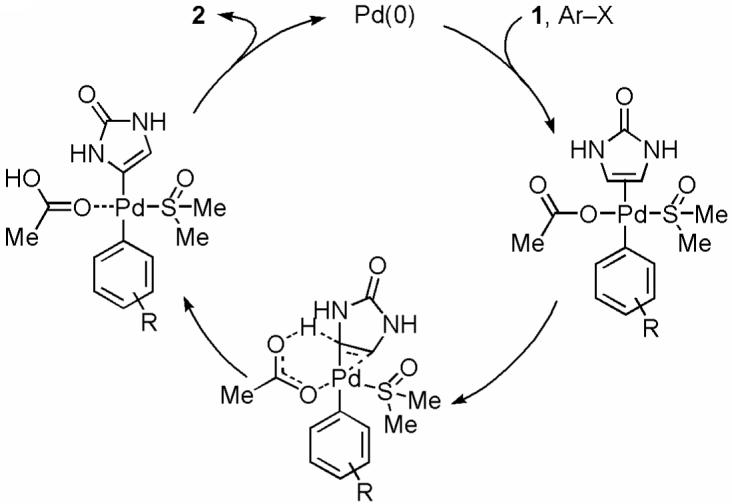
Abstract
The direct C-H functionalization of imidazolinone is achieved with Pd(OAc)2/NaOAc in DMSO. Dibromophakellstatin can be synthesized in 5 steps with 40% overall yield using this new C-H activation method.
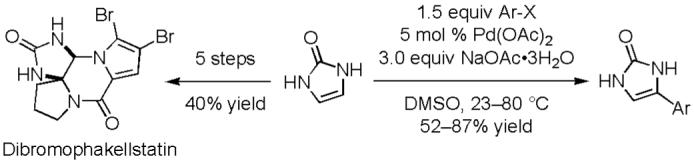
The popularity of transition metal-catalyzed C-H activation chemistry has grown rapidly in the recent years.1 Both direct2 and functional group-directed3 C-H insertion reactions have been developed to form a C-X or a C-C bond using transition metal catalysts. This reaction is particularly useful in heterocycle derivatization and several protocols have been reported.4 Despite its potentials, the application to natural product synthesis is rare.5 The limitation is partly due to the relatively harsh reaction conditions and limited functional group compatibility. We describe herein a C-H functionalization reaction of imidazolinone (1) under mild conditions (eq 1). We also demonstrate its synthetic utility with dibromophakellstatin synthesis.6,7
 |
(1) |
Our study started with the coupling of 1 and iodobenzene. We systematically studied the effects of palladium source, ligand, additive and solvent.8 The reaction proceeds most efficiently with 5 mol % Pd(OAc)2 and 3 equiv NaOAc·3H2O in DMSO at 80 °C (Table 1, entry 1). A small amount of water (<5% v/v) can be tolerated. The reaction can also be carried out with 2 mol % Pd(OAc)2 (entry 2), 20 mol % Pd/C (entry 3), or at room temperature (entry 4).
Table 1.
Scope of the direct arylation of imidazolinonea
| entry | Ar | X | catalyst loading | time | yield |
|---|---|---|---|---|---|
| 1 | Ph | I | 5 mol % | 6 h | 78% |
| 2 | Ph | I | 2 mol % | 30 h | 71% |
| 3 | Ph | I | 20 mol % Pd/C | 16 h | 63% |
| 4 | Ph | I | 10 mol % at 23 °C | 5 d | 68% |
| 5 | 2-Me-Ph | I | 5 mol % | 6 h | 75% |
| 6 | 4-F-Ph | I | 5 mol % | 12 h | 62% |
| 7 | 4-Cl-Ph | I | 5 mol % | 12 h | 61% |
| 8 | 4-Br-Ph | I | 5 mol % | 12 h | 52% |
| 9 | 3-MeO-Ph | I | 5 mol % | 6 h | 77% |
| 10 | 4-MeO-Ph | I | 5 mol % | 6 h | 85% |
| 11 | 2-HOCH2-Ph | I | 5 mol % | 6 h | 62% |
| 12 | 3-HOCH2-Ph | I | 5 mol % | 6 h | 87% |
| 13 | 4-HO-Ph | I | 5 mol % | 6 h | 85% |
| 14 | 2-NO2-Ph | Br | 10 mol % | 24 h | 68% |
| 15 | 3-MeCO-Ph | Br | 10 mol % | 36 h | 62% |
| 16 | 4-CF3-Ph | Br | 10 mol % | 36 h | 64% |
| 17 | Ph-CH=CHBr | 10 mol % | 36 h | 68% |
Conditions: 1.5 equiv Ar-X, cat. Pd(OAc)2, 3.0 equiv NaOAc·3H2O, degassed DMSO, 80 °C.
The scope of this palladium-catalyzed imidazolinone C-arylation reaction is shown in Table 1. Substitution at the 2-position of aryl iodide does not affect the reaction (entry 5). Both electron-deficient (entry 6-8) and electron-rich (entry 9-13) aryl iodides can be used. However, electron-deficient aryl iodides are less reactive. Extending the reaction time leads to a small amount of bis-arylation product. Notably, hydroxyl and phenol groups are tolerated (entry 11-13). Aryl bromides are also less reactive; however, good results can be obtained with electronic-deficient aryl bromides (entry 14-16).8 Finally, the direct vinylation of 1 can also be achieved (entry 17).
We have carried out a series of mechanistic studies and found the C-H insertion pathway most consistent with the experimental data. If the reaction proceeds through the Heck-type mechanism, the migratory insertion intermediate 3 would bear no syn-β-H for the subsequent β-hydride elimination (Scheme 1).
Scheme 1.
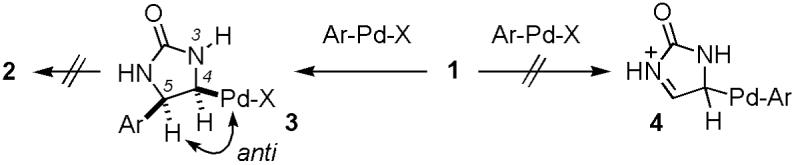
Although a β-H is present at the N-3 position in 3, the reaction does not proceed through N-H β-hydride elimination, as 5 also reacts (Scheme 2). We next carried out the linear free-energy relationship (LFER) analysis to test if the reaction proceeds through the anti-β-H elimination pathway.9 The anti-β-H elimination is believed to be promoted by base via an E2 or E1cb mechanism and a non-negative ρ value is expected with 3. However, we have obtained a strongly negative ρ value (ρ = -1.1) in the LFER analysis.8
Scheme 2.
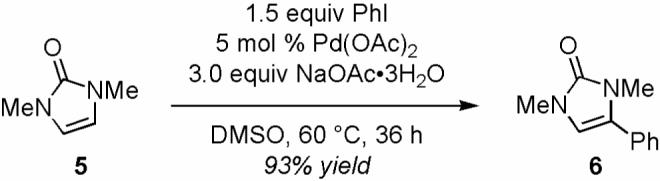
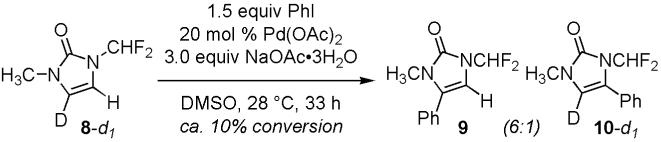
We then considered the possibility of Pd(0)-mediated SN2-inversion10 of the C-4 stereogenic center in 3 followed by syn-β-hydride elimination to give 2. While a secondary kinetic isotope effect (KIE) is expected for this sterically encumbered SN2-inversion, we have observed a primary KIE (kH/kD = 4.5) in the competing experiment of 1 with 4,5-dideuterated 1 (94% D) (Scheme 3).
Scheme 3.
The Busacca-Farina mechanism, which involves an α-H elimination of 3 followed by a 1,2-H shift,11 would be consistent with the experimental primary KIE; however, we did not observe significant amounts of H/D shift with 8-d1 (91% D) (Scheme 4).12 The absence of crossover products further suggests that H-5 in 3 does not undergo a Wacker-type depalladative 1,2-H shift (C5→C4)13 to give 2 after losing a proton.
Scheme 4.
We have also ruled out the possibility of electrophilic palladation mechanism.14 A secondary,4f instead of the observed primary KIE (Scheme 3) with 1 is expected for the electrophilic pathway. The regioselectivity of 8 (Scheme 4) is also opposite to that of the electrophilic reactions. Only the C-H insertion mechanism (Scheme 5) is consistent with all the experimental data. Our DFT calculations (B3LYP/LACVP**++)15 support the acetate ligand-assisted C-H insertion pathway.
Scheme 5.
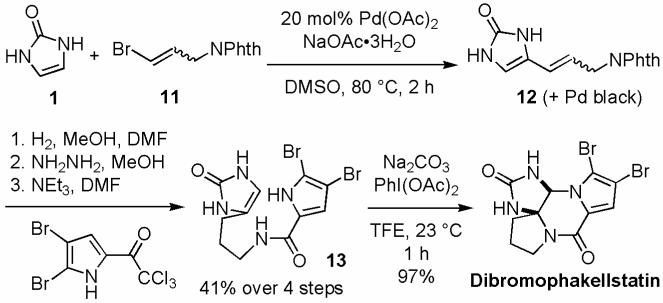
The synthetic utility of this catalytic C-H activation reaction is illustrated with the synthesis of dibromophakellstatin (Scheme 6). The direct coupling of 1 and vinyl bromide 11 proceeded smoothly. Imidazolinone 12, together with the resultant palladium black, was subjected to hydrogenation, phthalimide deprotection, and acyl pyrrole installation to give 13.8 While the bromine oxidants were reported to promote the biomimetic oxidative cyclization7a,f of 13 with only modest efficiency, we have found that oxidation of 13 with PhI(OAc)2 gave dibromophakellstatin in nearly quantitative yield.
Scheme 6.
In summary, we have developed a palladium catalyst system that allows the direct arylation and vinylation of imidazolinone under mild conditions. This method is proved useful in natural product synthesis. Dibromophakellstatin was synthesized with significantly improved efficiency (40% overall yield).
Supplementary Material
Acknowledgment
Financial supports are provided by NIH (NIGMS R01-GM079554), Welch Foundation (I-1596) and UT Southwestern. C.C. is a Southwestern Medical Foundation Scholar in Biomedical Research.
References
- (1).For reviews on transition metal-catalyzed C-H functionalization:Dick AR, Sanford MS. Tetrahedron. 2006;62:2439–2463.Yu J-Q, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041–4047. doi: 10.1039/b611094k.Daugulis O, Zaitsev VG, Shabashov D, Pham Q-N, Lazareva A. Synlett. 2006:3382–3388.Goj LA, Gunnoe TB. Curr. Org. Chem. 2005;9:671–685.Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731–1769. doi: 10.1021/cr0104330.Dyker G. Angew. Chem. Int. Ed. 1999;38:1698–1712. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6.
- (2).(a) Takemiya A, Hartwig JF. J. Am. Chem. Soc. 2006;128:10480–10481. doi: 10.1021/ja058299e. [DOI] [PubMed] [Google Scholar]; (b) Murphy JM, Lawrence JD, Kawamura K, Incarvito C, Hartwig JF. J. Am. Chem. Soc. 2006;128:13684–13685. doi: 10.1021/ja064092p. [DOI] [PubMed] [Google Scholar]; (c) Paul S, Chotana GA, Holmes D, Reichle RC, Maleczka RE, Jr., Smith MR., III J. Am. Chem. Soc. 2006;128:15552–15553. doi: 10.1021/ja0631652. [DOI] [PubMed] [Google Scholar]; (d) Shi Z, He C. J. Am. Chem. Soc. 2004;126:13596–13597. doi: 10.1021/ja046890q. [DOI] [PubMed] [Google Scholar]
- (3).(a) Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; (b) Delcamp JH, White MC. J. Am. Chem. Soc. 2006;128:15076–15077. doi: 10.1021/ja066563d. [DOI] [PubMed] [Google Scholar]; (c) Huang Q, Fazio A, Dai G, Campo MA, Larock RC. J. Am. Chem. Soc. 2004;126:7460–7461. doi: 10.1021/ja047980y. [DOI] [PubMed] [Google Scholar]; (d) Hennessy EJ, Buchwald SL. J. Am. Chem. Soc. 2003;125:12084–12085. doi: 10.1021/ja037546g. [DOI] [PubMed] [Google Scholar]
- (4).For examples:Deprez NR, Kalyani D, Krause A, Sanford MS. J. Am. Chem. Soc. 2006;128:4972–4973. doi: 10.1021/ja060809x.Chen X, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:12634–12635. doi: 10.1021/ja0646747.Beck EM, Grimster NP, Hatley R, Gaunt MJ. J. Am. Chem. Soc. 2006;128:2528–2529. doi: 10.1021/ja058141u.Bressy C, Alberico D, Lautens M. J. Am. Chem. Soc. 2005;127:13148–13149. doi: 10.1021/ja054472v.Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f.Lane BS, Brown MA, Sames D. J. Am. Chem. Soc. 2005;127:8050–8057. doi: 10.1021/ja043273t.Rieth RD, Mankad NP, Calimano E, Sadighi JP. Org. Lett. 2004;6:3981–3983. doi: 10.1021/ol048367m.Wiedemann SH, Lewis JC, Ellman JA, Bergman RG. J. Am. Chem. Soc. 2006;128:2452–2462. doi: 10.1021/ja0576684.Chotana GA, Rak MA, Milton R, Smith I. J. Am. Chem. Soc. 2005;127:10539–10544. doi: 10.1021/ja0428309.
- (5).For examples:Bowie J, A. L, Hughes CC, Trauner D. Org. Lett. 2005;7:5207–5209. doi: 10.1021/ol052033v.Baran PS, Richter JM, Lin DW. Angew. Chem. Int. Ed. 2005;44:609–612. doi: 10.1002/anie.200462048.Garg NK, Caspi DD, Stoltz BM. J. Am. Chem. Soc. 2004;126:9552–9553. doi: 10.1021/ja046695b.Hinman A, Du Bois J. J. Am. Chem. Soc. 2003;125:11510–11511. doi: 10.1021/ja0368305.For the use of stoichiometric amounts of Pd, see:Johnson JA, Li N, Sames D. J. Am. Chem. Soc. 2002;124:6900–6903. doi: 10.1021/ja026130k.García-Granados A, López PE, Melguizo E, Parra A, Simeó Y. J. Org. Chem. 2007;72:3500–3509. doi: 10.1021/jo070116e.
- (6).Pettit GR, McNulty J, Herald DL, Doubek DL, Chapuis J-C, Schmidt JM, Tackett LP, Boyd MR. J. Nat. Prod. 1997;60:180–183. doi: 10.1021/np9606106. [DOI] [PubMed] [Google Scholar]
- (7).For previous synthesis:Wiese KJ, Yakushijin K, Horne DA. Tetrahedron Lett. 2002;43:5135–5136.Poullennec KG, Romo D. J. Am. Chem. Soc. 2003;125:6344–6345. doi: 10.1021/ja034575i.Chung R, Yu E, Incarvito CD, Austin DJ. Org. Lett. 2004;6:3881–3884. doi: 10.1021/ol0490532.Feldman KS, Skoumbourdis AP. Org. Lett. 2005;7:929–931. doi: 10.1021/ol0500113.Jacquot DEN, Zöllinger M, Lindel T. Angew. Chem. Int. Ed. 2005;44:2295–2298. doi: 10.1002/anie.200462252.For synthesis of the related alkaloid dibromophakellin:Foley LH, Büchi G. J. Am. Chem. Soc. 1982;104:1776–1777.
- (8).See Supporting Information for further discussions.
- (9).(a) Lautens M, Fang Y-Q. Org. Lett. 2003;5:3679–3682. doi: 10.1021/ol035354k. [DOI] [PubMed] [Google Scholar]; (b) Maeda K, Farrington EJ, Galardon E, John BD, Brown JM. Adv. Synth. Catal. 2002;344:104–109. [Google Scholar]; (c) Takacs JM, Lawson EC, Clement F. J. Am. Chem. Soc. 1997;119:5956–5957. [Google Scholar]
- (10).The SN2 inversion of a Pd-bound sp3 carbon stereogenic center by Pd(0) is rare and sensitive to steric factors:Lau KSY, Wong PK, Stille JK. J. Am. Chem. Soc. 1976;98:5832–5840.On the other hand, inversion of a π-allyl palladium complex is more common:Trost BM, Verhoeven TR. J. Am. Chem. Soc. 1976;98:630–632. doi: 10.1021/ja00418a063.
- (11).(a) Farina V, Hossain MA. Tetrahedron Lett. 1996;37:6997–7000. [Google Scholar]; (b) Busacca CA, Swestock J, Johnson RE, Bailey TR, Musza L, Rodger CA. J. Org. Chem. 1994;59:7553–7556. [Google Scholar]; (c) Holtcamp MW, Henling LM, Day MW, Labinger JA, Bercaw JE. Inorg. Chim. Acta. 1998;270:467–478. [Google Scholar]; (d) Schrock RR, Seidel SW, Mösch-Zanetti NC, Shih K-Y, O’Donoghue MB, Davis WM, Reiff WM. J. Am. Chem. Soc. 1997;119:11876–11893. [Google Scholar]
- (12).The formation of bis-arylation products with extended reaction time also indicates that the α-H elimination is not operating, as there is no H alpha to the Pd in the second arylation step.
- (13).(a) Cornell CN, Sigman MS. J. Am. Chem. Soc. 2005;127:2796–2797. doi: 10.1021/ja043203m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smidt J. Chem. Ind. (London) 1962:54–61. [Google Scholar]
- (14).(a) Dupont J, Consorti CS, Spencer J. Chem. Rev. 2005;105:2527–2571. doi: 10.1021/cr030681r. [DOI] [PubMed] [Google Scholar]; (b) Ryabov AD. Chem. Rev. 1990;90:403–424. [Google Scholar]
- (15).(a) Hay PJ, Wadt WR. J. Chem. Phys. 1985;82:299. [Google Scholar]; (b) Keith JA, Oxgaard J, Goddard WA., III J. Am. Chem. Soc. 2006;128:3132–3133. doi: 10.1021/ja0533139. [DOI] [PubMed] [Google Scholar]
- (16).(a) Lafrance M, Fagnou K. J. Am. Chem. Soc. 2006;128:16496–16497. doi: 10.1021/ja067144j. [DOI] [PubMed] [Google Scholar]; (b) Lafrance M, Rowley CN, Woo TK, Fagnou K. J. Am. Chem. Soc. 2006;128:8754–8756. doi: 10.1021/ja062509l. [DOI] [PubMed] [Google Scholar]; (c) García-Cuadrado D, Braga AAC, Maseras F, Echavarren AM. J. Am. Chem. Soc. 2006;128:1066–1067. doi: 10.1021/ja056165v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.