Abstract
Individuals with the birth defect synpolydactyly (SPD) have 1 or more digit duplicated and 2 or more digits fused together. One form of SPD is caused by polyalanine expansions in homeobox d13 (Hoxd13). Here we have used the naturally occurring mouse mutant that has the same mutation, the SPD homolog (Spdh) allele, and a similar phenotype, to investigate the molecular pathogenesis of SPD. A transgenic approach and crossing experiments showed that the Spdh allele is a combination of loss and gain of function. Here we identify retinaldehyde dehydrogenase 2 (Raldh2), the rate-limiting enzyme for retinoic acid (RA) synthesis in the limb, as a direct Hoxd13 target and show decreased RA production in limbs from Spdh/Spdh mice. Intrauterine treatment with RA restored pentadactyly in Spdh/Spdh mice. We further show that RA and WT Hoxd13 suppress chondrogenesis in mesenchymal progenitor cells, whereas Hoxd13 encoded by Spdh promotes cartilage formation in primary cells isolated from Spdh/Spdh limbs, and that this was associated with increased expression of Sox6/9. Increased Sox9 expression and ectopic cartilage formation in the interdigital mesenchyme of limbs from Spdh/Spdh mice suggest uncontrolled differentiation of these cells into the chondrocytic lineage. Thus, we propose that mutated Hoxd13 causes polydactyly in SPD by inducing extraneous interdigital chondrogenesis, both directly and indirectly, via a reduction in RA levels.
Introduction
Limb malformations are a relatively common human birth defect. From a clinical point of view, they can be subdivided into brachydactylies (short digits), reduction defects, and duplications, the latter affecting most frequently the digits, in which case they are called polydactyly (1, 2). Polydactylies may occur at the anterior side (thumb, preaxial), the posterior side (little finger, postaxial), or between fingers (central). Early transplantation experiments have shown that a complete duplication of the autopod can be induced by implanting an additional zone of polarizing activity (ZPA) to the anterior (opposite) region of the limb bud. In this case, the bud receives 2 signals, one from the posterior side and an additional one from the anterior side, resulting in an autopod with mirror image duplication. The signaling molecule sonic hedgehog (Shh) was identified as the major signal from the ZPA that is necessary and sufficient to confer these effects (reviewed in refs. 3–5).
Misregulation of Shh signaling and misexpression of Shh at the anterior region of the limb bud was shown to be a major factor in the pathogenesis of polydactyly (3, 6). For example, mutations in the so called ZPA regulatory sequence (ZRS) of Shh result in ectopic expression of Shh at the anterior limb bud, thus inducing polydactyly (7–9). Mutations in Gli3, one of the transcriptional effectors of Shh signaling, result in polydactyly in mice and humans by disturbing the balance between the activator and the suppressor function of Gli3 (10). Dysfunction of the cilia can lead to polydactyly, for example in Bardet-Biedl, oral-facial-digital, Senior-Loken, and Meckel-Gruber syndromes, probably reflecting a role for cilia in hedgehog signaling (11), as studies in polydactylous mouse mutants with abnormal intraflagellar transport proteins were shown to have defective Gli3 processing (12). Genes such as Alx4 interfere with Shh signaling and produce polydactyly also by inducing Shh misexpression (13). Curiously, misregulation of another member of the hedgehog family, Indian hedgehog (Ihh), results in polydactyly in doublefoot mice through expression of Ihh in the anterior limb margin (14).
Duplications of digits are frequently associated with cutaneous or osseous webbing of the adjacent fingers, resulting in syndactyly, hence the name synpolydactyly (SPD) for these conditions (15). One type of SPD has been shown to be caused by mutations in homeobox d13 (Hoxd13), the most 5′ homeobox (Hox) gene of the D cluster. Hox genes of the A and D clusters are expressed in a graded overlapping fashion throughout the posterior limb. Through this specific pattern, positional cues are thought to be conferred to the cells (16). For example, in the absence of Hoxa13 and Hoxd13, the autopods fail to develop (17) and removal of Hoxa11 and Hoxd11 function leads to truncations of the zeugopod (18), whereas a deficiency of groups 9 and 10 affects the stylopod (19). While these results point to an important role for Hox genes in early patterning of the limb, an additional role in the subsequent process of skeletal organogenesis and growth is likely (20).
Interestingly, the mutations associated with SPD have been shown to be expansions of an Ala repeat located within the N-terminal part of the protein. These mutations result in an expansion of a 15-Ala repeat by an additional 7 to 14 Ala (21, 22). The specificity of these mutations was underlined by the identification of a mouse mutant with an identical mutation and a similar phenotype, which was consequently termed SPD homolog (Spdh) (23), and the lack of similar changes in Hoxd13 knockout mice (17). Similar mutations were subsequently described in a number of other genes. All mutations affect transcription factors and result in expansions with a total length of more than 21 Ala (24). For some of these mutations, a loss-of-function mechanism is likely, as similar phenotypes occur in association with (for example) nonsense mutations in the same gene. For Hoxd13, however, the mechanism appears to be different, as shown by breeding experiments of Spdh mice with various Hox mutants (25). The expansion of the Ala repeat in Hoxd13 results in degradation of the mutant protein in the proteasome. Insufficient degradation leads to the accumulation of aggregates within the cell. These effects correlate with the length of the repeat, i.e., the longer the repeat, the more protein is degraded and the more aggregates are present. This mechanism appears to apply for all polyalanine repeat expansions and can thus be considered as the pathomechanism behind the disease (26). In spite of these advances in understanding the pathology of polyalanine-related conditions, it remains unclear why the mutations in Hoxd13 result in SPD and how the dominant mechanism can be explained.
Using screening approaches we identify the retinoic acid–producing (RA-producing) enzyme retinaldehyde dehydrogenase 2 (Raldh2) as downregulated in Spdh limb buds. We show that Hoxd13 regulates Raldh2 and that RA represses chondrogenesis in the interdigital space, preventing the formation of cartilage condensations. In the Spdh mutant, less Raldh2 results in lower RA levels in the limb and thus less inhibition of chondrogenesis. In addition, we show that WT Hoxd13 is a strong repressor of chondrogenesis, whereas Hoxd13 with expanded Ala repeats has a prochondrogenic effect, contributing to the accelerated and uncontrolled differentiation of interdigital cells into chondrocytes. We propose that Hoxd13-associated polydactyly in Spdh mice is due to increased chondrogenesis in the interdigital mesenchyme.
Results
Spdh is a combination of partial loss of function and gain of function.
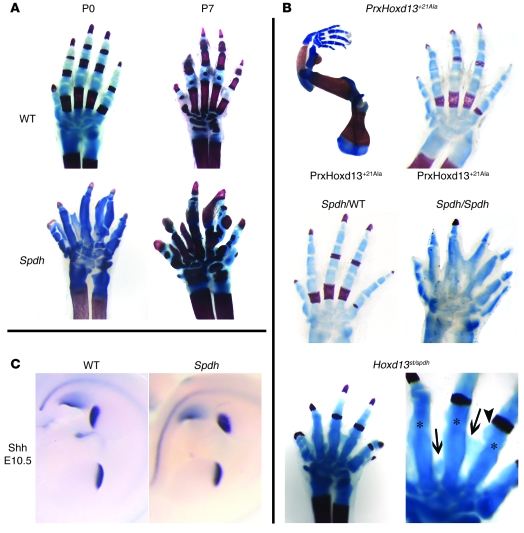
Figure 1A shows representative skeletal preparations stained with Alcian blue (cartilage) and Alizarin red (bone) of WT and Spdh/Spdh forelimbs at postnatal days P0 and P7. Spdh/Spdh mice exhibited additional digits at varying locations, fused joints, and a severe delay in ossification. To test whether polyalanine expansions result in a dominant phenotype we expressed a Hoxd13 mutant containing a +21 Ala expansion under the control of the limb-specific Prx1 promoter (PrxHoxd13+21Ala mice). Two mouse lines were obtained, both showing the same phenotype: bowing of the ulna and, apart from a delay in ossification, normal digits (Figure 1B). This phenotype did not change significantly after introducing 1 Spdh allele. These results indicate that even a very long polyalanine is not sufficient to induce polydactyly on a WT or heterozygous Spdh background. However, crossing PrxHoxd13+21Ala mice on a homozygous Spdh background resulted in a much more severe phenotype than that found in single Spdh/Spdh mice. To further investigate interactions of mutant Hoxd13 with other Hoxd genes, we crossed Spdh/+ mice with mice in which 1 allele of Hoxd13 was inactivated (Hoxd13st/+) (27). Double heterozygous (Hoxd13st/Spdh) mice showed an intermediate phenotype overlapping with, but also distinct from, the single homozygous (Spdh/Spdh, Hoxd13st/st) mice. Similar to our observations of Spdh/Spdh mice, we observed severe abnormalities of joint formation and a delay in ossification. However, central polydactyly did not occur. Instead, postaxial polydactyly was occasionally present, similar to what has been described in Hoxd13st/st mice (27). Close examination of the interdigital space, however, revealed ectopic cartilage formation between the digits and irregular boundaries of the digital cartilage. Neither abnormality is present in Hoxd13st/st mice (27) but were part of the Spdh/Spdh phenotype (Figure 1B).
Figure 1. The Spdh allele acts as a loss and gain of function.
(A) Skeletal preparations of WT and Spdh/Spdh mice at birth (P0) and 1 week of age (P7). Spdh/Spdh mice exhibited multiple additional incompletely formed digits, fused joints, and a severe delay in ossification. Cartilage was stained with Alcian blue, bone with Alizarin red. (B) Skeletal preparations of transgenic PrxHoxd13+21Ala mice at P0 showed a severe malformation of the radius and bending of the ulna. No major changes were seen in the digits. Introducing 1 Spdh allele (Hoxd13+21Ala; Spdh/WT) did not change the phenotype. Mice expressing Hoxd13+21Ala on a homozygous Spdh background (Hoxd13+21Ala; Spdh/Spdh) showed a severe phenotype with excessive fusions and polydactyly. Mice with 1 Spdh allele and 1 inactivated Hoxd13 allele (Hoxd13st/Spdh) showed a delay in bone formation, fusion of joints, occasional postaxial polydactyly, and a short digit 2. In addition, ectopic cartilage formation between the digits, and an uneven surface of the digit cartilage (magnification of interdigital space shown on right), was present. Original magnification, ×3.2 (left), ×4.0 (right). (C) Shh expression in E10.5 WT and Spdh embryos.
Polydactyly frequently involves Shh misexpression, and Shh was shown to regulate Hox expression (28). We thus investigated Shh expression in WT and Spdh limbs at various stages without observing significant differences in distribution or intensity of Shh mRNA (Figure 1C).
Raldh2 is downregulated in Spdh limbs.
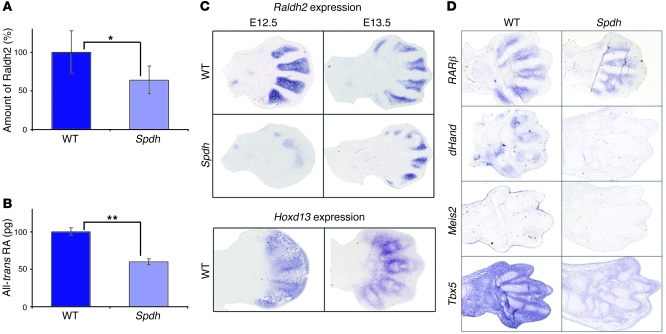
To identify pathways that were misregulated in Spdh limbs, we used a dual approach and screened for differences in protein and mRNA expression levels between WT and mutant limbs. We observed downregulation of genes known to be part of the RA signaling pathway (data not shown), including Raldh2. In addition, we performed 2D gel electrophoresis to screen for changes at the protein level, and Raldh2 was identified as being downregulated (63% of WT levels) (Figure 2A). Since Raldh2 converts retinol into RA (29), we measured RA content in E13.5 limb buds. This quantification revealed a reduction of all-trans RA in Spdh/Spdh limbs to 57% of WT values (Figure 2B).
Figure 2. Dysregulation of RA pathway in Spdh.
(A) 2D gel electrophoresis of limb bud tissue (stage E13.5) demonstrated a reduction (63% of WT) of Raldh2 protein levels in Spdh mice (*P < 0.05). (B) Quantification of RA in E13.5 autopod limb tissue. Spdh limbs show a reduction to 57% when compared to WT (**P ≤ 0.005). (C) In situ hybridization against Raldh2 on forelimbs at E12.5 and E13.5 demonstrated a reduction of Raldh2 mRNA in Spdh limbs. In the WT limbs, Raldh2 was expressed in the interdigital space but not the cartilaginous condensations. At E13.5, Raldh2 expression was mainly found in the perichondrium. Expression overlapped with Hoxd13. (D) In situ hybridizations of WT and Spdh forelimbs at E13.5 of RA downstream targets RARβ, dHand, Meis2, and Tbx5. All showed reduced expression in Spdh limbs.
To investigate the Raldh2 expression pattern and level, we performed in situ hybridization on serial sections of limb buds at different developmental stages (Figure 2C). At E12.5, we observed a distinct expression pattern of Raldh2 within the interdigital space between the condensations. With further development (E13.5), Raldh2 continued to be expressed in the interdigital mesenchyme, but the strongest expression was present in the perichondrium. In the mutant Raldh2, expression was almost absent at E12.5 and strongly reduced at E13.5. No expression was observed in the perichondrium. Raldh2 and Hoxd13 showed overlapping expression patterns in the mouse autopod at E12.5 and E13.5 (Figure 2C).
To further test our hypothesis of altered RA signaling, we performed expression analysis of different established RA signaling targets (Figure 2D). In our studies, RARβ was expressed more weakly in Spdh/Spdh than in WT forelimbs, and expression in the perichondrium was not observed at all. dHand, normally expressed in the digital chondrogenic regions, was downregulated. Likewise, Meis2 expression was reduced in Spdh/Spdh mutants. In WT mice, Tbx5 was expressed ubiquitously all over the limb bud (Figure 2D). In Spdh/Spdh mutant limbs, we observed a downregulation of Tbx5 expression.
Raldh2 is regulated by Hoxd13.
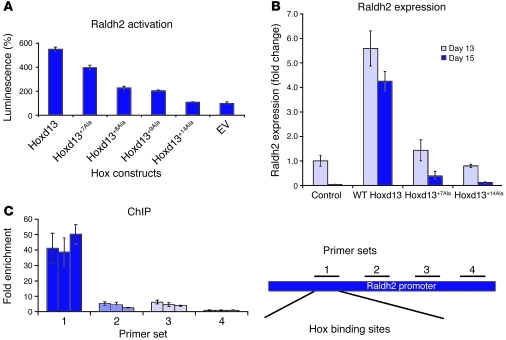
To test whether Raldh2 is directly regulated by Hoxd13, we performed different promoter assays. In silico analysis revealed a conserved region 3,300 bp upstream from the transcription start site of murine Raldh2. Within a 1,300-bp region, several Hox binding sites were identified that were conserved between Homo sapiens and rat. This region was cloned into pGL2, and enhancer and luciferase reporter assays were performed in Cos7 cells transfected with Raldh2 reporter and different Hoxd13 constructs. WT Hoxd13 activated the Raldh2 promoter (Figure 3A). Mutated Hoxd13 showed a reduction in activation depending on the length of the repeat. To test a possible regulation in cells naturally expressing Hoxd genes, quantitative PCRs were performed on chicken micromass (chMM) cultures after infection with RCAS virus carrying the WT or mutant Hoxd13 (Figure 3B). Hoxd13 was shown to upregulate Raldh2, whereas the mutated forms were comparable with uninfected controls. Direct binding of Hoxd13 to the endogenous Raldh2 promoter was shown by ChIP. Using 4 primer pairs covering different regions containing putative Hox binding sites of the Raldh2 promoter, we found the product of primer pair 1 (–4,900 to –4,849 bp relative to the transcription start site) to be enriched 38- to 50-fold compared with primer pair 4 (–1,169 to –1,115 bp relative to the transcription start site) (Figure 3C). Primer pairs 2 and 3 were only enriched 2.5- to 6-fold.
Figure 3. Regulation of Raldh2 by Hoxd13.
(A) Luciferase reporter assay of a conserved region of murine Raldh2. Cos7 cells were transfected with Raldh2 reporter and Hoxd13 expression constructs carrying different Ala repeat length mutations (+7, +8, +9, +14). WT Hoxd13 activated Raldh2. Increasing length of the Ala repeat results in loss of activation. Error bars show SEM. EV, empty vector. (B) Quantitative PCR of chMM cultures showed upregulation of Raldh2 by WT Hoxd13. Infections with Hoxd13+7Ala and Hoxd13+14Ala were comparable with uninfected controls. Error bars show SD. (C) ChIP confirmed binding of WT Hoxd13 to the Raldh2 promoter. ChIP from chMM cultures was done with 4 different primer pairs. Real-time PCR with primer set 1 showed an approximately 45-fold enrichment of the Raldh2 promoter fragment precipitated with Hoxd13. Sets of 3 bars represent results from 3 independent experiments. Error bars show SD.
RA suppresses chondrogenesis.
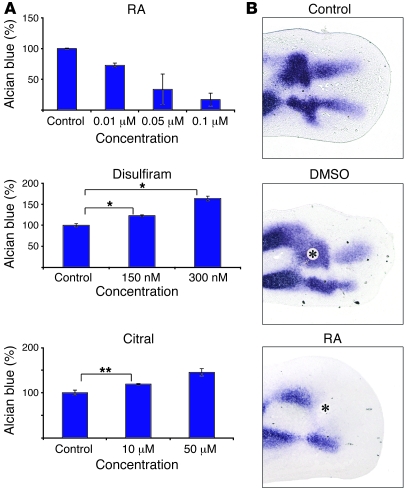
To investigate of the influence of RA on primary cells with a potential to differentiate, we used the chMM system. In this model system, mesenchymal limb bud cells are seeded at high density, inducing spontaneous differentiation into various cell lineages, including cartilage. The degree of cartilage formation was measured by staining with Alcian blue and subsequent quantification using AxioVision Outmess software (Figure 4A). Cultures were treated with increasing doses of RA or, alternatively, with increasing doses of the following 2 inhibitors of RA synthesis: disulfiram, known to block Raldh (30), and citral, which blocks both steps of vitamin A oxidation (31). Our experiments demonstrate an antichondrogenic dose-dependent effect of RA on mesenchymal cells. Disulfiram and citral enhanced chondrogenesis in chMM cultures, indicating that RA negatively regulates chondrogenic differentiation in limb mesenchymal cells.
Figure 4. RA inhibits chondrogenesis.
(A) ChMM cultures were treated with various concentrations of RA or RA inhibitors (disulfiram, citral), cultured, stained with Alcian blue, and quantified. Intensity of staining in controls is 100%. Note the reduction of chondrogenesis after RA treatment and induction after treatment with RA antagonists. Results are presented as mean ± SD. *P < 0.05; **P < 0.005. (B) Bead implantations in chicken limb buds at Hamburger Hamilton (HH) 27 with subsequent Col2a1 section in situ hybridization after 20 hours of incubation. Top: Control without bead. Middle: DMSO-soaked control bead with unchanged Col2a1 expression. Bottom: Limb after implantation of RA-soaked beads. Note the suppression of Col2a1 expression. Asterisks indicate beads.
To test the effect of RA on chondrogenesis in vivo, we implanted RA-soaked beads in chicken limb buds, harvested those 24 hours later, and performed section in situ hybridization with a Col2a1-specific probe to detect cartilage using collagen type II expression as a marker. The results showed that RA treatment results in a complete inhibition of chondrogenesis in the digits (Figure 4B).
Intrauterine treatment of Spdh mice with RA rescues the polydactyly phenotype.
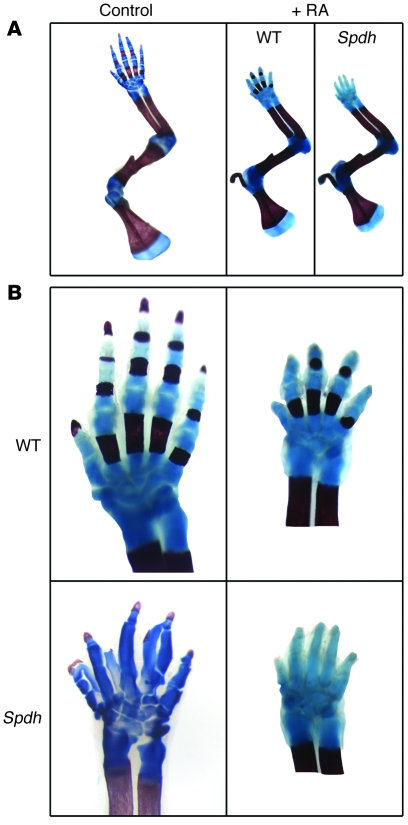
Our results indicated that dysregulation of RA signaling plays a role in the Spdh phenotype. To verify this hypothesis, we aimed at adjusting the level of RA by intrauterine supplementation. Pregnant females were treated with orally administered 5 μg RA/g body weight once per day from E8.5 to E14.5. Newborn animals were analyzed by skeletal preparation, and WT littermates served as controls (Figure 5). RA, a known teratogene, led to a reduction in size of the entire limb both in WT and in Spdh/Spdh animals (Figure 5A). All mutant offspring showed exactly 5 digits instead of the usual 6 or 7 condensations (Figure 5B). For statistics, 30 offspring animals were analyzed, including 8 WT, 8 mutant, and 14 heterozygous animals, fitting to the expected ratio. The size reduction was similar between the animals, and the observed effects were reproducible and stable.
Figure 5. RA treatment rescues the polydactyly phenotype.
(A) Newborn animals were analyzed with skeletal preparations. WT littermates served as controls. Bone was stained in red, cartilage in blue. RA led to an overall reduction in bone size both in WT and Spdh compared with an untreated control. Size reduction was comparable in WT and Spdh/Spdh mice. (B) Comparison of the autopod of untreated controls (left) with RA-treated animals (right) demonstrated an overall reduction in size but normal formation of digits, cartilage, and bone in WT animals. Treated Spdh/Spdh offspring had 5 digits, demonstrating a partial rescue of the Spdh phenotype.
Hox genes modulate chondrogenesis.
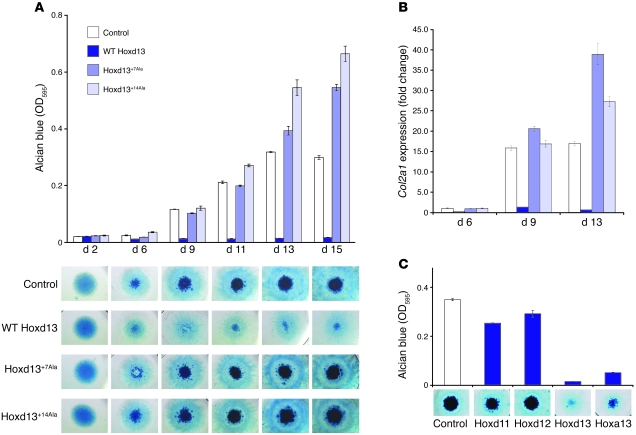
To test the influence of Hoxd13 on chondrogenesis, we expressed WT and mutant Hoxd13 in chMM culture using the NCI RCAS retroviral system. This system resulted in a moderate expression of the target gene in close to 100% of cells. Western blot analysis (data not shown) demonstrated that the expression levels were moderate and that mutant Hoxd13 was partially degraded, as previously shown (26). Expression of WT Hoxd13 had a profound effect on cartilage formation, as evidenced by the almost complete suppression of chondrogenesis (Figure 6A). Control chMM cultures began to form cartilaginous nodules on day 6, followed by a continuous increase of Alcian blue–positive tissue. In contrast, cells treated with WT Hoxd13 produced minute amounts of Alcian blue–positive matrix. To test whether this effect could be inhibited by blocking RA, we treated infected cells with disulfiram. We observed a moderate increase in chondrogenesis (data not shown), indicating that other pathways in addition to RA are important for Hoxd13-induced suppression of chondrogenesis in this system. To test the effect of mutant Hoxd13 on chondrogenesis, we performed the same experiments expressing Hoxd13 with an expanded polyalanine repeat (+7 Ala and +14 Ala). At day 9, induction of chondrogenesis was comparable with controls, indicating a loss of inhibition. Thereafter, the mutant showed an increase in cartilage formation when compared with the control. This effect became apparent at day 11 and was most pronounced on days 13 and 15. Using quantitative PCR we analyzed the expression of Col2a1, the predominant collagen of cartilage (Figure 6B). We observed an almost complete suppression of Col2a1 in cells with WT Hoxd13 expression throughout the tested period. The 2 mutant forms, i.e., Hoxd13+7Ala and Hoxd13+14Ala, showed no suppression of Col2a1 expression on day 9 and, as expected from the results obtained with Alcian blue staining, increased Col2a1 expression at day 13. To test whether other Hox genes had a similar effect, we expressed Hoxd11, Hoxd12, and Hoxa13 in chMM cultures and measured Alcian blue incorporation (Figure 6C). Hoxd11 and Hoxd12 had no major effect on cartilage formation, indicating that the observed antichondrogenic effect was specific for Hoxd13. In contrast, Hoxa13, similar to Hoxd13, is also a strong suppressor of chondrogenesis.
Figure 6. Hoxd13 modulates chondrogenesis.
(A) WT and mutant Hoxd13 were expressed in chMM cultures using the NCI RCAS retroviral system. Alcian blue staining was used to detect cartilage-specific nodules starting at day 6 (bottom) and subsequently quantified (top). WT Hoxd13 inhibited cartilage formation, while mutant Hoxd13 with +7Ala or +14Ala expansions had a prochondrogenic effect. (B) Quantitative PCR analysis of chMM cultures. Values were normalized to day 6 control. WT Hoxd13 strongly suppressed Col2a1 expression, while Hoxd13 with expanded Ala repeats showed increased expression. (C) The effects of other autopod-expressed Hox genes were analyzed in chMM cultures. Hoxd11 and Hoxd12 showed only a mild inhibitory effect on chondrogenesis. In contrast, Hoxa13 had an effect similar to Hoxd13. Results are presented as mean ± SD.
Enhanced chondrogenesis and polydactyly formation in Spdh/Spdh limbs.
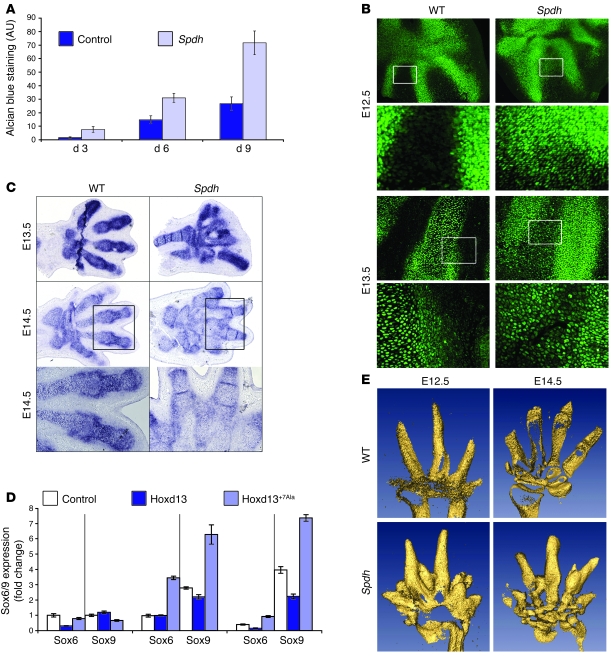
To further investigate the chondrogenic potential of mesenchymal cells in Spdh/Spdh autopods, we isolated cells at E13.5, the stage at which the mutant limbs can be visibly distinguished from WT, and cultured them at high density. Chondrogenesis was determined at days 3, 6, and 9 by staining with Alcian blue and image quantification. Cells from Spdh/Spdh autopods showed a much stronger potential to form cartilaginous nodules compared with cells from control animals, indicating that the Hoxd13 mutation has a prochondrogenic effect (Figure 7A).
Figure 7. Enhanced chondrogenesis in Spdh/Spdh limbs.
(A) Primary mesenchymal cells of E13.5 Spdh/Spdh autopods grown in vitro at high density, stained for Alcian blue, and quantified. Note the increased chondrogenesis in mutant limbs. Results are presented as mean ± SEM. (B) Immunohistochemistry with anti-Sox9 antibody on E12.5 and E13.5 limb sections. WT showed Sox9 in the cartilaginous anlagen but not in the interdigital mesenchyme. In Spdh limbs, no exact border could be determined, and cells within the interdigital mesenchyme were Sox9 positive. Boxed areas show magnification. Original magnification (from top to bottom), ×100, ×400, ×200, ×400. (C) In situ hybridization for Sox9 at stages E13.5 and E14.5 demonstrated expression in the interdigital mesenchyme of Spdh limbs. (D) Regulation of Sox6 and Sox9 in chMM cultures at days 6, 9, and 13 (left to right). RNA was extracted from chMM cultures, and transcribed cDNA was measured by quantitative PCR. Values were normalized to day 6 control. Mutant Hoxd13 activated Sox6/9 expression. Results are presented as mean ± SD. (E) 3D models of WT and Spdh/Spdh limbs stained for Col2a1 expression after in situ hybridization. Note additional condensations within interdigital spaces in Spdh limbs (arrows).
To further investigate the chondrogenic potential of interdigital mesenchyme in Spdh/Spdh limbs, we analyzed mutant limbs at E12.5, the stage at which the first cartilage condensation forms that will later develop into the digits. At these stages chondrogenesis has to be repressed in the interdigital mesenchyme and promoted at the sites where the digits form. In WT mice this results in restricted expression of Sox9 (Figure 7B), the gene that is pivotal for cartilage formation (32, 33), to the cartilaginous condensations. In contrast, Spdh/Spdh mice showed Sox9-positive cells dispersed throughout the interdigital mesenchyme (Figure 7B). Furthermore, the border between positive and negative cells is blurred, both at E12.5 and E13.5. These findings are consistent with our in situ hybridization data, which show expression of Sox9 mRNA (Figure 7C) at the same sites. Quantitative PCR of chMM cultures revealed that mutant Hoxd13 activated Sox9 and Sox6 expression (Figure 7D).
To document the nature and time point of the additional condensations, 3D models of developing limbs were generated (Figure 7E). Serial paraffin-embedded sections of E12.5 and E14.5 were hybridized with a Col2a1 probe to visualize cartilaginous condensations, photographed, and reconstituted with Amira software. In contrast to the WT limbs, Spdh/Spdh limbs showed multiple additional condensations in the proximal parts of the interdigital region. Some of these condensations developed in the interdigital mesenchyme independent of regular digit condensations, while others were connected to future digits. Thus, we concluded that cartilage formation in Spdh mutants does not represent a true digit formation but rather an uncontrolled process of enhanced differentiation at multiple sites.
Discussion
Our results indicate that RA signaling is disturbed in Spdh mice. RA was initially identified as a crucial signaling factor in limb bud development based on the observations that exogenous RA produces duplications in regenerating axolotl limbs (34). Furthermore, administration of RA to anterior chicken limb buds results in anterior-posterior duplications (35). RA signaling appears to be important for at least 2 different time points during development (36). In the early phase, RA is essential for the initiation of limb budding from the lateral plate mesoderm. Later, the main role of RA appears to be as a proximodistal signal during apical ectodermal ridge formation and in the induction of Shh expression (37). The latter function partly depends on RA-mediated induction of Hox genes, which in turn activate Shh (38). However, in spite of these known functions of RA, its definite role in limb development remains controversial. Active RA is produced by retinaldehyde dehydrogenases (Raldh1–3), enzymes that catalyze the second oxidative step in the biosynthesis of RA from retinol (29). Raldh2 is responsible for most of the RA-synthesizing activity and for initiating RA signaling during early mouse embryogenesis, as shown in Raldh2–/– embryos that have severe midline defects and do not form limb buds at all (36, 37). Due to the severe phenotype, late effects of Raldh2 deficiency were not investigated. We show that Raldh2 is expressed at E12.5 and E13.5 when the digital anlagen are formed. The expression is confined to the interdigital mesenchyme and the perichondrium, thus overlapping with Hoxd13 expression at these stages. Our results show that Raldh2 expression in the interdigital mesenchyme is reduced in Spdh/Spdh mice. Furthermore, the expression at the margins of the condensations (perichondrium) is completely missing in mutant mice, and residual activity is restricted to the most distal regions of the developing limb.
The deficiency of Raldh2 in Spdh mice suggests the possibility of direct regulation of Raldh2 by Hoxd13. Direct promoter and ChIP analysis and the upregulation of Raldh2 in chMM that expressed Hoxd13 suggest a direct regulation of Raldh2 by Hoxd13. Reduced amounts of Raldh2 should result in reduced RA concentrations. This was verified by direct RA measurement in limb tissue demonstrating a reduction by approximately 40% in Spdh/Spdh limbs. Our hypothesis of reduced RA signaling is additionally supported by the downregulation of several known RA targets including the RA receptor RARβ, Tbx5, Meis2, and dHand (36, 39–41).
Besides its role in early patterning, RA signaling was shown to play a role in the regulation of chondrogenesis. Earlier studies have shown that RA is able to inhibit chondrogenesis in vitro (42). This effect was shown to be most pronounced in cells derived from the more proximal limb bud indicating that the late blastemal stage of chondrogenesis is most vulnerable to RA treatment (43). In vivo, low doses are reported to enhance chondrogenesis, whereas high doses result in inhibition of cartilage formation (44, 45). Loss of RA receptor–mediated signaling was shown to be necessary and sufficient for expression of the chondroblast phenotype (46). Part of this effect is likely to be transmitted via Sox9, a transcription factor required for cartilage formation (47, 48). Furthermore, RA signaling antagonizes the prochondrogenic effects of bone morphogenetic proteins (BMPs), and BMP4 downregulates Raldh2 expression, thus influencing RA levels (46). Our experiments in chMM cultures confirm these results and show that RA suppresses chondrogenesis, whereas RA antagonists enhance cartilage formation. Thus, RA appears to be an important factor in the organogenesis of the skeleton by negatively influencing the differentiation of mesenchymal cells into chondrocytes. This antichondrogenic effect is reduced in Spdh/Spdh mice due to lower levels of RA in the interdigital mesenchyme.
Our results indicated that RA levels in Spdh/Spdh mice are moderately downregulated. This may explain the lack of more severe phenotypes such as limb truncations. On the other hand, the timing of Raldh2 regulation by Hoxd13 in the phase of skeletal organogenesis is likely to be a key factor in the determination of the phenotype. The limb phenotype of Raldh2–/– mice can be partially rescued by oral administration of RA to pregnant females (37). We thus treated pregnant Spdh mice with low doses of RA via intragastric feeding to test whether the phenotype can be rescued. Our results show that the polydactyly phenotype of Spdh/Spdh mice can be rescued, indicating that RA deficiency indeed plays a significant role in the pathogenesis of Spdh-related polydactyly.
In previous studies it was described that RA depletion results in premature and ectopic expression of Hox genes in early limb buds (37). Activation of Hox gene transcription by RA response elements and RA has previously been documented, and RA has been shown to trigger collinear activation of Hox genes in cells (49, 50). RA is known to play a role in the sequential activation of Hox genes in limb development by promoting anterior Hox genes and delaying posterior ones (51). The expression of Hox genes has been extensively studied in Spdh mice (25, 52). No differences were identified, indicating that the level of RA dysregulation in Spdh mice is not sufficient to induce misexpression.
Polyalanine expansions as they occur in Spdh mice are unlikely to be pure loss-of-function mutations, as inactivation of Hoxd13 alone does not result in a SPD phenotype. However, inactivation of the most posterior Hoxd genes together (Hoxd11, -d12, and -d13) results in SPD, similar to that observed in Spdh mice, but less severe (53). This indicates that the mutant Hoxd13 acts in a dominant-negative manner, possibly by negatively interacting with other Hox genes (25). To investigate this hypothesis further, we created the PrxHoxd13+21Ala transgenic mouse line. These mice showed a major deformity of the radius and the ulna but only minor changes in their autopods. Thus, based on the alterations in the stylopod, Hoxd13+21Ala is not a complete loss of function but is insufficient to induce a distal limb phenotype on a WT Hoxd13 background. Even if 1 WT allele is replaced by a Spdh allele (PrxHoxd13+21Ala; Spdh/WT), no major effect was observed. On a homozygous Spdh background (PrxHoxd13+21Ala; Spdh/Spdh), however, we observed a very severe phenotype. This indicated that 1 WT Hoxd13 allele was sufficient to prevent the effect of even a very long Ala expansion mutant. Crossing Spdh mice with mice carrying 1 inactivated Hoxd13 allele resulted in a distinct phenotype consisting of features described for Hoxd13 inactivation, such as shortening of digit 2 and postaxial polydactyly (27), as well as features of the Spdh spectrum, including abnormal joints, irregularities of the cartilaginous digits, and a delay in ossification. In addition, we observed ectopic cartilage between the digits, sometimes bridging the digits, which we interpreted as a minimal Spdh phenotype. Thus, Spdh is likely to result in a loss of Hoxd13 function together with a residual gain of function. The latter is different from WT Hoxd13 and can thus be called neomorphic. This effect is dosage dependent and occurs only in the absence of a WT Hox allele.
In order to investigate the proposed neomorphic effect further, we expressed WT and mutant Hoxd13 in chMM cultures using the NCI RCAS retroviral system. Our experiments demonstrate that WT Hoxd13 and Hoxa13 are strong suppressors of chondrogenesis. In contrast to WT, mutant Hoxd13 with an expanded Ala repeat induces chondrogenesis. To investigate the reason of the observed effect, we quantified the expression of Sox9 and Sox6, 2 transcription factors known to be essential and sufficient to induce chondrogenesis. Our results indicated that mutant Hox directly or indirectly activates Sox6/9 genes, which in turn promotes chondrogenesis. This effect was also observed in vivo. Whereas WT limbs showed a very restricted Sox9 expression pattern strictly confined to the cartilaginous condensations, Spdh limbs showed aberrant expression of Sox9 in cells of the interdigital mesenchyme. The enhanced chondrogenic potential of cells expressing mutant Hoxd13 was also demonstrated in Spdh/Spdh derived mesenchymal limb cells, a finding that further substantiates our hypothesis.
We conclude that at least 2 mechanisms contribute to the Spdh phenotype that are likely to function in an combined manner. In WT, RA suppresses chondrogenesis in the interdigital mesenchyme. In Spdh/Spdh mice carrying a mutant Hoxd13 with expanded Ala repeats, Raldh2 is downregulated, resulting in less RA and, consequently, increased cell differentiation. This hypothesis is further supported by the phenotype of RA receptor compound knockouts that also present with additional interdigital cartilage anlagen (54). Hoxd13, which is expressed in the interdigital mesenchyme, functions as a repressor of chondrogenesis and cell differentiation. Mutants with Ala expansions lose this repressive activity. Instead, they show a neomorphic effect by inducing chondrogenesis. The combination of loss of suppression and gain of induction results in aberrant condensations in the interdigital mesenchyme and thus polydactyly. In contrast to other polydactylies such as those involving the Shh pathway, these condensations do not develop into true digits, but rather represent an uncontrolled process of enhanced differentiation at multiple sites. This is somewhat similar to the ectopic formation of cartilage anlagen with subsequent polydactyly observed after apical ectodermal ridge or ectoderm removal (55). Hence, we propose a pathomechanism for Hoxd13-related SPD, which is not the result of a defect in patterning, but rather of irregularities in differentiation.
Methods
Mice.
Spdh mice were obtained from The Jackson Laboratory (23). Mutant offspring were generated by breeding Spdh/WT females to Spdh/WT males. Timed matings were produced, and 12 pm on the day of vaginal plug was considered E0.5. DNA from tail tip, amnion, or liver was used for genotyping, which was performed as described previously (52).
Transgenic Hoxd13 mice carrying Hoxd13 with additional 21 Ala under the control of Prx promoter (PrxHoxd13+21Ala mice) were created by DNA injection into oocytes. Two stables lines carrying the transgene were obtained. Two PCR reactions specific for the Prx promoter construct and Ala repeat length served for genotyping. Skeletal preparations were performed as previously described (52).
All-trans RA (Sigma-Aldrich; catalog R2625) was suspended in 100% EtOH (50 mg/ml), diluted in sunflower oil to the required dose, and administered orally (5 μg/g body weight) to pregnant Spdh/+ females once a day from E8.5 to E14.5. RA was cooled on ice and protected from light during handling. Newborn litters were analyzed with skeletal preparations (described above), and WT littermates served as controls. All animal experiments were approved by the Landesamt für Gesundheit und Soziales (Berlin, Germany).
RNA extraction and quantification.
To minimize effects of random biological variation, limbs from different litters were frozen in liquid nitrogen and then pooled after genotyping (30 WT or Spdh/Spdh forelimbs for each hybridization). RNA was extracted with peqGold Trifast (peqLab Biotechnologie GmbH) according to the manufacturer’s instructions. Extraction was followed by DNase treatment (RNase-Free DNase Set; Qiagen), purified with RNeasy Mini-Kit (Qiagen), and analyzed by Affymetrix GeneChip analysis.
2D electrophoresis.
Tissue of 40 WT and Spdh limb buds each were collected at E13.5, and frozen in liquid nitrogen until genotyping was done. 2D gel electrophoresis was performed as previously described (56). Protein spots were identified with MALDI-TOF analysis, and differences in protein amount were scaled by volume.
RA measurement.
For RA measurement directly out of limb tissue limb buds of stage E13.5 were collected. Preparation was done in red light darkness; limbs were directly frozen in liquid nitrogen and stored at –80°C. After genotyping, 6 limbs were pooled per sample. Measurement was done by As Vitas, and 3 independent experiments were performed.
In situ hybridization.
For in situ hybridization, limbs were fixed in 4% PFA, dehydrated, and embedded in paraffin. Sections (5 μm) from different E12.5, E13.5, E14.5, and E16.5 were hybridized with specific probes. To avoid cross-hybridization, probes were generated from 3′ UTR with 2 specific primers each. Raldh2, forward, ATGGGTGAGTTTGGCTTACG, reverse, CCTGCTGGAAGGACTCAAAG; RARβ, forward, AGCAAGCCTCACATGTTTCC, reverse, TCTTTGCCATGCATCTTGAC; dHand (57); meis2, forward, GTCCAATGGGAATGGGTATG, reverse, TGAGGCAACATAACGGAGTG; Tbx5, forward, CAGACTGGCCTTCAGTCTCC, reverse, CAACACTCAGCGAGGCAATA; Sox9 (58); and Col2a1 (59). In situ hybridization was performed as described previously (60). Whole-mount in situ hybridization was performed as previously described (52). 3D reconstruction was performed from serial sections hybridized to Col2a1 probes using Amira software (Amira 3.0; Mercury Computer Systems TGS).
Luciferase reporter assays.
A conserved region 3,300 bases upstream from transcription start site of murine Raldh2 comprising approximately 1,350 bp was amplified with specific primers GATCGCTAGCAGCCGAAGATCATCCTTTC (forward) and GATCAGATCTTGTTGTAGACCCCCAGGA (reverse) from genomic mouse DNA and cloned into pGL2-Enhancer (Promega; catalog E1621). This vector contains its own transcription start site for luciferase. Cos7 cells were cultured in 24-well plates and transfected with ExGen500 (Fermentas) with 150 ng promoter construct, 300 ng of required Hoxd13 construct, and 15 ng renilla luciferase. Reporter assays were performed 24 hours after transfection with Dual-Glo Luciferase Assay System (Promega; catalog TM058) according to the manufacturer’s instructions.
ChMM culture.
ChMM cultures were prepared as described previously (61). For RA treatment, cells were grown in DMEM: F12 with 10% FCS, 0.2% chicken serum, 2 mM l-glutamine, and penicillin/streptomycin. Medium was replaced every day. RA (Sigma-Aldrich; catalog R2625) was dissolved in EtOH, disulfiram (Fluka; catalog 86720) in DMSO, and citral (Sigma-Aldrich; catalog C83007) in medium. Controls were treated either with EtOH or DMSO alone. Alcian blue staining of chMM cultures was performed in 1% Alcian blue in 1 N HCl after fixation in Kahles fixative (28.9% vol/vol EtOH; 0.37% formaldehyde; 3.9% vol/vol acetic acid). Histomorphometric quantification by was performed with AxioVision Outmess Software (Zeiss). For Hoxd13 analysis, cells were infected with concentrated viral supernatants containing the cDNA encoding WT Hoxd13, Hoxd13+7Ala, and Hoxd13+14Ala. Infection with empty RCAS virus (62) was used as a control. Culture medium (DMEM-F12, 2% chicken serum, 4 mM l-glutamine, and penicillin/streptomycin) was replaced every 2 days. For each condition, 4 replicates were performed in parallel. Real-time PCR was performed as previously described (63) with gene-specific primers. Quantification of incorporated Alcian blue was performed after extraction of the dye with 6M guanidium chloride by OD595.
Primary mesenchymal cell culture.
Murine primary mesenchymal cells were isolated at E13.5, when mutants can clearly be distinguished from normal littermates. Hand and food plates were collected and prepared as described above for chMM. Cells were grown in DMEM: F12 with 10% FCS, 1% penicillin/streptomycin, and L-glutamine. Medium was replaced every second day. Alcian blue staining of primary mesenchymal cultures was performed (see above), and quantification was done using AxioVision Outmess Software (Zeiss).
ChIP.
ChMM cultures were infected with RCAS-BP virus expressing an N-terminal FLAG-tagged Hoxd13 and cultured for 5 days as previously described (64). For IP, chMM cultures were digested with 0.02% collagenase type IA, 0.02% trypsine, and 1% FCS in PBS for 30 minutes at 37°C. Approximately 108 cells were pooled and washed once in PBS. Chromatin crosslinking and IP were performed as previously described (65) with an affinity-purified monoclonal anti-FLAG M2 antibody (Sigma-Aldrich; catalog F1804). Sonification conditions were 32 pulses with 30% output and 45 seconds between pulses on a Branson sonifier 450D using a 5-mm tip. Following IP and washing steps, crosslinks were reversed for 15 minutes at 98°C. DNA was digested for 30 minutes at 37°C with RNase A and subsequently with proteinase K for 20 minutes at 45°C, followed by purification with PCR purification (QIAquick; Qiagen) columns. Analysis was performed by real-time PCR using POWER SYBR Green PCR Master Mix (Applied Biosystems) and ABI 7900 HT real-time PCR Cycler (Applied Biosystems), as described before (63), with gene specific primers. Primers were as follows: 1 forward, AATTTAAACTTGCAAACTAGATCACACAT; 1 reverse, CCAGGTCACATTTATCATCACTAAATG; 2 forward, GAGTGCTGTGTGCAAGGTGG; 2 reverse, CCCATCCAGCTCACAGGTTC; 3 forward, AACCACAGTGTTAATTTTTGAAACTGA; 3 reverse, TCAGTGTTTGGGTTATTTTAGAAGTCA; 4 forward, AAATCTGCGGTCTCCTCCATT; 4 reverse, TCACCACGTGCACCTCTGTAA.
Bead implantation.
Chicken eggs were incubated for 5 days. Beads (Bio-Rad; catalog AGI-X2) were soaked with either 4 mg/ml RA (Sigma-Aldrich; catalog R2625) in DMSO, with 0.1 g/ml citral (Sigma-Aldrich; catalog C83007) in DMSO, or in DMSO alone. Beads were incubated at room temperature for 15 minutes and then directly used. Implanted chicken embryos were removed after 24 hours, fixed in 4% PFA overnight, and then prepared for paraffin embedding and section in situ hybridization as described above. A specific chicken ColII probe was used, as unimplanted limbs served as controls.
Immunohistochemistry.
Immunohistochemistry was performed on 5-μm paraffin sections at E12.5 and E13.5. Sections were rehydrated in a descending series of EtOH concentrations, boiled in citrate buffer twice for 3 minutes, permeabilized with 0.2% Triton X-100 for 15 minutes, and blocked with normal goat serum. Antibody incubation was done at 4°C overnight with anti-Sox9 (1:50; Santa Cruz Biotechnology Inc.; catalog 20095) and for 1 hour with Alexa Fluor 488–conjugated secondary antibody (Invitrogen). Sample examination was done with an AxioVert 200 fluorescence microscope and AxioVision software (both Zeiss).
Statistics.
For statistical analysis, the 2-tailed Student’s t test was used. P values less than 0.05 were considered significant.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to S. Mundlos. We thank D. Duboule (University of Geneva, Geneva, Switzerland) for supplying us with Hoxd13st/+ mutant mice. We thank N. Rösener for help with genotyping, J. Wetzel for work with the mice, and O. Hagens for cloning chHoxd11 and chHoxd12 constructs. We also thank the Ressourcenzentrum für Genomforschung for chip hybridization and S. Reijntjes for teaching us implanting techniques.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: chMM, chicken micromass; Hox, homeobox; Hoxd13, homeobox d13; RA, retinoic acid; Raldh2, retinaldehyde dehydrogenase 2; Shh, sonic hedgehog; SPD, synpolydactyly; Spdh, SPD homolog.
Citation for this article: J. Clin. Invest. 119:146–156 (2009). doi:10.1172/JCI36851
References
- 1.Kornak U., Mundlos S. Genetic disorders of the skeleton: a developmental approach. Am. J. Hum. Genet. 2003;73:447–474. doi: 10.1086/377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwabe G.C., Mundlos S. Genetics of congenital hand anomalies. Handchir. Mikrochir. Plast. Chir. 2004;36:85–97. doi: 10.1055/s-2004-817884. [DOI] [PubMed] [Google Scholar]
- 3.Hill R.E. How to make a zone of polarizing activity: insights into limb development via the abnormality preaxial polydactyly. Dev. Growth Differ. 2007;49:439–448. doi: 10.1111/j.1440-169X.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 4.Robert B., Lallemand Y. Anteroposterior patterning in the limb and digit specification: contribution of mouse genetics. Dev. Dyn. 2006;235:2337–2352. doi: 10.1002/dvdy.20890. [DOI] [PubMed] [Google Scholar]
- 5.Tickle C. Making digit patterns in the vertebrate limb. Nat. Rev. Mol. Cell Biol. 2006;7:45–53. doi: 10.1038/nrm1830. [DOI] [PubMed] [Google Scholar]
- 6.Masuya H., Sagai T., Wakana S., Moriwaki K., Shiroishi T. A duplicated zone of polarizing activity in polydactylous mouse mutants. Genes Dev. 1995;9:1645–1653. doi: 10.1101/gad.9.13.1645. [DOI] [PubMed] [Google Scholar]
- 7.Lettice L.A., et al. Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7548–7553. doi: 10.1073/pnas.112212199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klopocki E., et al. A microduplication of the long range SHH limb regulator (ZRS) is associated with triphalangeal thumb-polysyndactyly syndrome. J. Med. Genet. 2008;45:370–375. doi: 10.1136/jmg.2007.055699. [DOI] [PubMed] [Google Scholar]
- 9.Maas S.A., Fallon J.F. Single base pair change in the long-range Sonic hedgehog limb-specific enhancer is a genetic basis for preaxial polydactyly. Dev. Dyn. 2005;232:345–348. doi: 10.1002/dvdy.20254. [DOI] [PubMed] [Google Scholar]
- 10.Hill P., Wang B., Rüther U. The molecular basis of Pallister Hall associated polydactyly. Hum. Mol. Genet. 2007;16:2089–2096. doi: 10.1093/hmg/ddm156. [DOI] [PubMed] [Google Scholar]
- 11.Marshall W.F., Nonaka S. Cilia: tuning in to the cell’s antenna. Curr. Biol. 2006;16:R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Haycraft C.J., et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan D.C., Laufer E., Tabin C., Leder P. Polydactylous limbs in Strong’s Luxoid mice result from ectopic polarizing activity. Development. 1995;121:1971–1978. doi: 10.1242/dev.121.7.1971. [DOI] [PubMed] [Google Scholar]
- 14.Babbs C., Furniss D., Morriss-Kay G.M., Wilkie A.O.M. Polydactyly in the mouse mutant Doublefoot involves altered Gli3 processing and is caused by a large deletion in cis to Indian hedgehog. Mech. Dev. 2008;125:517–526. doi: 10.1016/j.mod.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik S., Grzeschik K. Synpolydactyly: clinical and molecular advances. Clin. Genet. 2008;73:113–120. doi: 10.1111/j.1399-0004.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 16.Zakany J., Duboule D. The role of Hox genes during vertebrate limb development. Curr. Opin. Genet. Dev. 2007;17:359–366. doi: 10.1016/j.gde.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Fromental-Ramain C., et al. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 18.Davis A.P., Witte D.P., Hsieh-Li H.M., Potter S.S., Capecchi M.R. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375:791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- 19.Wellik D.M., Capecchi M.R. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. . 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 20.Boulet A.M., Capecchi M.R. Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development. 2004;131:299–309. doi: 10.1242/dev.00936. [DOI] [PubMed] [Google Scholar]
- 21.Muragaki Y., Mundlos S., Upton J., Olsen B.R. Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science. 1996;272:548–551. doi: 10.1126/science.272.5261.548. [DOI] [PubMed] [Google Scholar]
- 22.Goodman F.R., et al. Synpolydactyly phenotypes correlate with size of expansions in HOXD13 polyalanine tract. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7458–7463. doi: 10.1073/pnas.94.14.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson K.R., et al. A new spontaneous mouse mutation of Hoxd13 with a polyalanine expansion and phenotype similar to human synpolydactyly. Hum. Mol. Genet. 1998;7:1033–1038. doi: 10.1093/hmg/7.6.1033. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht A., Mundlos S. The other trinucleotide repeat: polyalanine expansion disorders. Curr. Opin. Genet. Dev. 2005;15:285–293. doi: 10.1016/j.gde.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Bruneau S., Johnson K.R., Yamamoto M., Kuroiwa A., Duboule D. The mouse Hoxd13(spdh) mutation, a polyalanine expansion similar to human type II synpolydactyly (SPD), disrupts the function but not the expression of other Hoxd genes. Dev. Biol. 2001;237:345–353. doi: 10.1006/dbio.2001.0382. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht A.N., et al. A molecular pathogenesis for transcription factor associated poly-alanine tract expansions. Hum. Mol. Genet. 2004;13:2351–2359. doi: 10.1093/hmg/ddh277. [DOI] [PubMed] [Google Scholar]
- 27.Dollé P., et al. Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell. 1993;75:431–441. doi: 10.1016/0092-8674(93)90378-4. [DOI] [PubMed] [Google Scholar]
- 28.Zákány J., Kmita M., Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- 29.Zhao D., et al. Molecular identification of a major retinoic-acid-synthesizing enzyme, a retinaldehyde-specific dehydrogenase. Eur. J. Biochem. 1996;240:15–22. doi: 10.1111/j.1432-1033.1996.0015h.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang T., Zhang H., Parent J.M. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. 2005;132:2721–2732. doi: 10.1242/dev.01867. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M., Tamura K., Ide H. Citral, an inhibitor of retinoic acid synthesis, modifies chick limb development. Dev. Biol. 1996;175:239–247. doi: 10.1006/dbio.1996.0111. [DOI] [PubMed] [Google Scholar]
- 32.Bi W., Deng J.M., Zhang Z., Behringer R.R., de Crombrugghe B. Sox9 is required for cartilage formation. Nat. Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 33.Ng L.J., et al. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 34.Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982;295:672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- 35.Tickle C., Alberts B., Wolpert L., Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982;296:564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- 36.Mic F.A., Sirbu I.O., Duester G. Retinoic acid synthesis controlled by Raldh2 is required early for limb bud initiation and then later as a proximodistal signal during apical ectodermal ridge formation. J. Biol. Chem. 2004;279:26698–26706. doi: 10.1074/jbc.M401920200. [DOI] [PubMed] [Google Scholar]
- 37.Niederreither K., Vermot J., Schuhbaur B., Chambon P., Dollé P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- 38.Lu H.C., Revelli J.P., Goering L., Thaller C., Eichele G. Retinoid signaling is required for the establishment of a ZPA and for the expression of Hoxb-8, a mediator of ZPA formation. Development. 1997;124:1643–1651. doi: 10.1242/dev.124.9.1643. [DOI] [PubMed] [Google Scholar]
- 39.de Thé H., Vivanco-Ruiz M.M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 40.Allenby G., et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. U. S. A. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercader N., et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- 42.Weston A.D., Hoffman L.M., Underhill T.M. Revisiting the role of retinoid signaling in skeletal development. Birth Defects Res. C Embryo Today. 2003;69:156–173. doi: 10.1002/bdrc.10010. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann B., Tsambaos D. Evaluation of the sensitive step of inhibition of chondrogenesis by retinoids in limb mesenchymal cells in vitro. Cell Differ. 1985;17:95–103. doi: 10.1016/0045-6039(85)90475-0. [DOI] [PubMed] [Google Scholar]
- 44.Wedden S.E., Lewin-Smith M.R., Tickle C. The effects of retinoids on cartilage differentiation in micromass cultures of chick facial primordia and the relationship to a specific facial defect. Dev. Biol. 1987;122:78–89. doi: 10.1016/0012-1606(87)90334-4. [DOI] [PubMed] [Google Scholar]
- 45.Desbiens X., Meunier L., Lassalle B. Specific effects of retinoic acid on the skeletal morphogenesis of the 11-day mouse embryo forelimb bud in vitro. Biol. Cell. 1990;68:213–220. doi: 10.1016/0248-4900(90)90310-Y. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman L.M., et al. BMP action in skeletogenesis involves attenuation of retinoid signaling. J. Cell Biol. 2006;174:101–113. doi: 10.1083/jcb.200604150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weston A.D., Rosen V., Chandraratna R.A., Underhill T.M. Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J. Cell Biol. 2000;148:679–690. doi: 10.1083/jcb.148.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weston A.D., Chandraratna R.A.S., Torchia J., Underhill T.M. Requirement for RAR-mediated gene repression in skeletal progenitor differentiation. J. Cell Biol. 2002;158:39–51. doi: 10.1083/jcb.200112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serpente P., et al. Direct crossregulation between retinoic acid receptor {beta} and Hox genes during hindbrain segmentation. Development. 2005;132:503–513. doi: 10.1242/dev.01593. [DOI] [PubMed] [Google Scholar]
- 50.Simeone A., et al. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990;346:763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- 51.Zákány J., Fromental-Ramain C., Warot X., Duboule D. Regulation of number and size of digits by posterior Hox genes: a dose-dependent mechanism with potential evolutionary implications. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13695–13700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albrecht A.N., et al. The synpolydactyly homolog (spdh) mutation in the mouse — a defect in patterning and growth of limb cartilage elements. Mech. Dev. 2002;112:53–67. doi: 10.1016/S0925-4773(01)00639-6. [DOI] [PubMed] [Google Scholar]
- 53.Zákány J., Duboule D. Synpolydactyly in mice with a targeted deficiency in the HoxD complex. Nature. 1996;384:69–71. doi: 10.1038/384069a0. [DOI] [PubMed] [Google Scholar]
- 54.Lohnes D., et al. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 55.Hurle J.M., Gañan Y. Formation of extra-digits induced by surgical removal of the apical ectodermal ridge of the chick embryo leg bud in the stages previous to the onset of interdigital cell death. Anat. Embryol. (Berl.). 1987;176:393–399. doi: 10.1007/BF00310193. [DOI] [PubMed] [Google Scholar]
- 56.Klose J., Kobalz U. Two-dimensional electrophoresis of proteins: an updated protocol and implications for a functional analysis of the genome. Electrophoresis. 1995;16:1034–1059. doi: 10.1002/elps.11501601175. [DOI] [PubMed] [Google Scholar]
- 57.Niedermaier M., et al. An inversion involving the mouse Shh locus results in brachydactyly through dysregulation of Shh expression. J. Clin. Invest. 2005;115:900–909. doi: 10.1172/JCI200523675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Healy C., Uwanogho D., Sharpe P.T. Regulation and role of Sox9 in cartilage formation. Dev. Dyn. 1999;215:69–78. doi: 10.1002/(SICI)1097-0177(199905)215:1<69::AID-DVDY8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Kohno K., Martin G.R., Yamada Y. Isolation and characterization of a cDNA clone for the amino-terminal portion of the pro-alpha 1(II) chain of cartilage collagen. J. Biol. Chem. 1984;259:13668–13673. [PubMed] [Google Scholar]
- 60.Stricker S., Fundele R., Vortkamp A., Mundlos S. Role of Runx genes in chondrocyte differentiation. Dev. Biol. 2002;245:95–108. doi: 10.1006/dbio.2002.0640. [DOI] [PubMed] [Google Scholar]
- 61.Seemann P., et al. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J. Clin. Invest. 2005;115:2373–2381. doi: 10.1172/JCI25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Federspiel M.J., Hughes S.H. Retroviral gene delivery. Methods Cell Biol. 1997;52:179–214. doi: 10.1016/S0091-679X(08)60379-9. [DOI] [PubMed] [Google Scholar]
- 63.Hecht J., et al. Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2(–/–) mouse model. Gene Expr. Patterns. 2007;7:102–112. doi: 10.1016/j.modgep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 64.Lehmann K., et al. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12277–12282. doi: 10.1073/pnas.2133476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee T.I., Johnstone S.E., Young R.A. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]