Abstract
Neocortical neuritic plaques (NP) and neurofibrillary tangles (NFT) are hallmarks of Alzheimer’s disease (AD) and usually, both are present. The Honolulu-Asia Aging Study autopsy series includes a significant number of individuals with only one neocortical AD lesion type. These could represent an early phase of the AD process. If so, such individuals would be expected to share other clinical and pathological features of AD. We compared frequency of apolipoprotein epsilon E4 (APOE4) allele, average Braak stage, and burden of cerebral amyloid angiopathy (CAA) among the two single lesion type groups, a group without AD lesions, and groups with high and low frequencies of both AD lesions. Single AD lesion groups shared only the characteristics associated with their unique lesion type with the combined AD lesion group and did not have higher prevalence of dementia than the no AD lesion group. Only the NP+NFT group showed a “dose response” relationship with greater probability of dementia with higher neocortical frequencies of either AD lesion. The single neocortical AD lesion groups do not appear to represent early AD.
Keywords: Alzheimer’s disease, Neocortical neuritic plaques, Neocortical neurofibrillary tangles, Braak stage, Apolipoprotein epsilon E4, Cerebral amyloid angiopathy
1. Introduction
Neuropathologic criteria for Alzheimer’s disease (AD) rely on identification of neuritic plaques (NP) and neurofibrillary tangles (NFT) in the neocortex and ventromedial temporal lobe structures.[1] While most AD cases have both lesion types, there are reports of individuals with dementia who have only one isolated lesion type in the neocortex.[15, 16, 43] It is unclear if these individuals have a variant form of AD, or some other cause of dementia. In the Honolulu-Asia Aging Study (HAAS) cohort of Japanese-American men in Hawaii, there are numerous individuals in the autopsy series whose brains have either neocortical NP or NFT in isolation.[35]
A previous publication demonstrated that there was no increased probability of dementia for either of these two single lesion type groups or a group with low frequencies of both lesion types (early AD process) compared to those without AD lesions. However, when concurrent focal ischemic cerebrovascular (CV) lesions were found at autopsy, rates of dementia more than doubled for all three groups. In contrast, although men with no neocortical AD lesions had low probability of dementia, and those with high frequencies of both NP and NFT in the neocortex had a significantly higher probability of dementia, CV lesions had little effect on rates of dementia in either of these groups.[35]
This led to the speculation that the NP only and NFT only groups could represent early AD processes, similar to those who had low frequencies of both NP and NFT in the neocortex. If this were true, and if the pathogenesis of isolated neocortical NFT and NP were similar to that for combined lesions, the three groups might be expected to share other clinical and pathological features. In order to examine this expectation, we compared three indicators that are known to be positively associated with AD: the frequency of apolipoprotein epsilon E4 (APOE4) allele,[9] average Braak stage,[8] and average amount of cerebral amyloid angiopathy (CAA)[10] among the different groups based on neocortical AD pathology. Age, education level, cognitive function, and dementia were also assessed for each group.
2. Methods
2.1. The Study Population
The HAAS began in 1991 as a supplement to the Honolulu Heart Program, a longitudinal study of cardiovascular disease in Japanese-American men living on Oahu at the time of the baseline examination in 1965. All subjects were born in the years 1900 through 1919. A detailed description of the study design was previously published.[42] At the time of this analysis the original cohort of 8006 men had been followed for 39 years with repeated examinations supplemented by surveillance of hospital and death records.
2.2. Dementia case-finding methods
Evaluation and follow-up for dementia began at the 1991–1993 examination of the cohort when the men were 71–93 (average 78) years of age.[46] Participants signed informed consent forms at each examination and the study was approved by the Kuakini Medical Center Institutional Review Board. A three phase screening and sampling design was used to find cases of dementia in the cohort. The Cognitive Abilities Screening Instrument (CASI) was used for cognitive function screening. The final phase included neuropsychological testing and examination by a neurologist or specially trained geriatrician. Individuals participating in the 1994–1996, 1997–99, and 2000–2001 examinations were similarly screened and evaluated.[39, 46]
Dementia was defined according to the DSM III-R criteria.[2] Final diagnosis was assigned by a clinical consensus committee, including the study neurologist and at least one other investigator.
2.3. Apolipoprotein Epsilon E4 Genotype Assessment
Blood was drawn at the 1991 to 1993 examination. Genotyping was done at Duke University by standard PCR techniques.[40]
2.4. Neuropathologic Assessment
Autopsies were sought for all cohort deaths and were obtained on 22% of all participants who died between February 1992 and December 2001. Among those decedent participants who had been recognized as demented, the autopsy rate was approximately 34%. Permission for autopsy was obtained from the next of kin.
Autopsy and neuropathologic methods have been described previously.[36, 45] Brains were fixed by immersion in 10% buffered formalin for at least 10 days after removal. One pathologist identified large and small (lacunar) infarcts by gross examination of coronal sections of the cerebrum and serial sections of the brainstem and cerebellum.
One of three neuropathologists (WM, DD, and JN) evaluated the brain histopathologically and quantified microscopic lesions without knowledge of clinical information. Seventy-two percent (270) of the cases were read by DD who has been the only neuropathologist actively reading cases for the last seven years. Although interrater reliability has not been formally assessed, measures were taken to assure adequate agreement. The three neuropathologists participated together in a standardization training meeting and the pathological groupings for this study were based on semiquantitative modified Consortium to Establish a Registry for AD (CERAD) categories (see section 2.5 below).
Neocortical NP and NFT were counted in 5 microscopic fields from each of 4 regions of the left cerebrum (middle frontal gyrus, middle temporal gyrus, inferior parietal lobule, and the occipital association cortex along the calcarine sulcus). Sections were 8µm in thickness and were stained using a modified Bielschowsky method.[22] Fields were selected from areas observed to have the highest lesion densities on preliminary scanning. Neuritic plaques were defined as containing silver-positive dystrophic neurites and/or dense well defined amyloid cores. Maximum neocortical NP or NFT density was defined as the highest count among the 20 neocortical fields examined. Results were recorded as NP or NFT/mm2.[34]
CAA was assessed using sections stained immunohistochemically for beta amyloid peptide (Aβ) using an antibody (10D-5) generously provided by Athena Neurosciences, San Francisco, CA).[14] Summary CAA indices were generated for meningeal and parenchymal vessels (mostly small arteries and arterioles). The CAA parenchymal vessel index was used for the analyses reported here. A grade of “1” was given to sections containing one or two Aβ positive vessels. Sections with three to five positive vessels were designated as “2”, and sections with greater than five positive vessels were designated as “3”. If all vessels within an area were nonreactive the section was designated “0”. For this study CAA grades from the four neocortical areas, hippocampus, entorhinal cortex and amygdala were combined to yield an overall CAA numerical grade (maximum score was 27).
Braak staging was performed by one neuropathologist (DD). Sections of entorhinal cortex, the hippocampus, and amygdala were stained using the Gallyas method. Neurofibrillary changes in these sections and in Beilschowsky stained sections of neocortical association areas and occipital striate cortex were assessed and staged according to the procedure described by Braak and Braak.[8]
Lewy bodies were evaluated by examining sections of the midbrain at the level of the inferior colliculi and the rostral pons. Single hematoxylin and eosin stained sections from the substantia nigra and locus ceruleus were examined for the presence of Lewy bodies. When one or more Lewy bodies were found, additional counts were performed on alpha-synuclein stained sections from the neocortical regions (left frontal, temporal and parietal cortices) and limibic/paralimbic regions (left anterior cingulate cortex and transentorhinal regions). Evaluation of cases was done using the Newcastle criteria.[24, 25]
2.5. Assignment to Pathologic Groups
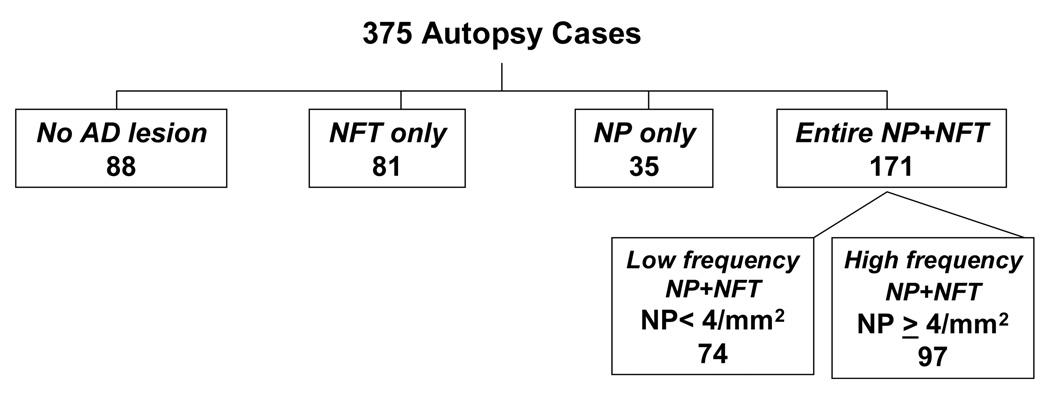
Decedents were assigned to one of five mutually exclusive pathologic groups based on neocortical AD pathology (figure 1). Those with both neocortical NP and neocortical NFT were divided into low and high frequency groups based on NP (not considering NFT) frequency. Those who had <4 NP per mm2 (“sparse NP” according to CERAD neuropathological criteria for AD), were defined as belonging to the low frequency NP+NFT group. Those with ≥ 4 NP/mm2 (“moderate or frequent NP” according to CERAD neuropathological criteria for AD), comprised the high frequency NP+NFT group. The remaining three groups were those with NP only, NFT only; and those with no AD lesions.
Figure 1.
Assignment of 375 autopsy cases to five mutually exclusive pathological groups by presence of neocortical neuritic plaques and neurofibrillary tangles
2.6. Statistical Methods
Because only three individuals were homozygous for the APOE4 allele (one in the NFT only group, two in the group with high frequency NP+NFT), we elected to define the APOE4 genotype as positive or negative. The Student’s t-test was used to compare continuous variables in each of the groups with lesions to the group with no lesions. Odds ratios were used to compare proportions with or without specific characteristics between the groups with lesions and the group with no lesions. None of the individuals in the group without neocortical lesions had Braak stage V or VI. Therefore, instead of an odds ratio, an exact Chi-square test was used to compare the presence of Braak stage V or VI between these groups.
3. Results
At the time of this analysis, 375 autopsy cases had completed microscopic data, among which 345 (92%) had cognitive function testing within four years of death. Analyses relating AD lesions to cognitive functioning or dementia were limited to this group of 345. Braak staging was complete for 210 of these cases. There were 126 (36.5%) who met DSM-III-R criteria for dementia among these 345. The mean interval between last cognitive function assessment and death was 622 days and the range was 20–1436. Table 1 contains information about age at death, education level, cognitive function during life, dementia status, APOE 4 status, level of CAA, and Braak stage for each of the five groups. As expected, both the high and low frequency NP+NFT groups had a significantly higher prevalence of APOE4 positively, and higher mean Braak scores, higher CAA scores, older age at death, lower cognitive function scores, and higher odds of having received a diagnosis of dementia during life compared to the group with no AD lesions. All of these relationships were strongest in the high frequency NP+NFT group. The NP only group shared the propensity of the combination NP+NFT groups to have high levels of amyloid-associated factors only: APOE4 and CAA. This group did not have higher Braak stage or worse cognitive function than the group with no AD lesions. The neocortical NFT only group had higher Braak stage and lower mean cognitive function test scores, but was indistinguishable from the group without AD lesions as regards CAA and APOE4.
Table 1.
Characteristics of 375 decedents divided among five mutually exclusive AD pathologic groups: associations with other factors
| Alzheimer Lesion Group (Neocortex) |
|||||
|---|---|---|---|---|---|
| No AD lesion | NFT only | NP only | Low frequency NP+NFT |
High frequency NP+NFT |
|
| N | 88 | 81 | 35 | 74 | 97 |
| Mean+SD of neocortical NP | 0 | 0 | 3.1+2.4 | 2.0+1.2 | 10.4+7.4 |
| Mean+SD of neocortical NFT | 0 | 4.0+9.0 | 0 | 3.8+3.8 | 18.7+20.1 |
| APOE4 alleles ≥1, #(%) | 6 (6.8%) | 6 (7.4%) | 8 (22.9%) | 16 (21.6%) | 27 (27.8%) |
| Odds ratio for APOE4 allele≥1 | 1 | 1.1 (0.3 – 3.5) | 4.0 (1.3-12.7)3 | 3.8 (1.4-10.2)2 | 5.3 (2.1-13.5)1 |
| Braak score - mean (N=210) | 2.6 + 0.9 | 3.3 + 1.03 | 2.9 + 0.8 | 3.7 + 1.21 | 4.6 + 2.01 |
| Braak stage ≥V, # (%) (N=210) | 0 | 5(15.2%)3 | 1 (3.6%) | 11 (26.8%)1 | 26 (55.4%)1 |
| CAA score - mean | 1.2 + 3.6 | 1.5 + 4.0 | 4.3 + 6.71 | 5.1 + 5.61 | 10.4 + 8.11 |
| CAA score ≥1, #(%) | 14 (15.9) | 16 (19.8) | 19 (54.3) | 54 (73.0) | 84 (86.6) |
| Odds ratio for CAA score ≥1 | 1 | 1.3(0.6-2.9) | 6.3(2.6-15.1)1 | 14.3(6.6-13.8)1 | 34.2(15.1-77.3)1 |
| Age at death – mean years | 84.2 + 5.0 | 84.6 + 6.0 | 85.4 + 4.9 | 86.4 + 4.92 | 86.7 + 4.71 |
| Education - mean years | 9.9 + 2.9 | 9.9 + 3.4 | 11.5 + 3.4 | 10.6 + 3.6 | 10.0 + 3.1 |
| Last CASI score-mean (N=345) | 69.5 + 24.4 | 60.2 + 28.53 | 69.5 + 23.8 | 59.2 + 30.13 | 45.3 + 32.71 |
| Last CASI < 74, # (%) (N=345) | 38 (45.2) | 43 (58.9) | 14 (45.2) | 38 (55.1) | 65 (73.9) |
| Odds ratio for CASI score < 74 (N=345) | 1 | 1.7(0.9-3.3) | 1.0(0.4-2.3) | 1.5(0.8-2.8) | 3.4(1.8-6.5)1 |
| Clinical dementia, # (%) (N=345) | 16 (19.1) | 23(31.5) | 7(22.6) | 26(37.7) | 54(59.1) |
| Odds ratio for dementia (N=345) | 1 | 2.0(0.9-4.1) | 1.2(0.5-3.4) | 2.6(1.2-5.3)3 | 6.1(3.1-12.2)1 |
p<0.001
p<0.01
p<0.05; for all comparisons, reference is no AD lesion group
AD = Alzheimer’s disease; SD = standard deviation; NFT = neurofibrillary tangle; NP = neuritic plaque; APOE4 = apolipoprotein epsilon 4 allele; CAA = cerebral amyloid angiopathy; CASI = Cognitive Abilities Screening Instrument.
There was no association between the occurrence or numbers of cortical Lewy bodies and either of the two neocortical single lesion groups: Cortical Lewy bodies were present in 8.8% (7/81) of the NFT only, and 8.6% (3/35) of the NP only groups, compared to 13.4–13.6% of all other groups including the no AD lesion group. The three decedents in the NP only group with cortical Lewy bodies had clinical diagnoses of PD without dementia (within 1 year of death), PD dementia, and diffuse Lewy body disease. All three cases met Newcastle Criteria for dementia with Lewy bodies and none met neuropathological criteria for AD.
To examine the possible impact of interrater differences, the analyses described above and reported in the table were repeated using only the 270 cases read by a single pathologist (DD). Except for wider confidence limits and fewer statistically significant relationships, results did not change.
To examine the possible impact of variable intervals between assessments of dementia or cognitive function and death, analyses of relationships between function and histopathologic observations were repeated using only those who expired within 2 years, and within 1 year of the cognitive function examination (not shown). Although some of the relationships were no longer statistically significant due to fewer individuals, the fundamental strengths and directions of the observed relationships were not altered.
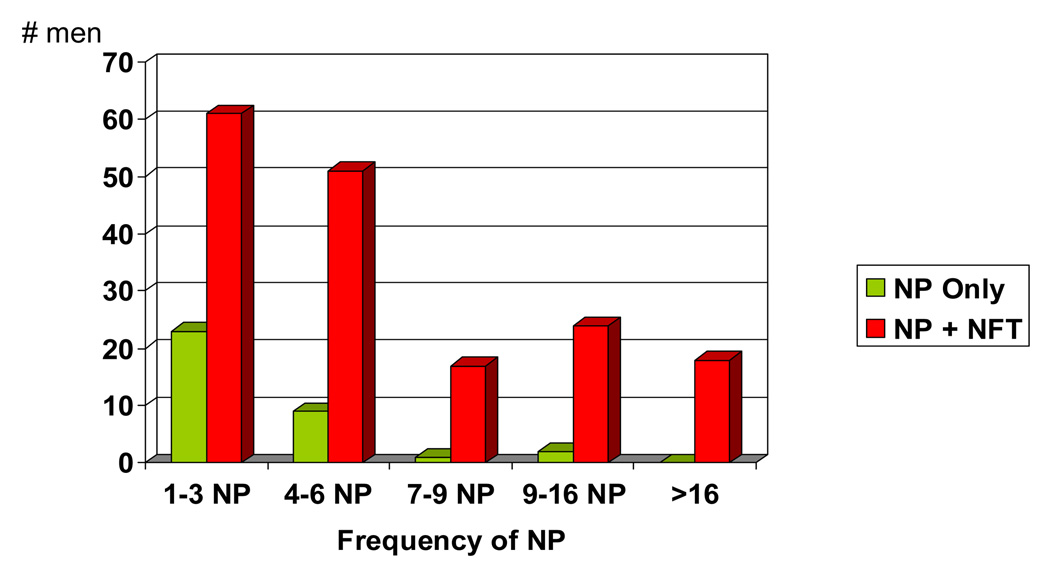
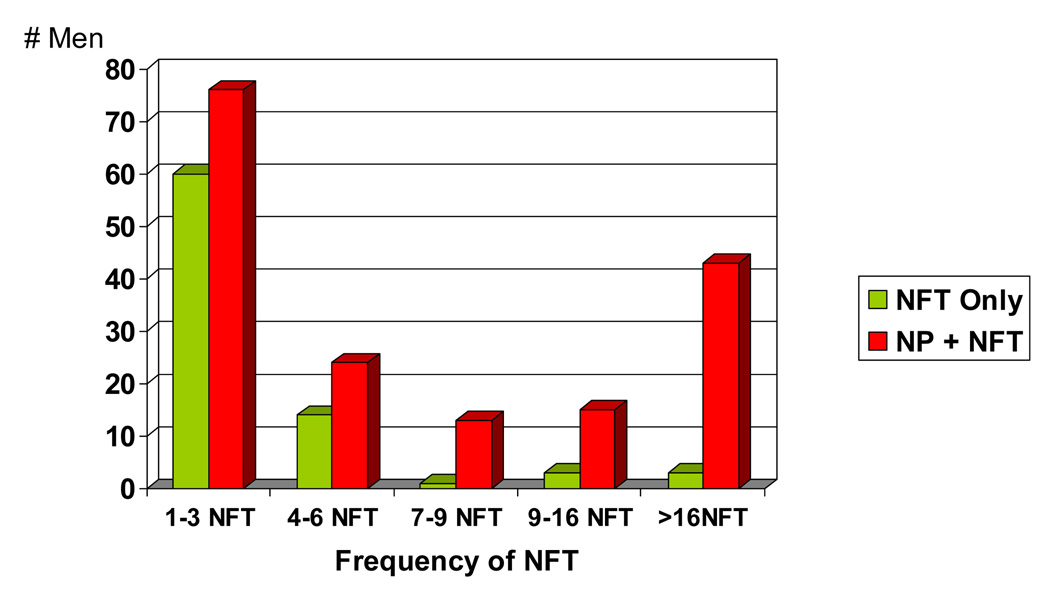
Figure 2 and Figure 3 demonstrate that both of the single AD lesion groups have relatively low frequencies of either NP or NFT compared to the group with both AD lesions. For both the NP only and NFT only groups, less than 10% of the individuals had NP or NFT counts > 6 per mm2. This is in contrast to the NP+NFT group where 35% have NP, and 42% have NFT >6 per mm2.
Figure 2.
The NP only group compared to the entire NP + NFT group: Frequency of NP per mm2
Figure 3.
The NFT only group compared to the entire NP + NFT group: Frequency of NFT per mm2
Regression analysis (not shown) demonstrated a “dose response” relationship between cognitive function and frequency per mm2 of neocortical NP (p<0.05) and NFT (p<0.001) in individuals with both lesion types, but no such relationship for either of the single neocortical AD lesion groups.
4. Discussion
As expected, individuals with typical AD pathology (neocortical NP+NFT) were more likely to possess an APOE4 allele,[9, 13, 17] have a higher Braak stage,[8] have more CAA,[14, 37] and to show lower cognitive function and higher odds of clinical dementia during life[5, 7] than decedents with no AD lesions. There was a significant “dose response” relationship between poorer cognitive function and frequencies of both AD lesions per mm2. These associations were noted even among decedents in whom NP and NFT densities were low.
In contrast, subjects with isolated neocortical NP or NFT (but not both lesions) had low rates of dementia during life and did not show “dose response” relationships between densities of the specific AD lesion and cognitive impairment or dementia. These individuals tended not to have high frequencies of the AD lesion in the neocortex.
Among the autopsied subjects with only neocortical NFT there was no association of Braak stage with cognitive test scores. In this same group (NFT only), we observed no increase in frequency of the APOE4 allele and no increase in vascular amyloid deposits, compared with decedents in whom neither AD lesion type was present in the neocortex. Conversely, the E4 allele and vascular amyloid deposition were increased in the neocortical NP only group and in decedents with both AD lesions. The NP only group had no increase in mean Braak stage above that displayed by the group with no AD lesions (table1). While definition of the NP only group precluded neocortical NFT being found at autopsy (as in the occipital lobe for Braak stage 6), individuals in this group were assigned Braak stages 1 through 5 (34 of 35 were assigned a stage of 4 or less). Further analysis demonstrated that the distribution of Braak stages in the NP only group (82% ≤ stage 3) was also similar to the no AD lesion group (86% ≤ stage 3) demonstrating that in the NP only group, development of NFT in the neocortex was probably not imminent. Preserved mental status[19] and lesser severity of dementia[43] in individuals with neocortical senile plaques only, compared to those with both lesions have been previously reported.
The relative importance and usual sequence of development of NP vs NFT in early AD pathology is uncertain. Several studies in human decedents have reported that senile plaques[27] and neocortical NP[12, 44] were more strongly associated with early AD than NFT are. In a triple transgenic mouse model, intraneuronal accumulation of Aβ was shown to be associated with deficits in synaptic plasticity before AD lesions developed.[33] Conversely, other autopsy studies[11, 26, 38] reported NFT to be the dominant lesion in AD and mild cognitive impairment.
Some of the results reported here suggest a more direct impact on cognitive functioning for NFT than for NP, consistent with published reports indicating stronger associations with duration and severity of dementia for NFT than for senile plaque frequencies.[3, 5, 6, 23] The term “senile plaques” is often used without further classification as to neuritic (NP) or diffuse (DP) subtypes. When the two plaque types have been distinguished, the linkage with cognitive impairment has usually been strongest for NP.[5, 31] The question of whether the presence of the subgroup NP in the neocortex is as strongly associated with cognitive decline as neocortical NFT is unresolved. Bennett reported that the association of amyloid load with AD severity is mediated by NFT.[4] Our finding that the NFT only group had poorer cognitive function than the NP only group may support the theory that NFT are most closely related to cognitive function level.
Alternatively, the lower mean cognitive function score in the NFT only group could be partly a result of more frequent CV or other coexisting neuropathologic lesions. In a recent report from the HAAS,[35] both the NP only and the NFT only groups had low proportions of demented individuals similar to the no AD lesion group unless a concomitant CV lesion was also present (6.3% demented for the NP only group, and 10.7% demented for the NFT only group, compared to 20% demented for the no AD lesion group). However when at least one lacune or infarct was present, 45% of the NP only group and 46% of the NFT only group were found to be demented. The proportion of demented individuals was not significantly increased with the addition of cerebrovascular lesions in the group without AD lesions. This indicates that although the burden of AD lesions in the single neocortical lesion type groups tends not to be enough to cause dementia, or significantly decreased cognitive function, the presence of low levels of these lesions is associated with an increased vulnerability to other neuropathology, such as CV lesions. This is similar to the situation in individuals with very low levels of both neocortical AD lesions.[35]
Additional analyses completed since that report, confirmed that both lacunes and large vessel infarcts were slightly more frequent in the NFT only group (49.4% had lacunes, and 39.5% had large infarcts) compared to the NP only group (42.9% had lacunes, and 31.4% had large vessel infarcts).
A limitation of our study is the relatively long time interval between cognitive evaluation and death in some individuals. Allowing up to 4 years between these events will result in misclassification of some individuals as cognitively normal who actually progressed to cognitive impairment or dementia before death. This would inflate the frequency with which apparently normal individuals tolerated a substantial burden of structural brain disease, thereby weakening the observable association between AD brain lesions at death and cognitive impairment or dementia during life. On the other hand, it is not expected that those who were demented at their last examination will have often regained normal functioning prior to death. This implies that it would be infrequent to find few or no brain lesions at death in persons who were identified as demented up to 4 years prior to death. Repeating the analyses relating the AD lesion groups to cognitive function and dementia using more limited intervals between examination and death did not alter the results. The failure to demonstrate stronger associations with shorter intervals seems consistent with the natural history of AD lesion accumulation being a protracted one that may be established years prior to clinical manifestations.
Another limitation is that progressive supranuclear palsy (PSP) or other tauopathy cannot be definitely ruled out using our current protocol which does not include routine Beilschowsky or tau immunohistochemistry of motor and premotor areas, thalamus, basal ganglia, brainstem, or cerebellum. The complete work-up for PSP is carried out when screening of these regions using the H&E stained sections reveals globose NFT in the subthalamic nucleus and/or brain stem nuclei or if PSP or other tauopathy is suspected from the clinical findings.
One individual in the NFT only group and one in the high frequency AD lesion group had symptoms suggestive of PSP during life. Neuropathology evaluation confirmed the diagnosis in the NFT only case. Although individuals in the neocortical NFT only group were not significantly more likely to be demented than individuals with no neocortical AD lesions, it is possible that rare individuals in this group represented PSP or other tauopathies (corticobasal degeneration, frontotemporal dementia, etc.) in the early or preclinical stages. These disorders are considered to be rare in this age group and our subjects undergo thorough neurological examinations making it unlikely that they account for many of the relatively large NFT only group (22% of the autopsy series).
An especially interesting finding was that education, well known to be associated with higher risk of dementia[28] and AD,[18, 21, 41, 47] was not associated with any of the AD lesion groups. This supports data from the Nun Study indicating that educational attainment was not related to fulfillment of neuropathologic criteria for AD.[30] It also supports the hypothesis that higher education decreases the ease of clinical detection of AD[20, 32] or strengthens “brain reserve”.[29] Years of education do not appear to have a direct influence on AD brain lesions.
The idea that AD has a single underlying pathogenesis manifested by the simultaneous appearance of NFT and NP that gradually progress from limbic regions into the neocortex as reflected in Braak staging is intuitively appealing. As the process first begins to enter the neocortex, it would be expected that neocortical lesions would be present in modest numbers, and one or the other could be dominant – corresponding to the single lesion groups described. Except for fewer clinical signs of impairment, this conceptualization would also lead one to expect persons with a single neocortical lesion type to be similar to persons with both lesions. The observations reported here do not support this expectation. Instead, the isolated occurrence of NP or NFT in the neocortex suggests that the mechanisms of their development in that region can be independent.
Most importantly, the essential mechanisms underlying the strong association between amyloid plaques and NFT, as well as between these lesions and cognitive impairment remain incompletely understood. Research on the phenomena responsible for the isolated occurrence of NP and NFT in the neocortex could provide insights into these and related questions.
Acknowledgments
This work was supported by the National Institutes of Health: National Institute on Aging, Grant Numbers: 1 U01 AG19349 and 5 R01 AG017155 and with resources from the Spark M. Matsunaga Veterans Affairs Medical Center, Honolulu, Hawaii. The authors would like to thank the Honolulu-Asia Aging Study/Honolulu Heart Program cohort members for their important and long term participation in the study and the Honolulu-Asia Aging Study staff who make the study possible.
Disclosure: Drs. Petrovitch, Ross, He, Nelson, and White receive salary support from one or more of the above grants. Drs. Davis and Markesbery are consultants. There are, otherwise, no real or potential financial conflicts of interest for any of the authors to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition. Washington, DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- 3.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch. Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 5.Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch. Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 6.Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch. Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 7.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey mater of elderly subjects. Brit. J. Psychiat. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer's Disease in Late Onset Families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 10.Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–1596. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 11.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch. Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 12.Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer's disease. Arch. Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- 13.Havlik RJ, Izmirlian G, Petrovitch H, Ross GW, Masaki K, Curb JD, Saunders AM, Foley DJ, Brock D, Launer LJ, White L. APOE-epsilon4 predicts incident AD in Japanese-American men: the honolulu-asia aging study. Neurology. 2000;54:1526–1529. doi: 10.1212/wnl.54.7.1526. [DOI] [PubMed] [Google Scholar]
- 14.Hyman BT, Tanzi RE, Marzloff K, Barbour R, Schenk D. Kunitz protease inhibitor-containing amyloid beta protein precursor immunoreactivity in Alzheimer's disease. J Neuropathol. Exp. Neurol. 1992;51:76–83. doi: 10.1097/00005072-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda K, Akiyama H, Arai T, Oda T, Kato M, Iseki E, Kosaka K, Wakabayashi K, Takahashi H. Clinical aspects of 'senile dementia of the tangle type'-- a subset of dementia in the senium separable from late-onset Alzheimer's disease. Dement. Geriatr. Cogn Disord. 1999;10:6–11. doi: 10.1159/000017091. [DOI] [PubMed] [Google Scholar]
- 16.Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia) Brain Pathol. 1998;8:367–376. doi: 10.1111/j.1750-3639.1998.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardaun JW, White L, Resnick HE, Petrovitch H, Marcovina SM, Saunders AM, Foley DJ, Havlik RJ. Genotypes and phenotypes for apolipoprotein E and Alzheimer disease in the Honolulu-Asia aging study. Clin Chem. 2000;46:1548–1554. [PubMed] [Google Scholar]
- 18.Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer's disease. Am J Epidemiol. 2004;159:175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- 19.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 20.Koivisto K, Helkala EL, Reinikainen KJ, Hanninen T, Mykkanen L, Laakso M, Pyorala K, Riekkinen PJ. Population-based dementia screening program in Kuopio: the effect of education, age, and sex on brief neuropsychological tests. J Geriatr. Psychiatry Neurol. 1992;5:162–171. doi: 10.1177/002383099200500306. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 22.Luna LG. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. New York: McGraw-Hill; 1992. [Google Scholar]
- 23.McKee AC, Kosik KS, Kowall NW. Neuritic pathology and dementia in Alzheimer's disease. Ann Neurol. 1991;30:156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers. Dis. 2006;9:417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 25.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han LY, Bienias JL, Lee VM, Trojanowski JQ, Bennett DA, Arnold SE. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer's disease. Ann Neurol. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol. Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 28.Mortel KF, Meyer JS, Herod B, Thornby J. Education and occupation as risk factors for dementias of the Alzheimer and ischemic vascular types. Dementia. 1995;6:55–62. doi: 10.1159/000106922. [DOI] [PubMed] [Google Scholar]
- 29.Mortimer JA. Brain reserve and the clinical expression of Alzheimer's disease. Geriatrics. 1997;52 Suppl 2:S50–S53. [PubMed] [Google Scholar]
- 30.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 31.Nagy Zs, Esiri MM, Jobst KA, Morris JH, King EMF, McDonald B, Litchfield S, Smith A, Barnetson L, Smith AD. Relative roles of plaques and tangles in the dementia of Alzheimer's disease: correlations using three sets of neuropathological criteria. Dementia. 1995;6:21–31. doi: 10.1159/000106918. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor DW, Pollitt PA, Treasure FP. The influence of education and social class on the diagnosis of dementia in a community population. Psychol Med. 1991;21:219–224. doi: 10.1017/s003329170001480x. [DOI] [PubMed] [Google Scholar]
- 33.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 34.Petrovitch H, Nelson J, Snowdon D, Davis DG, Ross GW, Li CY, White LR. Microscope field size and the neuropathologic criteria for Alzheimer's disease. Neurology. 1997;49:1175–1176. doi: 10.1212/wnl.49.4.1175. [DOI] [PubMed] [Google Scholar]
- 35.Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, Nelson J, Hardman J, Masaki K, Vogt MR, Launer L, White LR. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 36.Petrovitch H, White LR, Ross GW, Steinhorn SC, Li CY, Masaki KH, Davis DG, Nelson J, Hardman J, Curb JD, Blanchette PL, Launer LJ, Yano K, Markesbery WR. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57:226–234. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 38.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 39.Ross GW, Abbott RD, Petrovitch H, Masaki KH, Murdaugh C, Trockman C, Curb JD, White LR. Frequency and characteristics of silent dementia among elderly Japanese-American men. The Honolulu-Asia Aging Study. JAMA. 1997;277:800–805. [PubMed] [Google Scholar]
- 40.Saunders AM, Hulette C, Welsh-Bohmer KA, Schmechel DE, Crain B, Burke JR, Alberts MJ, Strittmatter WJ, Breitner JCS, Rosenberg C, Scott SV, Gaskell PC, Jr, Pericak-Vance MA, Roses AD. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer's disease. Lancet. 1996;348:90–93. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- 41.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 42.Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: introduction. Am J Epidemiol. 1975;102:477–480. doi: 10.1093/oxfordjournals.aje.a112185. [DOI] [PubMed] [Google Scholar]
- 43.Terry RD, Hansen LA, DeTeresa R, Davies P, Tobias H, Katzman R. Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol. Exp Neurol. 1987;46:262–268. doi: 10.1097/00005072-198705000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62:1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 45.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N. Y. Acad. Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 46.White L, Petrovitch H, Ross GW, Masaki K, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 47.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr. Soc. 2003;51:410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]