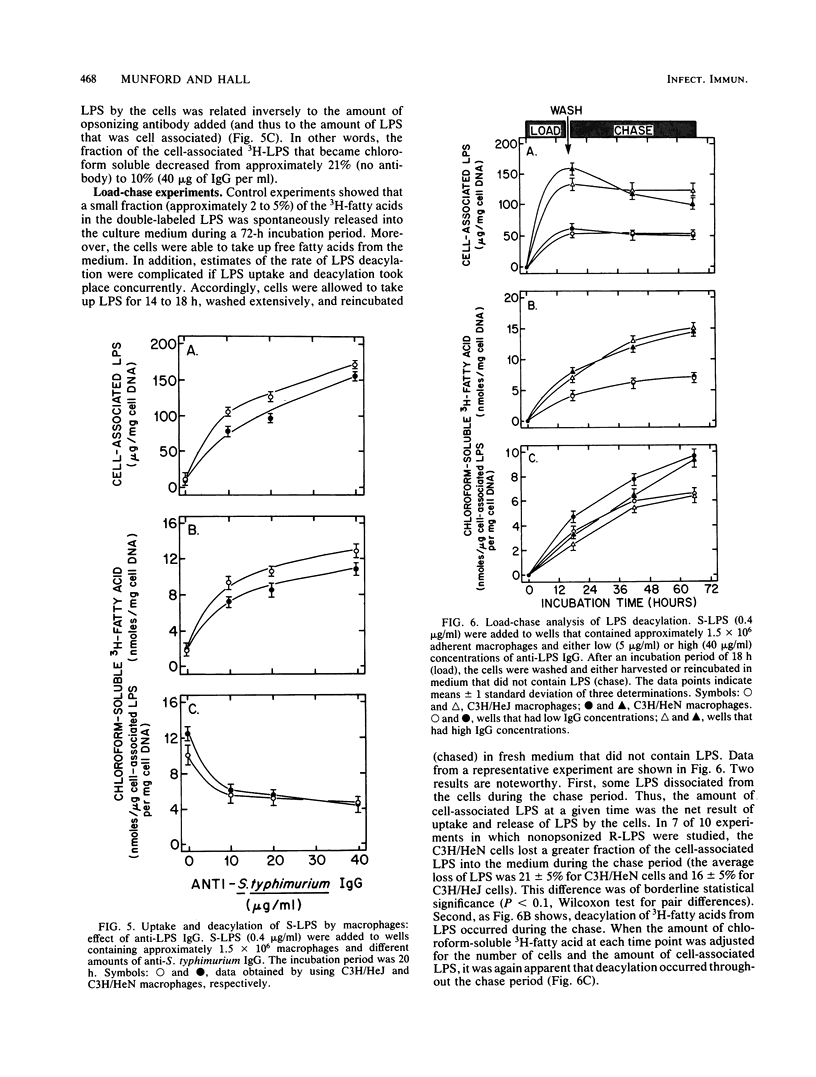
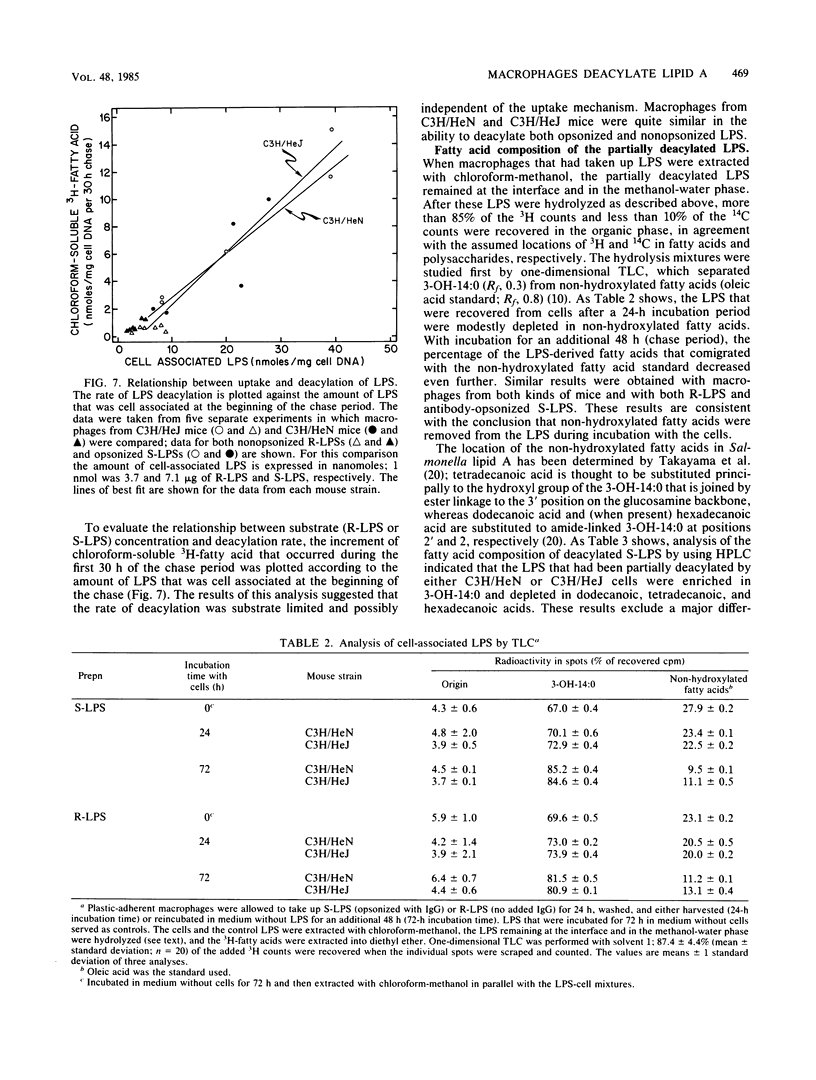
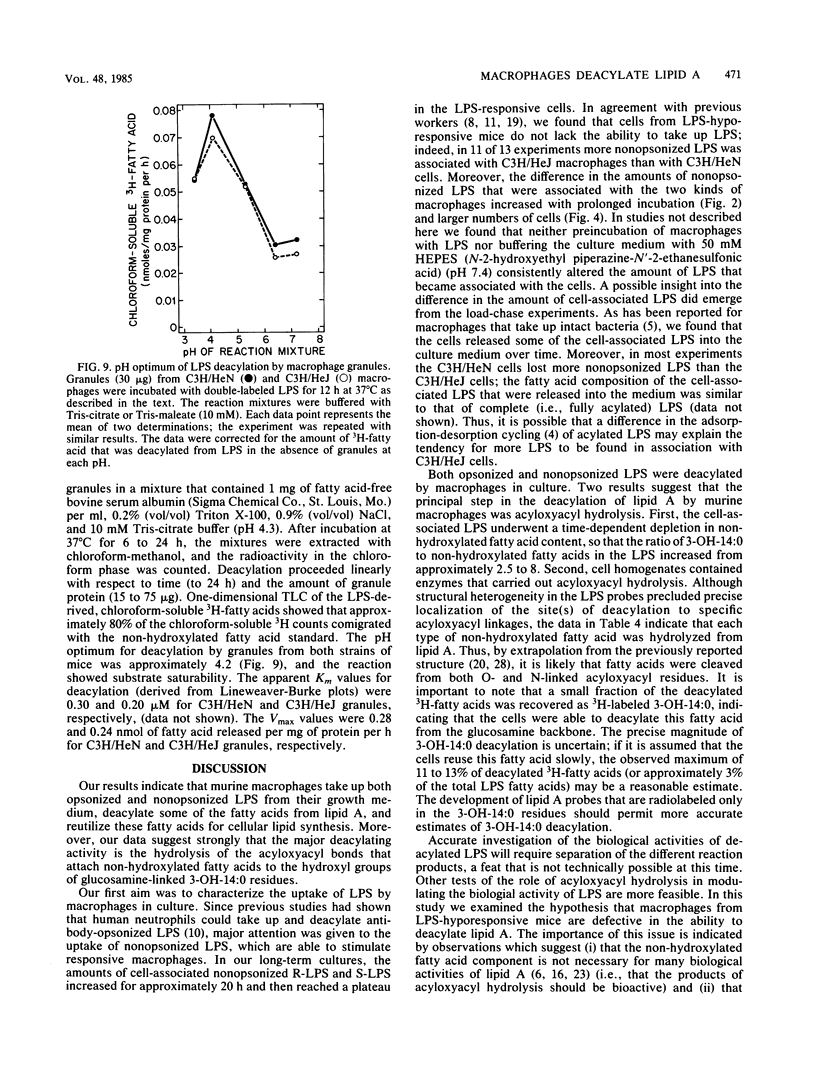
Abstract
Macrophages are thought to play a central role in the responses of animals to gram-negative bacterial lipopolysaccharides (LPS). Since nothing is known about the metabolism of LPS by these cells, we studied the uptake and deacylation of radiolabeled LPS by thioglycolate-elicited peritoneal macrophages from normal (C3H/HeN) and LPS-hyporesponsive (C3H/HeJ) mice. Macrophages from both kinds of mice took up and deacylated LPS that were added to the culture medium. Opsonization of the LPS with anti-LPS immunoglobulin G antibodies greatly increased LPS uptake; the opsonized LPS also underwent deacylation at rates that were directly related to the amount of cell-associated LPS. An analysis of the fatty acid composition of the cell-associated LPS indicated that the cells have one or more acyloxyacyl hydrolases that remove the non-hydroxylated fatty acids that are normally substituted to the hydroxyl groups of (glucosamine-linked) 3-hydroxytetradecanoate residues in lipid A; we also found evidence for deacylation of 3-hydroxytetradecanoate from the glucosamine backbone. LPS deacylation by macrophages from C3H/HeN and C3H/HeJ mice was qualitatively and quantitatively similar. Nonopsonized LPS are able to stimulate LPS-responsive cells; in these studies we established that animal cells can deacylate nonopsonized LPS, thus raising the possibility that LPS metabolism may play a role in modulating cellular stimulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. B., Gomez-Sanchez C. E., Adams B. V. Role of prostaglandins in angiotensin-induced steriodogenesis absence of an effect by prostaglandin E2. Hypertension. 1980 Jul-Aug;2(4):471–476. doi: 10.1161/01.hyp.2.4.471. [DOI] [PubMed] [Google Scholar]
- Cookson S. L., Adams D. O. A simple, sensitive assay for determining DNA in mononuclear phagocytes and other leukocytes. J Immunol Methods. 1978;23(1-2):169–173. doi: 10.1016/0022-1759(78)90120-5. [DOI] [PubMed] [Google Scholar]
- Davies M., Stewart-Tull D. E., Jackson D. M. The binding of lipopolysaccharide from Escherichia coli to mammalian cell membranes and its effect on liposomes. Biochim Biophys Acta. 1978 Apr 4;508(2):260–276. doi: 10.1016/0005-2736(78)90329-2. [DOI] [PubMed] [Google Scholar]
- Duncan R. L., Jr, Morrison D. C. The fate of E. coli lipopolysaccharide after the uptake of E. coli by murine macrophages in vitro. J Immunol. 1984 Mar;132(3):1416–1424. [PubMed] [Google Scholar]
- Galanos C., Lehmann V., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M. A., Hansen-Hagge T., Lüderitz T. Endotoxic properties of chemically synthesized lipid A part structures. Comparison of synthetic lipid A precursor and synthetic analogues with biosynthetic lipid A precursor and free lipid A. Eur J Biochem. 1984 Apr 16;140(2):221–227. doi: 10.1111/j.1432-1033.1984.tb08090.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gregory S. H., Zimmerman D. H., Kern M. The lipid A moiety of lipopolysaccharide is specifically bound to B cell subpopulations of responder and nonresponder animals. J Immunol. 1980 Jul;125(1):102–107. [PubMed] [Google Scholar]
- Haeffner N., Chaby R., Szabó L. Identification of 2-methyl-3-hydroxydecanoic and 2-methyl-3-hydroxytetradecanoic acids in the 'lipid X' fraction of the Bordetella pertussis endotoxin. Eur J Biochem. 1977 Aug 1;77(3):535–544. doi: 10.1111/j.1432-1033.1977.tb11696.x. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. M., Eldridge J. H. Surface phenotype of LPS-binding murine lymphocytes. Proc Soc Exp Biol Med. 1984 Apr;175(4):458–467. doi: 10.3181/00379727-175-41821. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Radioimmunoassay for Gram-negative bacterial lipopolysaccharide O antigens: influence of antigen solubility. Infect Immun. 1979 Oct;26(1):42–48. doi: 10.1128/iai.26.1.42-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Takayama K. Molecular requirements for B-lymphocyte activation by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4624–4628. doi: 10.1073/pnas.80.15.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Kempe J., Cohn Z. A. Prostaglandin synthesis by macrophages requires a specific receptor-ligand interaction. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4279–4282. doi: 10.1073/pnas.77.7.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain S. M., Fesik S. W., Armitage I. M. Structure and metal-binding properties of lipopolysaccharides from heptoseless mutants of Escherichia coli studied by 13C and 31P nuclear magnetic resonance. J Biol Chem. 1983 Nov 25;258(22):13466–13477. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic analysis of lymphocyte activation by lipopolysaccharide Endotoxin. Infect Immun. 1976 Jun;13(6):1579–1584. doi: 10.1128/iai.13.6.1579-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12801–12803. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. The accessory function of phagocytic cells in human T cell and B cell responses. J Immunol. 1982 Sep;129(3):1033–1040. [PubMed] [Google Scholar]
- Truffa-Bachi P., Kaplan J. G., Bona C. The mitogenic effect of lipopolysaccharide. Metabolic processing of lipopolysaccharide by mouse lymphocytes. Cell Immunol. 1977 Apr;30(1):1–11. doi: 10.1016/0008-8749(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Madonna G. S., Wahl L. M., Rick P. D. In vitro stimulation of C3H/HeJ spleen cells and macrophages by a lipid A precursor molecule derived from Salmonella typhimurium. J Immunol. 1984 Jan;132(1):347–353. [PubMed] [Google Scholar]
- Vogel S. N., Marshall S. T., Rosenstreich D. L. Analysis of the effects of lipopolysaccharide on macrophages: differential phagocytic responses of C3H/HeN and C3H/HeJ macrophages in vitro. Infect Immun. 1979 Jul;25(1):328–336. doi: 10.1128/iai.25.1.328-336.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Rosenstreich D. L., Glode L. M., Sandberg A. L., Mergenhagen S. E. Defective prostaglandin synthesis by C3H/HeJ mouse macrophages stimulated with endotoxin preparations. Infect Immun. 1979 Jan;23(1):8–13. doi: 10.1128/iai.23.1.8-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Kelly K., Largen M., Taylor B. A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978 Feb;120(2):422–424. [PubMed] [Google Scholar]
- Wollenweber H. W., Broady K. W., Lüderitz O., Rietschel E. T. The chemical structure of lipid A. Demonstration of amide-linked 3-acyloxyacyl residues in Salmonella minnesota Re lipopolysaccharide. Eur J Biochem. 1982 May;124(1):191–198. doi: 10.1111/j.1432-1033.1982.tb05924.x. [DOI] [PubMed] [Google Scholar]