Abstract
We measured antibodies to chondroitin sulfate A (CSA)-binding and placental Plasmodium falciparum-infected red blood cells (PRBCs) among pregnant women with or without placental malaria. Immunoglobulin G to PRBC surface antigens was rare in uninfected primigravidae (3.7%), more prevalent in infected primigravidae (70%; P < .001), and common in infected (77%) and uninfected (83%) multigravidae. Similar patterns were seen for agglutinating antibodies, and antibodies were similar among women with past or active placental infection. PRBC adhesion to CSA was inhibited 60% by cells obtained from infected primigravidae but 24% by cells obtained from uninfected primigravidae (P = .025), whereas infection did not alter adhesion inhibition by multigravidae (77% inhibition). There was substantial heterogeneity in antibody type and levels. Antibodies did not correlate with parasite density or pregnancy outcome. Comparisons between antibodies suggest that adhesion-inhibitory antibodies and those to PRBC variant antigens have distinct and overlapping epitopes, may be acquired independently, and have different roles in immunity.
During pregnancy, women are highly susceptible to Plasmodium falciparum malaria, despite preexisting immunity [1]. The risk of maternal malaria is highest in first pregnancies and is somewhat reduced in subsequent pregnancies and with increasing age [1, 2]. Coinfection with HIV-1, which is common in many settings, appears to increase susceptibility [3]. Complications of infection include maternal anemia, increased maternal mortality, and low birth weight and infant anemia, which are associated with high infant death rates [1].
The key pathological finding of maternal malaria is placental infection [1], which is characterized by the accumulation of parasitized red blood cells (PRBCs) [4]. They are predominantly mature asexual stages of PRBCs that express variant surface antigens (VSAs) and can adhere to host cells [5]. Placental infection appears to be mediated in part by the adhesion of PRBCs to chondroitin sulfate A (CSA), which is expressed on placental syncytiotrophoblasts [6-9] and may also involve adhesion to hyaluronic acid (HA) and the binding of immunoglobulins [10, 11].
During or before their first pregnancy, women lack antibodies to placental and CSA-binding PRBCs, which suggests that these parasites represent novel variants to which women have not been exposed previously [8, 12-14]. Multigravidae (MG) generally have a higher prevalence of antibodies specific to placental or CSA-binding PRBCs than do primigravidae (PG) or men, which reflects greater exposure [8, 12-15]. These antibodies may contribute to the protection or clearance of infection, and it has been suggested that adhesion-inhibitory antibodies may prevent parasite accumulation in the placenta [12]. Presumably, antibodies to CSA-binding PRBCs are acquired after placental infection. However, at present, the association between active or cleared placental infection and antibodies to CSA-binding PRBCs among women of different parities is unclear. An inverse association between adhesion-inhibitory antibodies and infection was reported among secundigravidae (SG) in Kenya [12], whereas no associations were found between adhesion-inhibitory antibodies and infection in Cameroon [16].
Antibodies to VSA expressed by PRBCs appear to be an important component of immunity to P. falciparum in non-pregnant individuals [17, 18]. A major target of these antibodies is P. falciparum erythrocyte membrane protein 1 (PfEMP1), which is expressed on the PRBC surface [19, 20]. PfEMP1 can undergo clonal antigenic variation [19], and it mediates the adhesion of PRBCs to a range of host molecules, including CSA [21, 22]. Total antibody to VSA may predominantly bind to different epitopes on the PRBC surface, rather than adhesion-inhibitory antibodies, which more specifically target receptor-binding sites on PfEMP1. These antibodies may have different associations with infection, clinical disease, and immunity, and understanding the relationship between the different antibody measurements is important for evaluating the nature and dynamics of immunity to placental malaria and the development of potential therapeutic or preventative interventions.
In the present study, we aimed to clearly elucidate the relationship between active or past placental infection and antibodies to CSA-binding and placental isolates, using strictly defined clinical samples and controlling for major confounding factors. We examined this association using measures of antibodies that differentiate between anti-VSA and adhesion inhibitory antibodies, to assess the nature of antibodies acquired after exposure to placental infection and to determine the relationship between antibodies to the surface of CSA-binding PRBCs and those that inhibit adhesion to CSA in the context of immunity and clinical disease.
SUBJECTS AND METHODS
Study population and sample collection
The population in the study area experiences year-round malaria transmission, with seasonal variation [2]. From January 1998 to November 2000, women attending the Labour Ward of the Queen Elizabeth Central Hospital, Blantyre, Malawi, for routine delivery were tested for peripheral, placental, and cord blood P. falciparum infection, by microscopy of Field’s-stained thick blood films. Peripheral blood plasma (in EDTA) and serum were separated within 1 h of collection, and placental biopsy samples were collected into neutral buffered formalin, fixed and processed routinely, and stained with Giemsa and/or hematoxylineosin [23]. Clinical and demographic data were collected for each donor. Placental histological results were classified as showing active infection (parasites visible), cleared or past placental infection (the presence of parasite pigment in fibrinoid deposits but no parasites visible), or no infection (no parasites or parasite pigment visible) [23]. All women were offered voluntary, confidential, HIV counseling and testing, as described elsewhere [23]. Fully informed consent was obtained from all participants in the study. Ethical approval for all aspects of the study was obtained from the College of Medicine Research Committee, University of Malawi, Blantyre, Malawi, and the Clinical Research Ethics Committee, Royal Melbourne Hospital Research Foundation, Victoria, Australia.
After all parasitological and histological data were available, a case-control study was designed. Case patients were defined as women with active placental infection, according to the results of examination of placental histological tests, together with positive peripheral and placental blood smears. All malaria infections were caused by P. falciparum. Infected women were individually matched by gravidity, age (±2 years), and date of delivery (±2 months) to women without evidence of current or previous placental infection according to the results of placental histological analysis and placental, peripheral, and cord blood smears, and/or to women with evidence of past placental infection (malaria pigment present by placental histological analysis but no parasites seen, together with negative placental, peripheral, and cord blood smears). Placental parasitemia was determined from counting at least 500 infected and uninfected RBCs during examination of histological sections and by examination of thick blood films, as described elsewhere [5]. All women were at ≥36 weeks gestation and had live births.
Serum samples were also collected from the peripheral blood of smear-negative women attending antenatal clinic for their first routine visit (16-24 weeks gestation) [8], to examine associations between specific antibody responses and gravidity. Plasma was also collected from men who were parents of children attending the hospital, malaria-smear negative, and of similar age to pregnant women in the study. Mean ± SD ages were 19.6 ± 2.1, 24.4 ± 2.2, and 29.3 ± 3.4 years for PG, MG, and men, respectively. Samples were also collected from non-malaria-exposed donors resident in Australia.
P. falciparum isolates and culture
P. falciparum PRBCs were isolated from infected placentas, as described elsewhere [8]. Placental biopsy tissue was collected immediately after delivery and incubated with PBS that contained 50 mmol/L EDTA. Isolated PRBCs were washed with PBS (pH 7.2) before use in agglutination or cytoadhesion assays without intervening culture.
Isolate E8B (or FAF-EA8) was derived from a clone of Brazilian P. falciparum isolate ItG2F6 by repeated selection for adhesion to endothelial cells in vitro [24]. Isolate CS2 was derived from E8B by repeated selection for adhesion to CSA [25]. E8B typically bound to CD36 and intercellular adhesion molecule (ICAM)-1 and only at low levels to CSA and HA [10, 25, 26]. CS2 typically adhered at high levels to CSA and HA but not to ICAM-1 or CD36 [10, 25-28]. E8B and CS2 expressed different forms of PfEMP1 [21]. Parasites were cultured in group O+ RBCs suspended in medium supplemented with 10% pooled human serum [8]. The identity of parasite lines was confirmed by genotyping, and cultures were free of Mycoplasma contamination, as shown by the results of polymerase chain reaction.
Parasite adhesion assays
Assays to measure the inhibition of parasite adhesion to CSA were performed as described elsewhere [8, 28], using P. falciparum trophozoite-infected erythrocytes at 1%-4% parasitemia. In brief, multiple circles of ∼10 mm diameter were drawn on the base of plastic petri dishes (Falcon 1058; Becton Dickinson) using a Dako Pen for Immunohistochemistry (Dako). CSA linked to phosphatidylethanolamine [25] was spotted (10 μL) into the circles and adsorbed onto the surface for 18-24 h at 4°C, and plates were subsequently blocked with 1% bovine serum albumin in PBS. Parasites, harvested from cultures or directly from placental tissue, were washed first in PBS (pH 7.4) or in RPMI-HEPES (pH 6.8) and then resuspended in RPMI-HEPES that contained test serum or plasma and incubated for 30 min at room temperature. Then, 30 μL of the parasite suspension with test serum was overlaid receptor spots and incubated for 20 min at 37°C without agitation. Unbound cells were removed by gentle washing with RPMI-HEPES (pH 6.8) before bound cells were fixed with glutaraldehyde, stained with Giemsa stain, and counted by light microscopy. Adhesion was expressed as a percentage of control (pooled serum from nonimmune donors resident in Australia) adhesion. Serum samples obtained from uninfected women (10 PG and 10 MG) at midpregnancy and from men (n = 6) were tested for the inhibition of adhesion to CSA, using 2 different placental isolates at 90% parasitemia and 0.5% hematocrit, with serum diluted 1:10. Matched samples obtained at term were tested for the inhibition of adhesion of CS2 parasites at 1:5 dilution, 4%-5% parasitemia, and 0.5% hematocrit over 2 separate experiments run in parallel with flow cytometry assays. A subset of samples was first tested to determine the ideal conditions for performing inhibitory assays. At 1:10 dilution, the range of inhibition by different samples was 0%-40%, and, at 1:5 dilution, inhibition was 0%-100%. Thus, the 1:5 dilution was chosen as the concentration to test all samples. All matched pairs (27 PG pairs and 12 MG pairs) were tested together on the same plate, in duplicate, with men’s samples (n = 16) and negative and positive control serum samples. Pools of samples were made and tested in duplicate or triplicate at 1:5 dilution. All samples were coded and tested blinded. The median level of inhibition of adhesion for all individual samples from women at term was 55%, relative to control adhesion, and samples that inhibited ≥55% were defined as highly inhibitory.
Parasite-infected cell agglutination assays
Mature trophozoite-stage PRBCs at 5%-10% parasitemia, from culture, were passed over Percoll density gradients (40%, 60%, and 80%) [8], to enrich samples to a parasitemia of 60%-90%. After washing cells 3 times with RPMI-HEPES or PBS, 22.5 μL of cell suspension was incubated in 96-well microtiter plates with 2.5 μL of test serum or plasma (final dilution, 1:10) in PBS for 45 min on a rotating wheel at room temperature. From each sample, 10 μL was taken and gently and evenly spread with a plastic micropipetting tip onto a glass slide, over an area of ∼15 mm diameter. Duplicate smears were made for each sample and allowed to dry over several minutes. Smears were fixed with methanol, stained with 10% Giemsa in water for 10 min, and mounted with a glass coverslip using DePeX (BDH) mounting compound. By light microscopy, the whole smear was initially examined for the presence of agglutinates of PRBCs at low power (10× objective lens) and confirmed at higher power. Samples were considered to be positive if at least 3 agglutinates of >5 PRBCs were observed in the absence of agglutinates of RBCs. This approach facilitated the testing of large numbers of samples in one experiment, to reduce the effect of interexperiment variation, and allowed samples to be reexamined or examined by a second observer. Results strongly agreed with those of assays in which cell agglutinates were examined without fixation and with conventional assays [29]. Positive slides was scored by 2 approaches: the proportion (percentage) of PRBCs that were in agglutinates, determined from representative areas of the smear, and on the size (number of PRBCs present) of the largest agglutinate observed. Scoring slides by both approaches significantly correlated with the dilution of test serum samples [29], and the 2 different scores were significantly correlated among positive samples (P < .01). Serum samples were tested for the agglutination of fresh placental isolates, as described elsewhere [8], at 2%-18% parasitemia, without enrichment or intervening culture. Samples were coded and tested blindly, and all matched pairs were run together with the inclusion of positive and negative controls and men’s samples.
Flow cytometry
P. falciparum trophozoite-infected erythrocytes at 3%-4% parasitemia, from culture, were washed 3 times in PBS with 1% fetal calf serum (PBS-FCS). Cells were incubated (100 μL, 107 cells/mL) with test serum or plasma diluted 1:20 in PBS-FCS for 30 min in 96-well round-bottom microtiter plates (Falcon 3077; Becton Dickinson). After 3 washes in PBS-FCS, cells were incubated with goat anti-human IgG (Fc-specific; Caltag) at 1:50 dilution for 30 min (50 μL), washed 3 times with PBS-FCS, then incubated with fluorescein isothiocyanate (FITC)-conjugated swine anti-goat Ig (Caltag) at 1:20 dilution and 10 μg/mL ethidium bromide for 30 min in darkness. Cells were washed 3 times with PBS, resuspended to 200 μL, and analyzed using a Becton-Dickinson FACSCalibur flow cytometer. Incubations were performed at room temperature. All samples were coded and tested blindly. Matched pairs were run together with positive and negative controls and men’s samples, in parallel with adhesion inhibition assays. For each sample, the geometric mean fluorescence intensity (MFI) in channel FL1 of ethidium bromide-labeled trophozoite-infected PRBCs was calculated as a measure of IgG binding. In preliminary assays, we found that the calculated MFI for FITC closely correlated with the titer of positive serum and plasma samples. Samples were considered to have tested positive for specific IgG if the MFI was >3 SD above the mean of nonagglutinating men’s samples.
Statistical analysis
Data were entered into and managed in Microsoft Access and Excel. To assess statistical significance, χ2 tests and odd ratios (ORs) were used for comparisons between proportions, and Student’s t tests (for normally distributed data) or Mann-Whitney U tests were performed for comparing groups of ordinal data. P < .05 was considered to be statistically significant.
Characteristics of the study population
Fifty-one pregnant women of differing gravidities (28 PG, 11 SG, and 12 MG) with active placental infection (parasitemia >0.2%, positive peripheral blood film) were individually matched to 1 or 2 uninfected women (histological tests and blood films negative; 36 PG, 13 SG, and 19 MG) of the same parity and similar age, all being at term (table 1). There were no significant differences between the 2 groups in age, number of doses of antimalarials (sulfadoxine-pyrimethamine) taken during pregnancy, or HIV prevalence. Women with active placental infection had significantly lower hemoglobin concentrations (mean ± SD, 11.4 ± 1.8 vs. 12.4 ± 1.7; P < .05) and lower birth weight babies (2707 ± 459 vs. 3058 ± 448 g; P < .05) than uninfected women. Thirty-one women (16 PG, 6 SG, and 9 MG) with active placental infection were individually matched to 31 women of the same parity and similar age who had evidence of past placental infection on histological analysis (negative blood smears). No significant differences were observed between the groups regarding mean age, number of doses of antimalarials taken during the pregnancy, the prevalence of HIV, maternal hemoglobin, or birth weight.
Table 1. Characteristics of women enrolled at delivery in a study of placental malaria in Malawi.
| Characteristic | No. | Age, years | Birth weight, g | Hemoglobin, g/dL | HIV-1 positive, % | Antimalarials, no. of doses |
|---|---|---|---|---|---|---|
| Infecteda | 51 | 20.9 | 2707b | 11.4b | 35.4 | 1.3 |
| No infectionc | 68 | 21.5 | 3058b | 12.4b | 32.3 | 1.1 |
| Infecteda | 31 | 21.4 | 2780 | 11.1 | 33.3 | 1.2 |
| Past infectiond | 31 | 20.8 | 2897 | 11.5 | 28.5 | 1.1 |
Parasites present by examination of placental histology and peripheral blood film.
P < .05, infected vs. uninfected. The values shown are means. The antimalarial agent administered was sulfadoxine-pyrimethamine.
No parasites observed by placental histological analysis or blood films in women matched to 51 infected women.
Parasite pigment was visible in fibrinoid deposits by placental histological analysis, but no parasites were observed by placental histology or blood films in women matched to 31 infected women.
RESULTS
Antibodies to CSA-binding parasites are associated with current and past placental infection
Agglutinating antibodies to CS2 were clearly associated with placental infection at term (figure 1A). Thirty-one (61%) of 51 samples from women with active infection and 11 (16.2%) of 68 samples from matched uninfected women agglutinated CS2 (OR, 8.5; 95% confidence interval [CI], 3.6-20.0; P < .0001). Samples from 14 (50%) of 28 infected PG agglutinated CS2, whereas only 1 (2.8%) of 36 samples from uninfected PG had agglutinating antibodies (OR, 35.0; 95% CI, 4.2-292; P < .0001). Among SG and MG, antibodies were also significantly more prevalent among women with active placental infection (17/22; 77.3%), compared with uninfected women (10/32 or 31.3%; OR, 7.5; 95% CI, 2.2-26; P < .01). By contrast, only 2 (5.4%) of 37 samples from men agglutinated CS2, and serum from nonexposed donors consistently tested negative in the assays. Agglutination scores (proportion of PRBCs in agglutinates) were 0%-74% for PG and 0%-89% for SG/MG. Agglutination scores of positive samples were not substantially different between the groups: mean scores were 34.6%, 29.9%, 28%, and 22.2% for all PG, PG case patients, all SG/MG, and MG case patients, respectively.
Figure 1.
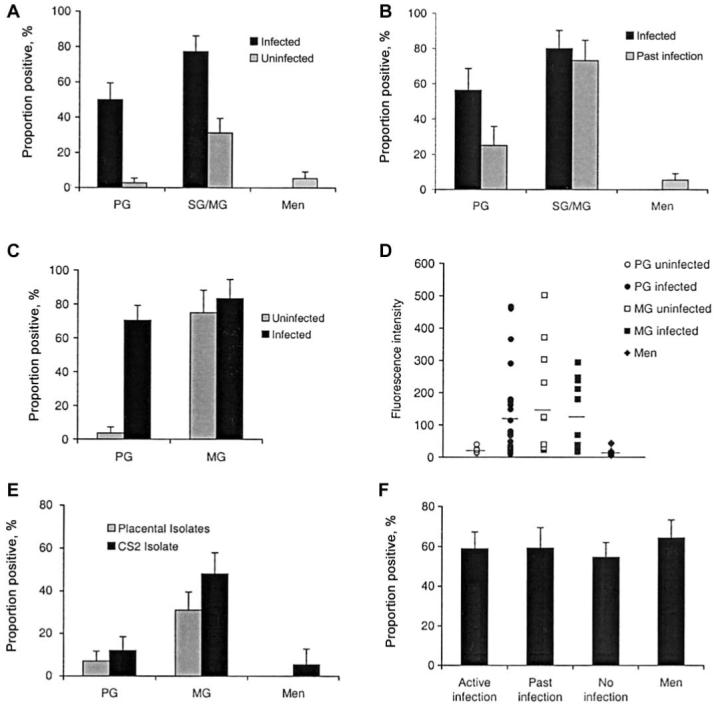
Associations between antibodies to the surface of chondroitin sulfate A (CSA)-binding Plasmodium falciparum and gravidity and placental infection. A, Proportion (±SE) of samples that tested positive for agglutination with CS2 among matched women with or without placental infection at term and men; P < .01, infected vs. uninfected groups. B, Proportion (±SE) of samples positive for agglutination with CS2 among matched women with active infection or past placental infection at term and men; differences between infected and past infection groups were not statistically significant. C, Proportion of samples (±SE), obtained at delivery, having detectable IgG binding among infected and uninfected primigravidae (PG) and multigravidae (MG); P < .001, for difference among PG samples. Samples with a mean fluorescence intensity value greater than the mean ±3 SD of nonagglutinating men’s samples were classified as positive for IgG to CS2 P. falciparum-infected red blood cells. D, Levels of the IgG binding (fluorescence intensity) of individual samples obtained at delivery from infected and uninfected PG, MG, and men. Horizontal lines represent the mean values; P < .01, infected vs. uninfected PG and MG vs. men. E, Proportion (±SE) of serum samples from smear-negative PG and MG at midpregnancy and men that were positive by agglutination using either placental isolates or the CSA-binding parasite isolate CS2; P < .05, for difference. F, Proportion (±SE) of samples obtained from women at term that were positive for agglutination using isolate E8B among pregnant women with active infection, no infection, or past placental infection, and men; differences were not statistically significant. SG, secundigravidae.
Women with evidence of cleared placental infection had a prevalence of antibodies similar to that of women with active placental infection (figure 1B); 21 (67.7%) of 31 and 15 (48.4%) of 31 samples from women with active or past placental infection, respectively, were positive for agglutination of CS2 (OR, 2.24; 95% CI, 0.8-6.3; P = .198). For PGs, 9 (56.3%) of 16 samples from infected women were positive, and 4 (25%) of 16 were positive among matched cleared infection samples (P = .149). For SG/MG, 12 (80%) of 15 women with active infection had antibodies, compared with 11 (73.3%) of 15 for cleared infection (P = .695). The prevalence of antibodies among women with past infection was higher than that among matched women without placental infection (60% vs. 22%; P < .01). Among PG, 4 of 12 past infected versus 1 of 14 uninfected women’s samples were positive. Among MG, 11 of 13 past infected versus 7 of 22 uninfected women’s samples were positive.
Similar results were obtained using flow cytometry to measure IgG binding to the surface of CS2 PRBCs (figure 1C and 1D). Among PG, IgG bound to CS2 at higher levels among samples from infected women (mean of MFI values, 116.4; range, 9.6-467) than individually matched PG without infection (mean, 20.1; range, 12.8-39.2; P < .001), and the proportion of positive samples was higher among infected (19/27 [70.4%]) than uninfected (1/27 [3.7%]) women (OR, 61.8; 95% CI, 7.11-536; P < .001). IgG binding of all MG samples and the proportion of samples positive were higher than uninfected PG or men (mean, 16.3; P < .001). There was no significant difference in IgG binding between infected (mean fluorescence [MFI], 118; range, 18-294; 83% of samples positive) and uninfected (MFI, 151; range, 22.4-501; 77% positive) MG. IgG binding was observed only with mature trophozoite PRBCs and not with ring-stage PRBCs (data not shown). MFI from uninfected RBCs in the assays was very low and varied only slightly among different samples. When this was accounted for by deducting the MFI of RBCs from that of PRBCs, there was no difference in the associations observed (data not shown).
We confirmed that the association between antibodies and gravidity is also present among women at midpregnancy and that similar results were obtained when placental isolates from Malawian women or CS2 were used (figure 1E). Of placental isolates, 31% of MG, 7% of PG, and 0% of men (P < .05) were positive, as reported elsewhere [8]. Similarly, with CS2 48% of MG, 12% of PG, and 11% of men were positive (P < .01). Testing additional samples, we confirmed that many MG (19/44) in midpregnancy without evidence of infection have antibodies to CSA-binding PRBCs, which were presumably acquired from exposure during prior pregnancies.
Using E8B PRBCs, there was no difference in the prevalence of agglutinating antibodies among women with active infection (20/34; 58.8%), past infection (13/22 [59.1%]), or uninfected placentas (24/44 [54.5%]) and men (18/28 [64.3%]; P = .879; figure 1F). In addition, there was no association between antibodies to E8B and gravidity (data not shown). Agglutination of E8B was observed among samples from pregnant women who did and did not agglutinate CS2; 26 (74.3%) of 35 samples that agglutinated CS2 also agglutinated E8B, and 26 (46.4%) of 56 samples that agglutinated E8B also agglutinated CS2. The difference in reactivity between CS2 and E8B may be attributed to different antigenic and adhesive phenotypes. The 2 isolates expressed different forms of the variant antigen PfEMP1 [21] and CS2 predominantly bound to CSA and HA, whereas E8B predominantly bound CD36 and ICAM-1 [10, 26] (data not shown).
Associations between placental infection and functional antibodies that inhibit parasite adhesion to CSA
The ability of samples to inhibit adhesion to CSA was highest among MG and infected PG, and there was little inhibition in men’s samples (figure 2A). The median adhesion score of CS2 PRBCs in the presence of samples from infected PG (40% of control; range, 0%-186%) was significantly lower than adhesion in the presence of corresponding matched uninfected samples (76%; range, 2%-146%; P = .0253). Among MG, there was little difference between infected and uninfected (median adhesion relative to control, 24%; range, 0%-158% vs. 21%; range, 0%-177%, respectively; P = .8182). Inhibition of adhesion by men’s samples (range of relative adhesion, 44%-135%; median, 81%) was significantly less than for MG or infected PG (P < .05). With some samples, adhesion to CSA was substantially higher (up to 179%) than the reference control, which suggests that there may be factors that enhance parasite adhesion to CSA in vitro; this was observed in all groups. The median inhibition of all pregnant women’s samples (n = 76) was 55%. Classifying samples that inhibited >55% as highly inhibitory, 15 (56%) of 27 samples from infected and 7 (26%) of 27 samples from uninfected PG were highly inhibitory (OR, 3.6; 95% CI, 1.13-11.3; P = .0525). Among MG, 10 (83%) of 12 infected and 8 (67%) of 12 uninfected women’s samples were highly inhibitory, whereas only 1 (6.3%) of 16 samples from men was highly inhibitory (P < 0.001 vs. MG). Among samples that tested positive by agglutination or IgG binding, there was no significant difference in inhibition of adhesion to CSA between PG and MG. Median adhesion was 8% and 6% for PG case patients and MGs that were agglutination positive, respectively, and 12% and 20% for PG case patients and MG that were IgG positive, respectively.
Figure 2.
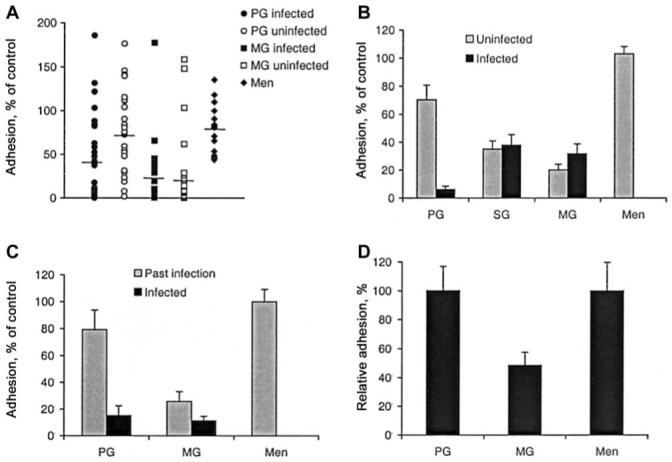
Associations between the inhibition of Plasmodium falciparum-infected red blood cell (PRBC) adhesion to chondroitin sulfate A (CSA) and gravidity and placental infection. A, The effect on adhesion of CS2 by samples from infected and uninfected primigravidae (PG) and multigravidae (MG) at term and men. Horizontal lines represent the median value; P < .05, infected vs. uninfected PG and MG or infected PG vs. men. B, Adhesion (mean + SE of 3 experiments) of CS2 in the presence of pooled samples from infected and uninfected PG, secundigravidae (SG), and MG at term or men; P < .01, infected vs. uninfected PG and SG or MG vs. men. C, Adhesion (mean ± SE of 3 experiments) of CS2 in the presence of pooled samples from PG and MG at term with active or past infection or men; P < .01, for the difference among PG and SG or MG vs. men. D, Adhesion (mean ± SE) of placental isolates in the presence of samples from smear-negative PG or MG at midpregnancy or men, tested individually; P < .05, for differences.
Associations between placental infection and adhesion inhibition were further evaluated using infection- and gravidity-stratified sample pools. Among pools of PG samples, adhesion was 7% ± 2% of control among infected samples and 68% ± 10% of control (P < .001) among uninfected samples (figure 2B). Among SG and MG pooled samples, inhibitory activity did not differ significantly between infected and uninfected groups but did inhibit to a greater extent than men and uninfected PG (P < .01; data not shown). Using pools of infected and past infection groups (figure 2C), PG with current infection inhibited adhesion significantly more than the past infection group (adhesion 15% ± 7% vs. 79% ± 15% of control, respectively; P < .01). The same trend was observed with MG pools; however, the difference was much less marked (adhesion 11% ± 3% for infected and 26% ± 7% for past infection; P < .073).
Using placental isolates, we confirmed that the association between antibodies and gravidity among women at midpregnancy and that similar gravidity associations are seen with placental isolates or CS2 (figure 2D). Using serum from MG, adhesion of placental isolates to CSA was 48.5% ± 9% (mean ± SE; range, 12.7%-98%) of adhesion observed with PG (100% ± 17%) and men (100% ± 20%; P < .05). We also observed that a proportion of MG during midpregnancy without evidence of infection effectively inhibited the adhesion of placental isolates and/or CS2 to CSA (data not shown). In adhesion inhibition assays, we found a high degree of correlation between repeat examination of samples in separate experiments (P < .001) and between duplicate samples within experiments (P < .001; data not shown).
Relationship among agglutinating antibodies, adhesion inhibition, and variant-specific IgG: separate and overlapping targets
Significant associations were observed between the different antibodies measured; however, comparisons highlight key differences between functional antibodies that inhibit adhesion and antibody binding to the PRBC surface (figure 3). Parasite adhesion among agglutinating samples was 22.4% ± 6.1% (mean ± SE; range, 0%-148% relative to control) and 76.2% ± 6.8% (range, 1%-177%) for nonagglutinating samples (P < .001; figure 3A). Of note, 6 (20%) of 30 samples that agglutinated CS2 were weakly or noninhibitory, and, of nonagglutinating samples, 17 (33.3%) of 51 were highly inhibitory (>55% inhibition), and 3 inhibited adhesion by >90%.
Figure 3.
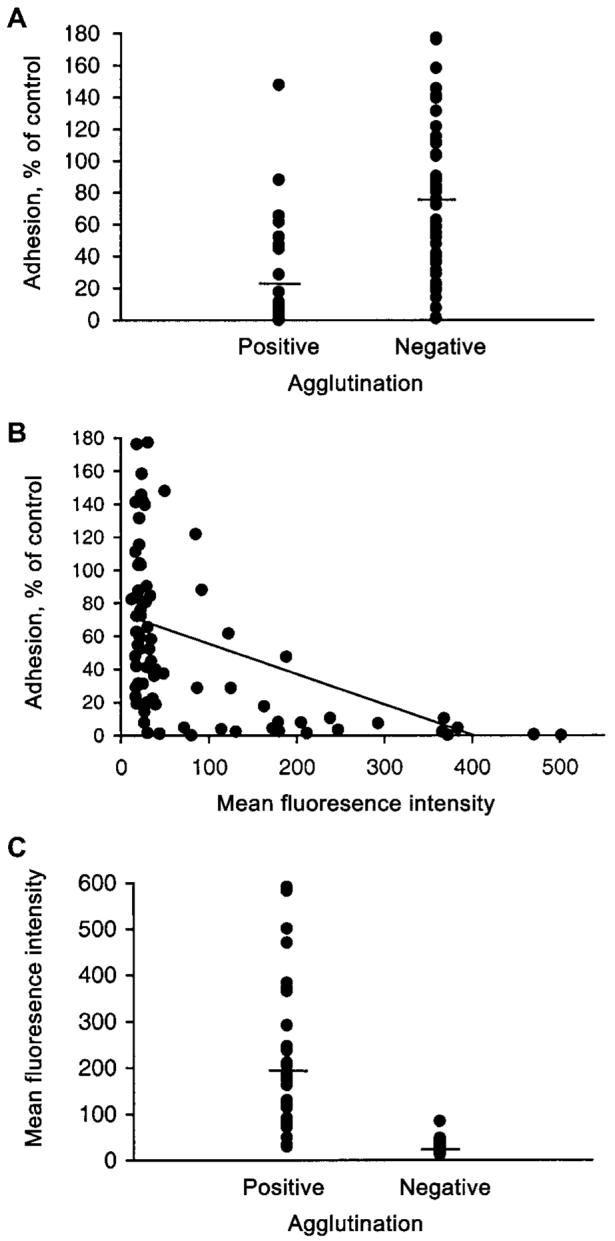
The relationship between different measures of antibodies to the surface of CS2 Plasmodium falciparum-infected red blood cells (PRBCs). A, The effect on adhesion to chondroitin sulfate A (CSA) by samples from pregnant women that were positive or negative in parasite agglutination assays; P < .001, for differences. B, The relationship between the effect on adhesion to CSA and IgG binding (fluorescence intensity by flow cytometry) among samples from pregnant women (r = 0.50; P < .001). C, Levels of IgG binding of individual samples from infected and uninfected pregnant women that were either positive or negative in parasite cell-agglutination assays; P < .001, for differences.
A significant correlation was observed between inhibition of adhesion to CSA and IgG labeling of PRBCs (r = 0.50; n = 80; P < .001; figure 3B). Most samples with high levels of fluorescence substantially inhibited adhesion to CSA. However, among samples with no detectable IgG, relative adhesion was 2%-177%, and, of those samples with detectable IgG binding, adhesion was 0%-148% that of control samples. Of note, several samples had no detectable agglutinating antibody or IgG to CS2 PRBCs but were highly inhibitory. Samples of this type formed 4 (14.8%) of 27 tested from uninfected PG, 1 (3.7%) of 27 from infected PG, 2 (14.3%) of 14 from uninfected MG, and 2 (16.7%) of 12 from infected MG, respectively. Of these, some agglutinated isolate E8B, and others did not. Although most samples with detectable IgG binding significantly inhibited adhesion to CSA, 12 (28.6%) of 42 IgG-positive samples were weakly or noninhibitory (7/19 [36.8%] IgG-positive PG samples and 4 [19%] of 21 IgG-positive MG samples had this property).
There was a strong association between IgG binding of CS2 PRBCs detected by flow cytometry and parasite-infected cell agglutination (figure 3C): 28 (96.6%) of 29 agglutination positive samples were regarded as positive by flow cytometry. The mean ± SE fluorescence for agglutination positive samples (198.9 ± 25.4; range, 23.9-501) was markedly higher than that for nonagglutinating samples (23.6 ± 1.4; range, 9.6-67.1; P < .001). There was also a significant correlation between level of fluorescence and agglutination score (using either the percentage of cells in agglutinates [r = 0.488; P = .011] or the size of agglutinates [r = 0.433; P = .027]). However, some samples with low levels of specific IgG detected did not induce cell agglutination; 11 (22.4%) of 49 nonagglutinating samples were considered positive by flow cytometry.
Antibodies are not associated with measures of pregnancy outcome or density of placental parasitemia
No significant associations were observed between agglutinating antibodies and birth weight, maternal hemoglobin, or donor age (table 2). The mean parasitemia of infected donors with agglutination positive samples was lower than for agglutination-negative samples, but the difference was not statistically significant (P = .695). No significant correlation was observed between adhesion inhibition and birth weight, maternal hemoglobin, donor age, or placental parasite density (determined by histological examination [figure 4A] or thick blood films), among all samples or separate gravidity or infection groups. Significant associations were not observed between the level of specific IgG to CS2 PRBCs measured by flow cytometry and birth weight, hemoglobin, or placental parasitemia (determined by histological testing [figure 4B] or thick blood films). Among infected PGs, specific IgG was weakly and inversely associated with age (borderline statistical significance, r = −0.357, n = 26; P = .0734). HIV infection was associated with significantly lower binding of IgG to CS2 PRBCs (P < .05), among PGs and MGs and a significantly lower prevalence of agglutinating antibodies (P < .05) that were not explained by differences in age. There was also a trend (not statistically significant) toward lower adhesion-inhibitory activity among HIV infected women. Associations with HIV are presently being further investigated. The prevalence of HIV was not significantly different between women with or without infection and did not explain the differences observed in antibodies between infected and uninfected women or women of different parities. Of note, no antibody type was universally present among any group of women. In all groups, there was substantial heterogeneity in the nature and levels of antibodies (figures 1, 2A, and 3B).
Table 2. Associations between antibodies and clinical parameters among pregnant women.
| Clinical parameter | No. | Parasitemia | Birth weight | Hemoglobin | Maternal age |
|---|---|---|---|---|---|
| Correlations with adhesion inhibitiona | |||||
| All cases | 39 | 0.076 | −0.127 | −0.174 | 0.0821 |
| PG cases | 27 | 0.033 | −0.172 | −0.259 | −0.013 |
| All MG | 25 | NA | 0.288 | 0.083 | 0.166 |
| PG and MGb | 52 | NA | −0.132 | −0.074 | −0.026 |
| Correlations with IgG bindinga | |||||
| All cases | 43 | −0.151 | 0.130 | −0.201 | −0.106 |
| PG cases | 30 | −0.095 | 0.166 | −0.234 | −0.337c |
| All MG | 27 | NA | 0.107 | 0.090 | −0.204 |
| PG and MGb | 57 | NA | 0.152 | −0.091 | −0.133 |
| Agglutinating antibodiesd | |||||
| Positive | 36 | 9.8% | 2826 g | 10.9 g/dL | 20.9 years |
| Negative | 23 | 11.3% | 2576 | 11.2 g/dL | 21.2 years |
NOTE. CS2 Plasmodium falciparum-infected red blood cells (PRBCs) were used for all assays. MG, multigravida; NA, not applicable; PG, primigravida.
Data are correlation coefficients.
Does not include uninfected PG who had a very low prevalence of antibodies.
Borderline statistical significance (P =.069). All other correlations and associations were not statistically significant (P> .1).
Data are means.
Figure 4.
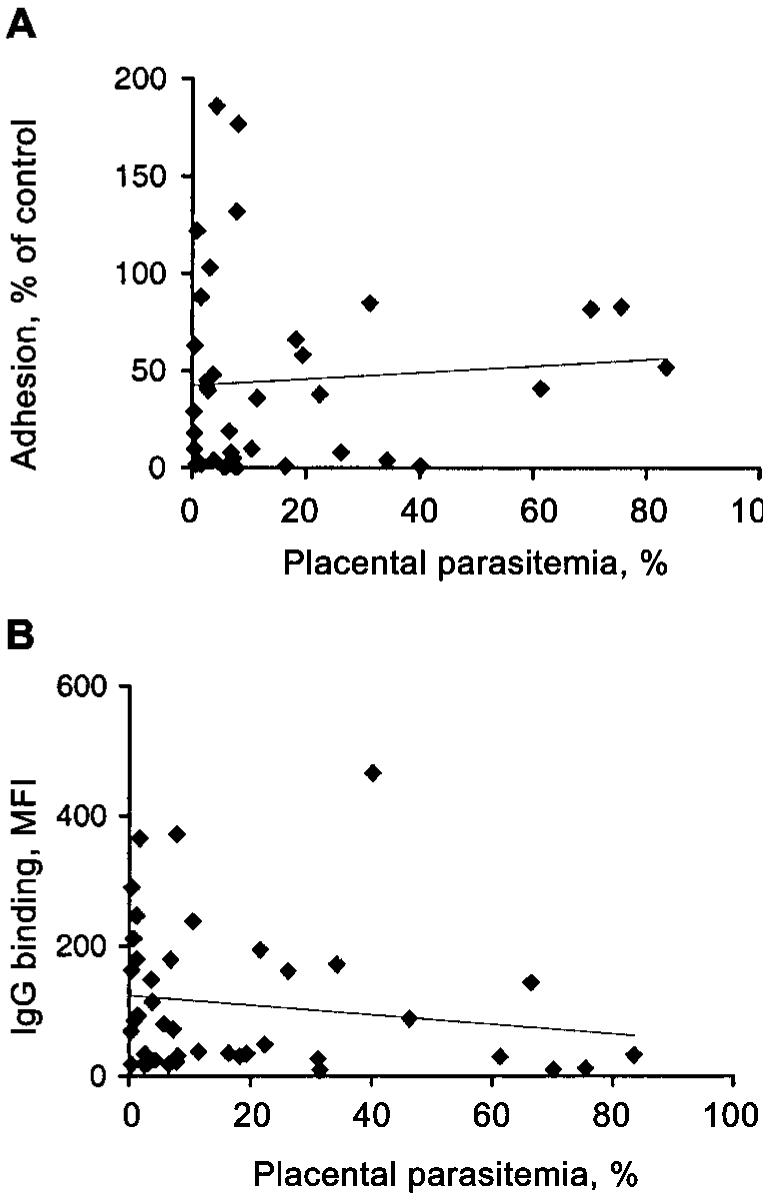
Relationship between antibodies to CS2 Plasmodium falciparum-infected red blood cells (PRBCs) and placental parasitemia. A, Relationship between level of adhesion of CS2 PRBCs to chondroitin sulfate A (CSA) in the presence of samples obtained from women at delivery and the placental parasitemia of each donor (P = .652). B, Relationship between IgG binding (mean fluorescence intensity [MFI] by flow cytometry) to CS2 PRBCs in samples obtained from women at delivery and the placental parasitemia of the donor (P = .33). Placental parasitemias were determined by histological examination.
DISCUSSION
Antibodies to CSA-binding PRBCs among PG were strongly associated with placental infection, being commonly detected at high levels in women with active or past placental infection but rarely among PG with no evidence of current or past infection or among men. After exposure to placental infection, women appear to acquire antibodies to VSA of PRBCs and adhesion-inhibitory antibodies, and similar associations with infection and gravidity were seen with each antibody type. This is consistent with the hypothesis that pregnant women develop placental infection with parasite variants able to sequester in the placenta and evade existing variant-specific antibodies [8, 12-14]. Prior reports have differed in the associations between infection and antibodies, with one reporting some association between agglutinating antibodies and the presence of parasites or pigment on placental blood smears [13] and others reporting either an inverse association with infection among SG [12] or no association among PG or MG [16] using adhesion-inhibitory antibody measurements. Our results clarify that the association between PRBC surface antibodies or adhesion-inhibitory antibodies and gravidity is only clearly seen among uninfected women.
To elucidate associations between placental infection or gravidity and specific antibodies we used a case-control design, individually matching women with active, past, or no placental infection, to reduce the influence of the major confounding factors—age, gravidity, and season of delivery—that are associated with malaria risk in this population [2]. Associations with infection and gravidity could not be explained by HIV coinfection, which is known to influence susceptibility to maternal malaria [3]. Placental infection, as an entity of malaria in pregnancy, was rigorously diagnosed by placental histological examination and blood smears, to maximize specificity when selecting cases and controls. Among women with negative placental blood smears, placental histological testing can identify those with recent placental infection [23, 30, 31]. Additionally, placental infection is sometimes observed histologically in women with negative peripheral and placental blood smears; rarely, women without evidence of infection by histological examination of the placenta have detectable peripheral parasitemia [30, 32], which emphasizes the need for strict definitions of infection.
A high proportion of PG with active or past placental infection had adhesion-inhibitory or PRBC-surface antibodies, and the adhesion-inhibitory activity of samples positive for PRBC surface antibodies (by agglutination or flow cytometry) was not different to MG. This suggests that placental infection in one pregnancy is sufficient to induce a substantial antibody response to CSA-binding PRBCs. In subsequent pregnancies, antibody levels may be boosted or the repertoire of specific antibodies diversified, or the balance of Ig subtypes may change, given that adhesion inhibition and IgG binding by MG was somewhat higher than infected PG. Other findings suggest the acquisition of antibodies is more rapid in MG than PG [16]. Consistent with our findings, antibodies to novel infections in childhood appear to be acquired rapidly [33, 34], and antibodies to heterologous isolates are more common in chronic and asymptomatic infections [35], which has implications for longitudinal studies of immunity. Antibodies may wane somewhat after delivery or the resolution of infection; antibodies were slightly lower among women with past versus active infection, which is consistent with prior reports of slightly lower antibody levels among women 6 months after delivery [13, 15]. Furthermore, many MG without evidence of malaria at midpregnancy had specific antibodies, which suggests these had been acquired in an earlier pregnancy and persisted.
Our study highlights similarities and key differences in agglutinating antibody, adhesion inhibition, and specific IgG, which suggests separate and overlapping epitopes of different antibodies. Adhesion-inhibitory and agglutinating antibodies or specific IgG may be acquired independently during infection and have different roles in immunity. There was a significant association between CSA-adhesion-inhibitory antibodies and specific IgG or agglutination, but many samples that tested negative or weakly positive by flow cytometry and agglutination were strongly inhibitory, which may reflect the presence of low levels of highly specific antibodies. Some samples that agglutinated CS2 or had specific IgG were not inhibitory. These findings suggest that adhesion inhibition is dependent on the quality, and not just the quantity, of antibodies. Understanding this relationship will be important for evaluating the dynamics of immunity to placental malaria and the development of future vaccines. Agglutination and flow cytometry probably measure antibodies to a range of targets on the PRBC surface (predominantly PfEMP1), whereas adhesion inhibition likely measures a specific subset targeting CSA-binding sites or sites that interfere with adhesion. The binding of antibody may also induce conformational changes in proteins that influence receptor binding activity. It is possible that differences in the conditions under which the assays were performed may have contributed to different results using the same samples.
Flow cytometry and agglutination assays appear to predominantly measure the same antibodies, with flow cytometry generally being more sensitive. The results also serves to establish that agglutination assays are practically useful to measure immune responses to placental malaria, which has implications for resource-limited settings typical of countries where malaria is endemic, where flow cytometry is often unavailable. IgM was not measured by flow cytometry but may be an important component of the agglutinating activity with some samples. Others have demonstrated that samples strongly positive by agglutination [13] or flow cytometry [14] more effectively inhibit adhesion than samples without detectable antibodies. However, bringing results from all measures of antibodies together here in an extended analysis has revealed a general relationship and highlighted important differences. IgG-binding and agglutination are reasonable surrogates of adhesion-inhibitory antibodies, but they should not assumed to be identical.
The strong association between antibodies to CSA-binding parasites and gravidity and infection was in contrast to the lack of associations when using a genetically identical isolate selected for adhesion to endothelial cells that bound CD36 and ICAM-1. Antibodies to E8B, which represent an endothelial binding-type isolate, were apparently not boosted by placental infection, which may indicate that placental infection does not boost antibody levels to other isolates not associated with maternal infection. E8B and CS2 expresses different forms of PfEMP1 [21], a target of agglutinating antibodies [19] and the parasite ligand for CSA [21]. In this population, the prevalence of agglutinating antibodies to children’s isolates among the same pregnant women was similar to men [8].
In all groups of women exposed to placental malaria, there was a broad range in the levels and nature of antibodies. Any particular antibody type was not universally observed in any group. This may reflect antigenic diversity of parasite populations in placental malaria stimulating different responses in different individuals and the variant-specific nature of antibodies or a lack of appropriate responses in some women. It is possible that antibodies were present among some women at levels below the limit of detection of the assay rather than being truly absent. The high prevalence of antibodies among infected women to a single isolate suggests that CSA-binding variants may be antigenically restricted, suggested by cross-inhibitory effects of serum [12, 36] and sequence conservation between var genes expressed by placental isolates [37, 38]. However, CSA-binding parasites may not always be the predominant phenotype in placental infection [8]. In addition, placental parasite phenotypes may change over time; we previously noted that a greater proportion of peripheral blood isolates from women at midpregnancy bound to CSA than those collected at term [8]. Other potential mechanisms of placental infection include parasite binding to HA and other receptors, binding nonimmune Ig, and clumping of PRBCs [10, 11, 26, 39].
Antibodies that develop to CSA-binding PRBCs in placental infection may contribute to clearance of parasitemia and preventing future infection by opsonizing PRBCs and inhibiting placental parasite adhesion. Antibodies to variant surface antigens have previously been associated with protection from infection among children [18, 40, 41]. The high prevalence of antibodies among uninfected MG may have contributed to protection from infection and is associated with a reduced, but not absent, risk of malaria in this population [2]. In one study, adhesion inhibition was reportedly greater among SG without placental infection than those with infection and adhesion-inhibitory antibodies were generally absent from PG with or without infection [12]. These findings were not replicated here or elsewhere [16], which may reflect differences in the populations, level of malaria transmission and/or in parasites properties. Some samples had an apparent enhancing effect on adhesion to CSA in vitro, which may warrant further investigation. In our study, adhesion-inhibitory or PRBC surface antibodies were not associated or correlated with parasite density, birth weight, or maternal hemoglobin. However, the number of samples may have been insufficient to detect these associations. An inverse correlation was found between inhibition of adhesion to CSA and placental parasite density in Cameroon [16], although this was not corrected for gravidity. Others reported an inverse correlation between IgG binding and parasitemia among some MG but not among PG [15].
In conclusion, knowledge of the development and nature of specific antibodies acquired after placental infection is fundamental to understanding the immunology and pathogenesis of malaria in pregnancy, a major cause of maternal and infant mortality. The findings presented here will help guide future studies needed to identify the role of specific antibodies in protection from placental malaria and the possible development of preventative or therapeutic interventions.
Acknowledgments
We thank Ebby Chalaluka, Labes Njiragoma, Maxwell Kanjala, and Patrick Mkundika, for assistance with sample collection and processing in Blantyre; Tim Byrne and Aphro Caragounis, for laboratory support in Melbourne; the staff of the Antenatal Clinic and Delivery Suite of the Queen Elizabeth Central Hospital, Blantyre; Terrie Taylor, for helpful discussions; and all individuals who participated in the study. Human erythrocytes and serum for in vitro culture were kindly provided by the Australian Red Cross Blood Service, Melbourne, Australia.
Financial support: National Health and Medical Research Council of Australia (grants 145677 and 215201 and Travel Award for Research Training to J.G.B.); The Wellcome Trust, UK (Career Development [046012] and Senior Research [063215] Fellowships to S.J.R.); the Royal Australasian College of Physicians (Cottrell Fellowship to J.G.B); ANZ Charitable Trust.
Footnotes
Presented in part: American Society of Tropical Medicine and Hygiene annual meetings, Atlanta, 11-15 November 2001 (abstract 289), and Denver, 10-14 November 2002 (abstract 490).
References
- 1.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull WHO. 1983;61:1005–16. [PMC free article] [PubMed] [Google Scholar]
- 2.Rogerson SJ, van den Broek NR, Chaluluka E, Qonqwane C, Mhango CG, Molyneux ME. Malaria and anemia in antenatal women in Blantyre, Malawi: a twelve month survey. Am J Trop Med Hyg. 2000;62:335–40. doi: 10.4269/ajtmh.2000.62.335. [DOI] [PubMed] [Google Scholar]
- 3.Steketee RW, Wirima JJ, Bloland PB, et al. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55:42–9. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 4.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria: a histologic and ultrastructural study. Am J Pathol. 1982;109:330–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Beeson JG, Amin N, Kanjala M, Rogerson SJ. Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect Immun. 2002;70:5412–5. doi: 10.1128/IAI.70.10.5412-5415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 7.Maubert B, Guilbert LJ, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun. 1997;65:1251–7. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J Infect Dis. 1999;180:464–72. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gysin J, Pouvelle B, Fievet N, Scherf A, Lepolard C. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect Immun. 1999;67:6596–602. doi: 10.1128/iai.67.12.6596-6602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beeson JG, Rogerson SJ, Cooke BM, et al. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat Med. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flick K, Scholander C, Chen Q, et al. Role of nonimmune IgG bound to PfEMP1 in placental malaria. Science. 2001;293:2098–100. doi: 10.1126/science.1062891. [DOI] [PubMed] [Google Scholar]
- 12.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–2. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 13.Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect Immun. 1999;67:5367–71. doi: 10.1128/iai.67.10.5367-5371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricke CH, Staalsoe T, Koram K, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–16. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 15.Staalsoe T, Megnekou R, Fievet N, et al. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis. 2001;184:618–26. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 16.O’Neil-Dunne I, Achur RN, Agbor-Enoh ST, et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun. 2001;69:7487–92. doi: 10.1128/IAI.69.12.7487-7492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh K, Howard RJ. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–3. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 18.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–60. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biggs BA, Goozé L, Wycherley K, et al. Antigenic variation in Plasmodium falciparum. Proc Natl Acad Sci USA. 1991;88:9171–4. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newbold CI, Pinches R, Roberts DJ, Marsh K. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol. 1992;75:281–92. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 21.Reeder JC, Cowman AF, Davern KM, et al. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by PfEMP1. Proc Natl Acad Sci USA. 1999;96:5198–202. doi: 10.1073/pnas.96.9.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buffet PA, Gamain B, Scheidig C, et al. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc Natl Acad Sci USA. 1999;96:12743–8. doi: 10.1073/pnas.96.22.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–9. [PubMed] [Google Scholar]
- 24.Biggs BA, Anders RF, Dillon HE, et al. Adherence of infected erythrocytes to venular endothelium selects for antigenic variants of Plasmodium falciparum. J Immunol. 1992;149:2047–54. [PubMed] [Google Scholar]
- 25.Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeson JG, Brown GV. Plasmodium falciparum-infected erythrocytes demonstrate dual specificity for adhesion to hyaluronic acid and chondroitin sulfate A and have distinct adhesive properties. J Infect Dis. 2004;189:169–79. doi: 10.1086/380975. [DOI] [PubMed] [Google Scholar]
- 27.Beeson JG, Rogerson SJ, Brown GV. Evaluating specific adhesion of Plasmodium falciparum-infected erythrocytes to immobilised hyaluronic acid with comparison to binding of mammalian cells. Int J Parasitol. 2002;32:1245–52. doi: 10.1016/s0020-7519(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 28.Beeson JG, Chai W, Rogerson SJ, Lawson AM, Brown GV. Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A. Infect Immun. 1998;66:3397–3402. doi: 10.1128/iai.66.7.3397-3402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann EJ, Rogerson SJ, Beeson JG. An alternative agglutination assay to measure antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes. Trans R Soc Trop Med Hyg. doi: 10.1016/s0035-9203(03)80111-6. in press. [DOI] [PubMed] [Google Scholar]
- 30.Shulman CE, Marshall T, Dorman EK, et al. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health. 2001;6:770–8. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 31.Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. Placental malaria: I. Pathological classification. Histopathology. 1993;22:211–8. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 32.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: a comparison of blood film preparation methods, and of blood films with histology. J Clin Microbiol. 2003;41:1370–4. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ofori MF, Dodoo D, Staalsoe T, et al. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect Immun. 2002;70:2982–8. doi: 10.1128/IAI.70.6.2982-2988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinyanjui SM, Bull P, Newbold CI, Marsh K. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J Infect Dis. 2003;187:667–74. doi: 10.1086/373994. [DOI] [PubMed] [Google Scholar]
- 35.Bull PC, Lowe BS, Kaleli N, et al. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J Infect Dis. 2002;185:1688–91. doi: 10.1086/340420. [DOI] [PubMed] [Google Scholar]
- 36.Lekana Douki J-B, Traore B, Costa FTM, et al. Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood. 2002;100:1478–83. doi: 10.1182/blood-2002-01-0315. [DOI] [PubMed] [Google Scholar]
- 37.Khattab A, Kun J, Deloron P, Kremsner PG, Klinkert MQ. Variants of Plasmodium falciparum erythrocyte membrane protein 1 expressed by different placental parasites are closely related and adhere to chondroitin sulfate A. J Infect Dis. 2001;183:1165–9. doi: 10.1086/319288. [DOI] [PubMed] [Google Scholar]
- 38.Salanti A, Staalsoe T, Lavstsen T, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–91. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 39.Beeson JG, Reeder JC, Rogerson SJ, Brown GV. Parasite adhesion and immune evasion in placental malaria. Trends Parasitol. 2001;17:331–7. doi: 10.1016/s1471-4922(01)01917-1. [DOI] [PubMed] [Google Scholar]
- 40.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 41.Dodoo D, Staalsoe T, Giha H, et al. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect Immun. 2001;69:3713–8. doi: 10.1128/IAI.69.6.3713-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


