Abstract
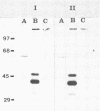
Mucosal nasopharyngeal immunoglobulin A (IgA) and IgG responses to proteins of Streptococcus equi were studied in horses after the experimental production of strangles. S. equi-specific IgA and IgG titers in nasopharyngeal mucus were much higher in samples from animals 1 to 2 weeks after challenge than in samples from control animals. Although IgA was the major immunoglobulin in nasal mucus, there was more antibody activity associated with IgG as measured by radioimmunoassay. Great differences between the specificities of antibodies in nasal mucus and in serum were detected. IgA and IgG of mucus origin recognized only two major proteins with molecular weights of about 41,000 and 46,000 in acid extracts of S. equi and gave no detectable reaction with culture supernatant proteins. Only one protein of about 62,000 molecular weight was recognized in acid extracts of an equine strain of S. zooepidemicus. In contrast, immunoglobulins in serum recognized a great variety of proteins in culture supernatants and acid extracts of S. equi and S. zooepidemicus which did not include those proteins recognized by immunoglobulins in mucus. These findings provide good evidence for the independence of the local and systemic immune responses of the horse to S. equi. Horses rechallenged shortly after recovery from the first infection were resistant to challenge with an inoculum of S. equi 10 times greater than that to which they were originally susceptible. This resistance appeared to be independent of the levels of bactericidal antibody in serum. We therefore suggest that immunity to S. equi infection is mediated by locally produced nasopharyngeal antibodies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bey R. F., Shade F. J., Goodnow R. A., Johnson R. C. Intranasal vaccination of dogs with liver avirulent Bordetella bronchiseptica: correlation of serum agglutination titer and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am J Vet Res. 1981 Jul;42(7):1130–1132. [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht H., Dolan M. Vaccination of swine for jowl abscesses. Oral administration of group E streptococcus vaccine (live culture-modified). Vet Med Small Anim Clin. 1968 Sep;63(9):872–875. [PubMed] [Google Scholar]
- Engelbrecht H. Vaccination against strangles. J Am Vet Med Assoc. 1969 Jul 15;155(2):425–427. [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R., Ogra P. L., Regas S., Waldman R. H. Rubella immunization of volunteers via the respiratory tract. Infect Immun. 1973 Oct;8(4):497–502. doi: 10.1128/iai.8.4.497-502.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R., Waldman R. H. Local immunity and local immune responses. Prog Allergy. 1980;27:1–68. [PubMed] [Google Scholar]
- Gearhart P. J., Cebra J. J. Differentiated B lymphocytes. Potential to express particular antibody variable and constant regions depends on site of lymphoid tissue and antigen load. J Exp Med. 1979 Jan 1;149(1):216–227. doi: 10.1084/jem.149.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., Helms C. M., Grizzard M. B., James W. D., Horswood R. L., Chanock R. M. Immunoprophylaxis of experimental Mycoplasma pneumoniae disease: effect of route of administration on the immunogenicity and protective effect of inactivated M. pneumoniae vaccine. Infect Immun. 1977 Apr;16(1):88–92. doi: 10.1128/iai.16.1.88-92.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith I. P. Immediate visualization of proteins in dodecyl sulfate-polyacrylamide gels by prestaining with Remazol dyes. Anal Biochem. 1972 Apr;46(2):402–412. doi: 10.1016/0003-2697(72)90313-2. [DOI] [PubMed] [Google Scholar]
- Guirguis N., Fraser D. W., Facklam R. R., El Kholy A., Wannamaker L. W. Type-specific immunity and pharyngeal acquisition of group A Streptococcus. Am J Epidemiol. 1982 Dec;116(6):933–939. doi: 10.1093/oxfordjournals.aje.a113495. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Wicher K., Arbesman C. E. Distribution of immunoglobulins in palatine and pharyngeal tonsils. Int Arch Allergy Appl Immunol. 1972;43(6):801–812. doi: 10.1159/000230898. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of type-specific M antigen isolated from a group A, type 1 hemolytic streptococcus. J Exp Med. 1952 Jul;96(1):71–82. doi: 10.1084/jem.96.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matthews J. B., Basu M. K. Oral tonsils: an immunoperoxidase study. Int Arch Allergy Appl Immunol. 1982;69(1):21–25. doi: 10.1159/000233140. [DOI] [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knope H. L., Wenzel R. P., Hornick R. B., Kapikian A. Z., Chanock R. M. Evidence for protective effect of an inactivated rhinovirus vaccine administered by the nasal route. Am J Epidemiol. 1969 Oct;90(4):319–326. doi: 10.1093/oxfordjournals.aje.a121076. [DOI] [PubMed] [Google Scholar]
- Polly S. M., Waldman R. H., High P., Wittner M. K., Dorfman A. Protective studies with a group A streptococcal M protein vaccine. II. Challange of volenteers after local immunization in the upper respiratory tract. J Infect Dis. 1975 Mar;131(3):217–224. doi: 10.1093/infdis/131.3.217. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Barnum D. A. Adherence of Streptococcus equi on tongue, cheek and nasal epithelial cells of ponies. Vet Microbiol. 1983 Oct;8(5):493–504. doi: 10.1016/0378-1135(83)90043-3. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Barnum D. A. The serological response of foals to vaccination against strangles. Can J Comp Med. 1981 Jan;45(1):20–25. [PMC free article] [PubMed] [Google Scholar]
- Woolcock J. B. Immunity to Streptococcus equi. Aust Vet J. 1975 Dec;51(12):554–559. doi: 10.1111/j.1751-0813.1975.tb09379.x. [DOI] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]