Abstract
Purpose
Serum levels of the inflammatory markers YKL-40 and IL-6 are increased in many conditions, including cancers. We examined serum YKL-40 and IL-6 levels in patients with Hodgkin lymphoma (HL), a tumor with strong immunologic reaction to relatively few tumor cells, especially in nodular sclerosis HL.
Experimental Design
We analyzed Danish and Swedish patients with incident HL (N=470) and population controls from Denmark (N= 245 for YKL-40; N= 348 for IL-6). Serum YKL-40 and IL-6 levels were determined by ELISA, and log-transformed data were analysed by linear regression, adjusting for age and sex.
Results
Serum levels of YKL-40 and IL-6 were increased in HL patients compared to controls (YKL-40: 3.6-fold, IL-6: 8.3-fold; both p<0.0001). In samples from pre-treatment HL patients (N=176), levels were correlated with more advanced stages (ptrend 0.0001 for YKL-40 and 0.013 for IL-6) and in those with B symptoms, but levels were similar in nodular sclerosis and mixed cellularity subtypes, by EBV status, and in younger (<45 years old) and older patients. Patients tested soon after treatment onset had significantly lower levels than pre-treatment patients, but even >6 months after treatment onset, serum YKL-40 and IL-6 levels remained significantly increased, compared to controls. In patients who died (N=12), pre-treatment levels for both YKL-40 and IL-6 were higher than in survivors, although not statistically significantly.
Conclusions
Serum YKL-40 and IL-6 levels were increased in untreated HL patients and those with more advanced stages but did not differ significantly by HL histology. Following treatment, serum levels were significantly lower.
Hodgkin lymphoma (HL) is a cancer with relatively few tumor cells in which the reactive response is thought to play an important role in the pathogenesis1. Although the Hodgkin/Reed Sternberg (HRS) cells appear to attract an immunological response, that response is apparently ineffective in controlling the tumor2. However, the higher risk of HL and changes in the distribution of HL subtypes in persons with immunosuppression indicate that immunity does play a role in disease incidence and histology3. It would be of interest if circulating biomarkers of immunity correlated with either this tumor or its subtypes. YKL-40 (also called chitinase 3-like-1 protein) is one such potential biomarker, but its value in HL has not been described. A member of mammalian chitinase-like protein family, YKL-40 is a lectin that binds heparin, chitin and collagen and is produced by many cell types, including leucocytes and macrophages. It appears to be important in host defense mechanisms4-6 and serum YKL-40 levels are increased in patients with diverse illnesses, including cancer. High levels have been reported in patients with primary or metastatic carcinomas, glioblastoma, melanoma, acute myeloid leukemia and multiple myeloma and may predict recurrence and short survival4. High serum YKL-40 levels are also found in patients with diseases characterised by inflammation, tissue remodelling and fibrosis5,6.
Although its biological function in cancer is unknown, YKL-40 could play a role in proliferation and differentiation of tumor cells, angiogenesis, cell adhesion, and metastatic potential. YKL-40 could also be a marker of tissue destruction or remodelling resulting from the tumor or the vascular and immunologic reactions involved in these processes4-6. YKL-40 expression may be regulated by interleukin 6 (IL-6) (Johansen, personal observation), a cytokine produced by a variety of cells, including tumor cells, macrophages, and lymphocytes. IL-6 plays a dominant role in the immune system and the acute phase response7, and production is also increased in HRS cells1,8-10, the neoplastic cell in HL. We therefore examined YKL-40 and IL-6 levels in patients with HL, hypothesizing that levels of these two markers might correlate with the immune reaction to the malignant cells, as manifest by histology, stage or prognosis.
Subjects and Methods
The HL subjects were enrolled in a population-based case-control study in Sweden and Denmark. Details of the study design have been previously reported11,12. Briefly, the lymphoma study enrolled HL patients aged 18−74 years diagnosed from January 1999 to mid-200212. Patients with a history of organ transplantation, HIV infection, or other hematopoietic malignancies were excluded. Overall, 91% of eligible HL patients consented to participate in the study. In the present analysis, sera obtained from 470 (76%) of 618 HL cases were included. Stage, available on 83% of cases, was coded as Ann Arbor stages 1 through 4. Subjects had HL confirmed by direct pathology review (90%) or reports of histology classified using the International Classification of Disease-O-3 codes13. Follow-up through national population registries was truncated at death, emigration or January 2005 (Swedes) or June 2005 (Danes), whichever came first. At enrollment, participants were asked for a blood sample, obtained pre-treatment when possible. Fresh samples were sent by overnight mail for next day processing at central laboratories in Sweden and Denmark and serum was stored at −80° C. Small amounts of YKL-40 and IL-6 are reported to be released into serum, probably from degranulation of neutrophils, beginning as soon as 3 hours after venipuncture14,15. However, the amounts released would have had little impact on the high serum levels we observed in HL patients in this study.
Serum concentrations of YKL-40 were determined by a two-site, sandwich-type enzyme-linked immunosorbent assay (ELISA)16 in accordance with the manufacturer's instructions (Quidel, CA, USA). The sensitivity of the ELISA for serum YKL-40 was 20 ng/mL, and the intra- and inter-assay coefficients of variation were ≤5.0% and ≤10.2%, respectively14. Serum concentrations of IL-6 were measured by ELISA (catalogue number HS600, R&D Systems, Abingdon, Oxon, UK) in accordance with the manufacturer's instructions. The sensitivity of the ELISA for serum IL-6 was 0.01 pg/mL and the intra- and inter-assay coefficients of variation for IL-6 were ≤10.5% and ≤17.7%, respectively15. For reference, serum YKL-40 and serum IL-6 were determined in apparently healthy Danish adult volunteers (blood donors and residents of retirement homes) from 18 to 79 years who were not on medication, as previously described14,15. Measurements of serum YKL-40 and IL-6 concentrations in HL patients were done in the same laboratory using the same assay as for the normal sera panel.
We used linear regression on the log-transformed concentration measurements to determine the associations of various factors with serum YKL-40 and IL-6 concentrations. We modelled deviation from the expected concentration level in age- and sex-matched healthy persons, obtained by linear regression of age and sex on the log-transformed concentration measurements in the normal sera samples. For YKL-40 and IL-6 associations with survival, estimated by Cox regression analysis, the entry was at the date of blood sampling. Confidence intervals and two-sided significance tests were based on Wald tests.
Results
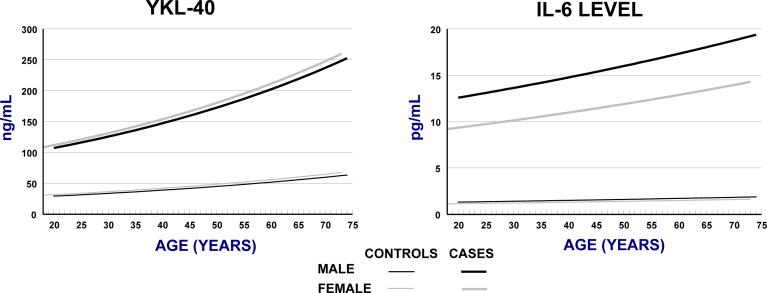
Levels in healthy individuals (N=245 for YKL-40, N=348 for IL-6) are presented in Figure 1. We used serum levels estimated in 35-year-old women as the reference value for statistical comparisons, 35 years being the median age of the HL cases. For YKL-40, this baseline serum level was 39 ng/mL. Serum YKL-40 levels increased by a factor of 1.15 per decade of age (95% CI: 1.12−1.19; ptrend=<0.0001), and the age-adjusted median level was lower in males than females by a factor of 0.91 (95% CI: 0.82−1.04). For serum IL-6, the baseline level was 1.3 pg/mL, and levels increased by a factor of 1.07 per decade of age (95% CI: 1.00−1.15; ptrend= 0.07). After age-adjustment, serum IL-6 levels in males were higher than females by a factor of 1.15 (95% CI: 0.98−1.34).
Figure 1.
Modelled levels of YKL-40 and IL-6 in cases and controls, by sex.
The characteristics of the 470 HL patients in the present study are provided in Table 1. Males and females were equally represented and the bimodal age distribution was typical of HL, as was the predominance (69%) of nodular sclerosis (NS) HL. For 388 patients, stage was known. For 98 patients, treatment status at the time of blood collection was not certain. The age, sex and histology distributions did not differ between cases with and without known treatment status, but YKL-40 and IL-6 levels were similar to those in treated patients, suggesting the majority of those without treatment information had been treated (data not presented).
Table 1.
Characteristics of 470 Hodgkin lymphoma cases diagnosed between 1999 and 2002 and examined for serum YKL-40 and IL-6.
| Stratum | Treatment status | ||||
|---|---|---|---|---|---|
| All | Pre-onset | After onset | Unknown | ||
| N | N | N | N | ||
| Total | 470 (100%) | 176 (100%) | 196 (100%) | 98 (100%) | |
| Country of residence | |||||
| Sweden | 288 (61%) | 121 (69%) | 109 (56%) | 58 (59%) | |
| Denmark | 182 (39%) | 55 (31%) | 87 (44%) | 40 (41%) | |
| Sex | |||||
| Male | 245 (48%) | 102 (48%) | 94 (48%) | 49 (50%) | |
| Female | 225 (52%) | 74 (52%) | 102 (52%) | 49 (50%) | |
| Age in years, median (25%−75%) | 35 (27−54) | 35 (27−54) | 35 (26−54) | 38 (29−54) | |
| Hodgkin lymphoma histology | |||||
| Nodular sclerosis | 326 (69%) | 116 (66%) | 148 (75%) | 62 (63%) | |
| Mixed cellularity | 86 (18%) | 37 (21%) | 31 (16%) | 18 (18%) | |
| Other/undefined | 58 (12%) | 23 (13%) | 17 (9%) | 18 (18%) | |
| Hodgkin lymphoma stage (Ann Arbor) | |||||
| 1 | 74 (16%) | 32 (18%) | 34 (17%) | 8 (8%) | |
| 2 | 187 (40%) | 82 (47%) | 74 (38%) | 31 (32%) | |
| 3 | 84 (18%) | 34 (19%) | 40 (20%) | 10 (10%) | |
| 4 | 43 (9%) | 18 (10%) | 16 (8%) | 9 (9%) | |
| Missing | 82 (18%) | 10 (6%) | 32 (16%) | 40 (41%) | |
Among 176 HL patients with pre-treatment samples, age- and sex-adjusted serum levels of both YKL-40 and IL-6 were significantly higher than in healthy persons at all ages (overall relative values: YKL-40: 3.6, 95% CI: 3.1−4.2, and IL-6: 8.3; 5.94−11.7; Figure 1). Relative serum levels of YKL-40 and IL-6 were not significantly different in 67 younger (<45 year old) vs. 129 older HL patients (p=0.86 and p=0.30, respectively) and the two groups were therefore combined for analysis. YKL-40 and IL-6 were moderately correlated (r=0.31; p<0.0001) with each other. After adjustment for age and sex, median serum levels of YKL-40 levels were 3.7-fold higher in patients with NS HL (95% CI: 3.1−4.5,) and 3.1-fold higher in patients with mixed cellularity (MC) HL (95% CI: 1.9−5.1), compared to the normal subjects. Similarly, serum IL-6 levels were increased 9.0-fold in NS HL (95% CI: 6.3−12.9) and 8.4-fold for MC HL (95% CI: 3.3−21.6). Other specified HL subtypes occurred too infrequently to allow robust comparisons. Patients with MC HL were older and more likely to be male than NS HL patients. After adjusting for the age and sex difference, serum YKL-40 and IL-6 levels did not differ significantly between NS and MC HL in pre-treatment samples (p=0-86 and p=0.37, respectively).
Pre-treatment serum levels were higher in patients with more advanced disease stages (Ptrend YKL-40 = 0.0001 and IL-6 = 0.013; Table 2). Data about B symptoms were available only for Danish patients. Of 55 subjects with pre-treatment samples and data, 18 (33%) had B symptoms. Relative serum YKL-40 levels were higher in patients with B symptoms (1.8; 95% CI: 1.3−2.3) than those without B symptoms. IL-6 levels were also higher in patients with B symptoms than those without B symptoms, although the difference was not statistically significant (1.3; 95% CI: 0.5−3.0). The 38 patients with Epstein-Barr virus-positive tumors had marginally lower serum YKL-40 levels (median relative level: 0.8; 95% CI: 0.6−1.0) but marginally higher serum IL-6 levels (1.3; 95% CI: 0.7−2.2) than 125 patients with EBV-negative tumors, after adjustment for age and sex.
Table 2.
Age- and sex-adjusted relative levels of serum YKL-40 and IL-6 in healthy subjects (N=245 for YKL-40 and N=348 for IL-6) and patients with pre-treatment (N=176) and treated (N=29) Hodgkin lymphoma. For reference, relative levels were compared to serum levels estimated for healthy 35 years old women: 39 ng/mL for YKL-40 and 1.3 pg/mL for IL-6.
| YKL-40 | IL-6 | |
|---|---|---|
| Healthy controls | ||
| Increase per decade | 1.15 (1.12−1.19) | 1.07 (1.00−1.15) |
| Males compared to females | 0.92 (0.82−1.04) | 1.15 (0.98−1.34) |
| Relative serum levels in pre-treatment patients with Hodgkin lymphoma compared to healthy controls* | ||
| Overall HL | 3.63 (3.10−4.24) | 8.32 (5.94−11.7) |
| Histology | ||
| Nodular sclerosis | 3.73 (3.13−4.46) | 9.00 (6.26−12.9) |
| Mixed cellularity | 3.15 (1.93−5.14) | 8.42 (3.28−21.6) |
| Other/undefined | 3.40 (2.32−4.98) | 6.06 (1.91−19.2) |
| Ann Arbor Stage | ||
| 1 | 3.42 (2.35−4.98) | 8.28 (2.60−26.4) |
| 2 | 3.28 (2.74−3.92) | 5.95 (3.91−9.06) |
| 3 | 4.23 (2.89−6.20) | 14.97 (7.28−30.8) |
| 4 | 4.20 (2.24−7.90) | 20.42 (6.58−63.4) |
| ptrend <0.0001 | ptrend = 0.013 | |
| Serum level ≥6 months after treatment onset compared to pre-treatment level** | ||
| Overall HL | 70% (53%−91%) | 47% (26%−83%) |
| Nodular sclerosis | 69% (50%−95%) | 30% (15%−58%) |
| Mixed cellularity | 79% (44%−141%) | 98% (33%−291%) |
| Other/undefined | 52% (24%−113%) | 112% (11%−1152%) |
Relative to age- and sex-adjusted levels in healthy controls.
Difference relative to median serum levels in untreated patients, after age- and sex-adjustment. For example, in HL patients, median levels for YKL-40 were 70% of those seen in pre-treatment patients, after age- and sex-adjustment.
In 196 samples obtained after treatment onset, serum levels of YKL-40 and IL-6 were also modestly correlated (r=0.27; p=0.001). The timing of the sample collection varied among the 196 patients with serum obtained after therapy had started. Serum levels of YKL-40 were lower in treated patients, even when the treatment had only been recently started, being 61%, 56%, and 70% of the pre-treatment levels at 0−2 (N=98), 3−5 (N=21) and ≥6 months (N=29) after therapy onset, respectively. Correspondingly, IL-6 levels were 66%, 42%, and 47% of pre-treatment levels at 0−2, 3−5 and ≥6 months, respectively (Table 2). Overall, serum levels ≥6 months after treatment onset were lower than in pre-treatment samples (p=0.007 for YKL-40 and p<0.01 for IL-6), but they remained 2.5 - fold (95% CI: 1.8−3.5, p<0.0001) higher for YKL-40 and 2.2-fold (95% CI: 1.0−5.1, p=0.06) higher for IL-6 than levels in healthy subjects.
Twelve HL patients with pre-treatment samples had died by the end of follow-up in 2005, including 11 with NS HL. Although both serum YKL-40 and IL-6 levels were higher in NS HL patients who died than survivors with NS HL, the differences were not statistically significant. The hazard ratios for death were 1.58 (95% CI: 0.75−3.33) for serum YKL-40 and 1.12 (0.75−1.66) for serum IL-6, per log unit change. Twelve additional subjects with samples collected after treatment onset died, and, in them, serum levels of YKL-40 and IL-6, while higher than in survivors, were also not statistically significant predictors of prognosis.
Discussion
Compared to healthy subjects, serum levels of YKL-40 and IL-6 were high in pre-treatment samples of patients with both NS and MC HL. In patients with HL, pre-treatment serum levels of YKL-40 and IL-6 were correlated directly with both disease stage and presence of B symptoms (YKL-40 only) but similarly increased in patients who were younger (<45 years) versus older at onset, had NS and MC HL subtypes, or by tumor EBV status. In treated patients, serum YKL-40 and IL-6 levels were significantly lower than in pre-treatment patients, but both levels still remained significantly higher than in healthy controls, even ≥6 months after therapy onset. Although levels were not significantly correlated with prognosis, we had limited power to evaluate prognosis since few people died during the follow-up period of this study.
The pathology and clinical symptoms of HL involve a complex interplay of chemokines and cytokines derived from both the HRS cells and the surrounding reactive cells1. This reactivity appears to involve the immune system2,3. Only a small percentage of the tumor mass in HL is composed of neoplastic cells, and the majority of the mass is created by the immunologic reaction surrounding the tumor cells2. The higher serum levels of YKL-40 might be mediated by tumor cells expressing IL-6 (known to be produced excessively by HRS and the surrounding reactive cells1,8-10), by the immunologic reaction induced by these cells2,13, or by tissue destruction and remodeling related to the tumor mass. A reasonably intact immune system appears to be essential for the manifestation of the sclerosing reaction characteristic of NS HL, since it is not seen in persons who are profoundly immunosuppressed3. In contrast, patients with MC HL may have less immunological reaction to their tumors. Since YKL-40 and IL-6 levels were similar in NS and MC HL, after adjustment for age and sex, we speculate that the differences in the immune responses to HRS cells are not driving YKL-40 or IL-6 production. Rather, cytokine signals from the malignant cells or tissue damage from the tumor mass (including the immunologic reaction to it) may be more important triggers. However, IL-6 levels were found to be increased in the unaffected identical twin mates of persons with HL, leading those authors to suggest that IL-6 response may be a marker of susceptibility to HL10.
Compared to pre-treatment samples, average serum levels of YKL-40 and especially IL-6 were lower in patients whose samples were obtained within weeks after therapy onset, possibly reflecting a rapid response to therapy. However, even ≥6 months after therapy onset, serum levels of YKL-40 and IL-6 both remained higher than in healthy persons. In treated patients, most samples would have been collected while they were still on therapy, and thus the declines may have been due either to clearing of tumor tissue or to therapy itself, such as by its effect on immunocytes or other cells. Consistent with studies in other cancer types4,5,7, serum YKL-40 and IL-6 levels in pre-treatment samples correlated with disease burden, as measured by stage, and were higher in individuals who later died than in survivors. However, few of these patients died during follow-up, and we cannot be confident about the relationship between level and survival. In addition, we were not able to identify whether deaths were related to therapy, disease progression or other reasons.
In summary, we found that serum levels of YKL-40 and IL-6 are higher in pre-treatment HL cases than healthy controls and in patients with more advanced stages of HL, but they were similar in NS and MC HL. Further work will be needed to determine whether these proteins act as direct mediators or surrogate markers of the disease process in HL.
Acknowledgement of research support
The study was supported by grants from “Direktør Jens Aage Sørensen og Hustru Edith Ingeborg Sørensens Mindefond”, the Nordic Cancer Union (16-02-D) and the National Institutes of Health (USA)(S-RO1-CA 69269). The YKL-40 ELISA kits were provided by Quidel Corporation (San Diego, CA). Quidel had no role in: 1) the design of the study, 2) the data collection, analysis and interpretation, 3) the preparation of the manuscript, and 4) no rights to approve, delay, or disapprove of publication of the work. Tonni Løve Hansen, Debbie Nadelmann, and Teresa Rozenfeld, Herlev and Hvidovre Hospitals are thanked for the measurement of YKL-40 and IL-6.
Footnotes
The authors have no conflict of interest in any aspect of this manuscript. The results are our work, have not previously been presented, and are not under consideration elsewhere. All authors have reviewed the manuscript and concur in its presentation.
Statement of Clinical Relevance:
Biological markers of cancer may help to reveal tumor pathogenesis and can serve to better define extent of disease and prognosis. Specifically, host immune responses contribute directly to disease manifestations of Hodgkin lymphoma, including both tumor mass and B symptoms. Therefore, markers related to immune response can be expected to be correlated with presence of this disease. High serum levels of both serum YKL-40 and IL-6 are non-specific markers of inflammation and both were found to be greatly increased in pre-treatment samples from HL cases, being 4-fold and 8-fold higher than in healthy age- and sex-matched controls, respectively. Both markers were associated with B symptoms and stage, but levels did not correlate with HL histology or EBV status. Prognosis could not be well evaluated because too few cases died during the follow-up period but likely will also be correlated with levels of these markers. Although HL can usually be successfully managed, levels of these markers continued to be increased at least 6 months after therapy was initiated. These and other markers should be explored for understanding the origins of B symptoms and to determine if hyper-reactivity, as represented by high levels, acts as mediators or surrogate markers in HL.
References
- 1.Ma Y, van den Berg A, Atayar C, Visser L, Poppema S. Cytokines, cytokine receptors, and chemokines in Hodgkin lymphoma. In: Hoppe RT, Mauch PT, Armitage JO, Diehl V, Weiss LM, editors. Hodgkin Lymphoma. Second Edition Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 87–98. [Google Scholar]
- 2.Poppema S. Dysregulated immune response in Hodgkin lymphoma. In: Hoppe RT, Mauch PT, Armitage JO, Diehl V, Weiss LM, editors. Hodgkin Lymphoma. Second Edition Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 99–107. [Google Scholar]
- 3.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–91. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price P. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 5.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 6.Dickey BF. Exoskeletons and exhalation. New Engl J Med. 2007;357:2082–3. doi: 10.1056/NEJMe0706634. [DOI] [PubMed] [Google Scholar]
- 7.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–65. [PMC free article] [PubMed] [Google Scholar]
- 8.Poppema S, van den Berg A. Interaction between host T cells and Reed-Sternberg cells in Hodgkin lymphomas. Semin Cancer Biol. 2000;10:345–50. doi: 10.1006/scbi.2000.0327. [DOI] [PubMed] [Google Scholar]
- 9.Aldinucci D, Lorenzon D, Olivo K, Rapanà B, Gattei V. Interactions between tissue fibroblasts in lymph nodes and Hodgkin/Reed-Sternberg cells. Leuk Lymphoma. 2004;45:1731–9. doi: 10.1080/10428190410001683633. [DOI] [PubMed] [Google Scholar]
- 10.Cozen W, Gill PS, Ingles SA, et al. IL-6 levels and genotype are associated with risk of young adult Hodgkin lymphoma. Blood. 2004;103:3216–21. doi: 10.1182/blood-2003-08-2860. [DOI] [PubMed] [Google Scholar]
- 11.Smedby KE, Hjalgrim H, Melbye M, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97:199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 12.Hjalgrim H, Smedby KE, Rostgaard K, Molin D, Hamilton-Dutoit S, Chang ET, Ralfkiaer E, Sundström C, Adami HO, Glimelius B, Melbye M. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 2007;67:2382–8. doi: 10.1158/0008-5472.CAN-06-3566. [DOI] [PubMed] [Google Scholar]
- 13.Stein H, Delsol G, Pileri S, et al. Hodgkin lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2001. pp. 237–53. [Google Scholar]
- 14.Høgdall EVS, Johansen JS, Kjaer SK, et al. Stability of YKL-40 concentration in blood samples. Scand J Clin Lab Invest. 2000;60:247–52. doi: 10.1080/00365510050184886. [DOI] [PubMed] [Google Scholar]
- 15.Knudsen LS, Christensen IJ, Lottenburger T, et al. Pre-analytical and biological variability in circulating IL-6 in healthy subjects and patients with rheumatoid arthritis. Biomarkers. 2008;13:59–78. doi: 10.1080/13547500701615017. [DOI] [PubMed] [Google Scholar]
- 16.Harvey S, Weisman M, O'Dell J, et al. Chondrex: new marker of joint disease. Clin Chem. 1998;44:509–16. [PubMed] [Google Scholar]