Abstract
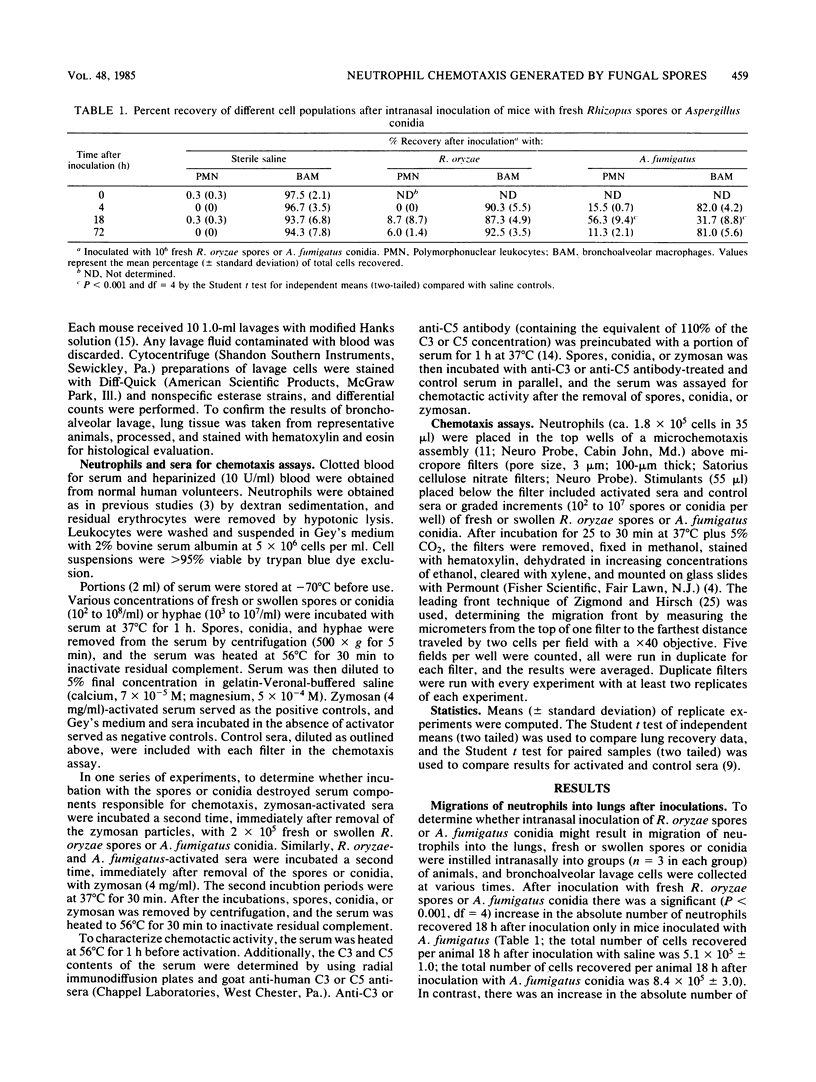
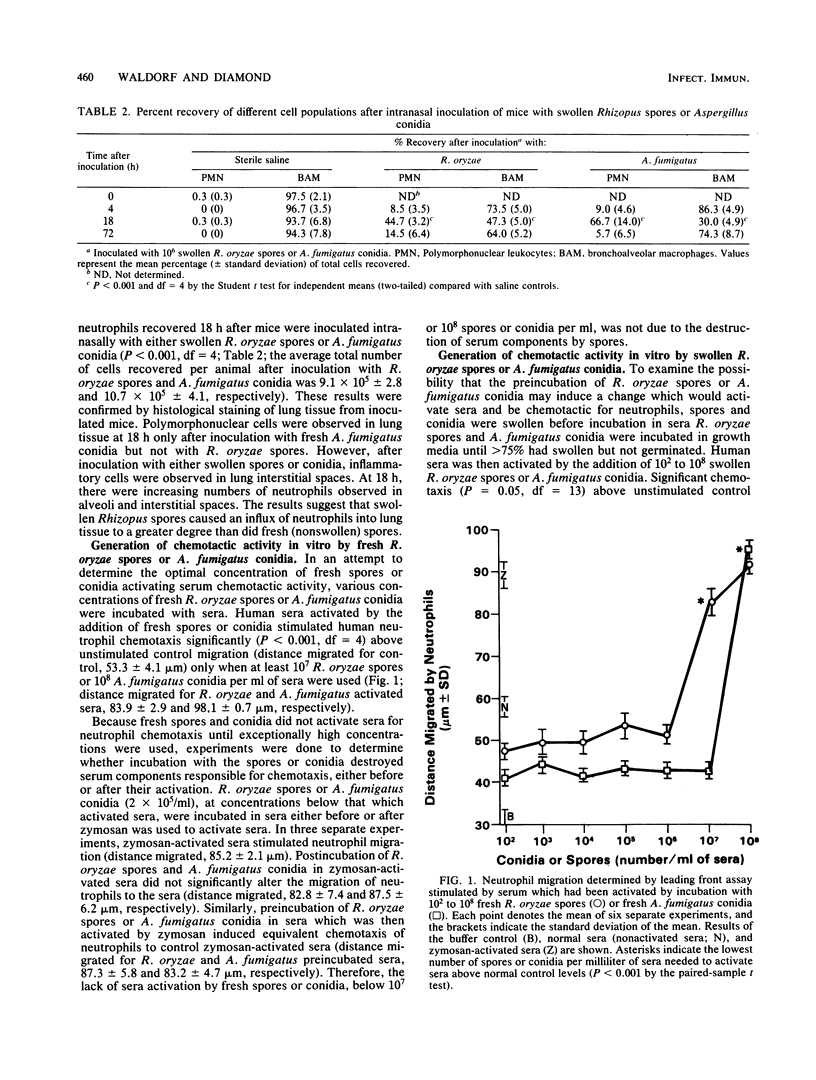
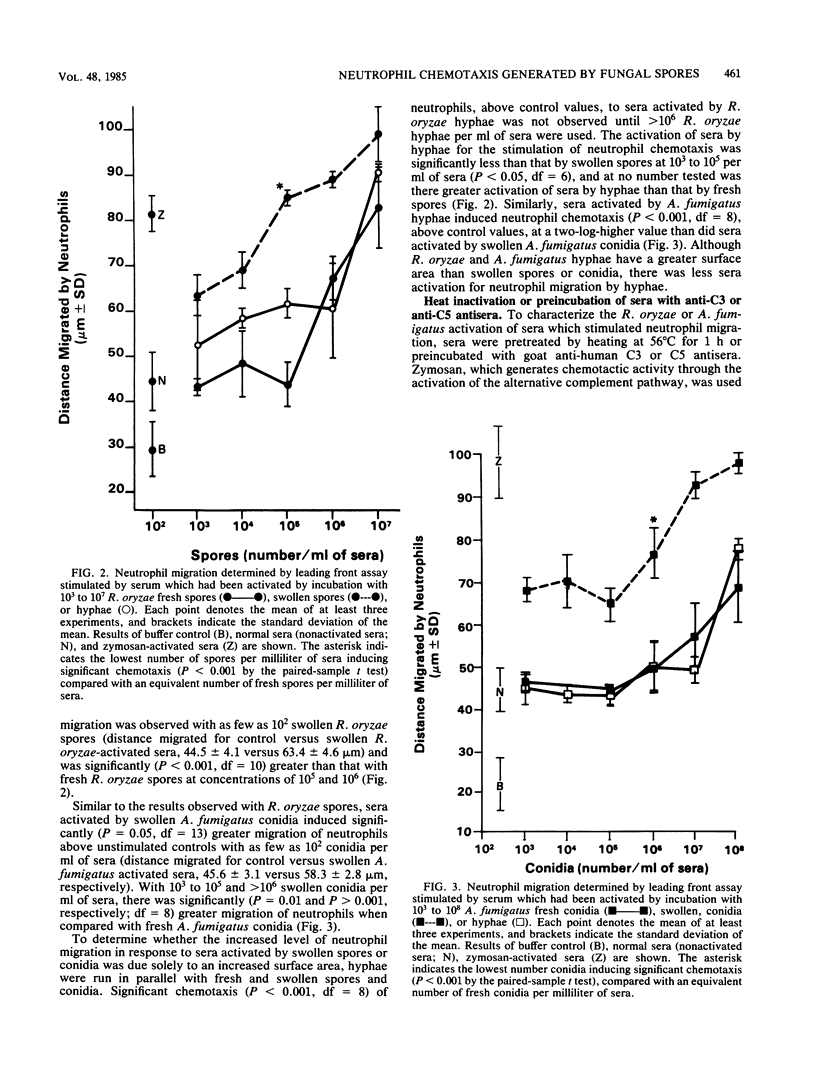
With the induction of germination, Rhizopus oryzae spores and Aspergillus fumigatus conidia activate the complement system and induce neutrophil chemotaxis. In contrast, freshly isolated R. oryzae spores did not induce neutrophil migration into lung tissue of mice after intranasal inoculation. Moreover, in microchemotaxis assays neither fresh R. oryzae spores nor A. fumigatus conidia activated sera to stimulate human neutrophil chemotaxis above control migration until at least 10(7) or 10(8) spores or conidia per ml of sera were used. The increased generation of chemotactic factors by swollen spores and conidia was not due to an increased surface area, as there was decreased neutrophil chemotactic response to Rhizopus or Aspergillus hyphae when compared with swollen spores or conidia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER H., FLANAGAN J. F., SHELDON W. H. Experimental cerebral mucormycosis in rabbits with alloxan diabetes. Yale J Biol Med. 1955 Sep;28(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- BAUER H., FLANAGAN J. F., SHELDON W. H. The effects of metabolic alterations on experimental Rhizopus oryzae (mucormycosis) infection. Yale J Biol Med. 1956 Sep;29(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- Chinn R. Y., Diamond R. D. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982 Dec;38(3):1123–1129. doi: 10.1128/iai.38.3.1123-1129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Gallin J. I., Kaplan A. P. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975 Dec 1;142(6):1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982 Nov;38(2):487–495. doi: 10.1128/iai.38.2.487-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C., Erickson N. F., 3rd Monocyte-mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun. 1982 Oct;38(1):292–297. doi: 10.1128/iai.38.1.292-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Huber E., Haudenschild C. C. Mechanisms of destruction of Aspergillus fumigatus hyphae mediated by human monocytes. J Infect Dis. 1983 Mar;147(3):474–483. doi: 10.1093/infdis/147.3.474. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- EKUNDAYO J. A., CARLILE M. J. THE GERMINATION OF SPORANGIOSPORES OF RHIZOPUS ARRHIZUS; SPORE SWELLING AND GERM-TUBE EMERGENCE. J Gen Microbiol. 1964 May;35:261–269. doi: 10.1099/00221287-35-2-261. [DOI] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis. 1981 Jul;144(1):61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. I. A simple technique for the preparation of high numbers of viable alveolar macrophages from small laboratory animals. J Immunol Methods. 1979;27(2):189–198. doi: 10.1016/0022-1759(79)90264-3. [DOI] [PubMed] [Google Scholar]
- Marx R. S., Forsyth K. R., Hentz S. K. Mucorales species activation of a serum leukotactic factor. Infect Immun. 1982 Dec;38(3):1217–1222. doi: 10.1128/iai.38.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Leukocytic function in hypogammaglobulinemia. J Clin Invest. 1970 Aug;49(8):1528–1538. doi: 10.1172/JCI106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELDON W. H., BAUER H. The development of the acute inflammatory response to experimental cutaneous mucormycosis in normal and diabetic rabbits. J Exp Med. 1959 Dec 1;110:845–852. doi: 10.1084/jem.110.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. I., Davis C. E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983 Dec;42(3):1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf A. R., Halde C., Vedros N. A. Murine model of pulmonary mucormycosis in cortisone-treated mice. Sabouraudia. 1982 Sep;20(3):217–224. doi: 10.1080/00362178285380321. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Levitz S. M., Diamond R. D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984 Nov;150(5):752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Peter L., Polak A. Mucormycotic infection in mice following prolonged incubation of spores in vivo and the role of spore agglutinating antibodies on spore germination. Sabouraudia. 1984;22(2):101–108. doi: 10.1080/00362178485380171. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Ruderman N., Diamond R. D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984 Jul;74(1):150–160. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Recognition and response in mononuclear and granular phagocytes. Clin Exp Immunol. 1976 Sep;25(3):355–366. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Recognition of protein structure in leukocyte chemotaxis. Nature. 1973 Aug 24;244(5417):512–513. doi: 10.1038/244512a0. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


