Abstract
The putative contribution of brain acetaldehyde (AcH) to ethanol (EtOH) tolerance and dependence (addiction) is reviewed. Although the role of AcH in EtOH addiction has been controversial, there are data showing a relationship. AcH can be formed in the brain tissues through the peroxidatic activity of catalase and by oxidation via other oxidizing enzymes such as cytochrome P-4502E1. Significant formation of AcH occurs in vitro in brain tissue at concentrations of EtOH that can be achieved by voluntary consumption of EtOH by rodents. AcH itself possesses reinforcing properties, which suggests that some of the behavioral pharmacological effects attributed to EtOH may be a result of the formation of AcH, and supports the involvement of AcH in EtOH addiction. Modulation of aldehyde dehydrogenase (ALDH) and brain catalase activity can change EtOH-related addictive behaviors presumably by changing AcH levels. Moreover, some condensation reaction products of AcH may promote some actions of EtOH and its consumption. On the basis of the findings, it can be concluded that AcH may mediate some of the CNS actions of EtOH including tolerance and dependence, although further exploration the involvement of AcH in EtOH addiction is warranted.
Keywords: Acetaldehyde, Ethanol, Brain, Addiction, reinforcement
Introduction
There are several problems associated with any study of acetaldehyde (AcH) physiological or biochemical effects in animals. The first is quantitative measures of blood and especially brain AcH. Jamal [1] has provided a technique to detect AcH in living animals by brain micro-dialysis in vivo. However, detection of AcH has been limited to the situation when the metabolism of AcH has been inhibited by cyanamide, an aldehyde dehydrogenase (ALDH) inhibitor. The second problem is the rapid removal of administered AcH by reduction to ethanol (EtOH) by alcohol dehydrogenase (ADH) in the liver and the rapid oxidation by ALDH2 in liver and brain. Thus, chronic treatment of animals with AcH administered peripherally is difficult and compromised by its rapid conversion to EtOH and acetate.
There are some possible ways around these problems. Close investigation of the rates and extent of tolerance and dependence in human subjects with inactive ALDH2 is one such technique. ALDH2 is responsible for converting AcH to acetate. In individuals with inactive ALDH2, there is a higher AcH level in blood after EtOH exposure. While the individuals that are heterozygous for this polymorphism, do not drink as much as individuals with active ALDH2 [2, 3], a close study of their chronic reactions would be a fruitful endeavor. Homozygous individuals are rare and consume little if any alcohol. The second possible way to study this issue would be a study of chronic tolerance and dependence on EtOH in knockout mice (Aldh2-/- mice) [4-7].
There is now ample evidence that anytime EtOH is present in the brain, AcH is formed in situ [8-12]. There is also extensive indirect evidence that AcH is responsible for a portion of the acute CNS effects of EtOH [reviewed in 13-16, 17]. The issue addressed in this review is whether or not AcH may also play a role in tolerance and dependence of EtOH, hallmarks of “addiction”.
Brain acetaldehyde production
Given that a significant amount of AcH is locally generated in the brain, there must be subsequent behavioral actions. Studies have shown that both astrocytes in culture and rat brain homogenates are able to produce such significant concentrations of AcH from EtOH [11, 17-20]. But ADH, the major EtOH oxidizing enzyme in liver, seems not physiologically active in the brain [10]. What pathways are responsible for the production of AcH is the focus of research. Indeed, there was evidence that catalase could oxidize brain EtOH to AcH in vivo after alcohol consumption [18]. By using catalase inhibitors or inducers, further studies demonstrated that AcH formation can be modified after EtOH addition [9, 20-25]. Lower levels of AcH were also found in catalase deficient (acatalasemic) mice when brain homogenates were incubated with EtOH, compared to those of normal mice [24, 25].
A recent study also demonstrated AcH formation from EtOH in the striatum of free-moving rats using brain microdialysis [26]. Catalase inhibition decreased AcH level when the accumulation of AcH was facilitated by pre-treating rats with an ALDH inhibitor. However it was also found that ADH inhibition decreased brain AcH concentration in the same study. This is contrary to what would be expected since it implies that AcH was entering the brain normally from the blood since ADH is not active in brain. However after ADH inhibition, blood AcH levels were decreased and entrance into the brain was very low. One explanation for this finding is that the dogma that AcH does not cross the blood brain barrier when blood AcH levels are low, is incorrect.
Brain catalase expression was studied by immunohistochemistry [27, 28], which has shown that catalase is localized in the body of catecholaminergic neurons of the forebrain and brain stem. Brain catalase staining was weak compared to that of staining in liver. The area of catalase staining was limited in contrast to ALDH staining, which is widely distributed in brain structures. However, AcH still could be generated locally in pharmacologically significant amounts.
In addition to catalase system, cytochrome P450 2E1 (CYP2E1) pathway might be involved in EtOH metabolism and AcH production within the brain. Different approaches were used to investigate the enzymatic mechanisms of EtOH oxidation in the brain homogenates of rats and mice [25]. CYP2E1 inhibitors significantly lowered the accumulation of the EtOH-derived AcH and acetate in brain homogenates. Inhibitors of ADH only significantly decreased acetate but not AcH accumulation. To explain this result, if one assumes that ADH is inactive in brain, is to postulate that the inhibitor used, 4-methylpyrazole, has an inhibitory effect on ALDH, or that it stimulates the removal of acetate.
EtOH-derived AcH accumulation in brain homogenates was further confirmed in a study utilizing CYP2E1(–/–) knockout mice, acatalasemic (catalase deficient) mice and double mutants (CYP2E1 knockout and acatalasemic) mice [25]. The CYP 2E1 mice had 91% of control AcH accumulation, acatalasemic mice had 47% of control but double mutants had 24% of control. One explanation of this synergistic effect of the double mutant is that CYP2E1 serves to generate hydrogen peroxide, the rate limiting factor in the metabolism of EtOH to AcH by catalase [25]. Subcellular fractions of brain homogenates were also investigated. It was found that the highest levels of EtOH oxidation were in microsomal and peroxisomal fractions, where CYP2E1 and catalase are located, respectively. It was also found that CYP2E1 (-/-) mice had an 11% increased sleep time due to ethanol, the acatalasemic mice had increased sleep time by 65% but in the double mutant the increase was 75%, or nearly exactly an additive effect. These data indicate that a presumed lower production of acetaldehyde in the brain leads to increased, rather than decreased sleep time as would be expected if acetaldehyde contributed to the sleep time effect of ethanol [29].
Correlatively, CYP2E1 is expressed in the neuronal cells in rat and human brain [30-32] though its mRNA and protein expression in rat brain was lower than that of liver [32]. It was seen in neurons within the cerebral cortex, Purkinje and granule cell layers of cerebellum, granule cell layer of dentate gyrus, and pyramidal neurons of CA1, CA2, and CA3 subfields of hippocampus. Like CYP2E1 in liver, brain CYP2E1 can also be induced by chronic ethanol treatments [33, 34]. Therefore, it can be postulated that there might be more AcH accumulation in alcoholics, which leads to its addiction and severe brain degeneration.
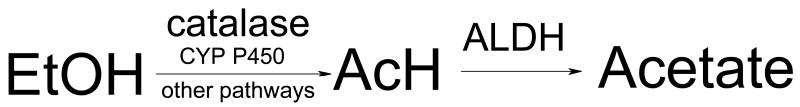
Hence, it can be concluded that in rodent brain AcH oxidation is mostly from catalase. CYP2E1 also plays an important role in EtOH oxidation, perhaps by furnishing hydrogen peroxide. However, not all brain AcH is from those two processes (Figure 1). There must be an additional, still unidentified, pathway that contributes to brain AcH production [11, 25].
Figure 1.
Schematic representation of ethanol (EtOH) metabolism in brain.
Effect of AcH related to addiction
Alcohol abuse and alcoholism have been severe social problems since alcohol was discovered. Great efforts have been made to prevent and treat those in the high risk group. However, it is complicated by the fact that not only EtOH itself but also AcH, the major metabolite of EtOH, could play a key role in alcohol abuse. However, it is difficult to clearly distinguish between the behaviors actions of alcohol which are attributed to EtOH in contrast to those due to AcH. This hinders the further research and the subsequent prevention and treatment of alcohol abuse and alcoholism. Indeed, studies have suggested that AcH mediates some of the behavioral effects of EtOH [reviewed in 15], such as narcosis [35], aversion [36], conditioned place preference [37], locomotor effect [38], and reinforcement [39]. These reinforcing properties increased EtOH consumption that leads to addiction though it remains extremely controversial.
A great deal of the evidence for the role of AcH in the tolerance and dependence on EtOH relies on indirect approaches. Thus the administration of ALDH inhibitors or use of mutants deficient in ALDH2 and pretreatment with catalase inhibitors or catalase deficient mice have all been used (Table 1). The results interpreted as to the presumed effects on AcH levels in the periphery or brain.
Table 1.
The acetaldehyde (AcH) role in ethanol (EtOH) addiction.
EtOH administration to rodents or humans after treatment with ALDH inhibitors leads to elevated blood AcH concentrations. This is the basis for treating alcoholics with disulfiram. Higher blood AcH is generally concluded to be aversive and deters voluntary EtOH consumption [15]. Although some people who were treated with disulfiram experienced AcH accumulation as pleasurable [40] and some ALDH2 heterozygous alcoholics have more positive psychological expectancies of alcohol drinking than ALDH2 deficient homozygous alcoholics [41], there was no difference for alcohol craving and consumption among alcoholics with different ALDH genotypes [41]. Instead, most people avoid EtOH consumption following ALDH inhibition. This is also seen in ALDH2 knockout mice [4]. EtOH preference was diminished by two thirds in a free drinking test though AcH concentration was not different in liver and brain between ALDH2 knockout and normal mice [42]. In the same study, AcH in brain could be increased after a high dose of EtOH in ALDH2 knockout mice but an EtOH consumption test was not performed [42]. It could be assumed that it is brain AcH level that determines AcH effects. At low concentrations, AcH might exert reinforcing effects, whereas at higher concentrations AcH would be predominantly aversive. This is also the case in UChA (low ethanol drinker) and UChB (high ethanol drinker) rats; the former has lower brain ALDH2 and subsequent higher AcH levels in contrast to the later. Indeed AcH really induced an enhancement of ethanol intake in UChB rats, but not in UChA rats [43].
Another approach to interfering with brain AcH production and EtOH consumption is to manipulate catalase activity. On one hand, there is a positive correlation between brain catalase activity and the natural propensity to drink EtOH in rodents [44-46], which was interpreted in the inverse in that lower brain catalase activity, would be associated with less EtOH consumption. In the same way, UChA and UChB rats showed attenuated acute tolerance to motor impairment and reduced voluntary EtOH consumption when pretreated with a catalase inhibitor [47]. On the other hand, a negative correlation between these measures was obtained in normal mice [48, 49] as well as in catalase deficient mice [24]. It seems that lower catalase activity, and thus lower brain AcH, is correlated with higher EtOH craving.
The reinforcing effect of AcH was observed in a tobacco animal study model. AcH, interacting with nicotine, enhanced the consumption in the self-administration acquisition whereas nicotine alone is only weakly reinforcing. These actions are more obvious in adolescent animals than in adults [50].
It could be concluded that altering EtOH metabolizing enzymes is not a useful strategy to induce AcH reinforcing effects. The difficulty is that when brain AcH is elevated, blood AcH is increased even much more. The conventional wisdom is that AcH has aversive effects in the periphery but reinforcing effects in the brain [15]. Thus to demonstrate a reinforcing effect of AcH by giving it peripherally, is a difficult task. To obviate this problem, investigators have given AcH intracerebroventricular (ICV) or directly into brain areas (reviewed by Quertemont) [15]. In the relatively few studies where AcH was given directly into the brain, it was found that there was stimulation of locomotor effects as opposed to depression when given intraperitoneally (IP). There was no conditioned taste aversion when given ICV as opposed to development of conditioned taste aversion when given IP in most cases. But in self administration and place conditioning studies, regardless of how it was administered, AcH induced reinforcing effects. For example, AcH voluntary self-administration is much easier to establish than that of EtOH [51] when AcH was given intravenously (IV), which is conflicting with commonly accepted view that blood AcH accumulation is highly aversive. More recently, the effect of D-penicillamine, a trapping agent for AcH, on the voluntary consumption of EtOH was assessed in rats [52]. Voluntary EtOH consumption was decreased after infusions of D-penicillamine. It seems that the presumed central sequestration of AcH blocks EtOH intake, which demonstrate further the role of AcH on the motivational properties of EtOH. In addition, AcH was similarly shown to be a 1,000 more potent reinforcer than EtOH when rats are trained to self-administer AcH and EtOH into the ventral tegmental area (VTA), a brain region strongly involved in EtOH reinforcing effects [53].
Several investigators have also administered AcH by inhalation [54-57] although this would cause irritation and still result in AcH which has to cross the blood brain barrier to reach neurons.
Mechanism of AcH's central effects
The exact mechanism underlies AcH's behavioral effects remain largely unknown. Even the mechanism of EtOH's behavioral actions are still debated although multiple molecular targets have been identified [58]. Nevertheless, there have been significant advancements in understanding of AcH's central effects in recent years.
Researches suggest that most abused drugs, including EtOH, stimulate the release of dopamine in several limbic regions [59], a brain reward system. Therefore, the reinforcing property of AcH may be mediated by increasing of dopamine in the system. Actually, the effect of AcH on the electrophysiological properties of DA-containing neurons in VTA of rats was investigated in vivo [60]. Similar to EtOH, AcH itself increased the firing rate, spikes/burst, and burst firing of VTA neurons. Furthermore, the stimulating actions of EtOH on the electrophysiological activity of dopamine VTA neurons were abolished when an ADH inhibitor was used [60]. Those authors claimed that AcH might play a role in the stimulation of the dopaminergic neuronal activity induced by EtOH. However, this is difficult to reconcile with what is known about the kinetics of AcH formation since there is no active ADH capable of oxidizing EtOH to AcH in brain [10]. Additionally, AcH could also be self-administrated into posterior VAT [61]. Co-administration of the D2 agonist quinpirole prevented the acquisition and extinguished the maintenance of EtOH self-infusion into the posterior VTA [62], again suggesting that dopamine as well as serotonin [53] is involved in AcH self-administration. AcH, in addition to EtOH, was also reported to affect β-endorphin secretion [63]. This may further modify central dopamine release and therefore mediate AcH reinforcing effects as application of the opioid receptor antagonist naloxone and buprenorphine reduced AcH self-administration [64]
Being a very reactive compound, AcH easily reacts with many other molecules by adduction, condensation and polymerization. This subject has recently been reviewed by Quertemont [15] and Marchitti [65]. AcH is able to react with endogenous neurotransmitters and proteins to form tetrahydro-β-carboline (THBC) and tetrahydroisoquinolines (TIQ) alkaloids, respectively. Two of the TIQ's are 1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquionoline (salsolinol) and tetrahydropapaveroline, which were extensively studied. Both were shown to be involved in EtOH voluntary consumption [66-69], leading to development of addiction. Multiple neurochemical mechanisms were speculated to explain the action [70], but a potentiation of the activity dopaminergic neurons in mesolimbric rewarding system was proposed.
Conclusion
Direct and indirect evidence indicates that AcH plays a role in EtOH tolerance, consumption, and reinforcement, which eventually leads to addiction. The behavioral data is plentiful, but not definitive and many interesting observations have been made. Should the role of AcH become definitive, the treatment of alcoholics would need re-examination, particularly the use of ALDH inhibitors would take on an entirely different perspective.
Acknowledgments
This work was supported from NIH grant # AA011464.
References
- 1.Jamal M, Ameno K, Kumihashi M, et al. Microdialysis for the determination of acetaldehyde and ethanol concentrations in the striatum of freely moving rats. J Chromatogr B Biomed Sci Appl. 2003;798:155–8. doi: 10.1016/j.jchromb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Deitrich RA, Petersen D, Vasiliou V. Removal of acetaldehyde from the body. Novartis Found Symp. 2007;285:23–40. doi: 10.1002/9780470511848.ch3. discussion 40-51, 198-9. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto K, Takeshita T. Low Km aldehyde dehydrogenase (ALDH2) polymorphism, alcohol-drinking behavior, and chromosome alterations in peripheral lymphocytes. Environ Health Perspect. 1996;104 3:563–7. doi: 10.1289/ehp.96104s3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isse T, Oyama T, Kitagawa K, et al. Dimished alcohol preference in transgeneic mice lacking aldehyde dehydrogenase activity. Pharmacogentics. 2002;12:621–6. doi: 10.1097/00008571-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto A, Ichiba M, Horita M, et al. Lack of aldehyde dehydrogenase ameliorates oxidative stress induced by single-dose ethanol administration in mouse liver. Alcohol. 2007;41:57–9. doi: 10.1016/j.alcohol.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez E, Koek W, Ran Q, Gerhardt GA, France CP, Strong R. Monoamine metabolism and behavioral responses to ethanol in mitochondrial aldehyde dehydrogenase knockout mice. Alcohol Clin Exp Res. 2006;30:1650–8. doi: 10.1111/j.1530-0277.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 7.Oyama T, Isse T, Ogawa M, Muto M, Uchiyama I, Kawamoto T. Susceptibility to inhalation toxicity of acetaldehyde in Aldh2 knockout mice. Front Biosci. 2007;12:1927–34. doi: 10.2741/2198. [DOI] [PubMed] [Google Scholar]
- 8.Aragon CMG, Stotland LM, Amit Z. Studies on ethanol-brain catalase interaction: Evidence for central ethanol oxidation. Alcohol Clin Exp Res. 1991;15:165–9. doi: 10.1111/j.1530-0277.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 9.Gill K, Menez JF, Lucas D, Deitrich RA. Enzymatic production of acetaldehyde from ethanol in rat brain tissue. Alcohol Clin Exp Res. 1992;16:910–5. doi: 10.1111/j.1530-0277.1992.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 10.Zimatkin SM, Deitrich RA. Ethanol metabolism in the brain. Addiction Biology. 1997;2:387–92. doi: 10.1080/13556219772444. [DOI] [PubMed] [Google Scholar]
- 11.Zimatkin SM, Liopo AV, Deitrich RA. Distribution and kinetics of ethanol metabolism in rat brain. Alcohol Clin Exp Res. 1998;22:1623–7. [PubMed] [Google Scholar]
- 12.Zimatkin SM, Liopo AV, Deitrich RA. Oxidation of ethanol to acetaldehyde in brain and the possible behavioral consequences. Adv Exp Med Biol. 1999;463:231–6. doi: 10.1007/978-1-4615-4735-8_28. [DOI] [PubMed] [Google Scholar]
- 13.Deitrich RA. Acetaldehyde: Deja Vu du Jour. J Stud Alcohol. 2004;65:557–72. doi: 10.15288/jsa.2004.65.557. [DOI] [PubMed] [Google Scholar]
- 14.Quertemont E, Grant KA, Correa M, et al. The role of acetaldehyde in the central effects of ethanol. Alcohol Clin Exp Res. 2005;29:221–34. doi: 10.1097/01.alc.0000156185.39073.d2. [DOI] [PubMed] [Google Scholar]
- 15.Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: A comprehensive review of animal studies. Prog Neurobiol. 2005;75:247–74. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Hunt WA. Role of acetaldehyde in the actions of ethanol on the brain - A review. Alcohol. 1996;13:147–51. doi: 10.1016/0741-8329(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 17.Aragon CMG, Rogan F, Amit Z. Ethanol metabolism in rat brain homogenates by a catalase- H2O2 system. Biochem Pharmacol. 1992;44:93–8. doi: 10.1016/0006-2952(92)90042-h. [DOI] [PubMed] [Google Scholar]
- 18.Cohen G, Sinet PM, Heikkila R. Ethanol oxidation by rat brain in vivo. Alcohol Clin Exp Res. 1980;4:366–70. doi: 10.1111/j.1530-0277.1980.tb04833.x. [DOI] [PubMed] [Google Scholar]
- 19.Eysseric H, Gonthier B, Soubeyran A, Bessard G, Saxod R, Barret L. Characterization of the production of acetaldehyde by astrocytes in culture after ethanol exposure. Alcohol Clin Exp Res. 1997;21:1018–23. [PubMed] [Google Scholar]
- 20.Hamby-Mason R, Chen JJ, Schenker S, Perez A, Henderson GI. Catalase mediates acetaldehyde formation from ethanol in fetal and neonatal rat brain. Alcohol Clin Exp Res. 1997;21:1063–72. [PubMed] [Google Scholar]
- 21.Correa M, Miquel M, Sanchis-Segura C, Aragon CM. Acute lead acetate administration potentiates ethanol-induced locomotor activity in mice: the role of brain catalase. Alcohol Clin Exp Res. 1999;23:799–805. [PubMed] [Google Scholar]
- 22.Sanchis-Segura C, Miquel M, Correa M, Aragon CM. The catalase inhibitor sodium azide reduces ethanol-induced locomotor activity. Alcohol. 1999;19:37–42. doi: 10.1016/s0741-8329(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 23.Escarabajal MD, Aragon CM. Concurrent administration of diethyldithiocarbamate and 4-methylpyrazole enhances ethanol-induced locomotor activity: the role of brain ALDH. Psychopharmacology (Berl) 2002;160:339–43. doi: 10.1007/s00213-001-0991-0. [DOI] [PubMed] [Google Scholar]
- 24.Aragon CM, Amit Z. Differences in ethanol-induced behaviors in normal and acatalasemic mice: systematic examination using a biobehavioral approach. Pharmacol Biochem Behav. 1993;44:547–54. doi: 10.1016/0091-3057(93)90165-p. [DOI] [PubMed] [Google Scholar]
- 25.Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30:1500–5. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 26.Jamal M, Ameno K, Uekita I, Kumihashi M, Wang W, Ijiri I. Catalase mediates acetaldehyde formation in the striatum of free-moving rats. Neurotoxicology. 2007 May 13; doi: 10.1016/j.neuro.2007.05.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Moreno S, Mugnaini E, Ceru MP. Immunocytochemical localization of catalase in the central nervous system of the rat. J Histochem Cytochem. 1995;43:1253–67. doi: 10.1177/43.12.8537642. [DOI] [PubMed] [Google Scholar]
- 28.Zimatkin SM, Lindros KO. Distribution of catalase in rat brain: aminergic neurons as possible targets for ethanol effects. Alcohol Alcohol. 1996;31:167–74. doi: 10.1093/oxfordjournals.alcalc.a008128. [DOI] [PubMed] [Google Scholar]
- 29.Vasiliou V, Ziegler TL, Bludeau P, Petersen DR, Gonzalez FJ, Deitrich RA. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genomics. 2006;16:51–8. doi: 10.1097/01.fpc.0000182777.95555.56. [DOI] [PubMed] [Google Scholar]
- 30.Hansson T, Tindberg N, Ingelman-Sundberg M, Kohler C. Regional distribution of ethanol-inducible cytochrome P450 IIE1 in the rat central nervous system. Neuroscience. 1990;34:451–63. doi: 10.1016/0306-4522(90)90154-v. [DOI] [PubMed] [Google Scholar]
- 31.Upadhya SC, Tirumalai PS, Boyd MR, Mori T, Ravindranath V. Cytochrome P4502E (CYP2E) in brain: constitutive expression, induction by ethanol and localization by fluorescence in situ hybridization. Arch Biochem Biophys. 2000;373:23–34. doi: 10.1006/abbi.1999.1477. [DOI] [PubMed] [Google Scholar]
- 32.Yadav S, Dhawan A, Singh RL, Seth PK, Parmar D. Expression of constitutive and inducible cytochrome P450 2E1 in rat brain. Mol Cell Biochem. 2006;286:171–80. doi: 10.1007/s11010-005-9109-z. [DOI] [PubMed] [Google Scholar]
- 33.Anandatheerthavarada HK, Shankar SK, Bhamre S, Boyd MR, Song BJ, Ravindranath V. Induction of brain cytochrome P-450IIE1 by chronic ethanol treatment. Brain Res. 1993;601:279–85. doi: 10.1016/0006-8993(93)91721-4. [DOI] [PubMed] [Google Scholar]
- 34.Montoliu C, Vallés S, Renau-Piqueras J, Guerri C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: effect of chronic alcohol consumption. J Neurochem. 1994;63:1855–62. doi: 10.1046/j.1471-4159.1994.63051855.x. [DOI] [PubMed] [Google Scholar]
- 35.Aragon CM, Spivak K, Amit Z. Effect of 3-amino-1,2,4-triazole on ethanol-induced narcosis, lethality and hypothermia in rats. Pharmacol Biochem Behav. 1991;39:55–9. doi: 10.1016/0091-3057(91)90397-k. [DOI] [PubMed] [Google Scholar]
- 36.Quintanilla ME, Tampier L, Sapag A, Gerdtzen Z, Israel Y. Sex differences, alcohol dehydrogenase, acetaldehyde burst, and aversion to ethanol in the rat: a systems perspective. Am J Physiol Endocrinol Metab. 2007;293:E531–7. doi: 10.1152/ajpendo.00187.2007. [DOI] [PubMed] [Google Scholar]
- 37.Font L, Aragon CM, Miquel M. Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with D -penicillamine, an inactivation agent for acetaldehyde. Psychopharmacology (Berl) 2006;184:56–64. doi: 10.1007/s00213-005-0224-z. [DOI] [PubMed] [Google Scholar]
- 38.Tambour S, Didone V, Tirelli E, Quertemont E. Locomotor effects of ethanol and acetaldehyde after peripheral and intraventricular injections in Swiss and C57BL/6J mice. Behav Brain Res. 2006;172:145–54. doi: 10.1016/j.bbr.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson CJ. The role of acetaldehyde in the actions of alcohol (update 2000) Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):15S–32S. doi: 10.1097/00000374-200105051-00005. [DOI] [PubMed] [Google Scholar]
- 40.Brown ZW, Amit Z, Smith BR, Sutherland EA, Selvaggi N. Alcohol-induced euphoria enhanced by disulfiram and calcium carbimide. Alcohol Clin Exp Res. 1983;7:276–8. doi: 10.1111/j.1530-0277.1983.tb05459.x. [DOI] [PubMed] [Google Scholar]
- 41.Hahn CY, Huang SY, Ko HC, et al. Acetaldehyde involvement in positive and negative alcohol expectancies in han Chinese persons with alcoholism. Arch Gen Psychiatry. 2006;63:817–23. doi: 10.1001/archpsyc.63.7.817. [DOI] [PubMed] [Google Scholar]
- 42.Isse T, Matsuno K, Oyama T, Kitagawa K, Kawamoto T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity shows high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol Clin Exp Res. 2005;29:1959–64. doi: 10.1097/01.alc.0000187161.07820.21. [DOI] [PubMed] [Google Scholar]
- 43.Quintanilla ME, Tampier L. Acetaldehyde-reinforcing effects: differences in low-alcohol-drinking (UChA) and high-alcohol-drinking (UChB) rats. Alcohol. 2003;31:63–9. doi: 10.1016/j.alcohol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Amit Z, Aragon CM. Catalase activity measured in rats naive to ethanol correlates with later voluntary ethanol consumption: possible evidence for a biological marker system of ethanol intake. Psychopharmacology (Berl) 1988;95:512–5. doi: 10.1007/BF00172965. [DOI] [PubMed] [Google Scholar]
- 45.Aragon CM, Sternklar G, Amit Z. A correlation between voluntary ethanol consumption and brain catalase activity in the rat. Alcohol. 1985;2:353–6. doi: 10.1016/0741-8329(85)90074-6. [DOI] [PubMed] [Google Scholar]
- 46.Gill K, Amit Z, Smith BR. The regulation of alcohol consumption in rats: the role of alcohol-metabolizing enzymes-catalase and aldehyde dehydrogenase. Alcohol. 1996;13:347–53. doi: 10.1016/0741-8329(96)00006-7. [DOI] [PubMed] [Google Scholar]
- 47.Tampier L, Quintanilla ME. Involvement of brain ethanol metabolism on acute tolerance development and on ethanol consumption in alcohol-drinker (UChB) and non-drinker (UChA) rats. Addict Biol. 2003;8:279–86. doi: 10.1080/13556210310001602185. [DOI] [PubMed] [Google Scholar]
- 48.Gill K, Liu Y, Deitrich RA. Voluntary alcohol consumption in BXD recombinant inbred mice: relationship to alcohol metabolism. Alcohol Clin Exp Res. 1996;20:185–90. doi: 10.1111/j.1530-0277.1996.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 49.He XX, Nebert DW, Vasiliou V, Zhu H, Shertzer HG. Genetic differences in alcohol drinking preference between inbred strains of mice. Pharmacogenetics. 1997;7:223–33. doi: 10.1097/00008571-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 51.Brown ZW, Amit Z, Rockman GE. Intraventricular self-administration of acetaldehyde, but not ethanol, in naive laboratory rats. Psychopharmacology (Berl) 1979;64:271–6. doi: 10.1007/BF00427509. [DOI] [PubMed] [Google Scholar]
- 52.Font L, Aragon CM, Miquel M. Voluntary ethanol consumption decreases after the inactivation of central acetaldehyde by d-penicillamine. Behav Brain Res. 2006;171:78–86. doi: 10.1016/j.bbr.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 53.Rodd ZA, Bell RL, Zhang Y, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005;30:330–8. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- 54.Shiohara E, Tsukada M, Chiba S, et al. Effect of chronic administration of acealdehyde by inhalation on (Na+ and K+)-activated adenosine triphosphatase activity of rat brain membranes. Toxicology. 2007;34:277–84. doi: 10.1016/0300-483x(85)90138-6. [DOI] [PubMed] [Google Scholar]
- 55.Bardina L, Pronko P, Santanovskaia VI, Kusmich AB. Effect of acetaldehyde on ethanol- and aldehyde-metabolising systems of the liver and brain of rats. Ukr Biokhim Zh. 2003;75:129–133. [PubMed] [Google Scholar]
- 56.Kharchenko N. Effect of acetaldehyde on the formation of alcohol dependence in animal experiments. Ukr Biokhim Zh. 1998;70:122–8. [PubMed] [Google Scholar]
- 57.Latge C, Lamboeuf Y, Roumec C, de Saint Blanquat G. Effect of chronic acetaldehyde intoxication on ethanol tolerance and membrane fatty acids. Drug Alcohol Depend. 1987;20:47–55. doi: 10.1016/0376-8716(87)90075-5. [DOI] [PubMed] [Google Scholar]
- 58.Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- 59.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 60.Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–6. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- 61.Rodd-Henricks ZA, Melendez RI, Zaffaroni A, Goldstein A, McBride WJ, Li TK. The reinforcing effects of acetaldehyde in the posterior ventral tegmental area of alcohol-preferring rats. Pharmacol Biochem Behav. 2002;72:55–64. doi: 10.1016/s0091-3057(01)00733-x. [DOI] [PubMed] [Google Scholar]
- 62.Rodd ZA, Melendez RI, Bell RL, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–7. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy BV, Boyadjieva N, Sarkar DK. Effect of ethanol, propanol, butanol, and catalase enzyme blockers on beta-endorphin secretion from primary cultures of hypothalamic neurons: evidence for a mediatory role of acetaldehyde in ethanol stimulation of beta-endorphin release. Alcohol Clin Exp Res. 1995;19:339–44. doi: 10.1111/j.1530-0277.1995.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 64.Myers WD, Ng KT, Singer G. Effects of naloxone and buprenorphine on intravenous acetaldehyde self-injection in rats. Physiol Behav. 1984;33:449–55. doi: 10.1016/0031-9384(84)90168-9. [DOI] [PubMed] [Google Scholar]
- 65.Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev. 2007;59:125–50. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- 66.Davis VE, Walsh MJ. Alcohol addiction and tetrahydropapaveroline. Science. 1970;169:1105–6. [PubMed] [Google Scholar]
- 67.Duncan C, Deitrich RA. A critical evaluation of tetrahydroisoquinoline induced ethanol preference in rats. Pharmacol Biochem Behav. 1980;13:265–81. doi: 10.1016/0091-3057(80)90083-0. [DOI] [PubMed] [Google Scholar]
- 68.McCoy JG, Strawbridge C, McMurtrey KD, Kane VB, Ward CP. A re-evaluation of the role of tetrahydropapaveroline in ethanol consumption in rats. Brain Res Bull. 2003;60:59–65. doi: 10.1016/s0361-9230(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 69.Myers RD, McCaleb ML, Ruwe WD. Alcohol drinking induced in the monkey by tetrahydropapaveroline (THP) infused into the cerebral ventricle. Pharmacol Biochem Behav. 1982;16:995–1000. doi: 10.1016/0091-3057(82)90059-4. [DOI] [PubMed] [Google Scholar]
- 70.Melchior CL. Interaction of salsolinol and tetrahydropapaveroline with catecholamines. Alcohol Clin Exp Res. 1979;3(4):364–7. doi: 10.1111/j.1530-0277.1979.tb05337.x. [DOI] [PubMed] [Google Scholar]