Abstract
Colonies of Neisseria gonorrhoeae JS3, each bearing a predominate protein II (PII) type, were derived from a progenitor transparent colony. Five distinct PIIs were identified and isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The PII bands were excised from gels of unlabeled whole cells and from gels containing lysates of surface-radioiodinated bacteria. These were subjected to alpha-chymotrypsin digestion and two-dimensional peptide mapping, which allowed for a comparison of both the primary structures of the PIIs and the identification of surface-exposed regions of the molecules. The results demonstrated that PIIs are unrelated to either Protein I or Protein III in structure but are closely related to one another, sharing about two-thirds of the peptides generated by alpha-chymotrypsin. The remaining third of the peptides varied with each PII, resulting in unique portions of the molecule being exposed on the bacterial surface. However, the variable peptides were not always among the exposed peptides, suggesting that the structural differences in the PIIs occur at a discrete site (or sites) of the PII molecule and not randomly throughout the protein. Such alterations can result in the exposure of distant, nonvariant portions of the molecule to the surface, perhaps by conformational changes. These bacteria can thus present a variety of new immunodeterminant sites to the host during the course of disease.
Full text
PDF
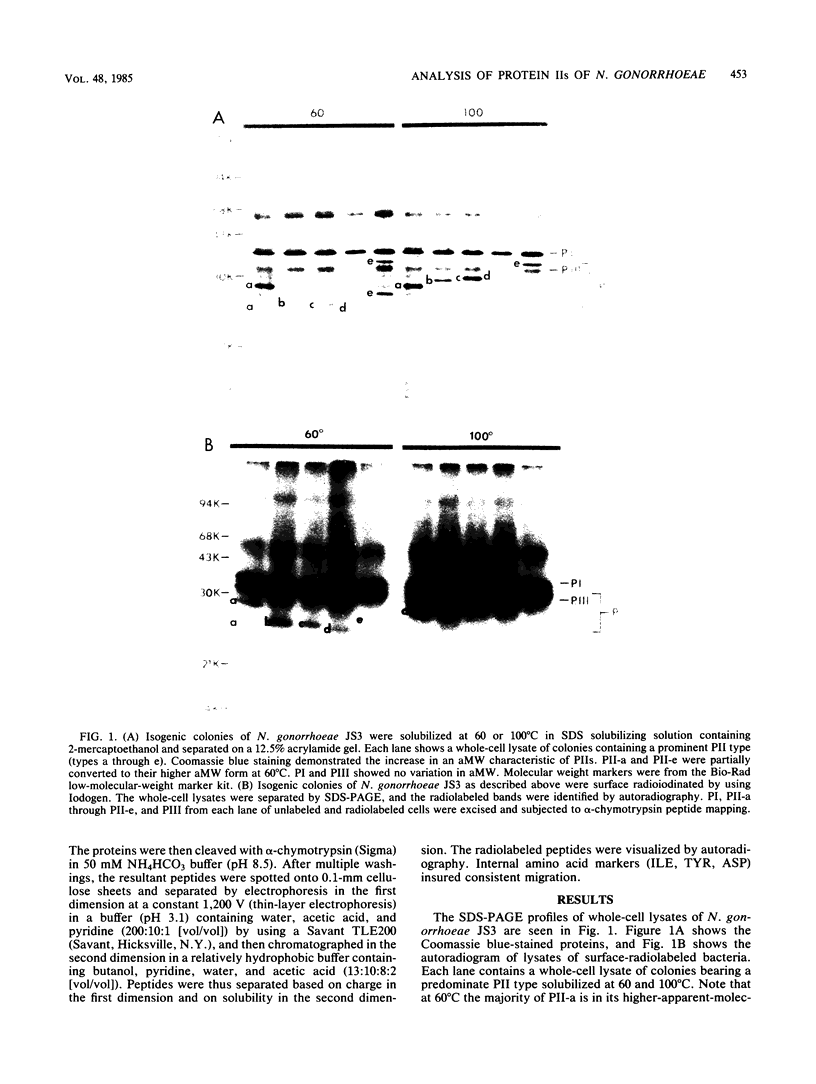
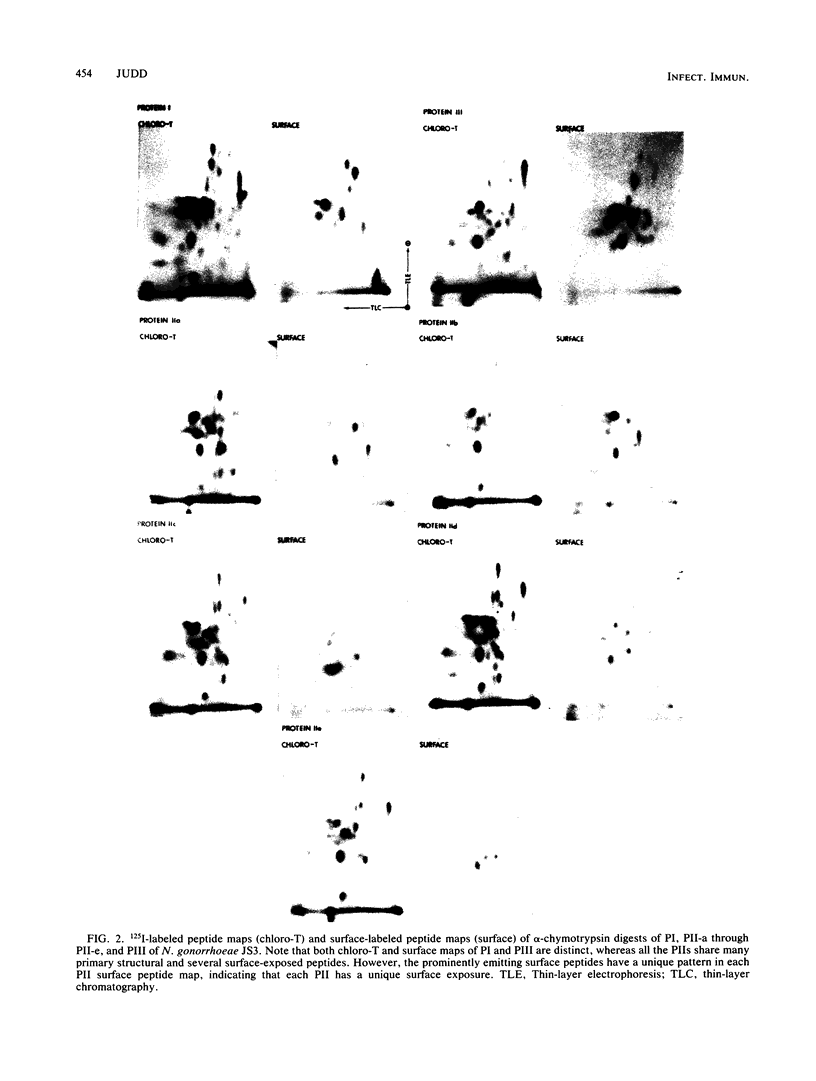
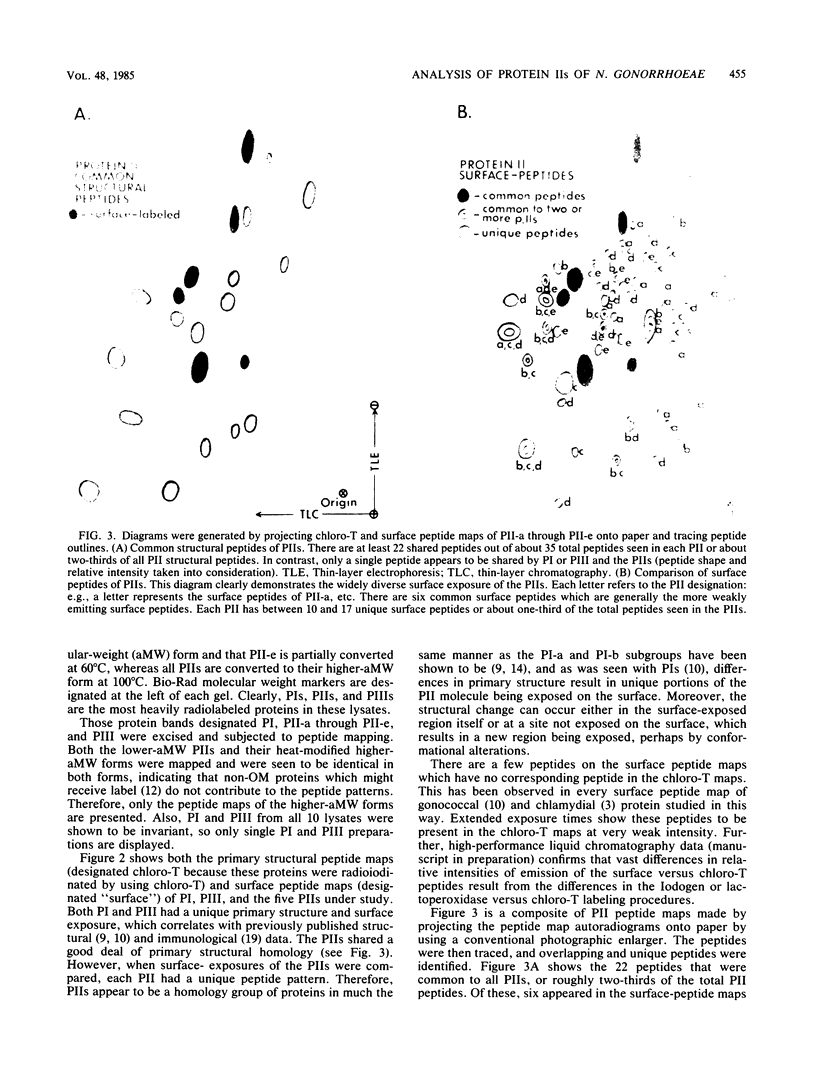


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Barrera O., Judd R. C. Structural analysis of the variable major proteins of Borrelia hermsii. J Exp Med. 1983 Dec 1;158(6):2127–2140. doi: 10.1084/jem.158.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Judd R. C. Structural analysis of chlamydial major outer membrane proteins. Infect Immun. 1982 Dec;38(3):960–968. doi: 10.1128/iai.38.3.960-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S. Regulation of the immune response by idiotypic-antiidiotypic interactions. N Engl J Med. 1981 Jul 2;305(1):25–28. doi: 10.1056/NEJM198107023050105. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):736–742. doi: 10.1128/jb.145.2.736-742.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. The immune system: a web of V-domains. Harvey Lect. 1974 1975;70(SERIES):93–110. [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Surface peptide mapping of protein I and protein III of four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):632–641. doi: 10.1128/iai.37.2.632-641.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Lactoperoxidase and Iodo-Gen-catalyzed iodination labels inner and outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Jul;155(1):443–446. doi: 10.1128/jb.155.1.443-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. 125I-labeled peptide mapping of some heat-modifiable proteins of the gonococcal outer membrane. Infect Immun. 1980 Apr;28(1):54–64. doi: 10.1128/iai.28.1.54-64.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983 May 1;157(5):1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Bach B. A., Dohi Y., Nisonoff A., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. I. Induction of suppressor T cells with anti-idiotypic antibodies. J Exp Med. 1979 Nov 1;150(5):1216–1228. doi: 10.1084/jem.150.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Everson J. S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981 Mar;31(3):965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]