Abstract
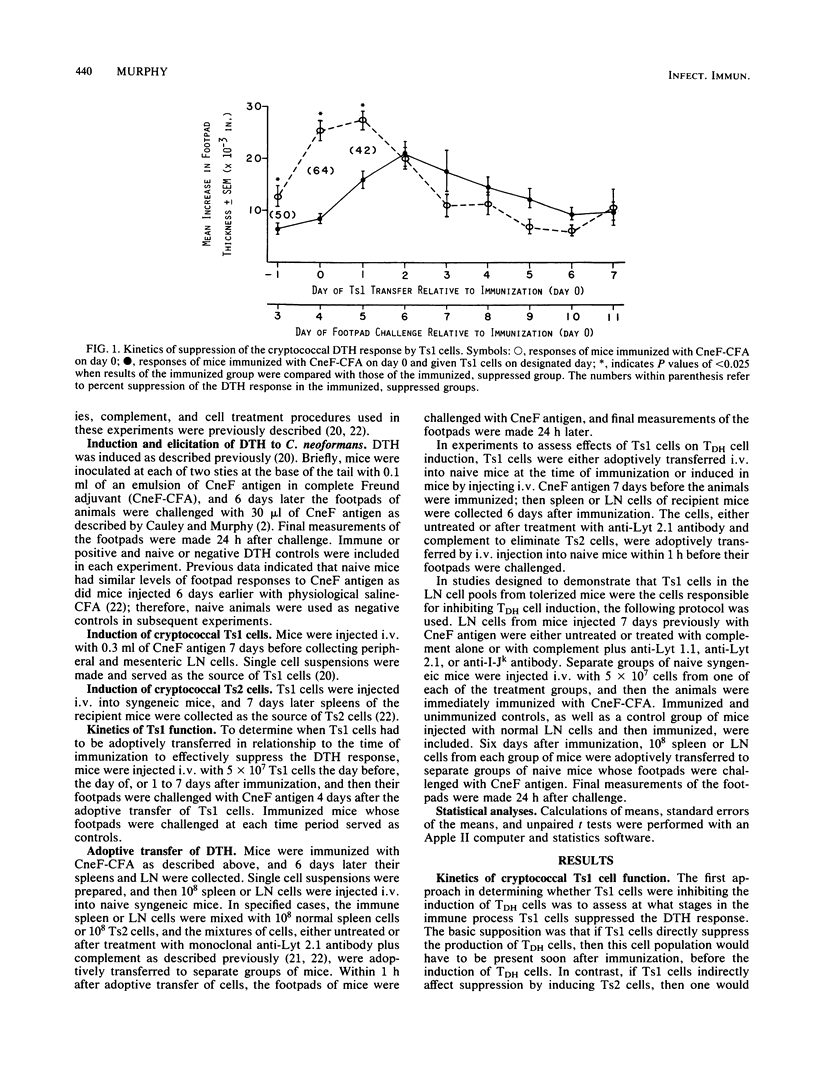
Cell-mediated immunity is an important aspect of host resistance against Cryptococcus neoformans. Using a CBA/J murine model, we demonstrated that injection of cryptococcal antigen (CneF) at dosages sufficient to stimulate the antigenemia observed in cryptococcosis patients induces specific T-cell-mediated suppression of the cryptococcal delayed-type hypersensitivity response. The purpose of this study was to establish whether Lyt 1+, first-order T-suppressor (Ts1) cells block the induction of T cells responsible for delayed-type hypersensitivity (TDH cells) or whether they function by inducing Lyt 2+, efferent suppressor (Ts2) cells. In one set of experiments, suppression was observed when Ts1 cells were adoptively transferred to recipient animals the day before, the day of, or the day after immunization; however, when Ts1 cells were transferred after TDH cells were present, no suppression occurred. In other experiments, putative TDH cells from lymph nodes (LN) or spleens were adoptively transferred from mice after immunization or after a suppressive dose of CneF or adoptive transfer of Ts1 cells and immunization. Delayed-type hypersensitivity could not be transferred with LN or spleen cells from mice receiving the suppressive dose of CneF or the Ts1 cells, even when the LN or spleen cells were treated with anti-Lyt 2.1 antibody and complement to remove any Ts2 cells. Delayed-type hypersensitivity was readily transferred with LN or spleen cells from immunized mice whether the cells were or were not treated with anti-Lyt 2 and complement. Furthermore, the cells in the tolerized LN cell pools responsible for suppression of TDH cell induction were Lyt 1+ 2-, I-J+ cells, which is the phenotype of the Ts1 cells. Taken together, these data indicate that Ts1 cells inhibit the induction of TDH cells. This finding, coupled with the previous demonstration that Ts1 cells or a Ts1 cell-derived soluble factor (TsF1) induces Ts2 cells, establishes that the cryptococcal Ts1 cells are bifunctional in the suppressive pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Colizzi V., Doria G., Ricciardi-Castagnoli P. Immunoregulation of lysozyme-specific suppression. II. Hen egg-white lysozyme-specific monoclonal suppressor T cell factor suppresses the afferent phase of delayed-type hypersensitivity and induces second-order suppressor T cells. Eur J Immunol. 1984 Sep;14(9):826–830. doi: 10.1002/eji.1830140911. [DOI] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Eng R., Chmel H., Corrado M., Smith S. M. The course of cryptococcal capsular polysaccharide antigenemia/human cryptococcal polysaccharide elimination kinetics. Infection. 1983 May-Jun;11(3):132–136. doi: 10.1007/BF01641291. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Theze J., Waltenbaugh C., Dorf M. E., Benacerraf B. Antigen-specific T cell-mediated suppression. II. In vitro induction by I-J-coded L-glutamic acid50-L-tyrosine50 (GT)-specific T cell suppressor factor (GT-T8F) of suppressor T cells (T82) bearing distinct I-J determinants. J Immunol. 1978 Aug;121(2):602–607. [PubMed] [Google Scholar]
- Goodman J. S., Kaufman L., Koenig M. G. Diagnosis of cryptococcal meningitis. Value of immunologic detection of cryptococcal antigen. N Engl J Med. 1971 Aug 19;285(8):434–436. doi: 10.1056/NEJM197108192850804. [DOI] [PubMed] [Google Scholar]
- Gordon M. A., Vedder D. K. Serologic tests in diagnosis and prognosis of cryptococcosis. JAMA. 1966 Sep 19;197(12):961–967. [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Kauffman C. A., Bergman A. G., Severance P. J., McClatchey K. D. Detection of cryptococcal antigen. Comparison of two latex agglutination tests. Am J Clin Pathol. 1981 Jan;75(1):106–109. doi: 10.1093/ajcp/75.1.106. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Butler L. D., Claman H. N. Suppressor T cell circuits in contact sensitivity. I. Two mechanistically distinct waves of suppressor T cells occur in mice tolerized with syngeneic DNP-modified lymphoid cells. J Immunol. 1982 Aug;129(2):461–468. [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. Suppressor T cell mechanisms in contact sensitivity. II. Afferent blockade by alloinduced suppressor T cells. J Immunol. 1978 Jul;121(1):274–280. [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFB in mice. VI. Inhibition of afferent sensitivity by suppressor T cells in adoptive tolerance. J Immunol. 1976 Sep;117(3):802–806. [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- NEILL J. M., SUGG J. Y., McCAULEY D. W. Serologically reactive material in spinal fluid, blood, and urine from a human case of cryptococcosis (torulosis). Proc Soc Exp Biol Med. 1951 Aug;77(4):775–778. doi: 10.3181/00379727-77-18924. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Dietz M. H., Germain R. N., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. IV. Idiotype-bearing I-J+ suppressor T cell factors induce second-order suppressor T cells which express anti-idiotypic receptors. J Exp Med. 1980 May 1;151(5):1183–1195. doi: 10.1084/jem.151.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold L. E., Roberts G. D., Brewer N. S., Zeller D. J., Fineman L. D., Paschall C. L., Rosenlof R. C. Massive antigenemia during disseminated cryptococcosis. Mayo Clin Proc. 1980 Aug;55(8):513–515. [PubMed] [Google Scholar]
- Young E. J., Hirsh D. D., Fainstein V., Williams T. W. Pleural effusions due to Cryptococcus neoformans: a review of the literature and report of two cases with cryptococcal antigen determinations. Am Rev Respir Dis. 1980 Apr;121(4):743–747. doi: 10.1164/arrd.1980.121.4.743. [DOI] [PubMed] [Google Scholar]


