HER2/neu gene is a member of family of genes encoding trans-membrane receptors for four growth factors, including the epidermal growth factor receptor (EGFR), HER2/neu, HER-3, and HER-4. The intracellular domain of HER2/neu has tyrosine kinase activity which regulates cell growth and proliferation [1-5]. Overexpression of HER2/neu can transform cultured cells into more aggressive phenotype and accelerate tumorigenesis [1, 6]. HER2/neu is overexpressed in 20−25% of invasive breast cancers and associated with an aggressive tumor, an early relapse and reduced survival rate [7-9].
It has been found that a tumor cell line overexpressing HER2/neu resulted in an increase in levels of choline-containing compounds (tCho) measured by in-vitro proton MR spectroscopy (MRS), including phosphocholine (PCho), glycerophosphocholine (GPC), and choline [10]. It was postulated that growth factor-mediated activation of the tyrosine kinase cascade can lead to an increase in phosphocholine levels [10]. The proton MRS has been proven very useful in differentiating between benign and malignant breast lesions based on elevated tCho [11-14]. Choline measured by MRS may provide an imaging marker for cell proliferation. Our recently published article [15] analyzing the MR imaging features with respect to HER2/neu overexpression in invasive breast cancer demonstrated a higher choline detection rate in HER2/neu positive compared to negative cancer. The number of patients in that study was however very small and conclusion could not be drawn. Here we reported a larger series study to further investigate the choline expression between HER2/neu +/− cancers.
Sixty-six breast cancer patients (range 32−76 years old, mean 51 years) enrolled from March 2005 to October 2006, who were scanned with the MRI/MRS protocol were included in this study. The inclusion criteria were patients with biopsy confirmed diagnosis of malignant lesions that measured 1.5 cm or larger on MR images. Of the 66 malignant lesions, 41 (77%) were invasive ductal carcinomas, 7 (11%) were invasive lobular carcinomas, and the other 8 (12%) were mixed invasive ductal and lobular carcinomas. HER2/neu status was determined initially using the immunohistochemical staining (IHC), positive in tumors with 3+ staining score, and negative for score of 0 and 1+. For those with IHC 2+ staining, fluorescent in situ hybridization (FISH) was conducted to determine the status.
The examinations were performed on a clinical 1.5T scanner (Eclipse; Philips Medical System, Cleveland, Ohio) with a dedicated four-channel phased-array breast coil. After the MRI study was completed, single-voxel MRS was performed using a point-resolved spin-echo sequence (PRESS). The spectroscopic voxel was carefully positioned to maximize the coverage of the contrast-enhanced lesions while minimizing the inclusion of adipose tissue. The voxel size was from 2.4 to 8.0 mL. The absolute tCho concentration was analyzed using as an internal reference method [16]. The tumor size was measured by a radiologist based on the maximum intensity projection (MIP) of the contrast subtraction images.
Of 66 cancers, 45 (68%) were HER2/neu negative, and 21 (32%) were HER2/neu positive. The mean size of 66 malignant tumors was 3.4 cm (range, 1.5 − 8.6 cm). The 1H-MRS result was positive for tCho in 53 (80%) of 66 patients. The measured Cho levels ranged from 0 to 8.5 mmol/kg (mean ± SD, 1.9 ± 1.9 mmol/kg), which were consistent with the previously published value by Bolan et al. [16]. Table 1 summarizes in vivo breast 1H-MRS results in HER2/neu positive and negative groups. The choline detection rate was higher in HER2/neu positive group (91%) than in HER2/neu negative group (76%), but not reaching significant level (p = 0.26, chi-square test).
Table 1.
Sensitivity and tCho Concentration in HER/neu Positive and Negative Breast Cancers Using In Vivo MR Spectroscopy
| No. of true positives | No. of false negatives | Sensitivity | tCho level (mmol/kg) | |
|---|---|---|---|---|
| HER2/neu | ||||
| Positive | 19 | 2 | 91% | 1.50 |
| Negative |
34 |
11 |
76% |
2.03 |
| Overall | 53 | 13 | 80% | 1.87 |
Note. tCho = total choline-containing compounds.
HER2 receptor mediates signaling to cancer cells and stimulates proliferation [3-5]. In vitro cell line study by overexpressing HER2/neu in MCF7 cells showed higher proliferation rate [17]. Overexpression/amplification of HER2 is associated with tumor aggressiveness and a poor prognosis in breast cancer. Limited literature is available correlating MR imaging features with HER-2 biomarkers expressed in invasive breast cancer. Tse et al. [18] reported 17 out of 19 breast cancer patients showing positive choline detection. The two false negative cases were negative for HER-2/neu oncogene expression, suggesting that a false-negative spectroscopic result may be related to an absence of Her-2 overexpression in carcinoma of the breast. Agrawal et al. [15] also showed more choline detection rate in HER-2/neu positive patients compared to Her-2/neu negative cohort in small number of patients (4/7 vs. 0/8, P< 0.05). The case number was too small in the two aforementioned studies. In this much larger series study, the sensitivity of in vivo 1H-MRS in HER2/neu negative group, although lower, was not significantly different from that of HER2/neu positive group, and also the absolute tCho levels did not appear to be related to HER2/neu overexpression. Our observation suggests that in vivo 1H-MR spectroscopy may play a very limited role for characterizing HER2/neu overexpression in carcinoma of the breast.
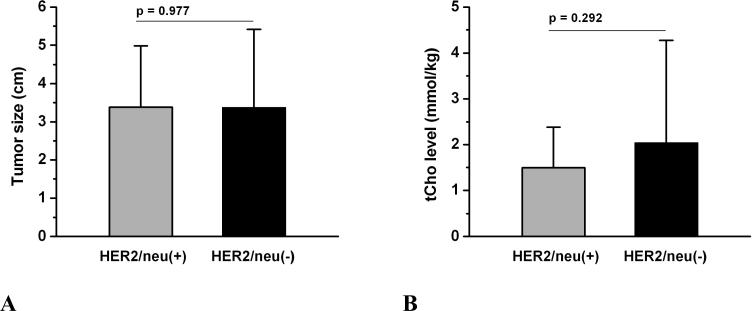
Figure 1.
Comparison of tumor size and total choline-containing compounds (tCho) in HER2/neu-positive and negative groups. There was no significant difference in tumor size (figure 1A) and tCho level (figure 1B) between these two groups.
Acknowledgements
This study was supported in part by NIH/NCI No. CA90437 and the California BCRP No. 9WB-0020 and No. 12FB-003.
REFERENCES
- 1.Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652–4. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 2.Brand FX, Ravanel N, Gauchez AS, et al. Prospect for anti-her2 receptor therapy in breast cancer. Anticancer Res. 2006 Jan-Feb;26(1B):715–22. [PubMed] [Google Scholar]
- 3.Ménard S, Casalini P, Campiglio M, Pupa SM, Tagliabue E. Role of HER2/neu in tumor progression and therapy. Cell Mol Life Sci. 2004 Dec;61(23):2965–78. doi: 10.1007/s00018-004-4277-7. [DOI] [PubMed] [Google Scholar]
- 4.Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- 5.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003 Mar 10;284(1):99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 6.Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12(Suppl 1):S3–8. doi: 10.1093/annonc/12.suppl_1.s3. Review. [DOI] [PubMed] [Google Scholar]
- 7.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–38. doi: 10.1016/j.canlet.2005.01.041. Review. [DOI] [PubMed] [Google Scholar]
- 8.Stefano R, Agostara B, Calabro M, et al. Expression levels and clinical-pathological correlations of HER2/neu in primary and metastatic human breast cancer. Ann N Y Acad Sci. 2004;1028:463–72. doi: 10.1196/annals.1322.055. [DOI] [PubMed] [Google Scholar]
- 9.Tokatli F, Altaner S, Uzal C, et al. Association of HER-2/neu overexpression with the number of involved axillary lymph nodes in hormone receptor positive breast cancer patients. Exp Oncol. 2005;27:145–9. [PubMed] [Google Scholar]
- 10.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–4. [PubMed] [Google Scholar]
- 11.Roebuck JR, Cecil KM, Schnall MD, Lenkinski RE. Human breast lesions: characterization with proton MR spectroscopy. Radiology. 1998;209:269–275. doi: 10.1148/radiology.209.1.9769842. [DOI] [PubMed] [Google Scholar]
- 12.Kvistad KA, Bakken IJ, Gribbestad IS, Ehrnholm B, Lundgren S, Fjosne HE, haraldseth O. Characterization of neoplastic and normal human breast tissues with in vivo 1H MR spectroscopy. J Magn Reson Imaging. 1999;10:159–164. doi: 10.1002/(sici)1522-2586(199908)10:2<159::aid-jmri8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Yeung DK, Cheung HS, Tse GM. Human breast lesions: characterization with contrast-enhanced in vivo proton MR spectroscopy-initial results. Radiology. 2001;220:40–60. doi: 10.1148/radiology.220.1.r01jl0240. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs MA, Barker PB, DPhil, Bottomley PA, Bhujwalla Z, Bluemke DA. Proton magnetic resonance spectroscopic imaging of human breast cancer: a preliminary study. J Magn Reson Imaging. 2004;19:68–75. doi: 10.1002/jmri.10427. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal G, Chen JH, Baik HM, Hsiang D, Mehta RS, Nalcioglu O, Su MY. MR imaging features of breast cancer: a correlation study with HER2/neu receptor. Ann of Oncol. 2007;18:1903–1904. doi: 10.1093/annonc/mdm477. [DOI] [PubMed] [Google Scholar]
- 16.Bolan PJ, Meisamy S, Baker EH, Lin J, Emory T, Nelson M, Everson LI, Yee D, Garwood M. In vivo quantification of choline compounds in the breast with 1H MR Spectroscopy. Magn Reson Med. 2003;50:1134–1143. doi: 10.1002/mrm.10654. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, Ren JQ, Chen Q, Zhang HP, Zhu HG. The effect of HER2/neu overexpression on p53 gene expression, cell proliferation and sensitivity to gamma-irradiation via the PI3K/Akt pathway in breast cancer cell MCF7. Zhonghua Zhong Liu Za Zhi. 2004 Oct;26(10):594–7. [PubMed] [Google Scholar]
- 18.Tse GM, Cheung HS, Pang LM, Chu WC, Law BK, Kung FY, Yeung DK. Characterization of lesions of the breast with proton MR spectroscopy: comparison of carcinomas, benign lesions, and phyllodes tumors. AJR Am J Roentgenol. 2003;181:1267–1272. doi: 10.2214/ajr.181.5.1811267. [DOI] [PubMed] [Google Scholar]