of PhIP adducts. Capillary LC-μESI-MS/MS was also used to detect MeIQx adducts in rat liver (15). We used column switching to perform on-line sample enrichment to reduce sample loss prior to analyte separation by capillary HPLC. Detection by μESI-MS/MS was optimized to increase sensitivity through the use of low capillary and cone voltages and low collision energies to cleave the glycosidic bond and detect the protonated adduct base.
Initial reports describing improved sensitivity of 32P-postlabeling analysis of DNA adducts included the use of 1-butanol to enrich samples (17) and the use of nuclease P1 to enhance detection through selective radiolabeling of adducted nucleotides (18). However, a comparison of specific nucleases to enhance sensitivity by increasing DNA digestion efficiency has not been reported. DNase I, micrococcal nuclease, and nuclease P1 are nonspecific endonucleases that digest single- and double-stranded DNA to oligodeoxyribonucleotides. Micrococcal nuclease and nuclease P1 are more active on single-stranded DNA, but the high temperatures (> 60°C) required to produce single-stranded DNA shortens the incubation time and inactivates most enzymes. The subsequent use of phosphodiesterases is required to liberate mononucleotides from the oligonucleotides. Spleen phosphodiesterase (phosphodiesterase II) is a 3′-exonuclease, and snake venom phosphodiesterase (phosphodiesterase I) is a 5′-exonuclease. The phosphate groups must be removed from the nucleotides before analysis by electrospray positive ionization. Alkaline phosphatase nonspecifically dephosphorylates phosphate monoesters, including nucleotides. Nuclease P1 also has phosphatase activity and can remove 3′-phosphates from 3′-nucleotides and oligonucleotides. An often overlooked requirement for the activity of all these nucleases is the presence of divalent cations. Micrococcal nuclease requires Ca2+; snake venom phosphodiesterase requires Mg2+; nuclease P1 and alkaline phosphatase require Zn2+.
The structure of the carcinogen determines its interaction with DNA and the conformation of the adducted DNA. The conformation of the adducted DNA affects the ability of DNA repair enzymes to repair the adducted base, thereby resulting in possible mutations and genotoxicity. Both DNA repair enzymes and nucleases interact with DNA. We propose here that the conformation of adducted DNA also affects the ability of nucleases to efficiently digest DNA. NMR structural studies performed on an 11-mer duplex DNA containing the dG-C8-PhIP adduct revealed that, in the main form, PhIP intercalates into the DNA helix by denaturing and displacing the modified base, causing significant bending of the double helix (19,20). Although NMR solution structures of MeIQx adducts are not experimentally available, energy minimization modeling and conformation analysis generated MeIQx DNA adduct structures in which the heterocyclic ring system of the amine is partly intercalated into the DNA helix (21). In contrast to MeIQx and PhIP, 4-ABP does not intercalate into the DNA helix and only weakly distorts DNA. A 4-ABP modified 15-mer duplex DNA adopted one major conformation in which the arylamine moiety was positioned in the major groove of the weakly distorted double helix (22). A 15-mer duplex DNA was also modified with 2-aminofluorene and was found to adopt essentially the same major conformation as dG-C8-ABP (23).
The difference in digestion efficiencies of micrococcal nuclease and nuclease P1 at hydrolyzing MeIQx and PhIP adducts, taken together with the distortion PhIP produces with duplex DNA, indicates that the active site of micrococcal nuclease may not be large enough to accommodate the bulky MeIQx and PhIP adducts. The micrococcal nuclease may be displaced from the DNA strand by the relatively large MeIQx and PhIP adducts before this nuclease is able to hydrolyze them. Based upon the ability of nuclease P1 to more efficiently hydrolyze MeIQx and PhIP adducts than micrococcal nuclease, the active site of nuclease P1 is probably larger than the active site of micrococcal nuclease. In contrast, both micrococcal nuclease and nuclease P1 are able to hydrolyze 4-ABP adducts to approximately the same extent due to the minimal distortion 4-ABP produces in the structure of the double-stranded DNA. DNase I increases digestion efficiency of all three adducts tested by hydrolyzing duplex DNA into shorter fragments that are better able to be completely digested by the other nucleases.
DNA adducts are useful biomarkers to measure exposure to carcinogens and potential genotoxic effects. Since the doses of 4-ABP, MeIQx, and PhIP administered to rats in this study far exceed human exposure levels, the use of DNA adducts to determine exposure levels and relate these levels to cancer risk in humans will require optimization of digestion methods to increase sensitivity for DNA adduct detection. Recently, this method was successfully utilized to measure MeIQx (23) and PhIP (24) DNA adducts in chinese hamster ovary cells transfected with human metabolic activation/deactivation enzymes and much lower doses of MeIQx (0.5 to 3 nM) and PhIP (0.5 to 1.5 nM) than used in the present study. Our DNA digestion results support the use of different hydrolytic schemes for the preparation of samples for analysis of DNA adducts to increase sensitivity for monitoring genetic damage from human environmental carcinogens.
Acknowledgments
This work was partially supported by USPHS grant CA034627 from the National Cancer Institute and postdoctoral fellowship grants from Kosair Charities and the James Graham Brown Cancer Center to Jason R. Neale.
References
- 1.Randerath K, Reddy MV, Gupta RC. 32P-labeling test for DNA damage. Proc Natl Acad Sci USA. 1981;78(10):6126–9. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips DH, Farmer PB, Beland FA, Nath RG, Poirier MC, Reddy MV, Turteltaub KW. Methods of DNA adduct determination and their application to testing compounds for genotoxicity. Environ Mol Mutagen. 2000;35(3):222–33. doi: 10.1002/(sici)1098-2280(2000)35:3<222::aid-em9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Sharma RA, Farmer PB. Biological relevance of adduct detection to the chemoprevention of cancer. Clin Cancer Res. 2004;10(15):4901–12. doi: 10.1158/1078-0432.CCR-04-0098. [DOI] [PubMed] [Google Scholar]
- 4.Opiteck GJ, Lewis KC, Jorgenson JW, Anderegg RJ. Comprehensive on-line LC/LC/MS of proteins. Anal Chem. 1997;69(8):1518–24. doi: 10.1021/ac961155l. [DOI] [PubMed] [Google Scholar]
- 5.Rindgen D, Turesky RJ, Vouros P. Determination of in vitro formed DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine using capillary liquid chromatography/electrospray ionization/tandem mass spectrometry. Chem Res Toxicol. 1995;8(8):1005–13. doi: 10.1021/tx00050a003. [DOI] [PubMed] [Google Scholar]
- 6.Ricicki EM, Soglia JR, Teitel C, Kane R, Kadlubar F, Vouros P. Detection and quantification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl adducts in human pancreas tissue using capillary liquid chromatography-microelectrospray mass spectrometry. Chem Res Toxicol. 2005;18(4):692–9. doi: 10.1021/tx049692l. [DOI] [PubMed] [Google Scholar]
- 7.National Toxicology Program. Report on Carcinogens. 11. U.S. Department of Health and Human Services, Public Health Service; Research Triangle Park, NC: 2005. [Google Scholar]
- 8.Landi MT, Sinha R, Lang NP, Kadlubar FF. Human cytochrome P4501A2. IARC Sci Publ. 1999;(148):173–95. [PubMed] [Google Scholar]
- 9.Turesky RJ. Heterocyclic aromatic amine metabolism, DNA adduct formation, mutagenesis, and carcinogenesis. Drug Metab Rev. 2002;34(3):625–50. doi: 10.1081/dmr-120005665. [DOI] [PubMed] [Google Scholar]
- 10.Hein DW. Acetylator genotype and arylamine-induced carcinogenesis. Biochim Biophys Acta. 1988;948(1):37–66. doi: 10.1016/0304-419x(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 11.Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat Res. 2002;506–507:65–77. doi: 10.1016/s0027-5107(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 12.Hein DW, Rustan TD, Bucher KD, Furman EJ, Martin WJ. Extrahepatic expression of the N-acetylation polymorphism toward arylamine carcinogens in tumor target organs of an inbred rat model. J Pharmacol Exp Ther. 1991;258(1):232–6. [PubMed] [Google Scholar]
- 13.Beland FA, Doerge DR, Churchwell MI, Poirier MC, Schoket B, Marques MM. Synthesis, characterization, and quantitation of a 4-aminobiphenyl-DNA adduct standard. Chem Res Toxicol. 1999;12(1):68–77. doi: 10.1021/tx980172y. [DOI] [PubMed] [Google Scholar]
- 14.Doerge DR, Churchwell MI, Marques MM, Beland FA. Quantitative analysis of 4-aminobiphenyl-C8-deoxyguanosyl DNA adducts produced in vitro and in vivo using HPLC-ES-MS. Carcinogenesis. 1999;20(6):1055–61. doi: 10.1093/carcin/20.6.1055. [DOI] [PubMed] [Google Scholar]
- 15.Paehler A, Richoz J, Soglia J, Vouros P, Turesky RJ. Analysis and quantification of DNA adducts of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in liver of rats by liquid chromatography/electrospray tandem mass spectrometry. Chem Res Toxicol. 2002;15(4):551–61. doi: 10.1021/tx010178e. [DOI] [PubMed] [Google Scholar]
- 16.Crosbie SJ, Murray S, Boobis AR, Gooderham NJ. Mass spectrometric detection and measurement of N2-(2′-deoxyguanosin-8-yl)PhIP adducts in DNA. J Chromatogr B Biomed Sci Appl. 2000;744(1):55–64. doi: 10.1016/s0378-4347(00)00227-9. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RC. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res. 1985;45(11 Pt 2):5656–62. [PubMed] [Google Scholar]
- 18.Reddy MV, Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986;7(9):1543–51. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- 19.Brown K, Guenther EA, Dingley KH, Cosman M, Harvey CA, Shields SJ, Turteltaub KW. Synthesis and spectroscopic characterization of site-specific 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine oligodeoxyribonucleotide adducts. Nucleic Acids Res. 2001;29(9):1951–9. doi: 10.1093/nar/29.9.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown K, Hingerty BE, Guenther EA, Krishnan VV, Broyde S, Turteltaub KW, Cosman M. Solution structure of the 2-amino-1- methyl-6-phenylimidazo[4,5-b]pyridine C8-deoxyguanosine adduct in duplex DNA. Proc Natl Acad Sci USA. 2001;98(15):8507–12. doi: 10.1073/pnas.151251898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauvin J, Broyde S, Shapiro R. The food mutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline: a conformational analysis of its major DNA adduct and comparison with the 2-amino-3-methylimidazo[4,5-f]quinoline adduct. Chem Res Toxicol. 2001;14(5):476–82. doi: 10.1021/tx000144r. [DOI] [PubMed] [Google Scholar]
- 22.Cho BP, Beland FA, Marques MM. NMR structural studies of a 15-mer DNA sequence from a ras protooncogene, modified at the first base of codon 61 with the carcinogen 4-aminobiphenyl. Biochemistry. 1992;31(40):9587–602. doi: 10.1021/bi00155a011. [DOI] [PubMed] [Google Scholar]
- 23.Cho BP, Beland FA, Marques MM. NMR structural studies of a 15-mer DNA duplex from a ras protooncogene modified with the carcinogen 2-aminofluorene: conformational heterogeneity. Biochemistry. 1994;33(6):1373–84. doi: 10.1021/bi00172a013. [DOI] [PubMed] [Google Scholar]
- 24.Bendaly J, Zhao S, Neale JR, Metry KJ, Doll MA, States JC, Pierce WM, Jr, Hein DW. 2-Amino-3,8-dimethylimidazo-[4,5-f]quinoxaline-induced DNA adduct formation and mutagenesis in DNA repair-deficient Chinese hamster ovary cells expressing human cytochrome P4501A1 and rapid or slow acetylator N-acetyltransferase 2. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1503–1509. doi: 10.1158/1055-9965.EPI-07-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metry KJ, Zhao S, Neale JR, Doll MA, States JC, McGregor WG, Pierce WM, Jr, Hein DW. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced DNA adducts and genotoxicity in Chinese hamster ovary (CHO) cells expressing human CYP1A2 and rapid or slow acetylator N-acetyltransferase 2. Mol Carcinog. 2007;46:553–563. doi: 10.1002/mc.20302. [DOI] [PubMed] [Google Scholar]