Abstract
Background
Little is known about how maternal zinc intake influences growth in utero and in postnatal life in humans.
Objective
We aimed to assess the effect of maternal zinc supplementation during pregnancy on infant growth through age 1 y.
Design
A double-blind, randomized controlled trial of prenatal zinc supplementation was conducted from 1995 to 1997 in Lima, Peru. Women (n = 1295) were enrolled at 15.6 ± 4.6 wk gestation and assigned to receive daily supplements with zinc (15 mg Zn + 60 mg Fe + 250 µg folic acid) or without zinc (60 Fe + 250 µg folic acid) through pregnancy to 1 mo after delivery. At birth, 546 infants were followed for 12 mo to assess growth. Anthropometric measures of body size and composition were collected monthly, and morbidity and dietary intake surveillance was carried out weekly.
Results
No differences in maternal socioeconomic characteristics by treatment group or follow-up period were found. Infants born to mothers prenatally supplemented with zinc had significantly (P < 0.05) larger average growth measures beginning in month 4 and continuing through month 12. In longitudinal regression modeling, prenatal zinc was associated with greater weight (by 0.58 ± 0.12 kg; P < 0.001), calf circumference (by 1.01 ± 0.21 cm; P < 0.001), chest circumference (by 0.60 ± 0.20 cm;P = 0.002), and calf muscle area (by 35.78 ± 14.75 mm2; P = 0.01) after adjustment for a range of covariates. No effect was observed for linear growth.
Conclusion
Maternal zinc supplementation in this population was associated with offspring growth, which is suggestive of lean tissue mass accretion.
INTRODUCTION
Pregnancy increases zinc requirements by 3 mg/d (1), an increment often difficult to attain in women living in resource-poor environments and having a chronic inadequate intake of zinc. Access to foods rich in bioavailable zinc may be limited, and dietary staples often contain phytates, which inhibit zinc absorption (2). The global prevalence of deficiencies in maternal zinc intake has been estimated to be as high as 82% (3), which is comparable to the 80–88% of pregnant women in the Peruvian population in the present cohort who were estimated to have inadequate zinc intakes (4). Zinc has a known association with growth during childhood, but the consequences of zinc deficiency in pregnancy (a critical phase of cell differentiation and proliferation for the developing fetus) for later offspring growth are underexplored.
Findings from studies of infants and children supplemented with zinc have relevance only to the extent that zinc acquired during fetal life is conserved for growth postnatally, eg, in lean tissue accumulation. The findings from a meta-analysis of randomized controlled trials investigating the effect of zinc supplementation on growth in children <12 y old found positive effects on both height and weight (5). The effect of zinc supplements on growth in young children appears to be influenced by many factors, including baseline anthropometry (6, 7), zinc status (8), and birth weight (9). Fewer studies examined the effects of zinc treatment on anthropometric measures of body composition; some evidence has been found for the effects of zinc on skinfold-thickness measures (10), mid-upper arm circumference (11), and fat-free mass accretion (12).
Animal studies in rhesus monkeys showed that zinc deprivation during pregnancy negatively affected offspring growth after 1 y of life (13). In humans, only one study (from Bangladesh) was identified in which the growth of infants whose mothers were prenatally supplemented with zinc was compared with the growth of infants in a control group; no growth-related differences were found at 6 mo of age (14). We conducted a zinc supplementation trial among 1295 Peruvian pregnant women from 1995 to 1997 and reported no effect on birth outcomes, including newborn anthropometry, by maternal prenatal supplement type (15). In the present analysis, we used follow-up data from the earlier trial involving a subset of offspring followed through age 12 mo to examine growth and body-composition effects associated with prenatal zinc as they emerged throughout infancy.
SUBJECTS AND METHODS
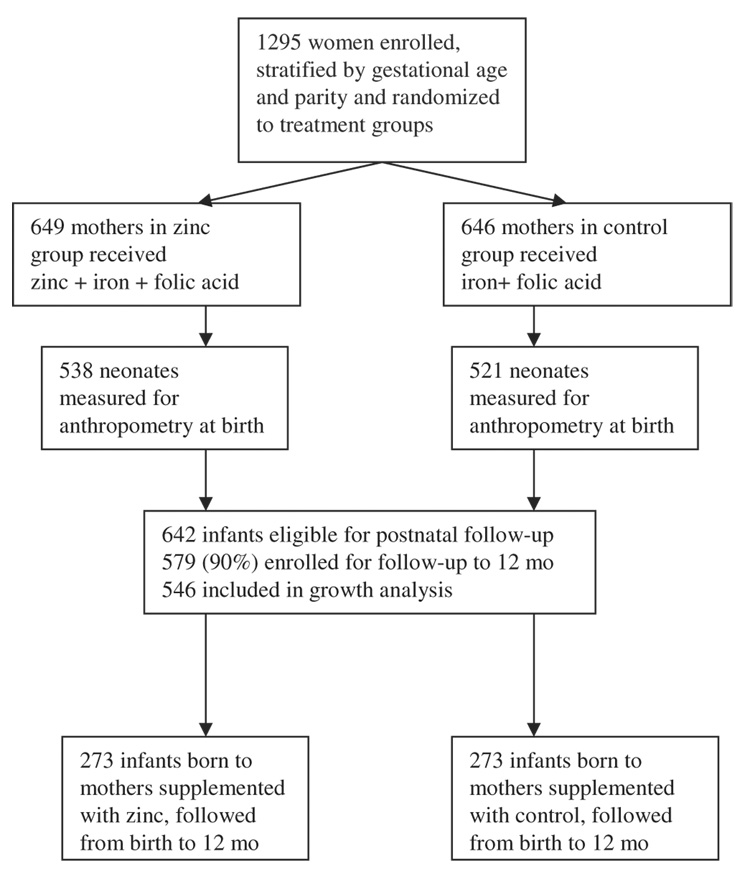
A double-blind randomized controlled trial of prenatal zinc supplementation was conducted from 1995 to 1997 in Villa El Salvador, a periurban community in the south part of Lima, Peru. Details of this study were reported elsewhere (16–18). Briefly, 1295 women were enrolled at entrance to prenatal care between 10–24 wk gestation, and they were randomly assigned to the treatment (zinc + iron + folic acid) or control (iron + folic acid) group. From this cohort, we had data at birth for 1059 infants. At the time the follow-up study was initiated, 642 infant candidates were available who met the criteria for entering into the follow-up study: singleton birth, healthy infant, and residence in the study community. Five hundred seventy-nine infants were enrolled; infants followed for a minimum of 1 mo during infancy (n = 546) were included in this analysis—273 in the zinc group and 273 in the control group (Figure 1). During the study, some families moved out of the area, and some mothers no longer wanted to participate when they returned to work; these 2 reasons for dropping out contributed to the lower numbers in the sample toward the end of infancy. There were no known infant deaths in the sample.
FIGURE 1.
Trial profile.
For each phase of the study (pregnancy and follow-up of infants), the protocol was fully explained to the mothers, and written informed consent was obtained. Approval of this study was granted by the Ethics Committee at the Instituto de Investigacion Nutritiónal and the Committee for Human Research at The Johns Hopkins Bloomberg School of Public Health.
Micronutrient supplements
In the primary study, pregnant women were randomly assigned to receive either 15 mg zinc sulfate, 60 mg ferrous sulfate, and 250 µg folic acid (zinc group) or 60 mg ferrous sulfate and 250 µg folic acid (control group). Supplementation began in both groups between 10 and 24 wk gestation (mean: 15.6 ± 4.6 wk) and continued through 4 wk after delivery. A local pharmaceutical company (Instituto Quimioterápico SA, Lima, Perú) produced both supplement types, which were similar in both color and shape, in coded blister packages. Compliance was monitored through biweekly fieldworker visits during pregnancy until delivery. Women took an average of 85% of the assigned number of tablets, and there were no significant differences by supplement type (15, 17).
Data collection: anthropometry, morbidity, and infant feeding
In the primary study, baseline information was collected from the mothers on a range of socioeconomic and demographic characteristics including the geographic area where they were raised, education level attained, housing material and amenities, house-hold ownership of assets, and marital status. Maternal clinical history and information about the pregnancy and delivery were also obtained. At enrollment, all participing women were considered to have a low-risk pregnancy according to local prenatal care guidelines (15).
A trained nutritionist used standard procedures to take anthropometric measures of the mothers at enrollment and at 28–32 and 36–38 wk gestation and of the infants at birth and every month until age 1 y (or the period of follow-up) (19). Maternal standing height was measured by using a stadiometer, and weight was recorded to the nearest 100 g on a SECA scale (SECA, Hamburg, Germany). Newborns were weighed at birth by hospital personnel using a SECA scale (± 10 g), and crown-heel length, circumferences (head, chest and mid-upper arm), and skinfold thicknesses (triceps, calf, and subscapular) were measured on day 1 by the study nutritionist. Anthropometric measures of the infant were then taken monthly, again using a SECA scale (±10 g) for weight and a wooden measuring board to obtain recumbent length to the nearest 0.1 cm. Skinfold thicknesses were measured with Lange precision calipers (Cambridge Scientific Instruments, Cambridge, MD).
Morbidity surveillance of infants in the follow-up study took place during weekly home visits by fieldworkers from birth to 12 mo. The methods for data collection were adapted from Penny et al (20, 21) and Lanata et al (22). Fieldworkers questioned care-givers about the child’s health—including questions about cough, fever, diarrhea, and appetite—since the last visit. The child was also examined by the fieldworkers if caregivers reported any new sign of illness or any worsening of existing illness or if no examination had taken place for 1 mo. Examinations included assessments of respiratory rate and signs of dehydration and measurement of rectal temperature. Referrals were given for any one of the designated signs of illness: fever lasting longer than 24 h, cough with elevated respiratory rate, persistent diarrhea, diarrhea with signs of dehydration, vomiting, or skin infection. Infant consumption of breast milk and complementary foods was collected by using a list of foods during these weekly morbidity surveillance visits by fieldworkers. Because funding for this portion of the study became available later, we focused the collection of morbidity and infant feeding information (98% of observation days) during the second half of infancy.
Definitions
Infants weighing less than the 10th percentile for gestational age were classified as small-for-gestational-age (23), and those weighing <2500 g at birth were classified as having low birth weight (LBW). Arm and calf muscle and fat areas were calculated with the following equations (24):
| (1) |
| (2) |
and
| (3) |
The 2006 growth standards of the World Health Organization (25) were applied to obtain z scores for length-for-age (LAZ), weight-for-age (WAZ), and weight-for-length (WLZ) and to classify infants as stunted (LAZ < −2 SD), underweight (WAZ < −2 SD), or wasted (WLZ < −2 SD).
Among the morbidity variables, diarrhea was defined as ≥3 liquid or loose stools in the previous 24 h. The longitudinal prevalence of diarrhea was defined as the number of days of diarrhea divided by the total number of days of observation for each child (26). Reported fever or rectal temperature > 38 °C was used to define fever. The term sick day was defined to include days on which infants were reported to have had cough, fever, or diarrhea. The longitudinal prevalence of sick days was similarly defined as the number of sick days divided by the number of days of observation per child. A child was categorized as receiving complementary foods if his or her mother reported consumption of solid foods (cereal, meat or fish, mixed or blended foods, stews, bread or other cereal products, and purées) plus breast milk. Those infants whose mothers reported consumption of meat, fish, eggs, or other milks besides breast milk were categorized as having animal-source foods in their diets. We assessed whether the foods and liquids were present in the infant’s diet at the time of the anthropometric measure or outcome, and we also created additional feeding variables for regression modeling. Global and child-specific prevalences for the infant feeding variables were calculated by summing all days with reported consumption and dividing by total recall days. To consider time-delayed effects of the feeding variables on growth outcomes in regression modeling, lagged variables were created by summing days of consumption for particular foods and food categories over the recall days during a previous period (eg, 1 mo) for individual children.
Principal components analysis was used to combine correlated variables and to create 2 indexes representing particularly influential social and environmental characteristics (27). Briefly, an asset-ownership factor represented housing material, electricity in the home, and type of cooking facility used. The second composite index, a sanitation and hygiene factor, incorporated the type of toilet facility, the number of persons residing in the home, and the source of water for the household.
Statistical analysis
The total sample size of this study (n = 546) was determined previously, by the number of infants available, eligible, and enrolled for follow-up from the primary prenatal supplementation study previously described. Assuming an α-level of 0.05 and a power (1-β) of 0.80, minimum detectable differences by supplementation type were ± 0.17 kg for weight and ± 0.42 cm for length. To check for selection biases in the group followed through infancy, differences in socioeconomic and demographic characteristics were compared by using t tests, Pearson chi-square tests of independence, and analysis of variance. Skinfold-thickness measures found to contain right-skewness were normalized by natural logarithmic transformation for various analyses and presented as geometric means.
Bivariate analyses of growth differences by treatment group were performed across several continuous indicators by one-way analysis of variance with Bonferroni multiple-comparisons test. Lowess curves were constructed to compare treatment and control groups for different growth outcomes by using 0.5 bandwidth. Longitudinal modeling of the treatment effect on growth outcomes was carried out by using panel regression by generalized least-squares with random effects (28, 29). This procedure was selected to account for heterogeneity in the sample and to partition out the variability observed within individual subjects. Several covariates representing infant and maternal biological factors, age, socioeconomic and environmental conditions, and infant morbidities and diet were examined in modeling for influence on the treatment effect for growth outcomes. Infant growth outcomes were modeled with covariates available in all panels. We also introduced interaction terms based on previous findings in the literature related to the multiplicative effects of treatment group in combination with different factors such as age, sex, and birth weight. To further investigate the varied influence of different covariates within subgroups, growth outcomes were compared by supplement type within sex, birth weight, and z score strata. Statistical significance was defined as P < 0.05, and all data analyses were performed with STATA software (version 8.0; Stata Corp, College Station, TX; 30).
RESULTS
Supplementation groups were compared for compositional differences vis-à-vis socioeconomic and demographic characteristics throughout infancy (Table 1). As in previous analyses, the baseline proportion of households with electricity was found to be significantly (P = 0.05) higher in the control group than in the zinc group. No other significant differences between treatment groups were noted in all of the months of infancy, despite losses to follow-up. Most women (>88%) in both groups had completed a primary school education. Many households were reported to have toilet facilities within the home, but more than one-third of the people in both groups reported still using a public latrine or the ground. Nearly one-half of the sampled mothers were primiparous, and, in both groups, the incidence of LBW was 2.2%, which is consistent with the low-risk nature of the sample selected for participation and which is a result of the possible effect of iron and folic acid supplementation on size at birth.
TABLE 1.
Baseline characteristics by follow-up period and treatment group
| Baseline (n = 1295) |
Growth analysis (n = 546) |
|||
|---|---|---|---|---|
| Zinc (n = 646) | Control (n = 649) | Zinc (n = 273) | Control (n = 273) | |
| Maternal age (y) | 24.1 ± 5.21 | 24.6 ± 5.3 | 23.8 ± 4.6 | 24.5 ± 5.3 |
| Gestational age at entry (wk) | 16.0 ± 4.6 | 15.9 ± 4.6 | 15.3 ± 4.7 | 15.8 ± 4.6 |
| Maternal education (%) | ||||
| <Primary | 5.7 | 9.2 | 5.5 | 11.0 |
| Primary, <secondary | 41.6 | 41.3 | 41.3 | 39.2 |
| Secondary | 30.3 | 30.6 | 34.9 | 34.4 |
| >Secondary | 22.3 | 18.9 | 18.0 | 15.4 |
| Primiparous (%) | 46.8 | 46.3 | 49.1 | 45.4 |
| Maternal BMI (in kg/m2) | 24.0 ± 3.4 | 24.0 ± 3.2 | 24.0 ± 3.2 | 24.0 ± 3.5 |
| Maternal height (cm) | 151.7 ± 5.6 | 151.2 ± 5.6 | 151.8 ± 5.4 | 151.5 ± 5.5 |
| Electricity in home (%)2 | 76.0 | 80.4 | 79.5 | 83.2 |
| Single (%) | 13.9 | 15.3 | 14.3 | 15.0 |
x¯ ± SD (all such values).
Significant difference in proportion with electricity in their home by treatment group at baseline, P = 0.05 (Pearson chi-square test of independence).
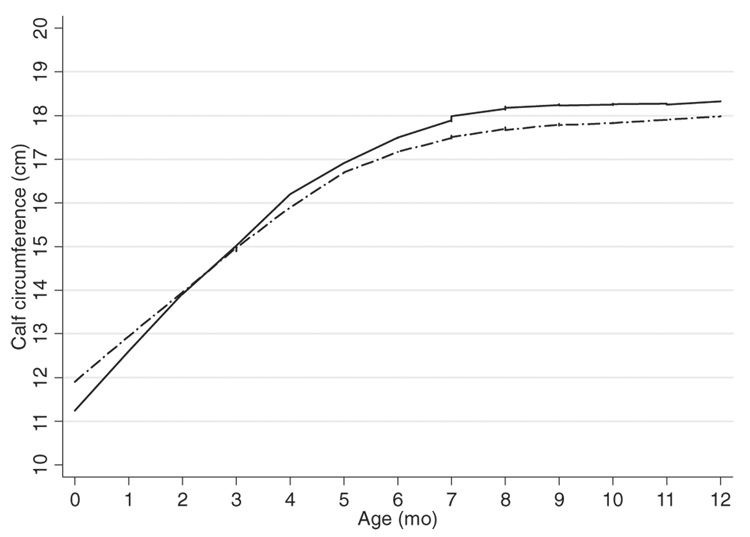
Anthropometric size and body-composition measures were similar in the first quarter of infancy by supplement type, except in month 1, when weight, head circumference, and triceps and subscapular skinfold thicknesses were significantly greater in the control group (Table 2). This growth advantage was reversed beginning in month 4, but it became more apparent by month 6 and continued so through month 12 for weight, calf, chest, and mid-upper arm circumferences, and skinfold thicknesses. No differences in length or head circumference were observed during this period. The growth trajectories for calf circumference in the zinc and control groups show the crossover effect around 2 mo of age (Figure 2).
TABLE 2.
Difference in mean anthropometric measures [zinc (Zn) – control (Ctrl)] of body size and composition in infants 0–12 mo old1
| Month (sample size by treatment group) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Growth outcome | (Zn, n = 262; Ctrl, n = 260) | (Zn, n = 224; Ctrl, n = 217) | (Zn, n = 220; Ctrl, n = 212) | (Zn, n = 227; Ctrl, n = 213) | (Zn, n = 217; Ctrl, n = 203) | (Zn, n = 197; Ctrl, n = 188) | (Zn, n = 211; Ctrl, n = 213) | (Zn, n = 178; Ctrl, n = 168) | (Zn, n = 157; Ctrl, n = 157) | (Zn, n = 160; Ctrl, n = 159) | (Zn, n = 136; Ctrl, n = 131) | (Zn, n = 130; Ctrl, n = 117) | (Zn, n = 115; Ctrl, n = 122) |
| Weight (kg) | 0.11 | −0.112 | −0.00 | 0.05 | 0.06 | 0.11 | 0.11 | 0.212 | 0.212 | 0.282 | 0.232 | 0.17 | 0.23 |
| Length (cm) | −0.12 | −0.39 | −0.24 | −0.11 | −0.06 | −0.13 | 0.00 | 0.34 | 0.29 | 0.27 | 0.16 | −0.11 | 0.16 |
| Circumference (cm) | |||||||||||||
| Head | −0.14 | −0.252 | −0.20 | −0.11 | −0.12 | −0.02 | −0.03 | 0.06 | −0.08 | −0.13 | 0.08 | −0.13 | −0.11 |
| Calf | −0.05 | −0.10 | 0.09 | 0.19 | 0.322 | 0.312 | 0.362 | 0.442 | 0.392 | 0.432 | 0.402 | 0.332 | 0.412 |
| Chest | −0.20 | −0.34 | −0.08 | 0.29 | 0.15 | 0.23 | 0.492 | 0.49 | 0.37 | 0.792 | 0.622 | 0.612 | 0.40 |
| MUAC (cm) | −0.03 | −0.14 | 0.10 | 0.08 | 0.15 | 0.15 | 0.20 | 0.19 | 0.19 | 0.292 | 0.272 | 0.16 | 0.18 |
| Skinfold thickness3 | |||||||||||||
| Triceps (mm) | −0.10 | −0.232 | 0.02 | −0.05 | 0.01 | 0.08 | 0.08 | 0.25 | 0.20 | 0.20 | 0.17 | 0.372 | 0.492 |
| Calf (mm) | −0.02 | −0.33 | −0.03 | 0.03 | 0.02 | 0.18 | 0.15 | 0.27 | 0.20 | 0.712 | 0.21 | 0.492 | 0.25 |
| Subscapular (mm) | −0.08 | −0.382 | −0.06 | −0.07 | 0.02 | −0.24 | −0.02 | 0.16 | 0.19 | 0.392 | −0.07 | 0.19 | 0.04 |
MUAC, mid-upper arm circumference.
P < 0.05 (one-way ANOVA with Bonferroni multiple-comparison test).
Geometric x¯ was used for all skinfold thickness measures.
FIGURE 2.
Lowess curves (bandwidth = 0.5) for calf circumferences of Peruvian infants by prenatal treatment group. —, Zinc; —-, control.
Infants experienced diarrhea on 7.2% of the total days of recall (2669/36 961). No statistically significant differences in the longitudinal prevalence of diarrhea by treatment group were observed here. Among the infants assessed primarily after 6 mo for feeding practices,95%were still breastfed. Infants were reported to be receiving breast milk on 92.9% of the days of observation. On 77.1% of the days, infants were consuming complementary foods; on 66.8% of days, they had some animal-source food or cow milk in their diet; and, on 18.9% of the days, they were reportedly fed meat or fish.
In regression modeling for longitudinal analysis, significant treatment differences in growth after adjustment for covariates were found for weight (P < 0.001), calf circumference (P < 0.001), chest circumference (P = 0.002), and calf muscle area (P = 0.01) (Table 3). The consistently important factors across models were the exact age of the infant (fitted as a quadratic), sex, corresponding birth and maternal anthropometric measures, the presence of breast milk in the infant diet, and diarrheal morbidity (the longitudinal prevalence of acute diarrhea). Inclusion of the diarrhea indicator removed a small amount of the treatment effect, although both prenatal zinc treatment and the longitudinal prevalence of diarrhea remained independently significant in the weight, calf circumference, and calf muscle area models. The proportion of days on which an infant had complementary foods in the diet in the previous month was positively associated with increases in the growth outcomes without removal of the treatment effect, as was the presence of breast milk in the diet for weight, calf circumference, and calf muscle area. Significant interaction was observed for longitudinal weight outcomes by offspring age and treatment (P < 0.001) and by offspring sex and treatment (P = 0.01). The negative interaction term for age and treatment in the models indicated a declining effect over the 12-mo follow-up period. No differences were found in treatment effect on growth outcomes by nutritional status including WAZ, LAZ, or WLZ.
TABLE 3.
Longitudinal regression models for prenatal treatment effect on growth in Peruvian infants
| Growth outcomes1 | Prenatal zinc | P |
|---|---|---|
| Weight (kg) | 0.58 ± 0.122 | <0.001 |
| Length (cm) | 0.13 ± 0.16 | 0.403 |
| Head circumference (cm) | 0.01 ± 0.09 | 0.939 |
| Mid-upper arm circumference (cm) | 0.09 ± 0.09 | 0.294 |
| Calf circumference (cm) | 1.01 ± 0.21 | <0.001 |
| Chest circumference (cm) | 0.60 ± 0.20 | 0.002 |
| Sum of skinfold thickness measures: triceps+calf+subscapular (mm) | 0.01 ± 0.01 | 0.456 |
| Arm fat area (mm2) | 3.07 ± 8.04 | 0.703 |
| Arm muscle (mm2) | 11.51 ± 14.91 | 0.440 |
| Calf fat area (mm2) | 8.64 ± 9.12 | 0.344 |
| Calf muscle area (mm2) | 35.78 ± 14.25 | 0.01 |
Longitudinal panel regression modeling with random effects, adjusted as appropriate for age; age quadratic; age × treatment interaction; sex; sex × treatment interaction; birth anthropometric measure; maternal anthropometry; primiparity; breastfeeding; complementary feeding in previous mo; diarrhea morbidity; and hygiene and sanitation index.
x¯ ± SE (all such values).
Neither the asset-ownership composite nor the separately considered socioeconomic and demographic factors (eg, maternal age, level of education, region of origin, toilet type, and electricity) were found to be confounding or effect-modifying factors. No significant sex × age, sex × birth measure, age × breast milk in the diet, or breast milk in the diet × child prevalence of diarrhea interactions for growth outcomes were found. Too few data were available for an analysis of differential effect of LBW infants compared with infants of normal or heavier birth weight. Other birth outcomes (small-for-gestational-age and ponderal index) and pregnancy factors (length of gestation) did not influence or modify treatment effect in our longitudinal models.
DISCUSSION
In this resource-poor population, ifants born to mothers prenatally supplemented with zinc together with iron and folic acid had significantly greater anthropometric measures from months 4 through 12 than did infants whose mothers were supplemented with iron and folic acid alone. The longitudinal effects of zinc treatment remained significant for weight, calf and chest circumferences, and calf muscle area after control for a range of covariates, including infant feeding practices and diarrheal morbidity. No effects were detected for linear growth or skinfold-thickness measures of adiposity.
On average, the infants from the zinc group were 0.58 ± 0.12 kg/mo heavier than were those from the control group, by slightly more than 0.5 of the SD of the weight distribution in the present sample. The positive association of prenatal zinc with weight outcomes was in small part mediated through diarrhea morbidity, but it remained significant, independent of this effect. A negative age × treatment interaction effect was observed in longitudinal modeling for weight, which suggested that the differences by treatment group were diminishing throughout infancy. We did not find that the treatment effect was influenced by birth weight, as was found in other studies (6, 7, 9), but the incidence of LBW in our population was small. In our view, insufficient data were available to address the question of LBW and growth outcomes, and, thus, that question must await future research.
The mechanisms whereby prenatal zinc nutriture would affect postnatal growth patterns are unknown at this time. It might be thought that zinc influenced growth through effects on immune system development, as stated above, but the effects we found on growth and body composition were independent from the morbidity factors considered in the present trial. Particular enzymes or growth hormones requiring zinc during pregnancy may be important in later growth pathways (31, 32). Placental alkaline phosphatase requires zinc to stimulate DNA synthesis and cell proliferation in pregnancy, and lower concentrations of placental alkaline phosphatase have been found in newborns born with intrauterine growth restriction (33, 34). Another trial carried out in Peru from 1998 to 2000 found greater femur diaphysis length among fetuses of mothers supplemented with 25 mg Zn than in control fetuses, and the investigators proposed the participation of this enzyme as a potential mechanism contributing to growth (35). In that same study, no differences in anthropometric size at birth were detectable by supplement type. Nutritional status is known to influence the synthesis of insulin-like growth factor-I (IGF-I), a cell growth and division–stimulating hormone that is especially important during the fetal period and infancy (36, 37). Animal studies have shown that zinc regulates IGF-I activity in osteoblast formation and particularly regulates bone growth (38). In humans, a 5-mo zinc supplementation of stunted infants in Vietnam showed higher IGF-I concentrations in the zinc than in the placebo group (P < 0.05) (32). These proposed mechanisms and others, discussed below, point to the role of zinc within the cell cycle as a contributing factor to later growth outcomes. The manifestation of growth in the months after birth could imply qualitative changes in fetal development in response to micronutrient nutrition (39–41).
Our findings suggest that prenatal zinc supplementation may have contributed to lean tissue mass accretion, particularly in the extremities of the infants, again independent of morbidity. The treatment effect initially found on measures of adiposity was removed with consideration of weight as a covariate, whereas differences in indicators more closely approximating muscle mass remained. In 75% of the follow-up months of infancy, calf circumference was significantly larger in infants whose mothers were treated with zinc than in those in the control group. Calf muscle area was significantly (P = 0.01) greater in the treatment group than in the control group, but calf fat area was not. It is possible that zinc contributes to motor development and activity patterns during infancy (42), thus preferentially affecting gains in muscle tissue. An alternative—and more likely, in our view—possibility is that zinc is required for muscle cell proliferation and maintenance early in life (43). Myoblasts fuse to form skeletal muscle fibers during embryogenesis, and at some subsequent point they lose their ability to divide (37). Thus, adequate zinc may be required during pregnancy to ensure hyperplasia in muscle cells, which later grow in size and become manifest in lean tissue mass. Arm muscle area was also greater in the zinc than in the control group, but the difference was not significant, perhaps because of the greater variability in this anthropometric outcome than in calf muscle area. The detected differences in chest circumference may be due in part to differences in fat-free tissue, but it likely involves other body compartment differences. Previous trials supplementing older children with zinc have shown differences in body composition (10, 44, 45) and, particularly among stunted infants, significant increases in fat free mass (11). The health advantages afforded by the lean tissue mass accretion are not completely understood, although some evidence suggests potential benefits for adult size, physical performance, and reductions in risk of chronic disease (46–48).
This study was limited by the fact that we considered morbidity and dietary surveillance data primarily from the second half of infancy. In view of the negative treatment × age interaction detected in longitudinal modeling for weight, more information pertaining to the earlier phase may have been useful. However, the incidence of diarrhea peaks between the ages of 6 mo to 11 mo (48), and infant feeding behaviors during this complementary feeding period are especially crucial to growth outcomes (49), which suggests that we considered, as possible confounding factors, the intervening effects of diet and morbidity during the more important period. The effect of prenatal zinc treatment was not modified by infant diet, although both the presence of breast milk in the diet and the proportion of days an infant consumed complementary foods in the previous month were found to be important predictors of growth outcomes. Finally, in recognition of the health and growth benefits that may be attributed to iron and folic acid in the supplements in both treatment and control groups, there may have been some attenuation of outcome effects in the present study.
There has been minimal inquiry into the effects of zinc nutrition during pregnancy on offspring growth and health outcomes. We found that prenatally supplementing pregnant women with zinc affected anthropometric growth and body composition of their infants through 12 mo of age. The study highlights the potential for programming prenatal nutrition interventions that could have positive effects for the infant. Further investigation is needed to replicate these findings and to evaluate the mechanisms through which maternal nutrition during pregnancy influences growth and development trajectories beyond gestation.
Acknowledgments
We are very grateful to all of the mothers and infants whose cooperation allowed us to undertake this study, to the health personnel of the Centro Materno Infantil Cesar López Silva, to the health authorities from the Ministry of Health, Dirección de Salud Lima Sur for their cooperation, and to the Instituto de Investigación Nutricional team for their dedication and professionalism in the implementation of this study.
The authors’ responsibilities were as follows—LEC, NZ, and AHS: design of the study, data collection and analysis, and writing of the manuscript; ZL: collection and interpretation of the growth data; LLI: conceptualized and performed the data analysis and wrote the manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Presented in part at the 2007 Experimental Biology Meeting, Washington, DC, April 28–May 2, 2007.
Supported by DAN-5116-A-00-8-51-00 and HRN-A-00-97-00015-00, cooperative agreements with USAID/Office of Health, Population, and Nutrition and The Johns Hopkins University; International Maternal and Child Health Training grant no. T32HD046405-01A from the National Institutes of Health (NIH) (2006); and grant no. HD 42675 from the NIH.
REFERENCES
- 1.Food and Nutrition Board/Institute of Medicine (IOM) Washington, DC: National Academies Press; Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. 2000 [PubMed]
- 2.Wise A. Phytate and zinc bioavailability. Int J Food Sci Nutr. 1995;46:53–63. doi: 10.3109/09637489509003386. [DOI] [PubMed] [Google Scholar]
- 3.Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr. 1998;68 suppl:499S–508S. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- 4.Sacco LM, Caulfield LE, Zavaleta N, Retamozo L. Dietary pattern and usual nutrient intakes of Peruvian women during pregnancy. Eur J Clin Nutr. 2003;57:1492–1497. doi: 10.1038/sj.ejcn.1601716. [DOI] [PubMed] [Google Scholar]
- 5.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002;75:1062–1071. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Duran C, Heresi G, Fisberg M, Uauy R. Controlled trial of zinc supplementation during recovery from malnutrition: effects on growth and immune function. Am J Clin Nutr. 1987;45:602–608. doi: 10.1093/ajcn/45.3.602. [DOI] [PubMed] [Google Scholar]
- 7.Umeta M, West CE, Haidar J, Deurenberg P, Hautvast JG. Zinc supplementation and stunted infants in Ethiopia: a randomised controlled trial. Lancet. 2000;355:2021–2026. doi: 10.1016/S0140-6736(00)02348-5. [DOI] [PubMed] [Google Scholar]
- 8.Osendarp SJ, Santosham M, Black RE, Wahed MA, van Raaij JM, Fuchs GJ. Effect of zinc supplementation between 1 and 6 mo of life on growth and morbidity of Bangladeshi infants in urban slums. Am J Clin Nutr. 2002;76:1401–1408. doi: 10.1093/ajcn/76.6.1401. [DOI] [PubMed] [Google Scholar]
- 9.Lira PI, Ashworth A, Morris SS. Effect of zinc supplementation on the morbidity, immune function, and growth of low-birth-weight, full-term infants in northeast Brazil. Am J Clin Nutr. 1998;68 suppl:418S–424S. doi: 10.1093/ajcn/68.2.418S. [DOI] [PubMed] [Google Scholar]
- 10.Cavan KR, Gibson RS, Grazioso CF, Isalgue AM, Ruz M, Solomons NW. Growth and body composition of periurban Guatemalan children in relation to zinc status: a longitudinal zinc intervention trial. Am J Clin Nutr. 1993;57:344–352. doi: 10.1093/ajcn/57.3.344. [DOI] [PubMed] [Google Scholar]
- 11.Kikafunda JK, Walker AF, Allan EF, Tumwine JK. Effect of zinc supplementation on growth and body composition of Ugandan preschool children: a randomized, controlled, intervention trial. Am J Clin Nutr. 1998;68:1261–1266. doi: 10.1093/ajcn/68.6.1261. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Gomez NM, Domenech E, Barroso F, Castells S, Cortabarria C, Jimenez A. The effect of zinc supplementation on linear growth, body composition, and growth factors in preterm infants. Pediatrics. 2003;111:1002–1009. doi: 10.1542/peds.111.5.1002. [DOI] [PubMed] [Google Scholar]
- 13.Golub MS, Gershwin ME, Hurley LS, Saito WY, Hendrickx AG. Studies of marginal zinc deprivation in rhesus monkeys. IV. Growth of infants in the first year. Am J Clin Nutr. 1984;40:1192–1202. doi: 10.1093/ajcn/40.6.1192. [DOI] [PubMed] [Google Scholar]
- 14.Osendarp SJ, van Raaij JM, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet. 2001;357:1080–1085. doi: 10.1016/s0140-6736(00)04260-4. [DOI] [PubMed] [Google Scholar]
- 15.Caulfield LE, Zavaleta N, Figueroa A, Leon Z. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. J Nutr. 1999;129:1563–1568. doi: 10.1093/jn/129.8.1563. [DOI] [PubMed] [Google Scholar]
- 16.Zavaleta N, Caulfield LE, Garcia T. Changes in iron status during pregnancy in peruvian women receiving prenatal iron and folic acid supplements with or without zinc. Am J Clin Nutr. 2000;71:956–961. doi: 10.1093/ajcn/71.4.956. [DOI] [PubMed] [Google Scholar]
- 17.Caulfield LE, Zavaleta N, Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. Am J Clin Nutr. 1999;69:1257–1263. doi: 10.1093/ajcn/69.6.1257. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J Nutr. 2000;130:2251–2255. doi: 10.1093/jn/130.9.2251. [DOI] [PubMed] [Google Scholar]
- 19.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 20.Penny ME, Marin RM, Duran A, et al. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. Am J Clin Nutr. 2004;79:457–465. doi: 10.1093/ajcn/79.3.457. [DOI] [PubMed] [Google Scholar]
- 21.Penny ME, Peerson JM, Marin RM, et al. Randomized, community-based trial of the effect of zinc supplementation, with and without other micronutrients, on the duration of persistent childhood diarrhea in Lima, Peru. J Pediatr. 1999;135:208–217. doi: 10.1016/s0022-3476(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 22.Lanata CF, Quintanilla N, Verastegui HA. Validity of a respiratory questionnaire to identify pneumonia in children in Lima, Peru. Int J Epidemiol. 1994;23:827–834. doi: 10.1093/ije/23.4.827. [DOI] [PubMed] [Google Scholar]
- 23.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 24.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34:2540–2545. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 25.Geneva, Switzerland: World Health Organization; ANTHRO 2005, beta version: software for assessing growth and development of the world’s children. 2006
- 26.Morris SS, Cousens SN, Kirkwood BR, Arthur P, Ross DA. Is prevalence of diarrhea a better predictor of subsequent mortality and weight gain than diarrhea incidence? Am J Epidemiol. 1996;144:582–588. doi: 10.1093/oxfordjournals.aje.a008968. [DOI] [PubMed] [Google Scholar]
- 27.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton L. Statistics with STATA. Belmont, CA: Brooks/Cole (a division of Thomson Learning, Inc); 2004. [Google Scholar]
- 29.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of longitudinal data. Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- 30.STATA statistical software, version 8.0. College Station, TX: Stata Corp; 2005. [Google Scholar]
- 31.Domenech E, Diaz-Gomez NM, Barroso F, Cortabarria C. Zinc and perinatal growth. Early Hum Dev. 2001;65 suppl:S111–S117. doi: 10.1016/s0378-3782(01)00213-4. [DOI] [PubMed] [Google Scholar]
- 32.Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM. Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am J Clin Nutr. 1996;63:514–519. doi: 10.1093/ajcn/63.4.514. [DOI] [PubMed] [Google Scholar]
- 33.She QB, Mukherjee JJ, Huang JS, Crilly KS, Kiss Z. Growth factor-like effects of placental alkaline phosphatase in human fetus and mouse embryo fibroblasts. FEBS Lett. 2000;469:163–167. doi: 10.1016/s0014-5793(00)01273-4. [DOI] [PubMed] [Google Scholar]
- 34.Rodin A, Duncan A, Quartero HW, et al. Serum concentrations of alkaline phosphatase isoenzymes and osteocalcin in normal pregnancy. J Clin Endocrinol Metab. 1989;68:1123–1127. doi: 10.1210/jcem-68-6-1123. [DOI] [PubMed] [Google Scholar]
- 35.Merialdi M, Caulfield LE, Zavaleta N, et al. Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. Am J Clin Nutr. 2004;79:826–830. doi: 10.1093/ajcn/79.5.826. [DOI] [PubMed] [Google Scholar]
- 36.Tortora G, Grabowski S. Principles of anatomy and physiology. Hoboken, NJ: Wiley & Sons, Inc; 2003. [Google Scholar]
- 37.Matsui T, Yamaguchi M. Zinc modulation of insulin-like growth factor’s effect in osteoblastic MC3T3–E1 cells. Peptides. 1995;16:1063–1068. doi: 10.1016/0196-9781(95)00067-t. [DOI] [PubMed] [Google Scholar]
- 38.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Dominici F, Dipietro JA. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. Am J Obstet Gynecol. 2004;190:1106–1112. doi: 10.1016/j.ajog.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 39.Martin H, Lindblad B, Norman M. Endothelial function in newborn infants is related to folate levels and birth weight. Pediatrics. 2007;119:1152–1158. doi: 10.1542/peds.2006-2706. [DOI] [PubMed] [Google Scholar]
- 40.Shankar A. Nutritional modulation of immune function and infectious disease. In: Bowman BA, Russell RM, editors. Present knowledge in nutrition. 9th ed. vol 2. Washington, DC: ILSI Press; 2006. pp. 604–624. [Google Scholar]
- 41.Black MM, Baqui AH, Zaman K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004;80:903–910. doi: 10.1093/ajcn/80.4.903. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Kutzler L, Killefer J, Novakofski J. Roles of zinc in skeletal muscle cell growth. FASEB J. 2006;20 A625-b (abstr). [Google Scholar]
- 43.Friis H, Ndhlovu P, Mduluza T, et al. The impact of zinc supplementation on growth and body composition: a randomized, controlled trial among rural Zimbabwean schoolchildren. Eur J Clin Nutr. 1997;51:38–45. doi: 10.1038/sj.ejcn.1600358. [DOI] [PubMed] [Google Scholar]
- 44.Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr. 2007;86:82–91. doi: 10.1093/ajcn/86.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–121. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 46.Rivlin RS. Keeping the young-elderly healthy: is it too late to improve our health through nutrition? Am J Clin Nutr. 2007;86 suppl:1572S–1576S. doi: 10.1093/ajcn/86.5.1572S. [DOI] [PubMed] [Google Scholar]
- 47.Black R, Lanata CF. Infections of the gastrointestinal tract. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. Epidemiology of diarrheal diseases in developing countries. [Google Scholar]
- 48.Caulfield LE, Huffman SL, Piwoz EG. Interventions to improve the complementary food intakes of 6–12 month old infants in developing countries: impact on growth, prevalence of malnutrition and potential contribution to child survival. Food Nutr Bull. 1999;20:183–200. [Google Scholar]