Abstract
Traumatic brain injury (TBI) is associated with brain volume loss, but there is little information on the regional gray matter (GM) and white matter (WM) changes that contribute to overall loss. Since axonal injury is a common occurrence in TBI, imaging methods that are sensitive to WM damage such as diffusion-tensor imaging (DTI) may be useful for characterizing microstructural brain injury contributing to regional WM loss in TBI. High-resolution T1-weighted imaging and DTI were used to evaluate regional changes in TBI patients compared to matched controls. Patients received neuropsychological testing and were imaged approximately 2 months and 12.7 months post injury. Paradoxically, neuropsychological function improved from Visit 1 to Visit 2, while voxel-based analyses of fractional anisotropy (FA), and mean diffusivity (MD) from the DTI images, and voxel-based analyses of the GM and WM probability maps from the T1-weighted images, mainly revealed significantly greater deleterious GM and WM change over time in patients compared to controls. Cross-sectional comparisons of the DTI measures indicated that patients have decreased FA and increased MD compared to controls over large regions of the brain. TBI affected virtually all of the major fiber bundles in the brain including the corpus callosum, cingulum, the superior and inferior longitudinal fascicules, the uncinate fasciculus, and brain stem fiber tracts. The results indicate that both GM and WM degeneration are significant contributors to brain volume loss in the months following brain injury, and also suggest that DTI measures may be more useful than high-resolution anatomical images in assessment of group differences.
Introduction
Traumatic brain injury (TBI) is a major health problem in the United States. In 2002, the estimated overall rate of TBI-related hospitalization was 79.0 per 100,000 (CDC, 2006) with approximately 1.5 to 2 million new cases per year (Kraus et al., 1996; NIH Consensus Statement, 1998; Thurman et al., 1999). TBI is the most commonly encountered serious brain disorder among young and middle-aged adults, exceeding the incidence of epilepsy, tumors, and stroke (Katz, 1997). Typical injury in TBI includes focal coupe and contre-coupe cortical damage due to impact, often occurring in frontal, temporal, and occipital areas in the case of motor vehicle accident. In addition, subcortical white matter damage results from stretching, straining and shearing of axons as the brain moves inside the skull. A number of studies suggest that a long term consequence of TBI is cerebral atrophy (Ariza et al., 2006; Bergeson et al., 2004; Bigler et al., 2002; Bigler et al., 1997; Blatter et al., 1997; Gale et al., 1993; Gale et al., 1995; MacKenzie et al., 2002; Tate and Bigler, 2000; Tomaiuolo et al., 2004). These studies suggest a protracted period of brain loss; however the regional pattern of loss is not well characterized with the fine resolution obtainable with voxel-based approaches. Furthermore, white matter is a likely candidate for the bulk of volume loss (Gale, 1994) but many brain imaging sequences are not sensitive to the microstructural white matter damage that occurs in TBI.
Alternative imaging methods such as diffusion tensor imaging (DTI) may be more sensitive to brain damage immediately following TBI, and may also be useful in monitoring changes that occur longitudinally. DTI, which is based on the principle that water molecule movement is restricted by barriers to diffusion that vary in the brain depending on tissue type, or pathology, [for review see (Le Bihan, 1991)], is finely tuned to changes in the microstructure of white matter. Several studies have shown that DTI is sensitive to damage in tissue that may appear normal when measured with conventional MRI (Arfanakis et al., 2002; Chan et al., 2003; Field et al., 2003; Filippi et al., 2001; Nakayama et al., 2006; Xu et al., 2007; Zhang et al., 2006). DTI has been used to identify specific fiber bundles such as the commissural fibers of the corpus callosum, and the long association fibers that form prominent fiber tracts in the brain, including the superior and inferior longitudinal fascicules, superior and inferior fronto-occipital fascicules, uncinate fasciculus, and the cingulum. Smaller fiber bundles, such as tracts in the brainstem, and projection fibers such as the corticothalamic fibers and the corticospinal tract can also be visualized. This capability makes DTI well suited for assessing the specific brain damage caused by TBI.
The goal of the current study was to characterize longitudinal structural change in the brain at approximately two months and one year post-TBI by using DTI, in addition to conventional T1-weighted imaging, neuropsychological testing, and voxel-based methods. We predicted that TBI patients would show changes in white matter tracts over time, indexed by regional declines in white matter volume, and changes to the DTI measures, specifically, decreased FA and increased MD. We predicted that gray matter would also show change over time in the TBI group, reflected as a decrease in gray matter volume, and an increase in gray matter MD. We also predicted that extent of WM loss one year post injury would be related to initial injury severity scores on the Glasgow Coma Scale in the TBI group. Finally, we expected that memory function and executive cognitive function would be correlated with brain damage one year post injury in TBI. Because TBI can affect widespread areas of the brain; we reasoned that using a voxel based morphometry (VBM) approach would also allow us to look for relationships with a high degree of spatial resolution.
Methods
Participants
All subjects gave written informed consent under a protocol approved by the University of Wisconsin Health Sciences Institutional Review Board. For both the TBI and control groups, exclusion criteria consisted of current major Axis I psychiatric disease or history of major medical conditions (cancer, diabetes, or diagnosed neurological condition; with the exception of traumatic brain injury in the patient group), as well as any previous diagnosis of substance dependence, or an undiagnosed pattern of behavior demonstrating longstanding maladaptive use of alcohol or other drugs (for the TBI group, intoxication at the time of the injury was not considered an exclusion criterion).
TBI Patients
Forty-six TBI patients participated in an initial MRI scan, forty-two returned for a second visit. Of these patients, thirty-five had usable DTI and high-resolution T1-weighted brain scans available from two visits (acquisition errors resulted in the loss of 6 participants, and excessive motion in one case). The mean age of the final group was 30.54±11.37 years; mean education was 13.26±1.62; and there were 26 men and 9 women. The majority of the brain-injured patients received acute treatment at the University of Wisconsin Hospital and Clinics level 1 trauma center and were referred from the departments of Neurosurgery, Trauma and/or Rehabilitation. The inclusion criteria for TBI consisted of involvement in a rapid impact injury to the brain (such as a motor vehicle accident or fall) causing a loss of consciousness. Evidence of brain injury included admittance for emergency medical attention following loss of consciousness, a Glasgow Coma Scale (GCS) score either at the emergency room (ER) or upon hospital admission of less than or equal to 13, and a post-resuscitation GCS score of 6 or above. All patients had day of injury CT scans that were positive for visible brain injury. All TBI patients were less than 3 months post-injury at their first visit, and most were studied between 8 and 12 weeks post-injury (average 56 days) depending on their availability and other medical issues related to their injury.
Controls
Thirty-six (mean age 28.47 ± 9.78 years; mean education 14.25 ± 1.96 years; 18 men, 18 women) control participants were recruited from the community and from the University of Wisconsin Madison campus via advertisement. These participants all had useable DTI and T1-weighted data acquired during the same scan session available for at least one visit. Acquisition and transfer errors resulted in the loss of single visit data in 4 cases, in 4 cases DTI was not collected at one of the visits due to time constraints, and nine cases were lost due to follow-up. The remaining nineteen right-handed age and education-matched control participants (mean age = 28.11±9.43 years; mean education 14.21±1.87 years; 8 men and 11 women) had complete data available for two visits and participated in the longitudinal portion of this study. Seventeen additional controls (mean age = 28.88±10.43 years; mean education 14.29±2.11 years; 10 men and 7 women) were included in the cross-sectional portion of the study (one examination).
Procedures
Volunteers in the longitudinal portion of the current study participated in two testing sessions, each consisting of MR imaging and neuropsychological testing. TBI patients were tested at two visits, Visit 1, acquired approximately 2 months post-injury (ranging from 28 to 81 days post injury) and Visit 2, approximately 1 year post-injury (ranging from 252 to 380 days post injury). There were approximately 326 days between the two scanning sessions (the scans ranged from 257 to 497 days apart). Nineteen controls participated in two visits during approximately the same time frame as patients. Control scans were approximately 286 days apart (ranging from 74 to 403 days apart). An additional 17 controls had high resolution T1-weighted and DTI data, available from one visit and were included in the cross-sectional portion of the current study.
Neuropsychological Testing
On the day of the scan, both patients and controls received an extensive battery of neuropsychological testing. Using principal component (PC) analysis, we calculated two scores, one representing memory function and a second representing executive function. The first PC score (MEM) was based on 4 scores of memory: CVLT (California Verbal Learning Test, 2nd Edition) total raw score and long delay recall, BVMT (Brief Visuospatial Memory Test, Revised) total raw score, and delayed recall. The second PC score (EXEC) was based on 3 scores of executive function, COWAT (Controlled Oral Word Association Test) raw score, Digit Symbol (from WAIS-III) and Trail Making Test B raw score. Since it is possible that motor function can affect function on Trails B, the Trails B score was adjusted by subtracting the Trails A score from the Trials B score. Participants also received WRAT-III (Wide Range Achievement Test) Reading; Finger Tapping Test; CPT (Continuous Performance Task); and WCST (Wisconsin Card Sorting Test). Scores on these tests were not available for all participants, and consequently were not included in the PC analysis. Participants were also administered several questionnaires: a detailed health history questionnaire; BDI-II (Beck Depression Inventory); State Trait Anxiety Inventory (STAI); PCRS (Patient Competency Rating Scale: Participant and Relative Forms); and DRS (Disability Rating Scale).
Magnetic Resonance Imaging
All participants underwent magnetic resonance on a General Electric 3.0 Tesla SIGNA (Waukesha, WI) MRI system with a quadrature birdcage head coil. Sequences included diffusion-weighted imaging, high resolution T1-weighted imaging, and T2* gradient echo imaging.
Diffusion tensor imaging was performed using a cardiac- gated, diffusion-weighted, spin-echo, single-shot, EPI pulse sequence. Diffusion tensor encoding was achieved using twelve optimum non-collinear encoding directions (obtained by minimum energy numerical optimization) with a diffusion weighting of 1114 s/mm2 and a non-DW T2-weighted reference image. The effective TR was 10–13 heartbeats (~10–15 s) dependent upon the subject’s heart rate. Other imaging parameters were TE = 78.2 ms, 3 averages (NEX: magnitude averaging), and an image acquisition matrix of 120 × 120 over a field of view of 240 × 240 mm2. The cerebrum was covered using 39 contiguous 3-mm thick axial slices. The acquired voxel size of 2 × 2 × 3 mm was interpolated to 0.9375 mm isotropic dimensions (256 ×256 in plane image matrix). The total acquisition time was between 6.5 and 8 min dependant upon the heart rate.
High order shimming was performed prior to the DTI acquisition to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions. Residual spatial distortions from B0 inhomogeneities were corrected using a B0 field map, which was obtained using a pair of non-EPI gradient echo images at two echo times: TE1 = 8 ms and TE2 = 11 ms. The other acquisition parameters were TR = 600 ms, flip angle = 60°, 256 × 128 acquisition matrix, 240 mm FOV, and 3 mm thick slices co-planar with the DTI images. The total time for field map acquisition was approximately 3 min.
A 3D T1-weighted image was obtained using an inversion recovery prepared fast gradient echo pulse sequence. The whole brain was imaged in the axial plane with the following parameters: TI = 600 ms; TR = 9 ms; TE = 1.8 ms; flip angle = 20°; acquisition matrix = 256 × 192× 124, interpolated to 256 × 256 × 124; FOV = 240 mm; slice thickness = 1.2 mm (124 slices); receiver bandwidth = ± 16 kHz; acquisition time ~7.5 min.
Additionally, a high resolution 2D axial T2* gradient echo sequence sensitive to both DAI and contusions, was collected for evaluation by a neuroradiologist who confirmed the presence of brain injury. Imaging parameters were as follows: gradient echo read-out with TR = 325 ms, TE = 20 ms; flip = 15°; acquisition matrix = 256×192×22 (axial 256×192 in plane, interpolated to 256×256); FOV= 240 mm; slice thickness = 5 mm, with a 1mm skip between slices; receiver bandwidth = ±15.83 kHz; acquisition time ~3.5 min (22 slices).
Diffusion tensor image processing
Image distortions in the DTI data caused by eddy currents were corrected using a 2D affine co-registration function, align linear, in the Automated Image Registration (AIR) software package (http://www.bishopw.loni.ucla.edu/AIR5/). Non-linear image distortion from static field (B0) inhomogeneities was corrected using the acquired field map and the methods of Jezzard and Balaban (Jezzard and Balaban, 1995) implemented in the prelude (Phase Region Expanding Labeller for Unwrapping Discrete Estimates) and fugue (FMRIB's Utility for Geometrically Unwarping EPIs) tools from the FSL software suite (Smith et al., 2004). After distortion corrections, three-dimensional maps of the diffusion tensor and derived measures, mean diffusivity (MD) and fractional anisotropy (FA), were calculated.
Voxel-based morphometry (VBM)
Processing of the T1-weighted images for the cross-sectional analysis was performed using Statistical Parametric Mapping software http://www.fil.ion.ucl.ac.uk/spm (SPM5). Segmentation in SPM5 employs a unified approach, combining: segmentation of the original anatomical images into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) images; normalization (12-parameter affine transformation and nonlinear deformation with a warp frequency cutoff of 25) of the segmented images to the Montreal Neurological Institute template (MNI); and bias correction, in one iterative process. A modulation step was also employed, which scales the final GM and WM images by the amount of contraction required to warp the images to the template. The final result is GM and WM volume maps for each participant, where the total amount of GM and WM remains the same as in the original images. Finally, the normalized maps were smoothed using an 8-mm isotropic Gaussian kernel to optimize signal to noise and facilitate comparison across participants. Analysis of gray and white matter volume employed an absolute threshold masking of 0.1 to minimize the inclusion of gray matter voxels in the white matter analysis, and vice versa for the gray matter analysis.
Processing of the DTI data for the cross-sectional analysis involved normalizing the FA map (via 12-parameter affine transformation and nonlinear deformation in SPM) to a custom FA template comprised of an average of 10 FA maps acquired from non-brain injured participants who matched the demographic composition of the current study sample. Sixteen nonlinear iterations were used for the nonlinear normalization, and 7 × 9 × 7 basis functions were used to generate the deformation field for normalization. Normalization parameters were derived from the normalization of each individual’s FA image, and then applied to both their FA and MD maps. All of the normalized DTI maps were checked to ensure that major tracts were normalized into a common space by displaying the images using “check registration” in SPM. The normalized images were smoothed using an 8-mm isotropic Gaussian kernel. White matter and gray matter masks generated during the group analysis of the T1-weighted images were used to restrict either the cross-sectional and longitudinal analysis of DTI data to white or gray matter only.
Processing of the T1-weighted images for longitudinal analyses employed essentially the same steps as those used for the cross-sectional data, with a few modifications: first, the two sets of T1-weighted images from each participant were registered to one another using a 6-parameter transform to correct for position. The images were then segmented, producing gray and white matter images in native space. The normalization parameters generated during the SPM5 segmentation were then used to create spatially normalized and modulated versions of both sets of images for each subject. Processing of the DTI images for longitudinal analysis involved registering both sets of DTI data to one another using a 6-parameter transform, followed by normalization. The normalization estimates were derived from the first FA scan only, and then applied to the FA and MD maps acquired at Visit 1 and Visit 2. Finally, the normalized T1-weighted and the DTI images were smoothed using an 8-mm isotropic Gaussian kernel.
Statistical Analysis
Statistical tests were performed on T1-weighted and the DTI images using Factorial ANOVA and multiple regression statistical modules in SPM5. We hypothesized that TBI patients would show damage to white matter tracts reflected as decreased white matter volume, decreased FA, and increased MD, one year post injury, compared to controls, in addition to smaller gray matter volume one year post injury, compared to controls. This hypothesis was tested using cross-sectional comparisons, i.e. two-sample t-tests performed on Visit 2 data (collected ~ one year post injury). We also hypothesized that TBI patients would show atrophy in the months following injury, reflected as a decline in gray and white matter volume, a decline in FA, and an increase in MD, and that controls would show no change. This was tested using a factorial design, with VISIT (Visit 1, Visit 2,) as the first factor, and GROUP (TBI, control), as the second factor. We hypothesized that there would be an overall group difference in brain change between patients and controls, manifesting as a significant interaction between GROUP and VISIT. Age, education, and gender were included as covariates because of the known or potential effects they may have on brain structure. Additionally, for the analyses of gray and white matter volume, total intracranial volume (TIV) was entered into the model as a proportional scaling factor to ameliorate brain size as a possible confounder of gray or white matter volume. Follow-up paired comparisons (t-tests) were used to investigate within-group longitudinal changes in white matter volume, gray matter volume, FA, and MD.
Multiple Regression
The relationships between WM volume, GM volume, FA, MD, GCS, and cognition (MEM and EXEC principal component scores) were assessed using linear regression on Visit 2 data using voxel-wise analysis. Age, education, and gender were included as covariates; in addition, TIV was included as a covariate for the gray and white matter volume analyses. In order to generate correlation coefficients to describe the strength of the relationships between the independent and dependent variables, FA, MD and volume data were extracted from the maxima of the regions that showed a significant relationship in the voxel-wise analysis, and correlations with GCS and cognition were performed in SPSS.
Statistical Threshold
False Discovery Rate (FDR) thresholds (p<.005) were originally used when producing SPMs. However, since FDR thresholds are adaptive depending on the amount of signal in a dataset, and one of the goals of this project was to compare the sensitivity of T1-weighted imaging to DTI, we decided to use a consistent threshold for all of the analyses. Specifically, we used a threshold of T = 4.03. This value corresponded to an FDR corrected probability of .005 for the FA factorial interaction (VISIT × GROUP) analysis.
Results
Behavioral and Demographic Results
Demographic results are shown in Table 1. There was no significant age difference between TBI patients and controls. Controls had slightly more education (M = 14.25 years) than TBI patients (M=13.26 years), t(69) = 2.32, p <.05. In addition, there was a significantly lower proportion of females in the TBI group compared to control, χ2(1, N = 71) = 4.44, p < .05. As can be seen from the group mean scores in Table 2, TBI patients’ neuropsychological test performance differed significantly from controls on a number of tests, both at Visit 1(Digit Symbol, Trails A & B, COWAT, BVMT total and delayed recall, and CVLT total and long delay) and Visit 2 testing (Digit Symbol, Trails A, COWAT, BVMT total and delayed recall, and CVLT long delay). This was also reflected in the significant difference between groups on the composite cognitive scores, with TBI patients showing a significantly lower mean memory PC score (M = −2.9536), t(57) = 2.43, p < .05, one year following their injury compared to controls (M = 4.0169) and lower mean executive PC score one year following their injury (M = −7.8928) compared to controls (M = 10.7342), t(57) = 2.807, p < .05. Also shown in Table 2, patients scored significantly lower than controls on the Patient Competency Rating Scale (PCRS Self & Other) at Visit 1, and on PCRS Self at Visit 2. Disability (as assessed by the Disability Rating Scale) was significantly higher in patients than controls at Visit 1, but not Visit 2. Scores did not differ significantly between patients and controls on either the State-Trait Anxiety Inventory, or on the Beck Depression Inventory (II) at Visit 1 or Visit 2.
Imaging Results
Unless otherwise reported below, imaging results are bilateral.
Cross-Sectional Comparison between TBI Patients and Controls at Visit 2
Fractional Anisotropy Compared between TBI and Control
As predicted, the TBI group had lower FA compared to controls in several white matter tracts, including the corpus callosum, forceps major and minor, the anterior region of the corona radiata, the anterior limb and retrolenticular part of the internal capsule, the cerebral peduncles, external capsule, cingulum, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus, the corticopontine tract, and portions of the thalamus.
Mean Diffusivity in White Matter Compared between TBI and Control
The TBI group had increased diffusivity compared to controls in virtually all the same tracts where they showed decreased FA. Figure 1 shows the regions where MD and FA differences were found between TBI and controls. The areas of increased MD were somewhat larger than those showing decreased FA and encompassed a larger portion of the corpus callosum, corona radiata, and internal capsule.
Figure 1.
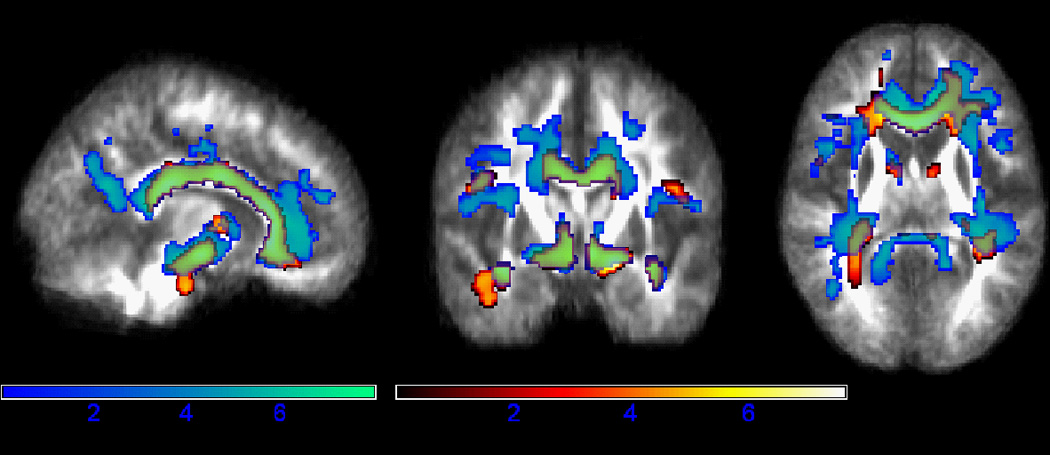
Fractional Anisotropy and White Matter MD: Cross-sectional Comparison. The TBI group had lower FA and higher MD compared to controls in several major white matter tracts. Regions of lower FA are shown in hot colors and regions of higher MD are shown in winter colors. Areas where the TBI group differed from controls on both MD and FA measures (primarily in the corpus callosum and cerebral peduncles) appear green. Results are shown in neurological orientation (left is left). Color bars reflect t-statistics.
Mean Diffusivity in Gray Matter Compared between TBI and Control
Greater diffusivity was present in the TBI group compared to controls in large areas of gray matter, particularly in inferior medial frontal gray matter, portions of parietal and occipital gray matter, and superior temporal gray matter. MD in patients was also greater in the thalamus, basal ganglia, and a small portion of anterior cerebellum.
White Matter Volume assessed with T1-weighted Imaging Compared between TBI and Control
In contrast to the widespread effects observed with diffusion tensor imaging, WM volume derived from structural T1-weighted imaging showed little difference between the 2 groups. As can be seen in Figure 2, TBI patients showed one small region of decreased frontal white matter (shown in cool colors), anterior to the left lateral ventricle when compared to controls.
Figure 2.

T1-derived Gray Matter and White Matter volume: Cross-sectional Comparison. TBI patients showed decreased GM volume compared to controls in the insula, thalamus, caudate, vermis, and small portions of right medial frontal gray matter, shown in hot colors. Patients showed one small anterior region of lower WM volume, shown in winter colors. Results are shown in neurological orientation (left is left). Color bars reflect t-statistics.
Gray Matter Volume assessed with T1-weighted Imaging Compared between TBI and Control
Figure 2 also depicts gray matter volume differences between the two groups. The TBI patients showed decreased volume in insula, caudate, anterior cingulum, pulvinar nucleus, thalamus, parahippocampal gray matter, small portions of the cerebellum, in particular the vermis, and a small region of medial frontal gray matter.
Longitudinal Analyses
Interactions Between VISIT (Visit 1, Visit 2) and GROUP (TBI, Control)
Fractional Anisotropy: Interaction between VISIT and GROUP
As predicted, TBI patients showed greater frontal and temporal WM decline in FA from Visit 1 to Visit 2 compared to controls. As is shown in Figure 3, short association fibers in bilateral inferior temporal white matter and left inferior frontal white matter showed significantly different change over time in the two groups. In addition, there was a significant difference in change over time in the left cerebral peduncle. A paired t-test performed in the TBI group indicated that FA decreased from Visit 1 to Visit 2 in several major white matter tracts including the inferior longitudinal fasciculus, corticospinal tract, middle cerebellar peduncle, corpus callosum, temporal stem WM, inferior frontal WM and ventral brain stem. A paired t-test in the control group also indicated small regions of change. Unlike the TBI group the regions of change were less localized to major white matter fiber bundles and appeared in one anterior region to the left of the genu of the corpus callosum, another to the right of the anterior limb of the internal capsule, and a third small cluster in right inferior temporal lobe.
Figure 3.

Fractional Anisotropy and White Matter MD: Longitudinal Interaction between VISIT and GROUP. TBI patients showed a greater decline in FA (winter colors) and a greater increase in MD (hot colors) over time, compared to controls. FA changes were more localized, while white matter MD changes were more diffuse and extended beyond the major white matter tracts, encompassing short association fibers in frontal, temporal, parietal, and occipital white matter. Results are shown in neurological orientation (left is left). Color bars reflect t-statistics.
Mean Diffusivity in White Matter: Interaction between VISIT and GROUP
TBI patients exhibited a greater increase in MD from Visit 1 to Visit 2 compared to controls in several white matter regions. Figure 3 shows those regions where TBI patients exhibited increased white matter MD over time, including the splenium of the corpus callosum, superior longitudinal fasciculus, and external capsule, the superior region of the corona radiata, and the superior cerebellar peduncles. MD also increased over time in the TBI group, in a small portion of the genu of the corpus callosum, the anterior cingulum, and in the region of ventral tegmentum.
Unlike FA changes, the white matter MD changes were more diffuse and extended beyond the major white matter tracts, encompassing greater amounts of short association fibers in frontal, temporal, parietal, and occipital white matter. Paired t-tests performed in TBI patients and controls indicated that TBI patients show an interesting pattern of diffusivity over time. MD in patient brains increased from Visit 1 to Visit 2 in the posterior corpus callosum, corticospinal tract, small portions of the middle cerebellar peduncles, and superior portions of the left corona radiata. However, MD also decreased from Visit 1 to Visit 2 over several areas of white matter, including internal capsule, superior and inferior longitudinal fasciculus, and portions of the corona radiata, anterior thalamic radiations, and small regions of occipital white matter. The control group showed no change in MD from Visit 1 to Visit 2. An interaction in the opposite direction was also tested (TBI patients showing a greater decline in MD from Visit 1 to Visit 2 compared to controls). This analysis yielded no regions of significant interaction.
Mean Diffusivity in Gray Matter: Interaction between Visit and GROUP
Gray matter MD in the TBI group increased from Visit 1 to Visit 2 in large areas of frontal, parietal, temporal, and occipital gray matter, compared to controls, manifesting as a significant interaction between VISIT and GROUP. Paired t-tests indicated that the TBI group showed an increase in MD from Visit 1 to Visit 2 in a large medial fronto-parietal region, right postcentral gyrus, thalamus, pulvinar, anterior cerebellum, and inferior occipital GM. TBI patients also showed a decrease in MD over time, apparent in the putamen, right posterior hippocampus, bilateral anterior hippocampus, and small regions of GM including the left parahippocampal gyrus, superior temporal gyrus, inferior frontal gyrus, and occipital GM. Control participants did not show a change from Visit 1 to Visit 2.
White Matter Volume assessed with T1-weighted Imaging: Interaction between VISIT and GROUP
As expected, the TBI patients showed signs of atrophy over time compared to the control group. As can be seen in Figure 4, TBI patients had greater volume decline over time compared to controls in corona radiata, corpus callosum, internal capsule, external capsule, the superior and inferior longitudinal fascicules, cingulum, inferior fronto occipital fasciculus, corticospinal tract, superior, middle, and inferior cerebellar peduncles, and small regions of cerebellar WM. Figure 5 shows volume from the splenium of the corpus callosum; TBI patients had lower WM volume than controls at Visit 1, and even lower volume at Visit 2. A paired t-test performed in the patients indicated a significant decline in WM volume from Visit 1 to Visit 2, including large regions of frontal, temporal, parietal, and occipital white matter and small portions of cerebellar WM. Specific fiber tracts that appeared affected included corpus callosum, internal and external capsule, thalamic radiations, corona radiata, superior and inferior longitudinal fascicles, uncinate fasciculus, cingulum, cerebellar peduncles, corticospinal tract, transverse pontine fibers, and forceps major (the occipital radiation of the corpus callosum). The control group showed one small cluster of white matter decline in left superior cerebellar peduncle.
Figure 4.

Gray Matter and White Matter Volume: Longitudinal Interaction between VISIT and GROUP. The TBI group showed extensive white matter volume decline over time (shown in hot colors), in addition to a few regions of GM volume decline, including the thalamus and bilateral pallidum (shown in winter colors). Patients also showed gray matter volume decline over time in the cingulum, right post central gyrus, supplementary motor area, right precentral gyrus, and bilateral putamen (areas not shown here). Results are shown in neurological orientation (left is left). Color bars reflect t-statistics.
Figure 5.

White Matter Longitudinal Interaction between VISIT and GROUP. TBI patients showed lower white matter volume in the splenium of the corpus callosum at Visit 1, compared to controls. While controls did not change from Visit 1 to Visit 2, TBI patients declined over the one year interval. The plot on the right was generated from WM volume values extracted from the region indicated by the red arrow on the glass brain (left).
Gray Matter Volume assessed with T1-weighted Imaging: Interaction between VISIT and GROUP
TBI patients had greater gray matter volume decline over time compared to controls, resulting in a significant interaction between VISIT and GROUP. As indicated in Figure 4, TBI patients had decreased GM volume in the thalamus and bilateral pallidum. Patients also showed small regions of decreased volume in the cingulum, right post central gyrus, supplementary motor area, right precentral gyrus, and bilateral putamen. A paired t-test performed in the patient group showed a significant decline in volume from Visit 1 to Visit 2, including a large area of medial fronto-parietal GM, a cluster in superior frontal GM, thalamus, caudate, pulvinar, nucleus accumbens and small portions of the cerebellum. The control group also showed small though significant declines in gray matter from Visit 1 to Visit 2, in small clusters of the left occipital lobe, and left cerebellum, bilateral inferior frontal white matter, parahippocampal gray matter, and a small medial parietal cluster.
Regression Analyses
Correlation between Visit 2 Brain measures and Glasgow Coma Score
As predicted, TBI patients showed a correlation between 24 hour GCS and all five brain measures assessed: FA of white matter, MD of white matter, MD of gray matter, white matter volume and gray matter volume. The FA analysis indicated that GCS was positively correlated with FA in the corpus callosum (r = .65) and the superior region of the coronal radiata (r = .57). It was negatively correlated with MD in the corpus callosum (r = −.66), the anterior limb of internal capsule (r = −.69), the superior fronto-occipital fasciculus (r = −.71), anterior thalamic radiations (−.62), the superior longitudinal fasciculus (r =−.71), left insula (r = −.69), right medial frontal GM (r = −.63), right cingulate (r = −.66), the tail of the hippocampus (r = −.63), and pulvinar (r = −.60). Analysis of white matter volume indicated that GCS was positively correlated with volume in the fornix and striatum adjacent to the hippocampus (r = .68), volume of the cerebellar peduncles (r = .58), inferior longitudinal fasciculus (r = .55), cingulum (r = .65), and superior regions of corona radiata (r = .68). The gray matter volume analysis showed small scattered regions of positive correlation including the caudate (r = .55), superior parietal GM (r = .69) and mammillary bodies (r = .633).
Correlation between Visit 2 Brain measures and the Memory Principal Component Score
Memory function was negatively correlated with MD. In gray matter, this negative correlation was present in bilateral dorsal cingulate (r = −.59), insula (r = −.50), and middle frontal gyrus (r = −.50). MD in white matter showed regions of negative correlation with the MEM PC score in dorsal posterior cingulate (r = −.53), and middle frontal gyrus (r = −.50), in addition to frontoparietal white matter (r = −.56). The negative relationship between MD and MEM PC was present in both patients and controls, and there was no significant interaction between GROUP and MEM PC. Figure 6 shows the negative relationship between memory function and MD in the dorsal posterior cingulate. Finally, there was no relationship between memory function and FA, GM volume, or WM volume.
Figure 6.

Negative relationship between Mean Diffusivity and Memory in posterior cingulate. Both TBI patients and controls showed a negative relationship between MD in the dorsal posterior cingulate and memory. MD data were extracted from the maxima of a significant cluster in dorsal posterior cingulate and plotted against the memory principal component score, which was based on 4 scores of memory function. Although there was no significant slope difference between TBI and controls, fit lines are shown by group for illustrative purposes. Controls are shown in blue and TBI are shown in red. Interestingly, the scatter indicates that the TBI group had lower MD than the controls in this region, but the cross-sectional voxel-wise comparison of TBI and controls did not show any regions where MD was significantly lower in controls.
Correlation between Visit 2 Brain measures and the Executive Principal Component Score
We predicted that the TBI group would show a stronger correlation between the EXEC PC score, brain volume, and the DTI measures, compared to controls. In contrast, controls showed a stronger correlation between EXEC and the gray matter measures compared to TBI. Controls showed a strong correlation between EXEC and gray matter volume in the cerebellum (r =.73) compared to TBI (r= .37). Controls also showed a strong negative correlation between EXEC and gray matter MD in left parahippocampal gray matter (r = −.81) compared to TBI (r = .03); with bilateral medial parietal cortex (r = −.77), compared to TBI (r = .01); with cerebellum (r = −.72), compared to TBI (r = .07), and in right superior temporal gyrus (r = −.62), compared to TBI (r = −.41). The relationship between gray matter MD and EXEC in the two groups is plotted in Figure 7. The TBI group showed a significantly stronger correlation between executive function and white matter volume in the calcarine fissure (r = .29) compared to controls (r = .03), and in dorsal posterior cingulate white matter (r = .35), compared to controls (r=.16). When the TBI patients and the controls were combined, there was little correlation between executive function and the brain measures. Only one small occipital brain region showed a simple correlation between the EXEC PC score and WM brain volume (r = .32).
Figure 7.

Interaction between Mean Diffusivity and Executive function in parahippocampus. Although we had hypothesized that TBI patients would show a stronger relationship between the cognitive measures and measures of diffusion, controls actually showed the stronger relationship. Shown here is a plot of mean diffusivity values against the executive principal component score which was based on 3 scores of executive function. Controls are shown in blue and TBI patients are plotted in red.
Discussion
A chief advantage of diffusion tensor imaging is the capacity to produce maps of the major white matter tracts in the brain. In the current study, TBI patients showed decreased FA and increased MD in several major fiber bundles including the superior longitudinal fasciculus, the uncinate fasciculus, inferior fronto-occipital fasciculus, corpus callosum and cingulum. Other regions affected included the internal and external capsule, the corticospinal tract and thalamus.
With respect to white matter differences between the groups in the cross-sectional analysis, the FA and MD maps were more revealing than the white matter probability maps. FA and MD changes were found in 1.79% and 3.91% of the total white matter respectively (calculated as a ratio of significant voxels in the longitudinal analysis to total white matter voxels), while only .04% of the total white matter showed a significant difference in the analysis of the white matter probability maps. MD measurements made in gray matter also showed more differences between TBI patients and controls than the analysis of T1-weighted data, with 2.12% of the total GM showing a difference in the MD analysis, compared to 1.38% in the analysis of the gray matter probability maps.
In contrast, the white matter probability maps revealed more longitudinal changes. The interaction between VISIT (Visit 1 and Visit 2) and GROUP (TBI, control) showed extensive areas of white matter volume that were differentially affected over time, (15.76% of the total WM voxels). Less white matter showed a change in the MD analysis (3.19%) and FA analysis (.06%). The gray matter MD analysis, affecting 4.35% of total gray matter voxels, showed more regions of change than the analysis of the gray matter probability maps, affecting only .26% of total gray matter voxels.
The results of the current study are similar to other recent studies using voxel-wise analysis of DTI data. For example, Xu et al. (Xu et al., 2007) compared nine TBI patients to 11 controls using voxel based analysis of FA and MD maps. They found significant differences in the corpus callosum, internal and external capsule, superior and inferior longitudinal fascicles, and the fornix in the TBI group. Salmond et al.(Salmond et al., 2006) used VBM to compare TBI patients to controls, and found significant decreases in anisotropy, both in major white matter tracts such as the corpus callosum and the internal and external capsule, as well as association fibers underlying cortex in the temporal, frontal, parietal, and occipital lobes. Diffusivity was increased in TBI in the cerebellum, insula, cingulate, frontal, temporal, occipital and parietal lobes. In the first study, time from accident was 2–6 years; in the second study patients were imaged approximately 6 months post injury. These two studies provide an indication of brain change following TBI, though they are both limited by their cross sectional nature.
Recently, Sidaros et al. (Sidaros et al., 2008) described longitudinal changes in DTI measures following TBI. Adopting an ROI approach, the authors found cross-sectional differences in FA between patients and controls in all regions studied (corpus callosum, posterior limb of internal capsule, centrum semiovale, and cerebral peduncles). A voxel-wise approach in the current study revealed decreased FA in the same regions, in addition to lower FA in several additional regions not sampled by Sidaros et al. (forceps major and minor, the anterior region of the corona radiata, the anterior limb and retrolenticular part of the internal capsule, external capsule, cingulum, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus, the corticopontine tract, and portions of the thalamus).
With regard to longitudinal changes suggestive of regeneration as opposed to degeneration, Sidaros et al. found an increase in FA in the TBI group, in centrum semiovale and internal capsule. In the current study, we found a decrease in MD over time in several regions including the internal capsule, superior and inferior longitudinal fasciculus, portions of the corona radiata, anterior thalamic radiations, and small regions of occipital white matter. To date, there is no clear model of diffusion change over time in the TBI brain. Immediately following injury, edema is primarily intracellular. As cells swell, the space in which extracellular water moves is decreased resulting in a reduction in diffusion. Intracellular edema often results in cell death, at which point diffusion may increase as water molecules have additional room to diffuse. It is unknown what cellular events took place in our patients over the next several months that would result in decreased MD. Patients may have possibly experienced residual extracellular edema at 2 months post-injury, with dissipation in the next few months resulting in decreased MD. However, taken with the findings of Sidaros et al., these results point toward the possibility of functional reorganization following TBI. Axonal sprouting is one mechanism that is known to decrease MD. For example Schwartz et al.(Schwartz et al., 2003), found decreased MD in rat spinal cord tissue corresponding to axonal sprouting following transplant of fibroblasts genetically modified to express brain derived neurotrophic factor. Regeneration and reorganization in the brain following serious injury are common, though few models have the capability of being evaluated in the human brain. The exact mechanisms will remain difficult to elucidate, except in those cases where histology can be performed and correlated with DTI measures.
The results from this study show that white matter loss continues to occur well after initial injury. In the longitudinal analysis, the white matter probability maps were somewhat superior to the FA and MD maps in demonstrating where TBI patients have greater decline over time than controls. In our data we observed white matter loss between 2 months and 12.7 months post injury that was most prominent in the corpus callosum, the internal and external capsule, the superior and inferior longitudinal fascicules, the cingulum, and several smaller areas of WM such as the corticospinal tract, cerebellar peduncles, and cerebellar WM. The most likely mechanism for this longitudinal change is Wallerian degeneration. Deafferentation of WM fiber tracts following injury results in downstream degeneration of the tract. Furthermore, axons that are damaged but not immediately disconnected continue to undergo subtle changes that include impaired axoplasmic transport, axonal swelling, and ultimate disconnection (Povlishock, 1992). The process of degeneration can last for several months post-injury in humans (Graham, 2002).
We suspect that the results found in the current study are also due to Wallerian degeneration. Pierpaoli et al. (Pierpaoli et al., 2001) have performed elegant investigations of water diffusion in Wallerian degeneration. Using DTI in patients with lacunar infarcts of the internal capsule, they found decreased diffusion anisotropy in white matter regions where fibers are arranged in isolated bundles of parallel fibers, such as the cerebral peduncles. In regions where fiber tracts cross, Wallerian degeneration is less likely to be detected by the anisotropy measures, though diffusivity does increase in these regions. In the present study we found that FA changes over time in the TBI group were indeed localized to major white matter tracts, while MD changes were more diffuse and extended beyond the major white matter tracts, encompassing short association fibers in frontal, temporal, parietal, and occipital white matter. The increases in MD observed in the present study are also likely to be indicative of Wallerian degeneration and loss of myelin that is part of the degeneration process following axonal injury.
Both cross-sectional and longitudinal differences in gray matter were found between TBI patients and controls. MD measurements made in gray matter were particularly sensitive, showing large areas of difference between patients and controls in frontal, parietal, occipital and temporal gray matter. Longitudinal changes in TBI gray matter MD were found in similar regions where cross-sectional differences were found. These effects are likely due to focal contusions in the TBI group, in addition to diffuse injury, both of which would result in cell death and increased water diffusion in areas of injury, reflected as higher MD. Although the cross-sectional analysis of gray matter volume did not show as extensive as a difference as the DTI analysis, the results do suggest loss of volume, likely related to primary injury and possibly retrograde cell death related to axonal injury.
The results of our volume analyses differ somewhat from Tomaiuolo et al. (Tomaiuolo et al., 2005) who found that TBI patients have lower white matter density values than control patients in a number of brain regions, including corpus callosum, fornix, internal capsule, superior frontal gyrus, parahippocampal gyrus, optic radiation, and chiasma. The cross-sectional VBM analysis of white matter in our analysis showed only one small frontal region where volume differed, though the gray matter analysis showed smaller volume in insula, caudate, anterior cingulum, pulvinar nucleus, thalamus, small portions of the cerebellum, and a small region of frontal gray matter. The only region found in common between our study and Tomaiuolo’s is parahippocampal gyrus. The likely reasons for these differences are methodological ones, such as differences in tissue classification methods, employing modulated vs. unmodulated images, the use of TIV as a covariate in the current study, and sample differences in the spatial variability and severity of the brain injuries.
We had hypothesized that cognitive function in TBI would correlate with DTI measures and brain volume. The relationship between cognition and brain measures in TBI is complex. Several studies have found a relationship (Ariza et al., 2004; Bergeson et al., 2004; Blatter et al., 1997; Himanen et al., 2005; Salmond et al., 2006; Serra-Grabulosa et al., 2005), but others have not (Anderson et al., 1995; Sherer et al., 2006; Yount et al., 2002). A number of patient characteristics increase the variability in TBI studies, including severity of injury; length of time since injury; the age of patients; and the nature of brain insult, such as presence of edema, hematoma, intracranial pressure, and closed vs. open head injury. Another factor affecting the discrepancy between studies is the variation in tests assessed and methods used for establishing indices of brain damage. In the current study, we used voxel based methods which enable analyses of the entire brain, as opposed to less focused methods such as lesion counting, or ratings of DAI severity, or narrowly focused methods that examine only one or two specific brain regions.
It is an interesting paradox that even though the TBI brain appears to undergo several disadvantageous changes over time (loss of volume, decreases in FA, increases in MD), neuropsychological function improves during the same time span. Clearly, there must be some brain mechanisms apart from degeneration that support the improved neuropsychological functioning over time. In the current study we found decreases in MD over time that may reflect a potential positive mechanism. Interestingly, the only measure that showed a correlation with the composite memory score was MD, with MEM PC showing a negative relationship with gray matter MD in bilateral dorsal cingulate, insula, and middle frontal gyrus, and a negative relationship with white matter MD in dorsal posterior cingulate, middle frontal gyrus, and fronto-parietal white matter. We had predicted that the TBI group would show a stronger correlation between the cognitive scores and the brain measures than controls. However, in several brain regions, including parahippocampal gray matter, medial parietal cortex, and superior temporal gyrus, it was the controls who showed a stronger correlation between cognition and the gray matter measures (MD in particular). One possibility is that the TBI group exhibited a floor effect in the brain measures compared to controls. For example, in the regions where controls showed a strong correlation between executive function and MD, the TBI group uniformly showed low MD, while controls showed a larger range of MD values.
The current study had some limitations. A potential limitation concerns the difference between the TBI group and the control group in the length of time between scans used for the longitudinal analysis. Five of the control subjects were scanned less than five months apart (though greater than 2 months apart), whereas the minimum interval in the TBI group was 8.5 months. It is unlikely however that our results are due to greater aging effects in the TBI group. Current research on aging suggests that the white matter changes found in the TBI group in this study are above and beyond what would be expected in a healthy brain. The mean age of the TBI group was 30.54 yrs. ± 11.37 years, a range when only small age-related changes in white matter would be expected. In fact, the pattern of white matter volume throughout the lifespan has been shown to increase during the earlier part of the lifespan, in particular throughout childhood and adolescence, continuing to increase (albeit at a slower rate) until reaching a plateau in the 4th decade of life (Courchesne et al., 2000). In addition to volume, DTI measures have also been characterized over the lifespan. Salat et al. (Salat et al., 2005) have found significant correlations between FA and age in frontal brain regions with relative preservation in temporal and occipital white matter regions. In the current study, the TBI patients showed significant white matter changes over time in frontal regions, but also temporal, parietal and occipital brain regions. Scanning at two time points is more important for control of measurement error, rather than for controlling effects due to aging.
Interestingly, a paired t-test performed in the control group showed a change in FA from Visit 1 to Visit 2. Unlike the FA findings in the TBI group, the significant clusters did not appear in major white matter tracts. These data suggest that there is some variability in the DTI signal from Visit 1 to Visit 2, an issue that has been relatively unaddressed in studies using DTI. Because the changes found in the TBI group were much larger than those found in the control group, it is unlikely that the results were due to variability in the DTI signal; and interaction analyses were used as the test of primary outcome to control for the variability in the controls.
When performing group comparisons between patient brains and control brains there is invariably an issue with how the processing methods may differentially affect the patient brains compared to the normal healthy brains. In order to ensure a template with well delineated tracts, healthy participants were used to construct the FA template. Accurate normalization of the patient brains was determined by individually inspecting each FA map following the normalization procedure. It has also been suggested that differences in FA and MD may be due to volumetric differences between groups that result in inaccurate normalization of the FA and MD maps. The analyses of volumetric changes compared to the FA and MD suggest that our FA and MD results can not be explained by volumetric differences alone. In particular, the cross-sectional comparison of FA and MD maps between TBI and controls showed extensive differences, while the comparison of the white matter probability maps showed little cross-sectional difference, suggesting that the diffusion results exceed volumetric differences.
Although voxel-wise analysis has advantages over an ROI based approach (particularly its capability of providing an analysis of the entire brain), it can not be performed in the absence of smoothing. A disadvantage of smoothing is the averaging of signals from different tissue types. This partial voluming can affect the final DTI results, in particular if there was greater inclusion of CSF values in the WM and GM measures acquired from the TBI group. The large sample size employed in the current study reduces these concerns, though does not eliminate them.
We have attempted to be specific in naming the tracts that were affected by TBI, but the final interpretation may also be affected by smoothing. Statistical differences appeared to be localized to specific tracts, but it is still possible that DTI changes were due to local differences surrounding the tracts. For example, changes that appeared in the corticospinal tract may have also been the result of damage to brain stem gray matter. Furthermore, certain fiber groups can be difficult to differentiate from one another (for example, corticospinal tract and thalamic radiations), and it is possible that apparent changes in one particular tract may have been due to changes in an adjacent tract.
A final limitation that should be mentioned was the greater ratio of women to men in the control group compared to the TBI group. We attempted to control for this disproportion by employing gender as a covariate in all of the analyses, in addition to employing TIV in the analyses of WM volume (TIV tends to be greater in men than in women). TBI is more common in men than in women, we are currently recruiting additional male controls for our on going longitudinal study of TBI.
Conclusion
In this study, we show that brain changes in TBI are apparent in the form of volume decline and FA and MD changes over one year, far in excess of the minimal changes expected in age-matched controls. Several studies have shown that volume decline occurs after TBI. These studies have either looked at whole brain volume, ventricular enlargement, or have used an ROI based morphometrical approach. By using a voxel-wise approach, our study was able to assess regional change over the entire brain. Furthermore, by employing DTI, our study was able to show which fiber tracts are affected by TBI, in addition to showing the extent of change that occurs over the months following TBI. Memory function and executive function were minimally related to brain volume, declines in FA, and increases in MD, suggesting that the imaging findings do not reflect evolving neurological dysfunction but rather the manifestation of the earlier insult to neurons that are already functionally impaired. Furthermore, it is possible that neural plasticity is accounting for some of the functional improvements over time. Future studies using analysis techniques that combine the results of DTI, morphometry, and fMRI, will yield greater insight into the networks underlying cognitive changes following TBI.
Acknowledgements
This study was supported by a Merit Review Grant from the Department of Veterans Affairs, the NIH MH65723 (SCJ), and by the facilities and resources at the William S. Middleton Memorial Veterans Hospital. The assistance of Britta Jabbar, Shelly Fitzgerald, Gemma Gliori, and Erik Kastman is greatly appreciated. We would also like to acknowledge the kind support of researchers and staff at the Waisman Center, University of Wisconsin, Madison, where MR imaging took place. Finally, we thank all the patients who took part in this study.
This study was undertaken with the understanding and written consent of each subject, under a protocol approved by the University of Wisconsin Health Sciences Institutional Review Board, and in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CV, Bigler ED, Blatter DD. Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatic brain-injured patients. J Clin Exp Neuropsychol. 1995;17:900–908. doi: 10.1080/01688639508402438. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Ariza M, Junque C, Mataro M, Poca MA, Bargallo N, Olondo M, Sahuquillo J. Neuropsychological correlates of basal ganglia and medial temporal lobe NAA/Cho reductions in traumatic brain injury. Arch Neurol. 2004;61:541–544. doi: 10.1001/archneur.61.4.541. [DOI] [PubMed] [Google Scholar]
- Ariza M, Serra-Grabulosa JM, Junque C, Ramirez B, Mataro M, Poca A, Bargallo N, Sahuquillo J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44:1956–1961. doi: 10.1016/j.neuropsychologia.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Bergeson AG, Lundin R, Parkinson RB, Tate DF, Victoroff J, Hopkins RO, Bigler ED. Clinical rating of cortical atrophy and cognitive correlates following traumatic brain injury. Clin Neuropsychol. 2004;18:509–520. doi: 10.1080/1385404049052414. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 2002;23:255–266. [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett BM. Hippocampal volume in normal aging and traumatic brain injury. American Journal of Neuroradiology. 1997;18:11–23. [PMC free article] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Ryser DK, Macnamara SE, Bailey BJ. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. American Journal of Neuroradiology AJNR. 1997;18:1–10. [PMC free article] [PubMed] [Google Scholar]
- CDC. Incidence rates of hospitalization related to traumatic brain injury--12 states, 2002. MMWR Morb Mortal Wkly Rep. 2006;55:201–204. [PubMed] [Google Scholar]
- Chan JH, Tsui EY, Peh WC, Fong D, Fok KF, Leung KM, Yuen MK, Fung KK. Diffuse axonal injury: detection of changes in anisotropy of water diffusion by diffusion-weighted imaging. Neuroradiology. 2003;45:34–38. doi: 10.1007/s00234-002-0891-y. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Field AS, Hasan K, Jellison BJ, Arfanakis K, Alexander AL. Diffusion tensor imaging in an infant with traumatic brain swelling. AJNR Am J Neuroradiol. 2003;24:1461–1464. [PMC free article] [PubMed] [Google Scholar]
- Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol. 2001;22:277–283. [PMC free article] [PubMed] [Google Scholar]
- Gale SD. White matter pathway degeneration following traumatic brain injury: morphometric and neuropsychologic correlates. Provo, Utah: Brigham Young University; 1994. [Google Scholar]
- Gale SD, Burr RB, Bigler ED, Blatter D. Fornix degeneration and memory in traumatic brain injury. Brain Research Bulletin. 1993;32:345–349. doi: 10.1016/0361-9230(93)90198-k. [DOI] [PubMed] [Google Scholar]
- Gale SD, Johnson SC, Bigler ED, Blatter DD. Nonspecific white matter degeneration following traumatic brain injury. Journal of the International Neuropsychological Society. 1995;1:17–28. doi: 10.1017/s1355617700000060. [DOI] [PubMed] [Google Scholar]
- Graham DI, Genarrelli TA, McIntosh TK. Trauma. In: Lantos GaPL., editor. Greenfield’s Neuropathology. Vol. 1. London: Arnold Publishers; 2002. pp. 823–898. [Google Scholar]
- Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Cognitive functions in relation to MRI findings 30 years after traumatic brain injury. Brain Inj. 2005;19:93–100. doi: 10.1080/02699050410001720031. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Katz D. Traumatic Brain Injury. In: Mills V, Cassidy J, Katz D, editors. Neurological Rehabilitation. Malden: Blackwell Science; 1997. [Google Scholar]
- Kraus J, McArthur D, Silverman T, Jayaraman M. Epidemiology of Brain Injury. In: Narayan R, Wilberger J, Povlishock J, editors. Neurotrauma. New York: McGraw-Hill; 1996. [Google Scholar]
- Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q. 1991;7:1–30. [PubMed] [Google Scholar]
- MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP, Grossman RI. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am J Neuroradiol. 2002;23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Yasokawa YT, Miwa K, Yoshimura SI, Iwama T. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry. 2006;77:850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Statement. Rehabilitation of Persons with Traumatic Brain Injury. Washington DC: NIH; 1998. [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, Pickard JD. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29:117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Schwartz ED, Shumsky JS, Wehrli S, Tessler A, Murray M, Hackney DB. Ex vivo MR determined apparent diffusion coefficients correlate with motor recovery mediated by intraspinal transplants of fibroblasts genetically modified to express BDNF. Exp Neurol. 2003;182:49–63. doi: 10.1016/s0014-4886(03)00036-0. [DOI] [PubMed] [Google Scholar]
- Serra-Grabulosa JM, Junque C, Verger K, Salgado-Pineda P, Maneru C, Mercader JM. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J Neurol Neurosurg Psychiatry. 2005;76:129–131. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer M, Stouter J, Hart T, Nakase-Richardson R, Olivier J, Manning E, Yablon SA. Computed tomography findings and early cognitive outcome after traumatic brain injury. Brain Inj. 2006;20:997–1005. doi: 10.1080/02699050600677055. [DOI] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tate DF, Bigler ED. Fornix and hippocampal atrophy in traumatic brain injury. Learn Mem. 2000;7:442–446. doi: 10.1101/lm.33000. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, Carlesimo GA, Di Paola M, Petrides M, Fera F, Bonanni R, Formisano R, Pasqualetti P, Caltagirone C. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F, Worsley KJ, Lerch J, Di Paola M, Carlesimo GA, Bonanni R, Caltagirone C, Paus T. Changes in white matter in long-term survivors of severe non-missile traumatic brain injury: a computational analysis of magnetic resonance images. J Neurotrauma. 2005;22:76–82. doi: 10.1089/neu.2005.22.76. [DOI] [PubMed] [Google Scholar]
- Xu J, Rasmussen IA, Lagopoulos J, Haberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J Neurotrauma. 2007;24:753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- Yount R, Raschke KA, Biru M, Tate DF, Miller MJ, Abildskov T, Gandhi P, Ryser D, Hopkins RO, Bigler ED. Traumatic brain injury and atrophy of the cingulate gyrus. J Neuropsychiatry Clin Neurosci. 2002;14:416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol. 2006;27:2000–2004. [PMC free article] [PubMed] [Google Scholar]


