Abstract
Live attenuated virus vaccines have shown the greatest potential to protect against simian immunodeficiency virus (SIV) infection, a model for human immunodeficiency virus (HIV). Immunity against the vaccine virus is thought to mediate protection. However, it is shown computationally that the opposite might be true. According to the model, the initial growth of the challenge strain, its peak load, and its potential to be pathogenic is higher if immunity against the vaccine virus is stronger. This is because the initial growth of the challenge strain is mainly determined by virus competition rather than immune suppression. The stronger the immunity against the vaccine strain, the weaker its competitive ability relative to the challenge strain, and the lower the level of protection. If the vaccine virus does protect the host against a challenge, it is because the competitive interactions between the viruses inhibit the initial growth of the challenge strain. According to these arguments, an inverse correlation between the level of attenuation and the level of protection is expected, and this has indeed been observed in experimental data.
Keywords: HIV, vaccine, live attenuated, protection, theoretical biology, dynamics, mathematical model
1. Introduction
Containment of the human immunodeficiency virus (HIV) epidemic would require an effective vaccine that blocks infection or significantly delays the rate of progression to AIDS (Duerr, Wasserheit, and Corey, 2006; Klausner et al., 2003; Letvin, 1998). While experimental vaccines have generally shown disappointing results, live attenuated virus vaccines have shown the best levels of protection in monkeys infected with simian immunodeficiency virus (SIV) (Almond et al., 1995; Daniel et al., 1992; Koff et al., 2006), although safety issues remain to be resolved (Hofmann-Lehmann et al., 2003; Ruprecht, 1999; Whitney and Ruprecht, 2004). While such attenuated viruses are replication impaired, they still establish a persistent infection in the patient, which carries a long-term risk of re-gaining pathogenic potential (Letvin, 1998; Ruprecht et al., 1996). At the same time, it is this persistence that is thought to promote protection in vaccinated animals. Even if the infection itself is not prevented, infection of vaccinated individuals can lead to relatively low virus loads and a slower rate of disease progression (Koff et al., 2006). The mechanisms underlying this protection are, however, unclear (Johnson and Desrosiers, 1998; Koff et al., 2006; Stahl-Hennig et al., 2007; Wyand et al., 1999). The attenuated virus is thought to induce immune responses that can down-modulate early viral replication upon infection with a challenge strain (Gauduin et al., 1998; Metzner et al., 2000). However, the level of immune responses has not been found to correlate with the level of protection observed (Marthas et al., 1992; Stahl-Hennig et al., 2007). In addition, other puzzling findings have been reported: for example that the level of protection correlates inversely with the level of vaccine attenuation (Johnson et al., 1999). Using an established mathematical model of virus dynamics (Nowak and Bangham, 1996; Perelson, 2002), it is shown that the extent of initial growth of the challenge virus is not limited by immunity, but predominantly by competition between the challenge and the vaccine virus for target cells. Counter-intuitively, it can be shown that stronger immunity against the vaccine virus results in a faster initial growth rate of the challenge strain, which is likely to correlate with a reduced probability of protection. These findings shed new light onto how live attenuated SIV/HIV vaccines work, and they can also reconcile some of the experimental data that have remained unexplained so far.
2. Results and Discussion
2.1. The basic model
A mathematical model is constructed that describes infection with a live attenuated virus and a challenge strain. Denote susceptible cells by x, cells infected by the vaccine virus by y1, cells infected by the challenge virus by y2, and a specific immune response by z. The infection dynamics model is given by the following set of differential equations, which are based on well-established mathematical models of virus dynamics (Nowak and Bangham, 1996; Perelson, 2002):
Susceptible cells are produced with a rate λ, die with a rate d, and become infected by the vaccine or challenge strain with a rate β1 and β2, respectively. Cells infected with the vaccine and the challenge strain die with a rate a1 and a2, respectively. The free virus is assumed to be in a quasi steady state, proportional to the number of infected cells. Therefore, the parameters β1 and β2 summarize not only the rate of infection, but the overall replication rate of the vaccine and the challenge virus. This includes the rate at which infected cells produce viruses that are transmitted to new cells. For details, see (Nowak and May, 2000; Wodarz, 2007). The challenge virus is assumed to replicate faster than the vaccine virus (β2>β1). Specific immune responses against the virus rise with a rate that can potentially be proportional to virus load and the number of immune cells. This can be described by a variety of mathematical functions, F(y1,y2,z). For simplicity, assume F(y1,y2,z)=(c1y1+c2y2)z, as described in (Nowak and Bangham, 1996), although more complex immune proliferation terms (De Boer and Perelson, 1998) do not alter basic results (see Methods). Such immune responses could be either T cell responses or B cell responses that directly and specifically respond to and attack the virus. For the analysis, complete immunological cross-reactivity between the virus strains is assumed, i.e. p1=p2 (this can be relaxed, see below). Assumptions and robustness are further described in Methods.
In this model, the vaccine virus can establish a persistent infection if β1λ/(da1) > 1, that is its basic reproductive ratio (Nowak and May, 2000; Wodarz, 2007) needs to be greater than one. The immune response against the virus becomes established if c(λ/a1−d/β1) > b, which is the invasion condition for the immune response z from low numbers. In this case, the infection will eventually equilibrate around the following levels: x(1) = λc1 / (dc1 + β1b), y1(1)=b/c1, y2(1)=0, z(1)=(β1x(1)−a1)/p. These are all basic properties of virus dynamics equations that have been worked out in the past (Nowak and Bangham, 1996; Perelson, 2002). So far, this type of mathematical framework has only been applied to live attenuated virus vaccines in one other study with a very different focus, in the context of post-exposure vaccination (Bonhoeffer and Nowak, 1995). Also, mathematical modeling has been used to examine the competition dynamics between wild-type and replication-impaired HIV strains (Altes and Jansen, 2000; Bagnoli, Lio, and Sguanci, 2006; Ball, Gilchrist, and Coombs, 2007; Smith and Wahl, 2005), again with a different focus in mind.
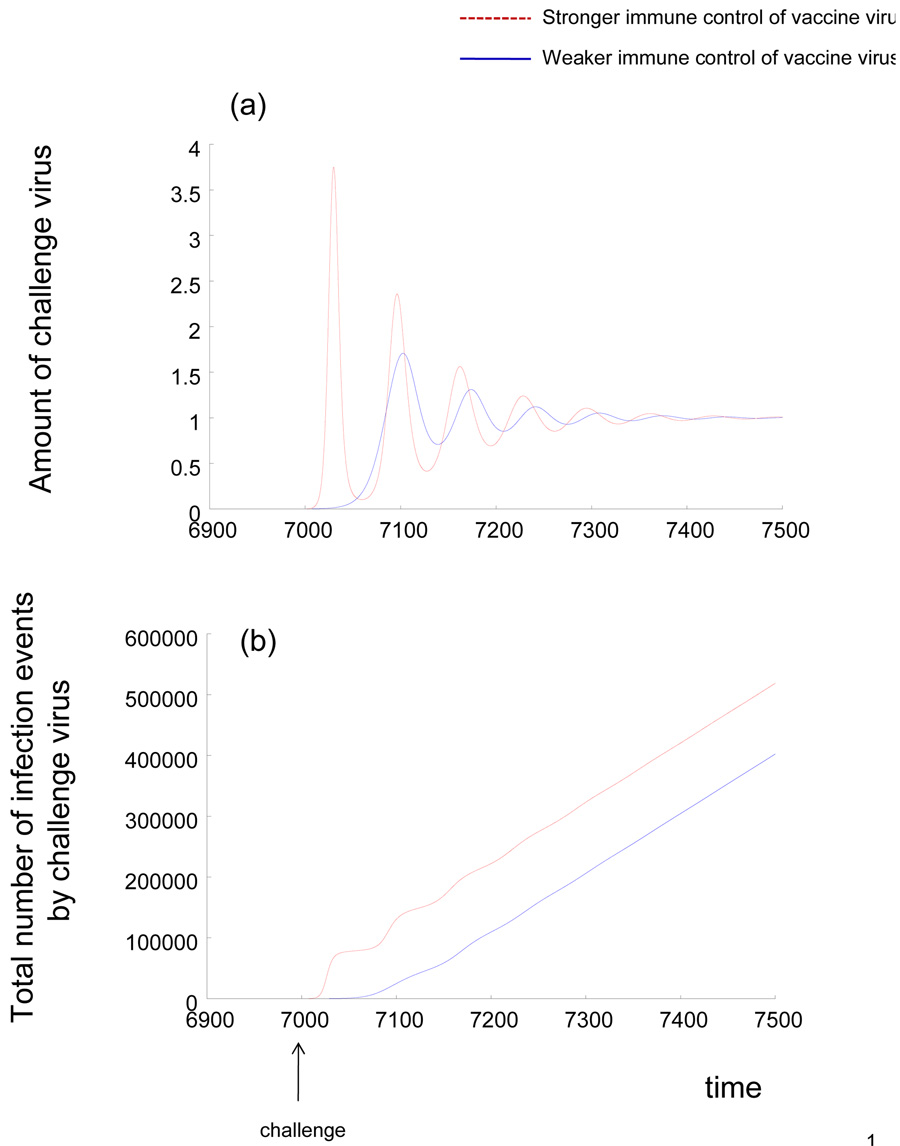
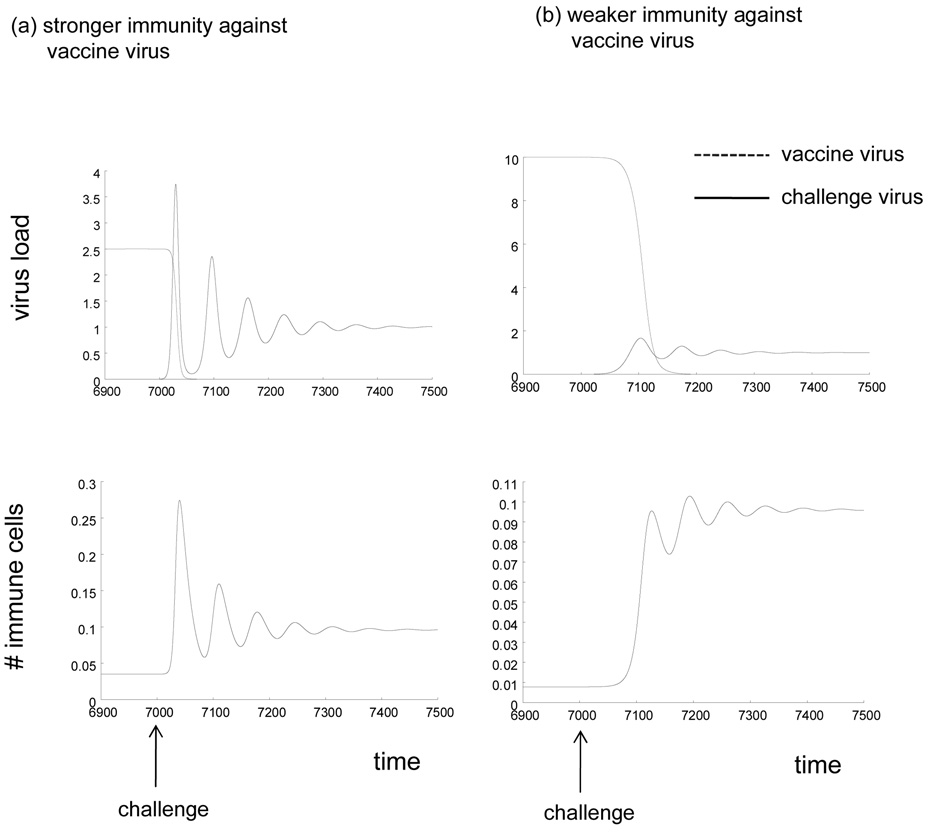
The initial growth rate of the challenge virus is given by r = x(1)(β2−β1) − a2 + a1, where x(1) denotes the equilibrium number of uninfected cells in the presence of the vaccine virus, as defined above. The challenge virus grows if r > 0. In this case, the following properties are observed. A higher number of uninfected cells in the presence of the vaccine virus, x(1), correlates with a faster initial growth rate of the challenge virus. Because x(1) = λc1 / (dc1 + β1b), a stronger immune response against the vaccine virus (higher value of c1) leads to a higher number of uninfected cells, and thus to a faster initial growth rate and a higher peak load of the challenge virus (Figure 1a, 3a). Because faster virus replication leads to a faster accumulation of mutations by the virus, stronger immunity against the vaccine virus leads to a higher average number of mutants generated by the challenge virus during its initial growth (Figure 1b). Therefore, the stronger the established immunity, the more likely it is that the challenge virus can escape or impair immunity during the initial growth, leading to reduced protection. This is because virus competition rather than immunity drives the initial growth rate of the challenge strain. The number of uninfected target cells left by the vaccine virus is the important determinant. This is also reflected by the fact that a faster replication rate of the vaccine strain slows down the growth rate of the challenge strain. Of course, if the virus fails to escape or impair immunity during the initial growth, then the previously established immunity contains virus load at relatively low levels in the long term, as shown by the convergence to equilibrium in Figure 1a.
Figure 1.
Dynamics of HIV/SIV challenge in a host infected with a live attenuated virus vaccine, according to the basic model. Stronger and weaker immune control of the vaccine virus is compared. (a) Kinetics of virus growth. Stronger immunity against the vaccine virus leads to faster initial virus growth and a higher peak virus load of the challenge strain. (b) Total number of infection events undergone by the challenge virus as a function of time, which correlates with the total number of mutants generated by the challenge strain (because mutation occurs during reverse transcription, which in turn occurs upon infection). The plot basically integrates over β2xy2. Stronger immunity against the vaccine virus leads to more infection events and thus to a higher chance that a mutant is generated that escapes the immune responses.
If an escape mutant is generated, virus load will rise to higher values (not shown here). If, on the other hand, no escape mutant arises during the initial growth phase, the virus will eventually converge to an equilibrium that describes immune control, as shown in part (a).
Parameters were chosen as follows. λ=0.1, d=0.001, a=0.002, β1=0.005, β2=0.05, b=0.05, p=1. For strong immune control of the vaccine virus, c1=0.02. For weak immunity against the vaccine virus, c1=0.005. The immune responsiveness against the challenge strain is assumed to be c2=0.05. The units of the axes are arbitrary and parameter values have been chosen for illustrative purposes, as most parameter values are unknown.
Figure 3.
The initial growth rate of the challenge strain (r, defined in text) as a function of the immune responsiveness c1 against the vaccine virus (i.e. the strength of specific immune response against the vaccine virus). This is done for three scenarios. (a) The basic model. A higher immune responsiveness correlates with a higher growth rate of the challenge strain. (b) Coinfection model. As the immune responsiveness is increased, the initial growth rate of the challenge virus first declines and then rises again. (c) Model which assumes that HIV activates its own target cells. At first, an increase in the immune responsiveness leads to a higher initial growth rate of the challenge virus, but for strong immunity, a further increase in the responsiveness leads to a decline in the growth rate of the challenge virus. This is because lower vaccine virus loads in this parameter region maintain a lower number of activated target cells. Parameters were chosen as follows. For panels (a) and (b): λ=0.1, d=0.001, a=0.002, β1=0.005, β2=0.05, b=0.05, p=1. For panel (c): η=1, ξ=1, f=0.01, r=0.5, d=0.1, β=1, a=0.1, p=1, b=0.1. The units of the axes are arbitrary and parameter values have been chosen for illustrative purposes, as most parameter values are unknown.
Figure 2 presents in more detail the dynamics of the vaccine and challenge strain, as well as the immune response. The figure plots the dynamics both for a strong and a weak immune response against the vaccine virus. Before the challenge, virus load and the immune response are at equilibrium, as described by the expressions x(1), y1(1), and z(1) above. Upon challenge, the new virus replicates to a peak and eventually converges to a steady state. At the same time, the vaccine virus declines to extinction in the model. This is because the two viruses compete for the same target cell pool, and the faster replicating challenge strain is superior and excludes the slower replicating vaccine strain. In vivo, it is possible that while the vaccine strain declines it does not go extinct or goes extinct more slowly because of reservoirs in which viruses can survive for prolonged periods of time, such as latently infected cells. These are not included in the model for simplicity. The immune response follows the dynamics of the challenge virus. It starts from the equilibrium level at which it controls the vaccine virus. It then expands and converges towards a new equilibrium. In the simulation shown in Figure 2, the number of immune cells at this new equilibrium is higher than before, because of the particular choice of parameters. That is, the immune response was assumed to react faster against the challenge strain, for example because attenuation might cause lack of immunogenicity. However, for other parameter values, the number of immune cells after infection with the challenge virus can also be lower than observed with the vaccine virus, for example because of increased immune-impairment caused by the challenge virus. Overall conclusions do not depend on these details. Other aspects of robustness will be discussed further below. The equilibrium, to which the dynamics converge after infection with the challenge strain is given by the following expressions: x(2) = λc2 / (dc2 + β2b), y1(2)=b/c2, y2(1)=0, z(2)=(β2x(2)−a2)/p. These patterns are observed both for weak and for strong immunity against the vaccine strain (Figure 2).
Figure 2.
Time series that documents that dynamics of the two viruses and the immune response upon challenge. The simulations are based on the basic model described in the text. The number of immune cells corresponds to specific immune effector cell populations that fight the virus, such as B cells or T cell. Simulations are shown for (a) stronger immunity against the vaccine virus (higher value of c1) and weaker immunity against the vaccine virus (lower value of c1). Parameters were chosen as follows: λ=0.1, d=0.001, a=0.002, β1=0.005, β2=0.05, b=0.05, p=1. For strong immune control of the vaccine virus, c1=0.02. For weak immunity against the vaccine virus, c1=0.005. The immune responsiveness against the challenge strain is assumed to be c2=0.05. The units of the axes are arbitrary and parameter values have been chosen for illustrative purposes, as most parameter values are unknown.
In the context of the simple model analyzed here, the behavior described above is observed over a wide parameter space, and it can be argued that it applies to the entire parameter space that is biologically realistic. The analysis so far was performed with the assumption that p1=p2 (the vaccine and challenge viruses are removed by immune responses with the same rate). Under this assumption, the arguments hold if β2>β1, i.e. the replication rate of the challenge strain β2 is faster than the replication rate of the vaccine strain β1. This is likely to be true, since the vaccine virus is attenuated while the challenge virus is not. In the more general case where the two viruses can be removed with different rates, the same arguments hold if p2/p1> β2 /β1. In words, the increase in the rate of immune attack of the challenge strain needs to be higher than the increase in the replication rate of the challenge strain relative to the vaccine virus. If this is the case, then stronger immunity leads to slower growth of the challenge strain and therefore to increased protection. For example, if the challenge strain replicates twice as fast as the vaccine virus, then the challenge strain needs to be removed by immune responses more than twice as fast as the vaccine virus for the results described here to break down. This might be a biologically unrealistic assumption in the light of data that report sub-optimal immune-mediated effector responses against HIV/SIV, for example in the context of CD8 T cell mediated killing (Asquith et al., 2006).
In summary, the results described here give rise to a tradeoff: The weaker the degree of vaccine attenuation (including immunological control), the higher the chances that the challenge virus will be controlled successfully, because the competitive ability of the vaccine strain is increased relative to that of the challenge strain. Such an inverse relationship between vaccine attenuation and protection has been reported in experimental data (Johnson et al., 1999). Other experimental data also support the theoretical notion that virus competition might be an important determinant of protection and that immune responses against the vaccine strain might not contribute significantly to the outcome of a challenge. For example, it has been found that CD8 T cell depletion before and upon challenge did not lower the degree of protection observed with a live attenuated vaccine (Stebbings et al., 2005; Stebbings et al., 1998; Whitney and Ruprecht, 2004), indicating that this branch of the immune system does not determine the level of protection observed. Similarly, protection by live attenuated SIV has been observed in the absence of neutralizing antibodies (Stebbings et al., 2004). In a recent study, the level of antibody or CD8 T cell responses did not correlate with the outcome of a challenge following vaccination with a live attenuated SIV strain (Mansfield et al., 2008). Several monkeys that were well protected did not show significant levels of adaptive immune responses. On the other hand, several animals that were characterized by the presence of a substantial amount of immunity against the vaccine strain were not protected well against a challenge. This is consistent with earlier work that also did not find a correlation between the level of immune responses and the degree of protection observed (Stahl-Hennig et al., 2007). If the established immune responses were protecting against challenges, then larger immune cell populations would be more protective. On the other hand, if virus competition drives the challenge dynamics, then no obvious correlation between the level of immunity and the level of protection is expected. Instead, the level of protection should correlate with the number of target cells available for infection by the challenge virus, which is determined by a variety of host and viral parameters (Nowak and Bangham, 1996; Nowak and May, 2000).
While such data leave open many questions about the determinants of challenge outcome after vaccination with a live attenuated virus, the theory presented here provides a first step to interpreting these data. At the moment it is not possible to further evaluate and validate the model against data, since this will require difficult new experiments, which directly tests the competition dynamics between the vaccine and the challenge virus in the context of protection. This is very hard to achieve with in vivo experiments because one has to rule out differences in immunity. The immune cell depletion experiments discussed above (Stebbings et al., 2005) are consistent with the model so far, but more extensive studies have to be performed for further validation, including more data points that document the growth of the challenge virus upon infection. However, the notion that virus competition and not immunity might be an important correlate of protection can help us think in a different way about the complex experimental results regarding the relationship between immunity and protection. At the same time, it raises questions about the feasibility of live attenuated HIV vaccines for clinical use. Making the vaccine virus more competitive by having too little attenuation raises serious safety concerns (Hofmann-Lehmann et al., 2003; Ruprecht, 1999; Whitney and Ruprecht, 2004). A higher vaccine virus load might directly lead to pathogenicity (Ruprecht et al., 1996), or it might enable the virus to acquire mutations that confer a heightened degree of virulence. This poses limitations for designing an optimal live attenuated vaccine against HIV.
2.2. Further considerations
The last section considered a basic model of virus infection in vivo and obtained insights about the dynamics of live attenuated virus vaccines and subsequent challenges. This section introduces more biological complexity into the model to examine how those insights hold up and under what circumstances the above obtained insights are altered. In particular, two features of HIV infection will be considered: (i) the ability of multiple HIV particles to infect the same target cell, and (ii) the fact that target cells for HIV are immune helper cells that themselves can be induced to divide by antigenic stimulation.
2.2.1. Coinfection
Consider multiple infection of target cells first, also known as coinfection (Chen et al., 2005; Dang et al., 2004; Jung et al., 2002; Levy et al., 2004). Importantly in this context, the challenge virus can infect not only uninfected cells, but also wild-type infected cells. A mathematical model that examines the basic kinetics of coinfection has been examined by (Dixit and Perelson, 2005), and a model that studies the competition between two HIV strains in the presence of coinfection has been studied by (Wodarz and Levy, 2007). Here, a very simple modeling approach is taken in order to investigate the impact of coinfection on the results described here. Coinfection will be considered in the context of two virus strains. The rate of spread of the challenge strain will be determined, assuming that the vaccine virus and immune cell populations have equilibrated. In the presence of coinfection, the equations for the growth of the challenge strain can be written as dy2/dt = β2y2(x+y1) − a2y2 − p2y2z. Instead of just replicating in uninfected target cells, x, the challenge virus can also replicate in vaccine virus infected cells, y1. Only the short term dynamics that describe the initial growth of the challenge strain will be described. The long term dynamics will not be considered, and so the effect of the vaccine virus replicating in cells infected by the challenge strain does not need to be included. Basically, coinfection changes the exact long-term competition dynamics. In the absence of coinfection, the faster replicating challenge strain outcompetes the slower vaccine strain. In the presence of coinfection, it is possible for the slower replicating vaccine virus to persist at relatively low levels rather than to go extinct. For a full analysis of how coinfection influences the outcome of competition, the reader is referred to (Wodarz and Levy, 2007). The initial growth rate of the challenge virus in the context of coinfection is proportional to the sum of uninfected and infected cells, x+y1. This shows the following dependence on the strength of the immune response against the vaccine virus (Figure 3b). As the strength of the immune response is increased, x+y1 can first decline to a minimum and can then rise again. If the immune response is weak (low value of c1), the number of uninfected cells is low, but the number of infected cells is higher. This leads to relatively fast challenge virus spread through coinfection (Figure 3b). If the immune response is relatively strong (high value of c1), many uninfected cells are available while the number of infected cells is low. Again, this leads to relatively fast challenge virus spread. For an intermediate strength of the immune system, the overall number of target cells is lowest and the spread of the challenge virus is slowest. Therefore, in this case, weakening immunity does not lead monotonically to better protection. Very weak immunity leads to high vaccine virus loads, and thus overall to more cells available for infection. Note, however, that this is not a universal pattern and only holds if the rate of virus-induced cell killing a1 lies below a threshold relative to the viral replication rate, β1, i.e. if a1 < (λβ1)1/2 (Wodarz and Krakauer, 2000). If this is not the case, i.e. if the virus kills its cells with a relatively fast rate, then reducing immunity monotonically leads to a reduction in the overall number of target cells, and the results remain qualitatively the same as in the simpler model without coinfection. Further note, that the dependence on the strength of the immune response described here does not translate into a dependence on the degree of attenuation in general. A higher vaccine virus attenuation through a reduction in the viral replication rate always leads monotonically to faster challenge virus growth and thus to less efficient protection, as in the context of the simple model. Finally, whether coinfection indeed turns out to be an important driving force in the dynamics of HIV infection remains to be determined by further experimental work, and the modeling effort presented here has to be interpreted with this in mind.
2.2.2. Infection of HIV-specific immune cells
Now a model extension is considered that is very relevant to HIV: the assumption that the target cells of the virus are immune cells that can themselves expand in response to antigenic stimulation. These are the CD4 T helper cells. In particular it has been shown that HIV might not infect all helper cells equally, but that it could preferentially infect HIV-specific T helper cells (Douek et al., 2002). These processes can be captured by models in a variety of ways. Here, a model described by (Wodarz et al., 1999) will be considered. Denote the population of resting T helper cells by s, the population of activated T helper cells by x, the population of infected helper cells by y, and an effector immune response by z. The model given by the following set of differential equations.
The model distinguishes between resting and activated T helper cells. It is further assumed that cell activation is stimulated by HIV itself. This is in accord with data that demonstrated preferential infection of HIV-specific T cells by the virus (Douek et al., 2002). However, the population x could also include other T cells that are activated by HIV through bystander effects. A more complete model would include a population of alternative target cells that do not require activation for infection, for example macrophages or dendritic cells. Such a more complete model is analyzed in (Wodarz et al., 1999). For the current purpose, a simplification is considered and it is assumed that in addition to the T cell infection described in the model, an influx of infected cells occurs with a rate ηi, arising from virus replication in those alternative target cells. This simplified model has the same basic properties as the more extensive model described in (Wodarz et al., 1999).
Thus, resting helper cells are produced with a rate ξ, die with a rate f, and become activated by antigen with a rate r. Activated T cells die with a rate d, and become infected by the different virus strains with a rate β1 and β2, respectively. Infected cells are further produced by replication in alternative target cells with a rate η1 and η2, respectively. Infected cells die with a rate a1 and a2 respectively. The immune response expands upon stimulation by the vaccine virus with a rate c1 and by the challenge virus with a rate c2. The immune response decays in the absence of antigen with a rate b. For simplicity, coinfection is not included in this model.
Parameter regions are considered in which the dynamics between the vaccine virus and the immune responses converge towards a stable equilibrium in which both populations persist. As in the simple model, the growth rate of the challenge strain is proportional to the number of uninfected susceptible target cells at the time of the challenge, when the dynamics between the vaccine virus and the immune response have equilibrated. In the current model, these are the cells which become activated through stimulation by HIV, x. In this case, the dependency of the number of activated cells on the strength of immune-mediated vaccine virus control is as follows (Figure 3c). If the degree of immune-mediated control is increased, the number of susceptible target cells, and thus the growth rate of the challenge virus, rise at first as in the simple model. Hence, stronger immunity again correlates with reduced protection. For stronger immune effector responses, however, a higher degree of immune-mediated vaccine virus control leads to a decline in the number of susceptible target cells and thus in the growth rate of the challenge virus (Figure 3c). Therefore, in this parameter region stronger immunity correlates with a higher degree of protection. The reason for this one-humped relationship is as follows. On the one hand, the virus population reduces the number of susceptible target cells. Hence, stronger immunity and lower vaccine virus load leads to less infection, which in turn leads to a higher number of target cells. On the other hand, the existence of susceptible target cells depends on cell activation and thus on stimulation by the virus. As vaccine virus control becomes very efficient, not enough virus is around to maintain the population of susceptible target cells at high levels, leading to a decline in this population. A one-humped relationship between virus load and the level of CD4 T helper cell responses has actually been observed in the context of SIV infected macaques that received anti-retroviral therapy during the acute phase of the infection (Wodarz et al., 2000). Animals treated 24 hours after infection showed an unusually high degree of virus control by HIV-specific immune responses. They were also protected both against homologous and heterologous re-challenges as long as one year after the initial infection (Lifson et al., 2003; Lifson et al., 2000). Re-challenge resulted in a blunted peak load of the challenge virus, followed by down-regulation to undetectable levels. On the other hand, animals that were treated 72 hours after infection showed much less efficient virus control and higher virus loads (Lifson et al., 2000). Among animals that controlled the virus less efficiently, a higher degree of immunological control and lower virus loads correlated with a higher number of HIV-specific CD4 T cells. According the model this is because more immune control leads to less T cell infection, which in turn leads to a higher number of T cells. Among the animals that controlled the virus very efficiently, however, the opposite correlation was observed: stronger immunological control and lower virus loads correlated with a lower number of HIV-specific helper T cells. According to the model, the reason is that the very low virus loads did not provide sufficient stimulation to maintain the specific helper T cell population at higher levels (Wodarz et al., 2000). These data (Wodarz et al., 2000), however, indicate that this pattern is only observed in the context of highly efficient virus suppression, which is not typically observed. These arguments can reconcile an apparent contradiction: While it has been found that the degree of vaccine attenuation is inversely correlated with the level of protection (Johnson et al., 1999), strong immune-mediated virus suppression resulted in very efficient protection against re-infection in the context of the monkeys that were treated 24 hours after infection and that showed exceptional degrees of virus control (Lifson et al., 2003; Lifson et al., 2000). In both cases, virus competition determines the degree of protection; the treatment 24 hours after infection pushed the animals into the unusual parameter space where not enough stimulation remained to maintain higher numbers of target cells, thus reducing the relative replication rate of the challenge virus.
Conclusions
In conclusion, the model analyzed here challenges some common lines of thought regarding the mechanisms underlying live attenuated HIV/SIV vaccines. According to the model, it is not the immunity established against the vaccine virus that can protect the host against a challenge. In fact, stronger immunity can lead to worse protection. Instead, if the vaccine virus protects the host against a challenge, it is because competitive interactions among the viruses inhibit the initial spread of the challenge virus. However, the degree of immunity does not always correlate negatively with the amount of protection in the model, depending on the exact assumptions of the model. If the challenge virus can productively infect cells that are already infected with the vaccine strain, then the growth rate of the challenge strain can go up at low levels of immunity against the vaccine strain, thus leading to reduced protection. In this case, slowest growth of the challenge virus, and thus the best protection, is observed for an intermediate strength of the immune response against the vaccine virus. If it is assumed that HIV predominantly infects cells that are activated by itself, then a strong degree of immunity can lead to a reduced growth rate of the challenge strain and thus to increased protection. This is because lower virus load, brought about by stronger immunity, can only maintain a few activated target cells, thus reducing the replicative potential of the challenge virus. However, data indicate that this parameter region only applies to cases of exceptional virus control, as has been observed in SIV infected monkeys treated 24 hours after infection. Perhaps HIV infected patients that are long term non-progressors also fall into this category. Despite these deviations, however, the result remains that virus competition determines the degree of protection. Even in the case where strong immunity leads to higher degrees of protection, this occurs because of a reduction in the number of target cells.
This notion should ideally be tested by experiments that can separate the effects of competition and immune-mediated protection, especially because the strength of the immune response determines the level at which the vaccine virus persists and how many uninfected cells remain available. Perhaps the degree of protection conferred by different vaccine viruses could be investigated in the context of long-term immune suppression in more detail, based on the experiments by (Stebbings et al., 2005). If the results described here are true, then life attenuated viruses do not present a feasible clinical option, even if the potential problems arising from the presence of a persisting vaccine virus could be overcome. The amount of protection correlates with the danger of the vaccine strain to the patient. However, it is important to note that the story would be very different for a life vaccine virus that is not based in HIV, but that is given by a relatively harmless virus which can persist and carry the relevant HIV epitopes. It would be important that such a virus does not infect the same target cells as HIV, so that no virus competition occurs. In such a scenario, the anti-HIV immune response generated by the vaccine virus would be responsible for protection. The persistence of the virus could keep the immune response in an alert state and contribute to immediate anti-viral activity upon infection, although the downside would be to harbor a persistent virus in the long term.
Methods
The mathematical model that was analyzed is described in the main text, along with a discussion of dependence on parameters and robustness. Here, some additional details are provided that are important to keep in mind when interpreting the model predictions.
One of the problems with all models that take into account immune responses is the fact that it is not exactly known how the process of immune expansion should be described mathematically. In persistent infections, it seems clear that the division of immune cells is promoted by the presence of antigen. In the simplest form, the rate of immune expansion can be described by (c1y1+c2y2)z, and this has been used here for illustrative purposes. However, simulations have been run with alternative immune expansion terms that contain various saturation terms, and results remained robust. Examples are (c1y1+c2y2)z/(z+ε), (c1y1+c2y2)/(y1+y2+ε), or (c1y1+c2y2)/(z+y1+y2+ε). Such more-complex models with saturation tend to show more stable dynamics and are probably more realistic, but are more difficult to analyze.
In certain parameter regions, where immunity is very weak, the challenge virus grows monotonically towards a steady state, instead of first growing towards a peak. In this case, the arguments about peak virus load are obviously not valid. Such parameter regions are, however, not likely to be realistic because infection of a host with SIV/HIV tends to involve growth towards a peak.
The difference in the growth rate of the challenge virus in the presence of stronger and weaker immunity diminishes as the rate of target cell production, λ, becomes faster. However, during the initial growth phase of the challenge virus, it is not likely that much target cell production occurs. Therefore, this is not a realistic regime.
Finally, a note on the figures that are presented in this paper: The parameter values used to plot the graphs are chosen for illustrative purposes, since most parameter values of this system are uncertain. Therefore, the axes are given in arbitrary units. However, the parameter regions in which the illustrated patterns hold, and when they break down, are discussed extensively in the text.
Acknowledgements
This study was funded in part by NIH grant R01AI058153-01A2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott EJ. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345(8961):1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- Altes HK, Jansen VA. Intra-host competition between nef-defective escape mutants and wild-type human immunodeficiency virus type 1. Proc Biol Sci. 2000;267(1439):183–189. doi: 10.1098/rspb.2000.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith B, Edwards CT, Lipsitch M, McLean AR. Inefficient cytotoxic T lymphocyte-mediated killing of HIV-1-infected cells in vivo. PLoS Biol. 2006;4(4):e90. doi: 10.1371/journal.pbio.0040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnoli F, Lio P, Sguanci L. Modeling viral coevolution: HIV multiclonal persistence and competition dynamics. Physica A. 2006;366:333–346. [Google Scholar]
- Ball CL, Gilchrist MA, Coombs D. Modeling within-host evolution of HIV: mutation, competition and strain replacement. Bull Math Biol. 2007;69(7):2361–2385. doi: 10.1007/s11538-007-9223-z. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer S, Nowak MA. Can live attenuated virus work as post-exposure treatment? Immunol Today. 1995;16(3):131–135. doi: 10.1016/0167-5699(95)80129-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Dang Q, Unutmaz D, Pathak VK, Maldarelli F, Powell D, Hu WS. Mechanisms of nonrandom human immunodeficiency virus type 1 infection and double infection: preference in virus entry is important but is not the sole factor. J Virol. 2005;79(7):4140–4149. doi: 10.1128/JVI.79.7.4140-4149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Q, Chen J, Unutmaz D, Coffin JM, Pathak VK, Powell D, KewalRamani VN, Maldarelli F, Hu WS. Nonrandom HIV-1 infection and double infection via direct and cell-mediated pathways. Proc Natl Acad Sci U S A. 2004;101(2):632–637. doi: 10.1073/pnas.0307636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- De Boer RJ, Perelson AS. Target cell limited and immune control models of HIV infection: a comparison. J Theor Biol. 1998;190(3):201–214. doi: 10.1006/jtbi.1997.0548. [DOI] [PubMed] [Google Scholar]
- Dixit NM, Perelson AS. HIV dynamics with multiple infections of target cells. Proc Natl Acad Sci U S A. 2005;102(23):8198–8203. doi: 10.1073/pnas.0407498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Duerr A, Wasserheit JN, Corey L. HIV vaccines: new frontiers in vaccine development. Clin Infect Dis. 2006;43(4):500–511. doi: 10.1086/505979. [DOI] [PubMed] [Google Scholar]
- Gauduin MC, Glickman RL, Means R, Johnson RP. Inhibition of simian immunodeficiency virus (SIV) replication by CD8(+) T lymphocytes from macaques immunized with live attenuated SIV. J Virol. 1998;72(8):6315–6324. doi: 10.1128/jvi.72.8.6315-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Vlasak J, Williams AL, Chenine AL, McClure HM, Anderson DC, O'Neil S, Ruprecht RM. Live attenuated, nefdeleted SIV is pathogenic in most adult macaques after prolonged observation. Aids. 2003;17(2):157–166. doi: 10.1097/00002030-200301240-00004. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Desrosiers RC. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr Opin Immunol. 1998;10(4):436–443. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Lifson JD, Czajak SC, Cole KS, Manson KH, Glickman R, Yang J, Montefiori DC, Montelaro R, Wyand MS, Desrosiers RC. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73(6):4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A, Maier R, Vartanian JP, Bocharov G, Jung V, Fischer U, Meese E, Wain-Hobson S, Meyerhans A. Multiply infected spleen cells in HIV patients. Nature. 2002;418(6894):144. doi: 10.1038/418144a. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Fauci AS, Corey L, Nabel GJ, Gayle H, Berkley S, Haynes BF, Baltimore D, Collins C, Douglas RG, Esparza J, Francis DP, Ganguly NK, Gerberding JL, Johnston MI, Kazatchkine MD, McMichael AJ, Makgoba MW, Pantaleo G, Piot P, Shao Y, Tramont E, Varmus H, Wasserheit JN. Medicine. The need for a global HIV vaccine enterprise. Science. 2003;300(5628):2036–2039. doi: 10.1126/science.1086916. [DOI] [PubMed] [Google Scholar]
- Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7(1):19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- Letvin NL. Progress in the development of an HIV-1 vaccine. Science. 1998;280(5371):1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. Dynamics of HIV-1 recombination in its natural target cells. Proc Natl Acad Sci U S A. 2004;101(12):4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson JD, Piatak M, Jr, Cline AN, Rossio JL, Purcell J, Pandrea I, Bischofberger N, Blanchard J, Veazey RS. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J Med Primatol. 2003;32(4–5):201–210. doi: 10.1034/j.1600-0684.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Arnaout R, Li L, Parks TL, Schneider DM, Kiser RF, Coalter VJ, Walsh G, Imming R, Fischer B, Flynn BM, Nowak MA, Wodarz D. Containment of SIV infection: cellular immune responses and protection from rechallenge following transient post-inoculation antiretroviral treatment. J. Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K, Lang SM, Gauduin MC, Sanford HB, Lifson JD, Johnson RP, Desrosiers RC. Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery. J Virol. 2008;82(8):4135–4148. doi: 10.1128/JVI.00015-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthas ML, Miller CJ, Sutjipto S, Higgins J, Torten J, Lohman BL, Unger RE, Ramos RA, Kiyono H, McGhee JR, et al. Efficacy of live-attenuated and whole-inactivated simian immunodeficiency virus vaccines against vaginal challenge with virulent SIV. J Med Primatol. 1992;21(2–3):99–107. [PubMed] [Google Scholar]
- Metzner KJ, Jin X, Lee FV, Gettie A, Bauer DE, Di Mascio M, Perelson AS, Marx PA, Ho DD, Kostrikis LG, Connor RI. Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques mmunized with a live, attenuated simian immunodeficiency virus vaccine. J Exp Med. 2000;191(11):1921–1931. doi: 10.1084/jem.191.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Bangham CR. Population dynamics of immune responses to persistent viruses. Science. 1996;272(5258):74–79. doi: 10.1126/science.272.5258.74. [DOI] [PubMed] [Google Scholar]
- Nowak MA, May RM. Virus dynamics. Mathematical principles of immunology and virology. Oxford University Press; 2000. [Google Scholar]
- Perelson AS. Modelling viral and immune system dynamics. Nature Rev Immunol. 2002;2(1):28–36. doi: 10.1038/nri700. [DOI] [PubMed] [Google Scholar]
- Ruprecht RM. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol Rev. 1999;170:135–149. doi: 10.1111/j.1600-065x.1999.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Ruprecht RM, Baba TW, Li A, Ayehunie S, Hu Y, Liska V, Rasmussen R, Sharma PL. Live attenuated HIV as a vaccine for AIDS: pros and cons. Seminars in Virology. 1996;7:147–155. [Google Scholar]
- Smith RJ, Wahl LM. Drug resistance in an immunological model of HIV-1 infection with impulsive drug effects. Bull Math Biol. 2005;67(4):783–813. doi: 10.1016/j.bulm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C, Eisenblatter M, Franz M, Stoiber H, Tenner-Racz K, Suh YS, Jasny E, Falkensammer B, Ugucchioni M, Georgsson G, Baroni C, Dierich MP, Lifson JD, Steinman RM, Uberla K, Racz P, Ignatius R. A single vaccination with attenuated SIVmac 239 via the tonsillar route confers partial protection against challenge with SIVmac 251 at a distant mucosal site, the rectum. Front Biosci. 2007;12:2107–2123. doi: 10.2741/2215. [DOI] [PubMed] [Google Scholar]
- Stebbings R, Berry N, Stott J, Hull R, Walker B, Lines J, Elsley W, Brown S, Wade-Evans A, Davis G, Cowie J, Sethi M, Almond N. Vaccination with live attenuated simian immunodeficiency virus for 21 days protects against superinfection. Virology. 2004;330(1):249–260. doi: 10.1016/j.virol.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Stebbings R, Berry N, Waldmann H, Bird P, Hale G, Stott J, North D, Hull R, Hall J, Lines J, Brown S, D'Arcy N, Davis L, Elsley W, Edwards C, Ferguson D, Allen J, Almond N. CD8+ lymphocytes do not mediate protection against acute superinfection 20 days after vaccination with a live attenuated simian immunodeficiency virus. J Virol. 2005;79(19):12264–12272. doi: 10.1128/JVI.79.19.12264-12272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings R, Stott J, Almond N, Hull R, Lines J, Silvera P, Sangster R, Corcoran T, Rose J, Cobbold S, Gotch F, McMichael A, Walker B. Mechanisms of protection induced by attenuated simian immunodeficiency virus. II. Lymphocyte depletion does not abrogate protection. AIDS Res Hum Retroviruses. 1998;14(13):1187–1198. doi: 10.1089/aid.1998.14.1187. [DOI] [PubMed] [Google Scholar]
- Whitney JB, Ruprecht RM. Live attenuated HIV vaccines: pitfalls and prospects. Curr Opin Infect Dis. 2004;17(1):17–26. doi: 10.1097/00001432-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Wodarz D. Killer Cell Dynamics: mathematical and computational approaches to immunology. New York: Springer; 2007. [Google Scholar]
- Wodarz D, Arnaout RA, Nowak MA, Lifson JD. Transient antiretroviral treatment during acute SIV infection facilitates long-term control of the virus. Phil. Trans. Roy. Soc. Lond. B. 2000;355:1021–1029. doi: 10.1098/rstb.2000.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz D, Krakauer DC. Defining CTL-induced pathology: implications for HIV. Virology. 2000;274(1):94–104. doi: 10.1006/viro.2000.0399. [DOI] [PubMed] [Google Scholar]
- Wodarz D, Levy DN. Human immunodeficiency virus evolution towards reduced replicative fitness in vivo and the development of AIDS. Proc Biol Sci. 2007;274(1624):2481–2490. doi: 10.1098/rspb.2007.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz D, Lloyd AL, Jansen VAA, Nowak MA. Dynamics of macrophage and T cell infection by HIV. Journal of Theoretical Biology. 1999;196:101–113. doi: 10.1006/jtbi.1998.0816. [DOI] [PubMed] [Google Scholar]
- Wyand MS, Manson K, Montefiori DC, Lifson JD, Johnson RP, Desrosiers RC. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73(10):8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]