Abstract
Infection with Plasmodium falciparum during pregnancy leads to the accumulation of parasite-infected erythrocytes in the placenta1, and is associated with excess perinatal mortality, premature delivery and intrauterine growth retardation in the infant, as well as increased maternal mortality and morbidity2,3. P. falciparum can adhere to specific receptors on host cells, an important virulence factor enabling parasites to accumulate in various organs4. We report here that most P. falciparum Bisolates from infected placentae can bind to hyaluronic acid, a newly discovered receptor for parasite adhesion that is present on the placental lining. In laboratory isolates selected for specific high-level adhesion, binding to hyaluronic acid could be inhibited by dodecamer or larger oligosaccharide fragments or polysaccharides, treatment of immobilized receptor with hyaluronidase, or treatment of infected erythrocytes with trypsin. In vitro flow-based assays demonstrated that high levels of adhesion occurred at low wall shear stress, conditions thought to prevail in the placenta. Our findings indicate that adhesion to hyaluronic acid is involved in mediating placental parasite accumulation, thus changing the present understanding of the mechanisms of placental infection, with implications for the development of therapeutic and preventative interventions.
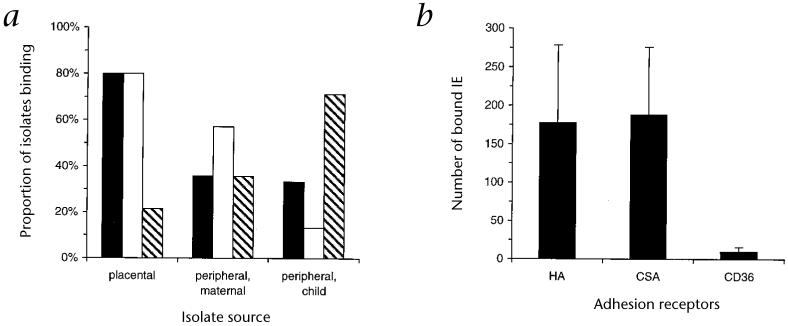
Syncytiotrophoblasts lining the intervillous spaces constitute an extensive area of fetal tissue in contact with the maternal circulation. Hyaluronic acid (or hyaluronan; HA) is prominent on the surface of this layer and may help protect against graft rejection, in combination with other mechanisms, by masking fetal antigens from the maternal immune system5-7. HA is a high-molecular-weight (up to several million daltons) glycosaminoglycan, consisting of repeating disaccharide units formed by N-acetylglucosamine and glucuronic acid, and differs from other glycosaminoglycans by its lack sulfation8. Adhesion to chondroitin sulfate A (CSA), which is also present on the placental lining, may be involved in placental infection9,10. We tested placental isolates directly for adhesion to immobilized, purified HA (Table 1), and compared these results with the adhesive characteristics of isolates from the peripheral blood of pregnant women and children (Fig. 1a). Twelve of fifteen (80%) placental isolates demonstrated substantial adhesion to HA, whereas only five of fourteen (36%) peripheral maternal isolates, and five of fifteen (33%) peripheral isolates from children did so. The level of binding of placental isolates to HA was much higher than to CD36 (178 ± 403 and 9.9 ± 23.5 infected erythrocytes (IEs) bound/mm2, respectively (mean ± s.d.); P < 0.01, Mann-Whitney U test; Fig. 1b) and was similar to binding to CSA (188 ± 351 IEs bound/mm2).
Table 1.
Adhesion of placental and laboratory P. falciparum isolates to purified receptors
| Adhesion Receptors | ||||
|---|---|---|---|---|
| HA | CSA | CD36 | ICAM-1 | |
| Placental isolates | ||||
| A | 53 | 0 | 0 | NA |
| B | 1 | 13 | 0 | NA |
| C | 23 | 933 | 0 | 0 |
| D | 11 | 59 | 0 | 0 |
| E | 103 | 386 | NA | NA |
| F | 38 | 31 | 25 | 0 |
| G | 1,254 | 17 | 18 | 0 |
| H | 3 | 0 | 1 | 0 |
| I | 33 | 13 | 0 | 0 |
| J | 1,075 | 64 | 1 | 0 |
| K | 13 | 140 | 0 | 0 |
| L | 34 | 1,100 | 87 | 0 |
| M | 10 | 19 | 4 | 1 |
| N | 0 | 40 | 1 | 1 |
| O | 11 | 6 | 2 | 0 |
| Laboratory isolates | ||||
| CS2 | 1,475 | 2,146 | 17 | 15 |
| E8B | 12 | 29 | 538 | 330 |
| E8B-HAsel3 | 1,527 | 1,963 | 12 | 1 |
| 3D7 | 30 | 35 | 663 | 1 |
| 3D7-HAsel3 | 817 | 518 | 72 | 0 |
Data represent mean number of bound IEs/mm2 above that recorded for bovine serum albumin controls (mean of triplicate spots; s.d. excluded for clarity). NA, not assessed.
Fig. 1.
Receptor-specific adhesion of P. falciparum isolates. a, The proportion of isolates collected from the placenta (n = 15), peripheral blood of pregnant women (maternal; n = 14) and peripheral blood of children (n = 15) that bound to immobilized purified HA (■), CSA (□) and/or CD36 (hatched bars) at a level of ≥ 10 IEs bound/mm2. b, The average density of adhesion of placental isolates to immobilized HA, CSA or CD36. Data represent mean (± s.e.m.) number of bound IEs per mm2.
Only three of fourteen (21.4%) placental isolates bound in substantial amounts to CD36 (Fig. 1a), which has not been detected on syncytiotrophoblasts, and these did so only at low levels. However, most isolates (ten of fourteen; 71%) from children bound CD36 at much higher levels than binding of the same isolates to HA (786 ± 1727 and 23.4 ± 43.6 IEs bound/mm2, respectively; P < 0.01, Mann-Whitney U test). As has been reported, no isolates from pregnant women bound intracellular adhesion molecule (ICAM)-1 (ref. 10), and there was little or no adhesion to heparan sulfate or heparin (data not shown). Most, but not all, placental isolates showed some binding to both HA and CSA, and the degree of adhesion to the two receptors differed considerably with many isolates (such as isolates A, C, G, J, K and L; Table 1), indicating separate specificities of isolates for each of HA and CSA. Among peripheral maternal isolates, both the proportion of isolates that bound CSA (eight of fourteen; 57%) and the mean level of binding of IEs to CSA (437.2 ± 837.9 IEs bound/mm2) were higher than with HA (five of fourteen (36%); mean binding, 20.1 ± 31.2), and several isolates bound at high levels to CSA with little or no adhesion to HA.
The P. falciparum isolate CS2 (ref. 11), propagated by in vitro culture, bound to immobilized HA at high levels (Table 1) in addition to CSA, but there was little or no adhesion to CD36 or ICAM-1. As for the clinical samples, adhesion to HA was isolate-specific and not a feature of all IEs. Parasite isolates E8B, the parent of CS2, and 3D7 bound only at low levels to immobilized HA (Table 1). E8B and 3D7 were separately passaged through three cycles of selection for adhesion to HA, thereby modeling a process thought to occur in vivo, to yield E8B-HAsel3 and 3D7-HAsel3 (Table 1). For each, adhesion to both HA and CSA increased considerably, indicating these isolates co-express ligands for the two receptors, with a corresponding reduction in adhesion to CD36 and ICAM-1 (Table 1).
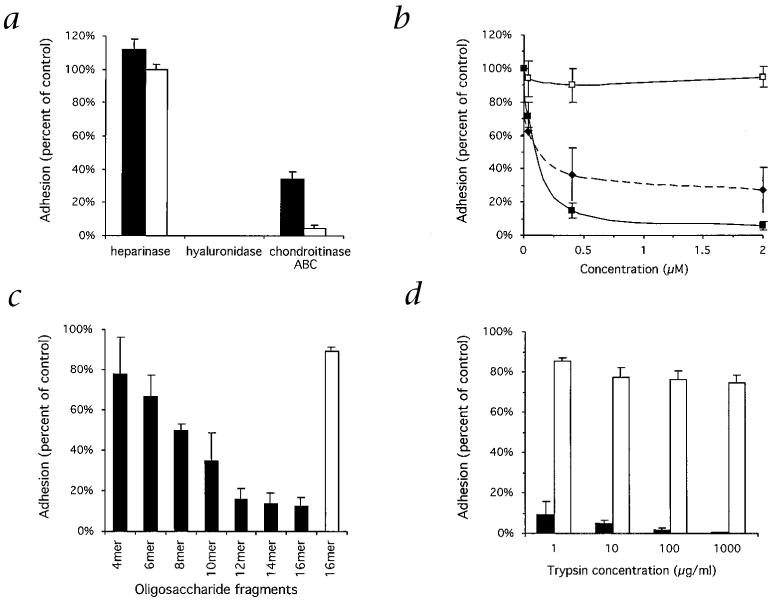
Confirming the specificity of the interaction with HA, there was no adhesion of the isolates to heparin/heparan sulfate or colominic acid (a sialic acid polymer that, like HA, carries charge through carboxylic acid groups and not sulfate groups), nor was there adhesion to a variety of other purified glycosaminoglycans and polysulfated sugars. Adhesion was maximal at physiologic pH of 7.2-7.6, and there was no adhesion of uninfected erythrocytes. Treatment of immobilized HA with hyaluronidase (which also digests chondroitin sulfates) before assessment of IE adhesion completely abolished binding, whereas there was no effect with heparinase (Fig. 2a). We used chondroitinase ABC (which digests chondroitin sulfates and, at a slower rate, HA) to treat immobilized receptors, to demonstrate a selective reduction of adhesion to immobilized CSA compared with adhesion to HA (Fig. 2a), supporting a specific interaction of IEs with HA.
Fig. 2.
Adhesion of P. falciparum-infected erythrocytes to HA and CSA. a, Adhesion of IEs is abolished by pre-treatment of immobilized HA (■) and CSA (□) with testicular hyaluronidase but not heparinase. Treatment with chondroitinase ABC reduces adhesion to CSA to a much greater degree than to HA. b, Soluble HA (■) but not colominic acid (□) added to IE suspensions before the adhesion assays competitively inhibits binding to immobilized HA in a concentration-dependent manner. There is some inhibition with soluble CSA (◆). c, Adhesion of IEs to immobilized HA (■) is effectively inhibited by oligosaccharide fragments of HA of dodecamer (12-mer) size or larger. There is no substantial inhibition of adhesion to CSA (□) (only the results for the hexadecamer (16-mer) fragments are shown). d, Surface proteins on intact IEs were cleaved with trypsin before adhesion to each of HA and CSA was tested. Trypsin treatment abolishes adhesion to immobilized HA (■) but not to CSA (□). All data represent the proportion of bound IEs expressed as a percentage of control (mean ± s.e.m. for multiple experiments).
Adhesion of CS2 IEs to immobilized HA was inhibited in a concentration-dependent manner by soluble HA (Fig. 2b); at a concentration of 0.4 μM, inhibition was 85.1 ± 1.9% (mean ± s.e.m.). There was no substantial inhibition of adhesion to HA with 0.4 μM soluble colominic acid (10 ± 10.5% inhibition), but there was some inhibition with 0.4 μM CSA and 0.4 μM chondroitin sulfate C (63.5 ± 6.3% and 27.1 ± 8.9%, respectively), which are sulfated and more highly charged than HA, a factor that may contribute to nonspecific inhibitory effects. To determine the chain length of HA required for interaction with IEs, we tested oligosaccharide fragments of HA ranging from four to sixteen monosaccharide units in length as competitive inhibitors of CS2 IE adhesion. The minimum length of fragment causing substantial inhibition of adhesion to HA was a dodecamer (12 monosaccharide units; Fig 2c), providing compelling evidence that the interaction of IEs with HA is not due to nonspecific charge effects, but to a specific receptor-ligand interaction. Adhesion to CSA was not inhibited substantially by the HA oligosaccharide fragments.
The variability in the level of adhesion to HA and CSA of the clinical isolates, which may consist of mixed parasite populations, indicates that adhesion is mediated by separate parasite ligands or binding sites. The adhesion of the HA-selected laboratory isolates indicates IEs can co-express ligands for HA and CSA, represented either by separate adhesive molecules or by different sites on the one molecule. However, this does not always occur in vivo, as many clinical isolates were found to bind to one receptor with little or no adhesion to the other. The presence of separate binding sites for HA and CSA is supported by additional experimental findings. Cleavage of CS2 IE surface proteins by treatment with 1 μg/ml trypsin reduced binding to HA by more than 90% (Fig. 2d), whereas adhesion to CSA was not substantially affected, even at a concentration 1,000-fold higher, as has been reported11. The different effects of trypsin on adhesion of IEs has been found with isolates that bind both CD36 and ICAM-1, activities thought to be mediated by different domains of the same adhesive molecule12. Furthermore, although there was some cross-inhibitory effect on binding to the two receptors using HA and CSA polysaccharides, this effect was clarified by the finding that HA oligosaccharide fragments effectively inhibited adhesion to immobilized HA with little or no effect on binding to CSA (Fig. 2c).
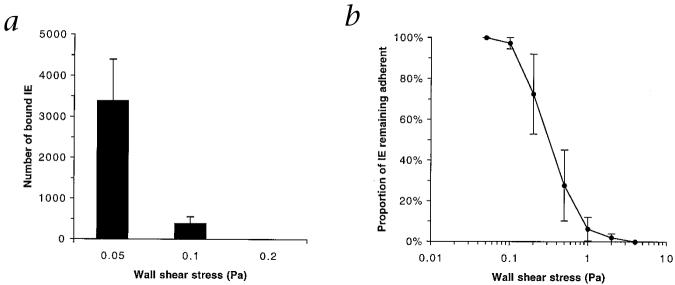
Results from studies using flow-based assays, modeling conditions that exist in vivo13, support a role for adhesion to HA in the placenta, and validate the results from static adhesion assays. High levels of adhesion from flow (Fig. 3a) occurred only at low wall shear stress (3,387 ± 1,006 IEs bound/mm2 per 1 × 107 IEs perfused, at 0.05 Pa), such as would be present in the placenta, where blood flow velocity is much reduced compared with other vascular beds14. At 0.1 Pa, the lower limit of shear stresses predicted to exist in the microvasculature of organs other than the placenta15, adhesion was greatly reduced, on average by 89.4 ± 2.4%. There was no binding at a wall shear stress of 0.2 Pa. Most (>90%) of erythrocytes that adhered were ‘parasitized’, and remained stationary, rather than rolling. The stress required to detach 50% of adherent cells was 0.38 ± 0.13 Pa (mean ± s.e.m.).
Fig. 3.
P. falciparum-infected erythrocytes adhere to immobilized HA under flow conditions. a, Adhesion of IEs from flow at increasing wall shear stresses. Data represent number of bound IEs per mm2 per 1 × 107 IEs perfused. b, The proportion of IEs remaining adherent at increasing levels of wall shear stress (expressed as a percentage of IEs initially adherent at 0.05 Pa). All data represent mean ± s.e.m. for multiple experiments.
Even at stresses higher than the range predicted for capillaries and post-capillary venules, a proportion of IEs resisted detachment (Fig. 3b) indicating that binding of substantial numbers of IEs can be maintained, provided conditions favor initial attachment. A particular subset of IEs that bind with lower affinity may preferentially adhere in the placenta rather than other vascular beds in which wall shear stresses are likely to be higher.
The identification of HA as a receptor for adhesion of P. falciparum-infected erythrocytes from the placenta has considerable implications for the understanding of adverse events at the fetal-maternal interface, indicating that parasite sequestration may subvert the proposed fetal-protective role of HA on syncytiotrophoblasts. The results challenge the existing paradigm that sequestration in the placenta is mediated solely by adhesion to CSA, as has been proposed in a report showing that all placental isolates demonstrate some binding to CSA (ref. 9). Instead, our findings indicate that adhesion to HA and CSA is involved in the process leading to the accumulation of IEs in the placenta. An ability to express different ligands for adhesion in the placenta would convey a survival advantage for P. falciparum by increasing the likelihood of sequestration and thus avoiding splenic clearance from the circulation, even in the face of possible host responses targeting one adhesive interaction. In the case of IEs with multiple receptor specificities, HA may be the receptor of initial attachment of IEs, as it has a much higher molecular weight than CSA (approximately 50 kDa) or its proteoglycan thrombomodulin, and may therefore extend further from the surface of the syncytiotrophoblast layer. Furthermore, the presence of at least two receptors for parasite adhesion may augment sequestration through synergy, as has been demonstrated with co-expression of CD36 and ICAM-1 on endothelial cells16, or allow sequestration where only one receptor is present. Such factors may favor the selection of particular parasite variants able to bind both receptors. Elements other than adhesion, such as placental immune function17, may influence susceptibility to infection with P. falciparum, but parasite adhesion is an essential determinant that could be addressed therapeutically. Approaches to the possible development of new adhesion-blocking therapies or vaccines may need to target both adhesive interactions to be effective.
Methods
P. falciparum culture and collection
P. falciparum was cultured in vitro as described10. Parasite line E8B (FAF-EA8) was derived from a clone of Brazilian P. falciparum isolate ItG2F6 as described18, and CS2 was generated by selection for adhesion to Chinese hamster ovary cells five times, followed by two cycles of selection to immobilized purified CSA (ref. 11). Isolate 3D7 was originally provided by D. Walliker. Clinical P. falciparum isolates were obtained from pregnant women attending the Labour Ward or Antenatal Clinic of the Queen Elizabeth Central Hospital, Blantyre, Malawi, and from children who either presented to outpatient clinics with uncomplicated malaria or were admitted with severe malaria at the same hospital. Informed consent was obtained in all cases. Peripheral blood was collected in tubes containing lithium heparin anticoagulant, centrifuged to remove plasma and buffy coat, washed three times in phosphate-buffered saline(PBS), pH 7.4, then cultured in vitro. P. falciparum-infected erythrocytes were collected from freshly delivered infected placentae by gentle washing of tissue sections in PBS with 50 mM EDTA on a tube roller, as described10.
All aspects of the study received ethical approval from the College of Medicine Research Committee of The University of Malawi, and the Ethics Committee of The Walter and Eliza Hall Institute of Medical Research.
Cytoadherence assays
The following chemicals were used in cytoadherence assays (all glycosaminoglycan and polysulfated sugars were obtained from Sigma): hyaluronic acid from bovine vitreous humor or human umbilical vein (potassium salt); chondroitin sulfate A from bovine trachea or porcine rib; CSA covalently linked to phosphatidylethanolamine11; CD36 (a gift from M. Berndt, Baker Medical Research Institute, Melbourne, Australia); soluble ICAM-1 from transfected Chinese hamster ovarian cells (a gift from A. Boyd, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia); chondroitin sulfate C from shark cartilage; heparan sulfate from bovine kidney; heparin from porcine intestinal mucosa; colominic acid (a sialic acid polymer); chondroitin sulfate B from intestinal mucosa; fucoidan sulfate; dextran sulfate with an average molecular weight of 500 kDa; and pentosan polysulfate.
Assays used P. falciparum trophozoite-infected erythrocytes as described10,19. Receptors were adsorbed for 24 h at 4 °C onto plastic Petri dishes (Falcon 1058; Becton Dickinson, Lincoln Park, New Jersey). In preliminary experiments, we found that HA derived from both bovine vitreous humor (molecular weight, about 250 kDa) and human umbilical vein (molecular weight, about 750 kDa) could support high levels of adhesion, which was maximum at concentrations of 50 μg/ml or 10 μg/ml of each, respectively, when coated on plastic surfaces. Before the assay, plates were blocked with 1% bovine serum albumin in PBS. Subsequently, immobilized receptors spots were overlaid with parasite suspensions, and adhesion was allowed to occur for 30 min at 37 °C. Unbound cells were removed by gentle washing with RPMI-HEPES, pH 6.8, before bound cells were fixed with glutaraldehyde, stained with Giemsa stain, and counted using light microscopy. Laboratory isolates were tested at a parasitemia of 5-10% and 2-5% hematocrit, suspended in RPMI-HEPES, pH 6.8, with 10% ‘pooled’ human serum (group O). Clinical isolates were tested for adhesion to HA, CSA and CD36 at 1% parasitemia or higher and 5% hematocrit, suspended in RPMI-HEPES, pH 6.8, containing 0.5% bovine serum albumin. Isolates obtained from peripheral blood were cultured for up to 24 h until mature trophozoite forms predominated. Placental isolates typically showed mostly mature forms at the time of collection and could be tested directly for adhesion. In some cases, the parasitemia of clinical isolates was enriched by passage over Percoll gradients20. For these, preparations were tested for adhesion at a hematocrit of 2.5%. Glycosaminoglycans were tested at varying concentrations for inhibition of IE adhesion to purified receptors by prior incubation with IE suspensions for 5 min before adhesion assays. Selection for high-level adhesion to HA was made, using a modification of a published procedure18, by repeated rounds of adhesion of IEs to immobilized HA, followed by culture of adherent cells.
Preparation of HA oligosaccharide fragments
HA from bovine vitreous humor was partially depolymerized by controlled digestion at 37 °C for 20 h with testicular hyaluronidase21 (from bovine testes; Sigma) in 0.1 M sodium acetate buffer, pH 5.0, containing 0.15 M NaCl. The extent of digestion was checked for a even distribution of the oligosacchride fragments of different sizes by fast protein liquid chromagraphy on a Superdex 75 column eluted by 0.2 M ammonium acetate at 15 ml/h. The digestion mixture was fractionated by gel filtration chromatography on a Bio-Gel P-6 column (1.6 × 90 cm) after being desalted by Sephadex G10. Bio-Gel P-6 fractions were desalted before quantitation by carbazole assay22 and analysis by negative-ion liquid secondary ion mass spectrometry23 to confirm the size of the fragments. Oligosaccharide fragments were tested for inhibition of adhesion at a concentration of 200μg/ml by incubation for 20 min at 37 °C with parasite suspensions, before adhesion assays were done.
Enzymatic digestion of glycosaminoglycans and protease treatment of IEs
HA and CSA immobilized on Petri dishes were treated with 10 μg/ml testicular hyaluronidase in PBS, pH 7.2, for 45 min at 37 °C; 0.025-0.5 units/ml chondroitinase ABC in Tris HCl, pH 8.6, for 10-30 min at 37 °C; 0.5 units/ml heparinase in PBS or without enzyme (control) before adhesion of IEs was tested. Enzymes were obtained from Sigma.
For trypsin digestion of IE surface proteins, parasite cultures were washed three times in PBS, pH 7.2, before being incubated for 15 min at 21 °C with various concentrations of trypsin (TPCK-treated; Worthington Biomedical, Lakewood, New Jersey) in PBS. Control samples were incubated with 100 μg/ml trypsin plus 1 mg/ml soybean trypsin inhibitor. Digestion was then stopped by the addition of 1 mg/ml soybean trypsin inhibitor (Worthington Biomedical, Lakewood, New Jersey) for 5 min, and IEs were washed three times in culture medium containing 10% human serum. The treated IE suspensions were then tested for adhesion to receptors as described above.
Flow-based adhesion assays
Adhesion of P. falciparum-infected erythrocytes to HA was visualized and quantified in flat, rectangular, glass microcapillary tubes (‘microslides’) connected to a flow control system and mounted on the stage of an inverted phase-contrast light microscope, as described13,24. Microslides were first washed with nitric acid and coated with 5 mg/ml poly-L-lysine, then coated overnight at 4°C with 100 μg/ml HA (from human umbilical vein) in PBS. Microslides were blocked with 1% bovine serum albumin in PBS before the assay. Parasite cultures containing 1.5 × 108 erythrocytes/ml at 3-8% parasitemia (mature trophozoite stage), were suspended in RPMI-HEPES medium containing 1% Albumax, pH 7.2, and ‘flowed through’ microslides at wall shear stresses of 0.05, 0.1 or 0.2 Pa for 5 min, followed by cell-free adhesion medium for 5 min to remove non-adherent cells. Adherent cells were then counted microscopically, and scores were standardized to number of bound IEs per mm2 per 1 × 107 IEs perfused. In separate assays, IEs were allowed to adhere at 0.05 Pa as described above, and then exposed to ‘step-wise’ increases in wall shear stress (each 5 min in duration) from 0.05 to 4.0 Pa. The numbers of IEs remaining adherent at each level of stress were counted microscopically.
Acknowledgments
Human erythrocytes and serum were provided by the Red Cross Blood Bank (Victoria, Australia). The authors thank E. Chaluluka and R. Tembenu for assistance with sample collection and processing; C. Nicoll for assistance with flow-based adhesion assays; R.A. Childs for FPLC analysis; and C. Bradshaw, V. Lema and T. Taylor for support. Thanks also go to C. Mhango and the staff of the Antenatal Clinic and Labour Ward of the Queen Elizabeth Central Hospital (Blantyre, Malawi) for cooperation, and to all individuals who participated in the study. This study was supported by funding from the National Health and Medical Research Council of Australia, the Wellcome Trust, UK, the District 9680 Rotary Against Malaria Programme, Australia, and the Medical Research Council, UK.
References
- 1.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. Am. J. Pathol. 1982;109:330–342. [PMC free article] [PubMed] [Google Scholar]
- 2.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull. WHO. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 3.McGregor IA. Epidemiology, malaria and pregnancy. Am. J. Trop. Med. Hyg. 1984;33:517–525. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 4.Newbold CI, et al. PfEMP1, polymorphism and pathogenesis. Ann. Trop. Med. Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 5.Martin BJ, Spicer SS, Smythe NM. Cytochemical studies of the maternal surface of the syncytiotrophoblast of human early and term placenta. Anat. Rec. 1973;178:769–786. doi: 10.1002/ar.1091780408. [DOI] [PubMed] [Google Scholar]
- 6.Sunderland CA, Bulmer JN, Luscombe M, Redman CWG, Stirrat GM. Immunohistological and biochemical evidence for a role for hyaluronic acid in the growth and development of the placenta. J. Reproduct. Immunol. 1985;8:197–212. doi: 10.1016/0165-0378(85)90041-5. [DOI] [PubMed] [Google Scholar]
- 7.Kirby DRS, Billington WD, Bradbury S, Goldstein DJ. The antigen barrier of the mouse placenta. Nature. 1964;204:548–549. doi: 10.1038/204548a0. [DOI] [PubMed] [Google Scholar]
- 8.Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 9.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 10.Beeson JG, et al. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 1999;180:464–472. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner JP, Pinches RA, Roberts DJ, Newbold CI. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke BM, Coppel RL. Cytoadhesion and falciparum malaria: going with the flow. Parasitol. Today. 1995;11:282–287. doi: 10.1016/0169-4758(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey EM, Donner MW. Placental Vasculature and Circulation:Anatomy, Physiology, Radiology, Clinical Aspects: Atlas and Textbook. Saunders; Philadelphia, Pennsylvania: 1980. 1980. [Google Scholar]
- 15.Chien S. Hemodilution. In: Messmer K, Schnid-Schönbein H, editors. Theoretical Basis and Clinical Application. S. Karger; Basel: 1972. pp. 1–45. [Google Scholar]
- 16.McCormick CJ, Craig A, Roberts D, Newbold CI, Berendt AR. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J. Clin. Invest. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lea RG, Calder AA. The immunology of pregnancy. Curr. Opin. Infect. Dis. 1997;10:171–176. [Google Scholar]
- 18.Biggs BA, et al. Adherence of infected erythrocytes to venular endothelium selects for antigenic variants of Plasmodium falciparum. J. Immunol. 1992;149:2047–2054. [PubMed] [Google Scholar]
- 19.Beeson JG, Chai W, Rogerson SJ, Lawson AM, Brown GV. Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A. Infect. Immun. 1998;66:3397–3402. doi: 10.1128/iai.66.7.3397-3402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutner S, Breuer WV, Ginsburg H, Aley SB, Cabantchik ZI. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J. Cell. Physiol. 1985;125:521–527. doi: 10.1002/jcp.1041250323. [DOI] [PubMed] [Google Scholar]
- 21.Cowman MK, Balazs EA, Bergmann CW, Meyer K. Preparation and circular dichroism analysis of sodium hyaluronate oligosaccharides and chondroitin. Biochem. 1981;20:1379–1385. doi: 10.1021/bi00508a053. [DOI] [PubMed] [Google Scholar]
- 22.Bitter T, Muir HM. A modified uronic acid carbozole reaction. Anal. Biochem. 1962;4:330–4. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 23.Chai W, Lawson AM, Gradwell MJ, Kogelberg H. Structural characterization of two hexasaccharides and an octasaccharide from chondroitin sulfate C containing the unusual sequence (4-sulpho)-N-acetylgalactosamine-1-4-(2sulpho)-glucuronic acid-1-3-(6-sulpho)-N-acetylgalactosamine. Eur. J. Biochem. 1998;251:114–121. doi: 10.1046/j.1432-1327.1998.2510114.x. [DOI] [PubMed] [Google Scholar]
- 24.Cooke BM, Nicoll CL, Baruch DI, Coppel RL. A recombinant peptide based on PfEMP1 blocks and reverses adhesion of malaria-infected red blood cells to CD36 under flow. Mol. Microbiol. 1998;30:83–90. doi: 10.1046/j.1365-2958.1998.01040.x. [DOI] [PubMed] [Google Scholar]